Abstract
Background
Shigella is a leading cause of childhood diarrhea and target for vaccine development. Microbiologic and clinical case definitions are needed for pediatric field vaccine efficacy trials.
Methods
We compared characteristics of moderate to severe diarrhea (MSD) cases in the Global Enteric Multicenter Study (GEMS) between children with culture positive Shigella to those with culture-negative, quantitative polymerase chain reaction (qPCR)-attributable Shigella (defined by an ipaH gene cycle threshold <27.9). Among Shigella MSD cases, we determined risk factors for death and derived a clinical severity score.
Results
Compared to culture-positive Shigella MSD cases (n = 745), culture-negative/qPCR-attributable Shigella cases (n = 852) were more likely to be under 12 months, stunted, have a longer duration of diarrhea, and less likely to have high stool frequency or a fever. There was no difference in dehydration, hospitalization, or severe classification from a modified Vesikari score. Twenty-two (1.8%) Shigella MSD cases died within the 14-days after presentation to health facilities, and 59.1% of these deaths were in culture-negative cases. Age <12 months, diarrhea duration prior to presentation, vomiting, stunting, wasting, and hospitalization were associated with mortality. A model-derived score assigned points for dehydration, hospital admission, and longer diarrhea duration but was not significantly better at predicting 14-day mortality than a modified Vesikari score.
Conclusions
A composite severity score consistent with severe disease or dysentery may be a pragmatic clinical endpoint for severe shigellosis in vaccine trials. Reliance on culture for microbiologic confirmation may miss a substantial number of Shigella cases but is currently required to measure serotype specific immunity.
Molecular methods identified Shigella more commonly than microbiologic culture in younger and stunted children. A simplified clinical score containing dehydration, hospitalization, and diarrhea duration could be used to stratify vaccine trial endpoints by severity based on its ability to predict death.
Shigella is a leading cause of diarrhea among children <5 years in resource-limited settings, associated with over 60 000 deaths in this age group per year [1]. Several Shigella vaccines are currently in development [2–4], and vaccine efficacy will be determined by the number of clinically relevant, Shigella-attributable diarrhea cases prevented by the vaccine [5].
Microbiological culture is the gold standard for Shigella-confirmation; however, the application of highly sensitive molecular tools, such as quantitative polymerase chain reaction (qPCR), has revealed a large burden of Shigella infections undetected by culture [6, 7]. This increased sensitivity of qPCR could make vaccine trials more efficient, but the clinical significance of culture-negative and PCR-attributable shigellosis is unclear.
Rotavirus vaccine is most efficacious in preventing severe rotavirus diarrhea [8], and the Vesikari score is commonly used to stratify clinical endpoints in vaccine trials [9]. There is no universally accepted clinical severity score for Shigella diarrhea in children, and the Vesikari score does not include severity indicators that may be specific to shigellosis, such as dysentery [5]. Identifying the ideal severity score to use in Shigella vaccine trials requires an empiric assessment of the performance of existing and new severity scores in disaggregating severe versus nonsevere cases of Shigella diarrhea.
The Global Enteric Multicenter Study (GEMS) was a multicountry case-control study that enrolled children seeking care for moderate-to-severe (MSD) diarrhea and matched controls [10]. Utilizing clinical and laboratory data from GEMS cases, we sought to inform a microbiologic and clinical case definition for severe, laboratory-confirmed Shigella diarrhea by answering 3 questions: (1) Does the clinical presentation of Shigella differ by culture versus qPCR? (2) What are risk factors for death among children with Shigella- attributed diarrhea? (3) Can we develop a clinical severity score specific to Shigella, and how does it compare to a modified Vesikari score (MVS) previously created to fit these data?
METHODS
Parent Study
Children aged 0–59 months presenting to health centers in Bangladesh, India, Kenya, Mali, Mozambique, Pakistan, and The Gambia with diarrhea were screened for eligibility into GEMS between 2007 and 2011 as described elsewhere [10]. Eligible children were those with an acute episode of MSD defined as 1 or more of the following: dehydration (sunken eyes, loss of skin turgor, intravenous rehydration recommended), dysentery, or hospital admission [11]. At enrollment, clinical history and sociodemographic information were ascertained using a standardized questionnaire, whole stool samples were collected, and a physical exam was performed. Length/height, weight, and mid-upper arm circumference (MUAC) were measured at enrollment and at a single follow-up visit that was performed 60 days later (acceptable range 50–90 days) at which time vital status was also ascertained.
Stool samples were originally processed using conventional enteric pathogen detection methods as described elsewhere [12, 13], and a portion of samples stored at −80°C. For Shigella diagnosis by bacterial culture, stool samples were transported in cold storage in buffered glycerol saline (BGS) transport media and were inoculated onto MacConkey and xylose lysine desoxycholate agar. Suspected Shigella colonies were confirmed using triple-sugar iron, motility indole ornithine (MIO lysine decarboxylase media), citrate and urea biochemical typing media. A random subset of stored stool samples and samples from all fatal cases (if not included in the random subset) were also tested by qPCR using a 32-enteropathogen TaqMan Array Card [7]. Cycle thresholds (Ct) required to detect the pathogen gene target, which are inversely related to nucleic acid quantity, of ≥35 were deemed negative. The gene amplified for Shigella detection, ipaH, is shared by enteroinvasive Escherichia coli (EIEC), but all ipaH detections were assumed to be Shigella based on metagenomic sequencing in a subset of samples [14].
Nested Study
In this secondary data analysis, we excluded controls as well as cases who did not have both culture and qPCR results available and then grouped MSD cases by Shigella culture results. Among culture-negative children, we further divided low- and high-quantity infections using the ipaH Ct cutoff (<27.9) associated with an odds ratio of 2.0 in the original qPCR GEMS analysis [7]. This grouping led to 4 mutually exclusive categories: (1) Shigella negative (no detection by culture or qPCR); (2) culture-negative/qPCR-unattributable (culture negative and 27.9 ≤ ipaH Ct <35); (3) culture-negative/qPCR-attributable (culture negative and ipaH Ct <27.9); and (4) culture-positive (culture-positive Shigella irrespective of qPCR value). The clinical and demographic characteristics of these 4 categories were compared using prevalence ratios determined from Poisson regression with culture-positive Shigella as the reference group and each dichotomous covariate of interest modeled separately in a model including site (indicator variable) and age (continuous variable). Characteristics of interest, ascertained at MSD presentation, included age, site, sex, dysentery (visibly bloody stool reported by caregiver, clinician, or laboratory technician), mucoid stool, caregiver-reported number of days of diarrhea prior to presentation, caregiver reported number of loose stools in previous 24 hours, axillary temperature, caregiver-reported vomiting, clinician-determined dehydration status (according to World Health Organization [WHO] IMCI guidelines [15]), stunting (length for age z-score [LAZ] <−2), wasting (mid-upper arm circumference [MUAC] < 12.5cm among children 6 months or older), and admission to hospital. We utilized clinical signs at presentation to recreate the MVS generated previously with these data [16]. This MVS totaled 16 points and was categorized as mild (1–5 points), moderate (6–8 points), and severe (9–16 points). To establish the likelihood of causes of diarrhea other than Shigella in each of the 4 diagnostic categories, we considered site-and age-adjusted attributable fractions ≥ 0.5, derived from qPCR Ct-values [17]. These pathogens were grouped as: viral (astrovirus, norovirus, rotavirus, sapovirus, adenovirus), parasitic (Cryptosporidium, Entamoeba histolytica, Cyclospora, Isospora), and other bacterial (Aeromonas Campylobacter, Helicobacter pylori, Salmonella, Vibrio cholerae, enteroaggregative Escherichia coli (E.coli) [EAEC], heat-stable enterotoxin-producing E. coli [ST-ETEC], heat-labile enterotoxin-producing E. coli [LT-ETEC], typical enteropathogenic E.coli [tEPEC], Shiga toxin producing E. coli [STEC]). The prevalence of other causes was compared between the 4 Shigella categories in age- and site-adjusted Poisson regression models.
To establish risk factors for death among children with Shigella-attributed diarrhea, we excluded children who were Shigella negative (by culture and qPCR) or had culture-negative/qPCR-unattributable Shigella and children without a 60-day follow-up visit in which vital status (and date of death, if applicable) was ascertained. Cox proportional hazards regression was used to identify univariate and adjusted risk factors for death in the first 14-days after presentation. We evaluated deaths in the 14-days to capture deaths most likely related to the MSD. Adjusted models included site (indicator variable) and age (continuous variable).
We derived a new severity score (model-derived score) based on risk of dying in the 14-days after MSD presentation using forward stepwise Cox proportional hazards regression and Akaike information criteria (AIC) for model-selection. The following clinical variables were considered in building this model: dysentery, mucoid stool, duration of diarrhea including and prior to the day of enrollment, maximum number of loose stools in last 24 hours, axillary temperature, caregiver-reported vomiting, WHO dehydration status, and clinician decision to hospitalize. Continuous variables were categorized to match that of the previously published MVS [16]. The final Cox model coefficients were used to calculate the new score using methods described elsewhere [18]. The total number of possible points were constrained to 16 and categorized as mild (<6), moderate [6–8], and severe (9+) to be consistent with the MVS [16].
The model-derived score and the MVS were compared using the area under the curve (AUC) calculated from a logistic model containing deaths in the first 14 days as the outcome and the continuous score as the independent variable with bootstrapped standard errors and a chi-square statistic. Finally, both scores’ ability to predict odds of death beyond 14-days (among those 14-day survivors) were also evaluated using logistic-regression based AUCs with bootstrapped standard errors.
To evaluate the robustness of our findings, we repeated all analyses in 3 subsets of data: (1) Excluding children with another possible etiology based on site and age-adjusted attributable fraction ≥0.5 in the culture-negative/qPCR-attributable and culture-positive groups; (2) excluding data from Bangladesh because of the uniquely high culture-positivity and dysentery rate at this site [7], and (3) excluding the fatal cases that were enriched in the sample (not qPCR-tested randomly).
Analyses were conducted in Stata 14.0 (Stata Corp, College Station, TX, USA) with an alpha of 0.05. The funder had no role in this manuscript’s design, data collection, analysis, writing, or submission.
RESULTS
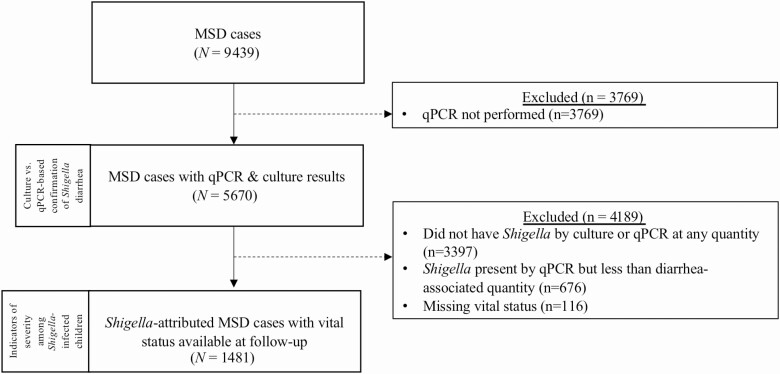
Of 9439 MSD cases enrolled in GEMS, 5670 had qPCR results and were included in the analysis of clinical presentation by Shigella diagnostic assay (Figure 1). Sixty percent (n = 3397) of children did not have Shigella detected by culture or by qPCR at any Ct value. Shigella was isolated from culture in 745 children (13.1%), the majority (727 [97.5%]) of which were detected by qPCR (697 [95.9%] at qPCR-attributable levels and 30 [4.1%] qPCR-unattributable). Of the culture-positive Shigella cases, 65.4% were S. flexneri, 24.0% S. sonnei, 5.5% S.dysenteriae, and 5.1% S. boydii, as described elsewhere [12]. Of 4925 culture-negative MSD cases, qPCR-attributable Shigella infections were identified in an additional 852 (17.3%) children and qPCR-unattributable infections in 676 (13.7%).
Figure 1.
Participant flow of children with MSD included in each analysis. Abbreviations: MSD, moderate to severe diarrhea; qPCR, quantitative polymerase chain reaction.
Culture Versus qPCR-Based Confirmation of Shigella Diarrhea
Accounting for potential confounding by age and site, compared to children with culture-positive shigellosis (Tables 1 and 2), cases with culture-negative/qPCR-attributable shigellosis were more likely to be under one year of age, stunted, and have had more than three days of diarrhea; they were also less likely to be febrile or have passed more than 6 loose stools in a day. The prevalence of a concomitant attributable viral pathogen was similar between culture-positive (13.3%) and culture-negative/qPCR-attributable (15.6%) shigellosis as was the likelihood of dysentery, mucoid stool, severe dehydration, vomiting, hospital admission, and a “severe” classification by the MVS. When removing episodes with other potentially attributable pathogens detected, the observed differences in clinical presentation of culture-positive and culture-negative/qPCR-attributable shigellosis did not change for any manifestation other than diarrhea duration, which became a more pronounced difference between the 2 diagnostic categories (Supplementary Table 1 [S1]). Exclusion of the Bangladesh site (Supplementary Table S2) resulted in a significantly lower prevalence of dysentery and negative/qPCR-attributable shigellosis compared to culture-positive shigellosis, and removal of fatal cases that were enriched in the data set (Supplementary Table S3) did not meaningfully change any comparisons.
Table 1.
Frequencies of Sociodemographic, Clinical, and Pathogen Characteristics by Diagnostic Categories
| Absent | Present | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Shigella Culture | Absenta (n = 3397) | qPCR-unattributableb (n = 676) | qPCR-attributablec(n = 852) | Any qPCR valued (n = 745) | ||||
| Shigella qPCR | n | (%) | n | (%) | n | (%) | n | (%) | |
| Sociodemographic | |||||||||
| Age | |||||||||
| 1–11 m | 1684 | (49.6) | 129 | (19.1) | 149 | (17.5) | 91 | (12.2) | |
| 12–23 m | 977 | (28.8) | 271 | (40.1) | 405 | (47.5) | 301 | (40.4) | |
| 24–59 m | 736 | (21.7) | 276 | (40.8) | 298 | (35.0) | 353 | (47.4) | |
| Female sex | 1422 | (42.5) | 276 | (40.8) | 371 | (43.5) | 339 | (45.5) | |
| Site | |||||||||
| Bangladesh | 357 | (10.5) | 35 | (5.2) | 98 | (11.5) | 410 | (55.0) | |
| India | 567 | (16.7) | 123 | (18.2) | 138 | (16.2) | 58 | (7.8) | |
| Kenya | 650 | (19.3) | 107 | (15.8) | 83 | (9.7) | 72 | (9.7) | |
| Mali | 556 | (16.4) | 150 | (22.2) | 163 | (19.1) | 18 | (2.4) | |
| Mozambique | 337 | (9.9) | 73 | (10.8) | 85 | (10.0) | 24 | (3.2) | |
| Pakistan | 481 | (14.2) | 110 | (16.3) | 153 | (18.0) | 86 | (11.5) | |
| The Gambia | 449 | (13.2) | 78 | (11.5) | 132 | (15.5) | 77 | (10.3) | |
| Clinical characteristics at enrollment | |||||||||
| Dysentery | 459 | (13.5) | 91 | (13.5) | 355 | (41.7) | 534 | (71.7) | |
| Caregiver reported mucoid stool | 765 | (22.5) | 148 | (21.9) | 255 | (29.9) | 364 | (48.9) | |
| Duration of diarrhea (including day of presentation) ≥3 d | 1748 | (51.5) | 348 | (51.5) | 489 | (57.4) | 351 | (47.1) | |
| ≥7 loose stools child in 24-h period | 1213 | (35.7) | 218 | (32.3) | 303 | (35.6) | 424 | (56.9) | |
| Temperature ≥ 38°C | 735 | (21.6) | 129 | (19.1) | 140 | (16.4) | 238 | (32.0) | |
| Caregiver reported vomiting > 3 times per day | 1509 | (44.4) | 289 | (42.8) | 222 | (26.1) | 162 | (21.7) | |
| Severe dehydration | 1908 | (56.2) | 390 | (57.7) | 437 | (51.3) | 221 | (30.0) | |
| Stunted (LAZ <−2) | 943 | (27.9) | 240 | (35.7) | 310 | (36.7) | 214 | (28.9) | |
| Wastede (MUAC <12.5cm) | 411 | (14.3) | 99 | (15.3) | 124 | (15.0) | 66 | (9.1) | |
| Hospitalized | 819 | (24.1) | 158 | (23.4) | 145 | (17.0) | 209 | (28.1) | |
| Severe by modified Vesikari scoref | 1544 | (45.5) | 314 | (46.5) | 298 | (35.0) | 304 | (40.8) | |
| Other etiologies | |||||||||
| Viralg | 1169 | (34.4) | 172 | (25.4) | 133 | (15.6) | 99 | (13.3) | |
| Parasitich | 280 | (8.2) | 41 | (6.1) | 54 | (6.4) | 15 | (2.0) | |
| Other bacteriai | 615 | (18.1) | 168 | (24.9) | 185 | (21.7) | 61 | (8.2) |
Abbreviations: E. coli, Escherichia coli; EAEC, enteroaggregative Escherichia coli; LAZ, length for age z-score; LT-ETEC, heat-labile enterotoxin-producing E. coli; MUAC, mid-upper arm circumference; qPCR; quantitative polymerase chain reaction; STEC, Shiga toxin producing E. coli; ST-ETEC, heat-stable enterotoxin-producing E. coli; tEPEC, typical enteropathogenic E. coli.
a ipaH Ct value ≥35.
b27.9 ≤ ipaH Ct <35.
c ipaH Ct value <27.9.
dAbsent by qPCR (n = 18 [2.4%]), present below diarrhea-associated quantity (n = 30 [4.0%]), present at, or above, diarrhea associated quantity (n = 697 [93.6%]).
eAmong those ≥6 months of age (in whom MUAC is validated).
fAs derived in Kotloff et al, Vaccine, 2017.
gSite and age-adjusted attributable fraction ≥.5 for any of the following: adenovirus, astrovirus, norovirus, rotavirus, sapovirus, adenovirus.
hSite and age-adjusted attributable fraction ≥.5 for any of the following: Cryptosporidium, Entamoeba histolytica, Cyclospora, Isospora.
iSite and age-adjusted attributable fraction ≥.5 for any of the following: H. pylori, Campylobacter, Aeromonas, Salmonella, V. cholerae, EAEC, ST-ETEC, LT-ETEC, tEPEC, STEC.
Table 2.
Sociodemographic, Clinical, and Pathogen Factors Associated With Shigella Diagnostic Categories
| Absent | Present | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Shigella Culture | Absenta (n = 3397) | qPCR-unattributableb(n = 676) | qPCR-attributablec(n = 852) | Any qPCR valued (n = 745) | ||||
| Shigella qPCR | aPRe | (95% CI) | aPRe | (95% CI) | aPRe | (95% CI) | aPRe | (95% CI) | |
| Sociodemographic | |||||||||
| Age <12 m | 4.52 | (3.63–5.63) | 1.76 | (1.33–2.32) | 1.57 | (1.20–2.05) | Ref | … | |
| Female sex | .89 | (.78–1.01) | .85 | (.72–1.01) | 0.91 | (.78–1.07) | Ref | … | |
| Clinical characteristics at enrollment | |||||||||
| Dysentery | .37 | (.32–.42) | .40 | (.31–.50) | 1.06 | (.91–1.22) | Ref | … | |
| Caregiver reported mucoid stool | .66 | (.57–.77) | .72 | (.58–.89) | 0.95 | (.80–1.13) | Ref | … | |
| Duration of diarrhea ≥3 d | 1.04 | (.92–1.19) | 1.09 | (.93–1.28) | 1.20 | (1.04–1.39) | Ref | … | |
| ≥7 loose stools child in 24-h period | .83 | (.73–.94) | .80 | (.67–.95) | 0.82 | (.70–.96) | Ref | … | |
| Temperature ≥ 38°C | .78 | (.66–.92) | .71 | (.56–.90) | 0.62 | (.50–.78) | Ref | … | |
| Caregiver reported vomiting >3 times per day | 1.84 | (1.55–2.20) | 1.85 | (1.51–2.27) | 1.13 | (.91–1.39) | Ref | … | |
| Severe dehydration | 1.22 | (1.05–1.41) | 1.22 | (1.03–1.45) | 1.14 | (.96–1.34) | Ref | … | |
| Stunted (LAZ <−2) | 1.05 | (.89–1.24) | 1.21 | (.99–1.48) | 1.27 | (1.05–1.53) | Ref | … | |
| Wastedf (MUAC <12.5cm) | .86 | (.65–1.14) | 1.19 | (.85–1.64) | 1.08 | (.79–1.48) | Ref | … | |
| Hospitalized | 1.29 | (1.08–1.54) | 1.37 | (1.10–1.71) | 0.90 | (.72–1.13) | Ref | …- | |
| Severe by modified Vesikari scoreg | 1.11 | (.95–1.25) | 1.16 | (.98–1.37) | 0.87 | (.74–1.03) | Ref | … | |
| Other etiologies | |||||||||
| Viralh | 2.24 | (1.80–2.79) | 2.56 | (1.53–2.56) | 1.18 | (.91–1.55) | Ref | … | |
| Parasitici | 1.94 | (1.14–3.32) | 2.93 | (.88–2.93) | 1.73 | (.97–3.10) | Ref | … | |
| Other bacteriaj | 2.24 | (1.69–2.97) | 3.88 | (2.09–3.88) | 2.54 | (1.88–3.44) | Ref | … |
Abbreviations: aPR, adjusted prevalence ratio; CI, confidence interval; E. coli, Escherichia coli; EAEC, enteroaggregative Escherichia coli; LAZ, length for age z-score; LT-ETEC, heat-labile enterotoxin-producing E. coli; MUAC, mid-upper arm circumference; qPCR; quantitative polymerase chain reaction; STEC, Shiga toxin producing E. coli; ST-ETEC, heat-stable enterotoxin-producing E. coli; tEPEC, typical enteropathogenic E. coli.
a ipaH C t value ≥35
b27.9 ≤ ipaH Ct <35
c ipaH Ct value <27.9
dAbsent by qPCR (n = 18 [2.4%]), present below diarrhea-associated quantity (n = 30 {4.0%}), present at, or above, diarrhea associated quantity (n = 697 [93.6%]).
e aPR from relative risk regression assuming Poisson distribution adjusting for site (considered as an indicator variable) and age (considered continuously) except age model adjusted only for site.
fAmong those ≥6 months of age (in whom MUAC is validated).
gAs derived in Kotloff et al, Vaccine, 2017.
hSite and age-adjusted attributable fraction ≥.5 for any of the following: astrovirus, norovirus, rotavirus, sapovirus, adenovirus.
iSite and age-adjusted attributable fraction ≥.5 for any of the following: Cryptosporidium, Entamoeba histolytica, Cyclospora, Isospora.
jSite and age-adjusted attributable fraction ≥.5 for any of the following: H. pylori, Campylobacter, Aeromonas, Salmonella, V. cholerae, EAEC, ST-ETEC, LT-ETEC, tEPEC, STEC.
In contrast, children with culture-negative/qPCR-unattributable shigellosis were more likely than children with culture-positive shigellosis to present with vomiting, severe dehydration, to be hospitalized, and to have a viral etiology (Tables 1 and 2). Similar associations were found when comparing Shigella negative (no detection by culture or qPCR) cases to culture-positive shigellosis. Subset analyses revealed similar findings, except the analysis excluding Bangladesh data, which found no differences in fever, severe dehydration, or hospitalization when comparing culture-positive Shigella cases to the other 2 groups (Supplementary Tables S1–S3).
Bacterial causes other than Shigella were more common in all 3 culture-negative groups (21.7% qPCR-attributable, 24.9% qPCR-unattributable, and 18.1% absent) compared to culture-positive Shigella (8.2%, Tables 1 and 2). Subset analyses led to similar findings (Supplementary Tables S1–S3). These associations did not appear to be driven by a single bacterial pathogen (Supplementary Table S4A/B).
Risk Factors for Death Among Children With Shigella-attributed Diarrhea
The clinical and demographic features of the 1481 children with either culture-negative/qPCR-attributable or culture-positive Shigella with known vital status at follow-up are presented, by age, in Table 3. Children <12 months comprised 14.9% of the Shigella-attributed cases and were less likely to present with dysentery and fever and more likely to present with vomiting and dehydration. This trend held true when limiting to qPCR-attributable cases only (Supplementary Table S5) and culture-positive shigellosis (Supplementary Table S6).
Table 3.
Age-stratified Characteristics of Shigella MSD Cases Defined as Culture Positive or Quantitative Polymerase Chain Reaction (qPCR)-Attributable (n = 1481)
| Characteristic | 0–5 m (n = 35) | 6–11 m (n = 185) | 12–23 m (n = 654) | 24–59 m (n = 607) | ||||
|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | |
| Sociodemographic | ||||||||
| Female sex | 14 | (40.0) | 74 | (40.0) | 307 | (46.9) | 263 | (43.3) |
| Site | ||||||||
| Bangladesh | 3 | (8.6) | 49 | (26.5) | 198 | (30.3) | 252 | (41.5) |
| India | 5 | (14.3) | 19 | (10.3) | 62 | (9.5) | 101 | (16.6) |
| Kenya | 9 | (25.7) | 26 | (14.1) | 58 | (8.9) | 56 | (9.2) |
| Mali | 1 | (2.9) | 16 | (8.7) | 87 | (13.3) | 55 | (9.1) |
| Mozambique | 0 | (0) | 11 | (6.0) | 49 | (7.5) | 35 | (5.8) |
| Pakistan | 15 | (42.9) | 32 | (17.3) | 92 | (14.1) | 60 | (9.9) |
| The Gambia | 2 | (5.7) | 32 | (17.2) | 108 | (16.5) | 48 | (7.9) |
| Clinical characteristics | ||||||||
| Dysentery | 13 | (37.1) | 88 | (47.6) | 345 | (52.8) | 390 | (64.3) |
| Caregiver reported mucoid stool | 13 | (37.1) | 70 | (37.8) | 253 | (38.7) | 247 | (40.7) |
| Duration of diarrhea (including day of presentation) | ||||||||
| 1–3 | 19 | (54.3) | 128 | (69.2) | 460 | (70.3) | 448 | (73.8) |
| 4–5 | 14 | (40.0) | 35 | (18.9) | 143 | (21.9) | 124 | (20.4) |
| 6+ | 2 | (5.7) | 22 | (11.9) | 51 | (7.8) | 35 | (5.8) |
| Max no. of loose stools child passed in 24-h period | ||||||||
| ≤6 | 20 | (57.1) | 95 | (51.4) | 361 | (55.2) | 321 | (52.9) |
| 7–10 | 11 | (31.4) | 66 | (35.7) | 196 | (30.0) | 182 | (30.0) |
| >10 | 4 | (11.4) | 24 | (13.0) | 97 | (14.8) | 104 | (17.1) |
| Axillary temperature at presentation | ||||||||
| <38°C | 30 | (85.7) | 152 | (82.2) | 510 | (78.0) | 431 | (71.0) |
| 38–38.9°C | 3 | (8.6) | 24 | (13.0) | 80 | (12.2) | 106 | (17.5) |
| ≥39°C | 2 | (5.7) | 9 | (4.9) | 64 | (9.8) | 70 | (11.5) |
| Caregiver reported vomiting ≥3 times per day | 12 | (34.3) | 63 | (34.1) | 159 | (24.3) | 124 | (20.4) |
| WHO-defined dehydration categories | ||||||||
| None | 11 | (31.4) | 53 | (28.7) | 208 | (31.8) | 255 | (42.0) |
| Some | 5 | (14.3) | 46 | (24.9) | 165 | (25.2) | 141 | (23.2) |
| Severe | 19 | (54.3) | 86 | (46.5) | 281 | (43.0) | 211 | (34.8) |
| Modified Vesikari scorea | ||||||||
| Mild | 6 | (17.1) | 24 | (13.0) | 78 | (11.9) | 93 | (15.3) |
| Moderate | 15 | (42.9) | 81 | (43.8) | 326 | (49.9) | 299 | (49.3) |
| Severe | 14 | (40.0) | 80 | (43.2) | 250 | (38.2) | 215 | (35.4) |
| Stunted (LAZ <−2) | 14 | (40.0) | 46 | (25.1) | 197 | (30.3) | 220 | (36.4) |
| Wasted (MUAC <12.5cm)b | -- | -- | 50 | (27.0) | 97 | (14.8) | 27 | (4.5) |
Abbreviations: LAZ, length for age z-score; MSD, moderate to severe diarrhea; MUAC, mid-upper arm circumference; WHO, World Health Organization.
aAs derived in Kotloff et al, Vaccine, 2017.
bAmong children ≥6 months.
Forty-two children (2.8%) with Shigella-attributable diarrhea died during follow-up, of whom 22 (52.4%) died in the first 2 weeks. There was no difference in risk of death between culture negative/qPCR-attributable shigellosis and culture-positive shigellosis (adjusted hazard ratio [aHR]: 1.1, 95% confidence interval [CI]: .5–2.6). Age <6 months, duration of diarrhea >3 days, vomiting >3 times per day, stunting, MUAC under 12.5 cm, and hospital admission were associated with risk of death in the first 14 days (Table 4). Severe dehydration was not statistically associated with death in adjusted models, but 20/22 (90.9%) of the deaths among Shigella cases that occurred in the first 2 weeks were in children with severe dehydration. Dysentery was not associated with death in adjusted models, nor was presence of a second attributable etiology. When excluding episodes with additional potentially attributable pathogens (Supplementary Table S7), we found all risk factors remained significantly associated with death, including vomiting. There were no differences in significant risk factors in the subset of children excluding Bangladesh (Supplementary Table S8). Finally, excluding enriched fatal cases (Supplementary Table S9), young age and diarrhea duration were no longer significantly associated with death, although the direction and magnitude of association were similar to primary analyses.
Table 4.
Characteristics of Shigella Moderate to Severe Diarrhea (MSD) Cases Defined as Culture Positive or Quantitative Polymerase Chain Reaction (qPCR)-Attributable (N = 1481) Who Died Versus Those Who Survived in the 14 Days Post enrollment
| Characteristic | Died (n = 22) | Survived (n = 1459) | Hazard Ratiob (95% CI) | Hazard Ratio (95% CI)c | ||
|---|---|---|---|---|---|---|
| n | (%)a | n | (%)a | |||
| Sociodemographic | ||||||
| Age | ||||||
| 0–5 m | 3 | (13.6) | 32 | (2.2) | 10.7 (2.6–45.0) | 6.3 (1.4–27.5) |
| 6–11 m | 4 | (18.2) | 181 | (12.4) | 2.6 (.7–9.8) | 1.6 (.4–5.8) |
| 12–23 m | 10 | (45.5) | 644 | (44.1) | 1.9 (.6–5.4) | 1.3 (.4–3.8) |
| 24–59 m | 5 | (22.7) | 602 | (41.3) | Ref | Ref |
| Sex | ||||||
| Female | 7 | (31.8%) | 651 | (44.6%) | 0.6 (.2–1.4) | 0.6 (.2–1.4) |
| Male | 15 | (68.2%) | 808 | (55.4%) | Ref | Ref |
| Clinical characteristics | ||||||
| Dysentery | ||||||
| Present | 7 | (31.8%) | 829 | (56.8%) | 0.4 (.2–.9) | 0.6 (.2–1.6) |
| Absent | 15 | (68.2%) | 630 | (43.2%) | Ref | Ref |
| Caregiver reported mucoid stool | ||||||
| Present | 8 | (36.4%) | 575 | (39.4%) | 0.9 (.4–2.1) | 1.2 (.5–3.0) |
| Absent | 14 | (63.6%) | 884 | (60.6%) | Ref | Ref |
| Duration of diarrhea (including day of presentation) | ||||||
| ≥3 | 19 | (86.4%) | 757 | (51.9%) | 5.8 (1.7–19.6) | 4.4 (1.3–15.3) |
| <3 | 3 | (13.6%) | 702 | (48.1%) | Ref | Ref |
| Max no. of loose stools child passed in 24-h period | ||||||
| ≥7 | 7 | (31.8%) | 677 | (46.4%) | 0.5 (.2–1.3) | 1.1 (.4–2.9) |
| <7 | 15 | (68.2%) | 782 | (53.6%) | Ref | Ref |
| Temperature | ||||||
| ≥38°C | 9 | (40.9%) | 349 | (23.9%) | 2.2 (.9–5.1) | 2.1 (.9–5.1) |
| <38°C | 13 | (59.1%) | 1110 | (76.1%) | Ref | Ref |
| Caregiver reported vomiting | ||||||
| >3 times per day | 12 | (54.6%) | 346 | (23.7%) | 3.8 (1.6–8.8) | 2.5 (1.1–5.9) |
| ≤3 times per day (or none) | 10 | (45.5%) | 1113 | (76.3%) | Ref | Ref |
| WHO-defined dehydration categories | ||||||
| Severe | 20 | (90.9%) | 577 | (39.7%) | 17.9 (2.4–133.3) | 7.9 (.8–79.7) |
| Some | 1 | (4.6%) | 356 | (24.4%) | 1.5 (.09–23.6) | 1.3 (.07–23.5) |
| None | 1 | (4.6%) | 526 | (36.1%) | Ref | Ref |
| Chronic malnutrition | ||||||
| Stunted (LAZ <−2) | 11 | (57.9%) | 466 | (32.1%) | 2.9 (1.2–7.2) | 3.0 (1.2–7.7) |
| Nonstunted | 8 | (42.1%) | 988 | (68.0%) | Ref | Ref |
| Acute malnutrition d | ||||||
| MUAC <12.5cm | 8 | (42.1%) | 166 | (11.6%) | 5.4 (2.2–13.4) | 3.3 (1.2–8.6) |
| MUAC ≥12.5cm | 11 | (57.9%) | 1261 | (88.4%) | Ref | |
| Admission status at enrollment visit | ||||||
| Hospitalized | 16 | (72.7%) | 319 | (21.9%) | 9.3 (3.6–23.8) | 14.5 (5.1–41.2) |
| Seen as outpatient | 6 | (27.3%) | 1140 | (78.1%) | Ref | Ref |
| Modified Vesikari scoree | ||||||
| Severe | 18 | (81.8%) | 541 | (37.1%) | 5.9 (2.0–17.4) | 4.4 (1.5–12.9) |
| Moderate | 4 | (18.2%) | 717 | (49.1%) | Ref | Ref |
| Mild | 0 | (0) | 201 | (13.8%) | Not estimable | Not estimable |
| Laboratory | ||||||
| Shigella culture results | ||||||
| Culture positive | 9 | (40.9%) | 698 | (47.8%) | 0.8 (.3–1.8) | 1.1 (.4–2.6) |
| Culture negative | 13 | (59.1%) | 761 | (52.2%) | Ref | Ref |
| Shigella qPCR Ct values f | ||||||
| <20.8 | 11 | (50.0%) | 635 | (44.9%) | 1.5 (.5–4.7) | 1.9 (.6–6.0) |
| 20.8–24.34 | 7 | (31.8%) | 436 | (30.8%) | 1.4 (.4–4.7) | 1.7 (.5–5.8) |
| 24.35–27.89 | 4 | (18.2%) | 343 | (24.3%) | Ref | Ref |
| Other potential etiologyg | ||||||
| Yes | 8 | (36.4%) | 429 | (29.4%) | 1.4 (.6–3.3) | 1.4 (.6–3.3) |
| No | 14 | (63.6%) | 1030 | (70.6%) | Ref | Ref |
Abbreviations: CI, confidence interval; E. coli, Escherichia coli; EAEC, enteroaggregative Escherichia coli; LAZ, length for age z-score; LT-ETEC, heat-labile enterotoxin-producing E. coli; MUAC, mid-upper arm circumference; STEC, Shiga toxin producing E. coli; ST-ETEC, heat-stable enterotoxin-producing E. coli; tEPEC, typical enteropathogenic E. coli; WHO, World Health Organization.
aColumn percentages.
bFrom Cox proportional hazards regression including only the variable of interest in the model.
cFrom Cox proportional hazards regression including the variable of interest, site as an indicator variable, and age as a continuous variable except age model adjusted only for site.
dAmong those ≥6 months of age in whom MUAC is validated.
eAs derived in Kotloff et al, Vaccine, 2017.
fAmong those with qPCR attributable-Shigella (n = 1436).
gBased on site and age-adjusted attributable fraction ≥0.5 for any of the following pathogens: astrovirus, norovirus, rotavirus, sapovirus, adenovirus, Cryptosporidium, E. histolytica, Cyclospora, Isospora, isospora, H. pylori, Aeromonas Campylobacter, Salmonella, V. cholerae, EAEC, ST-ETEC, LT-ETEC, tEPEC, STEC.
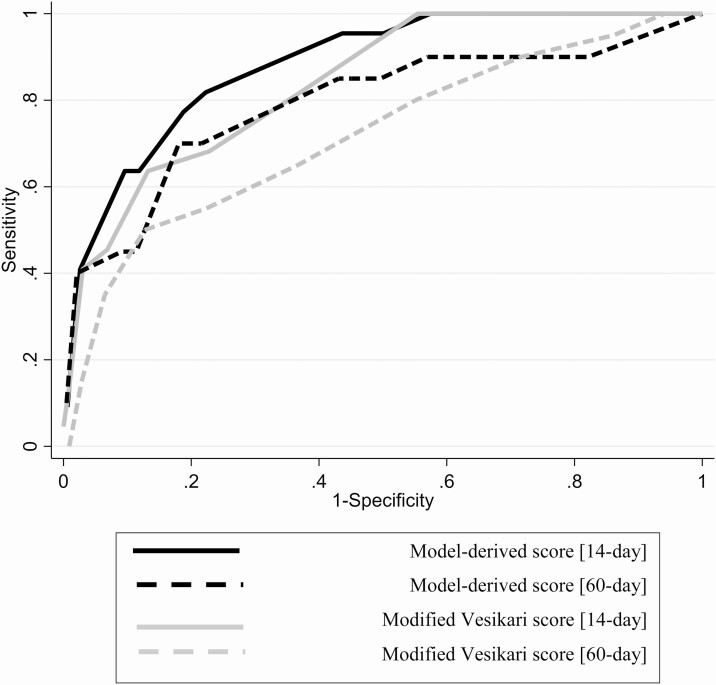
Based on model fit, 3 clinical features maximally predicted death: clinician decision to hospitalize, dehydration status, and diarrhea duration prior to presentation (Table 5, Supplementary Table S10). This model-derived score had an AUC of 0.85 for predicting 14-day mortality (95% CI: .76–.91). The MVS had a similar AUC (AUC = 0.80, 95% CI: .71–.88, P-valueAUC derived vs. AUC MVS = .077) (Figure 2). The model-derived score classified 564 (38.1%) of children as severe, 409 (27.6%) as moderate, and 729 (49.2%) as mild, whereas MVS classified 559 (37.7%) as severe, 721(48.7%) as moderate, and 201 (13.6%) as mild. Table 6 displays the median model-derived score and MVS across various characteristics. Against the outcome of death after 14 days among the 1459 14-day survivors, the 2 scores did not differ significantly (AUCmodel = 0.75, 95% CI: .59–.87 vs AUCMVS: 0.67, 95% CI: .53–.80, P = .064). Among children ≥12 months (n = 1261) or in those with culture-confirmed Shigella (n = 707), the AUC values were not meaningfully different (Supplementary Figures S1 and S2) other than a significantly higher AUC for the model-derived score in predicting 14-day mortality (P = .048) in children aged 12 months or older.
Table 5.
Shigella Model-derived Score and Modified Vesikari Score
| Predictor | Model-derived Score | Modified Vesikari Scorea |
|---|---|---|
| Days diarrhea prior to presentation (including day of presentation) | ||
| ≥6 | 3 | 3 |
| 4–5 | 2 | 2 |
| 1–3 | 0 | 1 |
| Max no. of loose stools child passed in 24-h period | ||
| >10 | -- | 3 |
| 7–10 | -- | 2 |
| ≤6 | -- | 1 |
| Max no. of vomiting episodes in 24-h period | ||
| >3 times per day | -- | 2 |
| ≤3 times per day (or none) | -- | 1 |
| WHO-defined dehydration categories | ||
| Severe | 8 | 3 |
| Some | 4 | 2 |
| None | 0 | 0 |
| Temperature | ||
| ≥39°C | -- | 3 |
| 38.5–38.9°C | -- | 2 |
| 37.1–38.4°C | -- | 1 |
| Hospitalizedb | ||
| Yes | 5 | 2 |
| No | 0 | 0 |
Abbreviation: WHO, World Health Organization.
aAs derived in Kotloff et al, Vaccine, 2017.
bIn sum, 28 participants received intravenous (IV) rehydration in a short-stay ward, and these were upgraded to “hospitalized.”
Figure 2.
ROC curves of model-derived score and modified Vesikari score predicting death in first 14 days and within 60 days (range 50–90 days) among 14-day survivors. Model-derived score: AUC0–14 of 0.85 (95% CI: .77–.92); AUC15–90 0.75 (95% CI: .60–.87). Modified Vesikari score: AUC0–14: 0.80 (95% CI: .70–.88); AUC15–90: 0.67 (95% CI: .53–.80). Abbreviations: AUC, area under the curve; CI, confidence interval; ROC, receiver operating characteristic.
Table 6.
Median and Interquartile Range (IQR) of Model-derived and Modified Vesikari Scores by Participant Characteristic Among 1481 Children With Shigella-attributed Diarrhea
| Characteristic | Model-derived Score | Modified Vesikari Scorea | ||
|---|---|---|---|---|
| Median | (IQR) | Median | (IQR) | |
| Sociodemographic | ||||
| Age | ||||
| 0–5 m | 8 | (2–10) | 8 | (6–11) |
| 6–11 m | 8 | (4–9) | 8 | (6–10) |
| 12–23 m | 6 | (4–8) | 8 | (6–9) |
| 24–59 m | 5 | (2–8) | 8 | (6–9) |
| Sex | ||||
| Female | ||||
| Male | 6 | (3–8) | 8 | (6–9) |
| Clinical characteristics | 6 | (3–8) | 8 | (6–9) |
| Dysentery | ||||
| Present | 4 | (0–8) | 7 | (6–9) |
| Absent | 8 | (5–10) | 8 | (7–10) |
| Caregiver reported mucoid stool | ||||
| Present | 5 | (2–8) | 8 | (6–9) |
| Absent | 8 | (4–9) | 8 | (7–9) |
| Duration of diarrhea (including day of presentation) | ||||
| ≥3 | 6 | (3–10) | 8 | (6–9) |
| <3 | 5 | (4–8) | 8 | (6–10) |
| Max no. of loose stools child passed in 24-h period | ||||
| ≥7 | 6 | (4–8) | 9 | (7–10) |
| <7 | 5 | (2–8) | 7 | (6–9) |
| Temperature | ||||
| ≥38°C | 6 | (4–9) | 10 | (8–11) |
| <38°C | 6 | (2–8) | 7 | (6–9) |
| Caregiver reported vomiting | ||||
| >3 times per day | 8 | (5–10) | 10 | (8–11) |
| ≤3 times per day (or none) | 5 | (2–8) | 7 | (6–9) |
| WHO-defined dehydration categories | ||||
| Severe | 8 | (8–13) | 9 | (8–11) |
| Some | 4 | (4–6) | 7 | (6–9) |
| None | 2 | (0–3) | 6 | (5–8) |
| Chronic malnutrition | ||||
| Stunted (LAZ <−2) | 7 | (3–9) | 8 | (7–9) |
| Nonstunted | 5 | (3–8) | 8 | (6–9) |
| Acute malnutrition b | ||||
| MUAC <12.5 cm | 8 | (7–11) | 9 | (7–11) |
| MUAC ≥12.5 cm | 5 | (2–8) | 8 | (6–9) |
| Admission status at enrollment visit | ||||
| Hospitalized | 9 | (5–13) | 10 | (9–12) |
| Seen as outpatient | 4 | (2–8) | 7 | (6–8) |
| Modified Vesikari scorea | ||||
| Severe | 9 | (8–13) | 10 | (9–11) |
| Moderate | 4 | (3–8) | 7 | (6–8) |
| Mild | 0 | (0–0) | 5 | (4, 5) |
| Laboratory | ||||
| Shigella culture results | ||||
| Culture positive | 5 | (2–8) | 8 | (6–10) |
| Culture negative | 8 | (4–9) | 8 | (6–9) |
| Shigella qPCR Ct valuesc | ||||
| <20.8 | 5 | (3–8) | 8 | (6–10) |
| 20.8–24.34 | 6 | (2–8) | 8 | (6–9) |
| 24.35–27.89 | 8 | (4–9) | 8 | (6–9) |
| Other potential etiologyd | ||||
| Yes | 7 | (4–9) | 8 | (7–9) |
| No | 5 | (2–8) | 8 | (6–9) |
Abbreviations: CI, confidence interval; E. coli, Escherichia coli; EAEC, enteroaggregative Escherichia coli; IQR, interquartile range; LAZ, length for age z-score; LT-ETEC, heat-labile enterotoxin-producing E. coli; MUAC, mid-upper arm circumference; qPCR, quantitative polymerase chain reaction; STEC, Shiga toxin producing E. coli; ST-ETEC, heat-stable enterotoxin-producing E. coli; tEPEC, typical enteropathogenic E. coli; V. cholerae, Vibrio cholerae; WHO, World Health Organization.
aAs derived in Kotloff et al, Vaccine, 2017.
bAmong those≥6 months of age in whom MUAC is validated.
cAmong those with qPCR attributable Shigella (n = 1436).
dBased on site and age-adjusted attributable fraction ≥.5 for any of the following pathogens: astrovirus, norovirus, rotavirus, sapovirus, adenovirus, Cryptosporidium, E. histolytica, Cyclospora, Isospora, H. pylori, Campylobacter, Salmonella, V. cholerae, EAEC, ST-ETEC, LT-ETEC, tEPEC, STEC.
Discussion
Our secondary analysis of children <5 years of age presenting to health centers with MSD found the clinical presentations of culture-positive shigellosis and culture-negative/qPCR-attributable shigellosis to differ in terms of fever, duration, and stool frequency but not in terms dehydration, vomiting, and MVS severity. Presuming culture-negative/qPCR-attributable Shigella infections are indeed Shigella diarrhea, as we routinely assume with culture-positive Shigella, culture missed half of Shigella-attributed MSD cases in this study, with a higher likelihood of missing the diagnosis in children <12 months and those who were stunted. Infants and malnourished children may require a lower inoculum to cause MSD which may go undetected by culture methods. Culture also missed over half of the Shigella-associated deaths. A simplified severity score, composed of dehydration status, diarrhea duration prior to presentation, and clinician decision to hospitalize performed similarly to a MVS at predicting mortality in the 2 weeks following Shigella-diarrhea presentation.
Although reliance on culture determination alone in vaccine trials will require larger trials, omission of culture-confirmation will not be conducive to Shigella serotyping, a critical consideration in assessing serotype-specific immunity. Antibiotic resistance determination in Shigella and other enteric bacteria, an important secondary endpoint of Shigella vaccine trials, will also require cultured isolates for interpretation. In the absence of methods for culture-independent serotyping and resistance testing, we suggest that vaccine trials be powered for culture-confirmation with a prespecified secondary molecular microbiologic endpoint that disaggregates low and high concentrations of Shigella DNA.
Care-seeking for diarrhea has been shown to correlate with severity, linear growth faltering, and mortality [19–21]. Medically attended diarrhea has been suggested as a key feature of vaccine trial endpoints [5] and, practically, centralizes clinical assessments of dehydration and other indicators of severity. With a 14-day Shigella case fatality rate of approximately 1.5%, an MSD definition among children seeking care (as used in GEMS) may be sufficient for clinical endpoint severity classification. Alternatively, the MVS or the simplified model-derived severity score could be used to stratify severity among care-seeking diarrhea cases. The 3 factors included in our model-derived score are all included in the MVS, and the 2 scores performed similarly at predicting immediate deaths, despite our score being derived within the same dataset in which its performance was validated. Dysentery did not end up in the model-derived score, possibly because of antibiotic management of dysentery according to WHO guidelines [22]. Despite this lack of association, we advocate for dysentery to be included in a severe Shigella case definition because it is a sign of intestinal inflammation and epithelial destruction, consequences of Shigella that likely lead to its long-term impact on growth.
Risk factors analyses revealed the host factors of young age, stunting, and wasting to be independently associated with death, as has been found previously [23–26]. The clinical presentation of Shigella in infants more commonly included vomiting, dehydration, and absence of dysentery, which is consistent with previous findings among infants in Bangladesh using culture-based diagnosis [23]. Decisions about when to introduce a Shigella vaccine must weigh the lower burden but higher risk of Shigella infection among infants against the difficulty of including new vaccinations in the early infant immunization schedule. Moreover, vaccination prior to 6 months may be more effective by predating nutritional deterioration attributed to Shigella.
There were a number of limitations to this analysis. Because of its exploratory nature, the limited number of deaths, as well as the use of AIC, rather than P-values, for developing a severity score, we did not account for multiple comparisons. We were limited by which and how clinical data were collected to inform the severity scores. For example, 2 previously validated MVS [27, 28] could not be generated with this data due to the unavailability or lack of finer categorization of some symptoms. Validation of our model-derived Shigella severity score in other cohorts where death or other poor outcomes are ascertained would strengthen this score’s utility and generalizability. We chose 14-day mortality as the gold standard measure of severity; however, children may have severe shigellosis and survive. We found bacteria other than Shigella to be more common in children with culture-negative/qPCR-attributable Shigella (but not viral etiologies) and cannot exclude the possibility that a small subset of this group of children had a bacterial etiology other than Shigella. Ultimately, vaccine trials that include both culture- and qPCR-based diagnostics can confirm culture-negative qPCR-attributable episodes are indeed Shigella.
The GEMS study design and extensive diagnostic testing provided a unique opportunity to compare clinical features of Shigella by diagnostic categories and to examine risk factors for death. A composite severity score consistent with severe disease or dysentery may be a pragmatic clinical endpoint (confirmed by culture and secondarily, by qPCR) in vaccine trials.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the children who participated in these studies and their families, and the dedicated physicians, nurses, scientists, and staff at each study site for their dedication and outstanding performance of clinical and laboratory study activities.
Financial support. This work was supported by the Bill & Melinda Gates Foundation (OPP1019093 and OPP1197861).
Potential conflicts of interest. P. P. and K. K. received funding from the Bill & Melinda Gates Foundation for part of the submitted work and outside the submitted work; D. S. reports employment and shares with GSK Vaccines, outside the submitted work. S. T. reports grants from the Bill & Melinda Gates Foundation during the conduct of the study. All other authors have no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Khalil IA, Troeger C, Blacker BF, et al. . Morbidity and mortality due to Shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990–2016. Lancet Infect Dis 2018; 18: 1229– 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mani S, Wierzba T, Walker RI. Status of vaccine research and development for Shigella. Vaccine 2016; 34:2887–94. [DOI] [PubMed] [Google Scholar]
- 3. Barry E, Cassels F, Riddle M, Walker R, Wierzba T. Vaccines against Shigella and enterotoxigenic Escherichia coli: a summary of the 2018 VASE Conference. Vaccine 2019; 37:4768–74. [DOI] [PubMed] [Google Scholar]
- 4. Anderson JDt, Bagamian KH, Muhib F, et al. . Potential impact and cost-effectiveness of future ETEC and Shigella vaccines in 79 low- and lower middle-income countries. Vaccine X 2019; 2:100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Porter CK, Gutierrez RL, Kotloff KL. Clinical endpoints for efficacy studies. Vaccine 2019; 37:4814–22. [DOI] [PubMed] [Google Scholar]
- 6. Liu J, Kabir F, Manneh J, et al. . Development and assessment of molecular diagnostic tests for 15 enteropathogens causing childhood diarrhoea: a multicentre study. Lancet Infect Dis 2014; 14:716–24. [DOI] [PubMed] [Google Scholar]
- 7. Liu J, Platts-Mills JA, Juma J, et al. . Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 2016; 388:1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soares-Weiser K, Bergman H, Henschke N, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev 2019; 3:CD008521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis 1990; 22:259–67. [DOI] [PubMed] [Google Scholar]
- 10. Kotloff KL, Nataro JP, Blackwelder WC, et al. . Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 11. Kotloff KL, Blackwelder WC, Nasrin D, et al. . The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin Infect Dis 2012; 55 Suppl 4:S232–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Livio S, Strockbine NA, Panchalingam S, et al. . Shigella isolates from the global enteric multicenter study inform vaccine development. Clin Infect Dis 2014; 59:933–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Panchalingam S, Antonio M, Hossain A, et al. . Diagnostic microbiologic methods in the GEMS-1 case/control study. Clin Infect Dis 2012; 55 Suppl 4:S294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu J, Almeida M, Kabir F, et al. . Direct detection of Shigella in stool specimens by use of a metagenomic approach. J Clin Microbiol 2018; 56:e01374–17. doi: 10.1128/JCM.01374-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization. Chart booklet: integrated management of childhood illness. Geneva, Switzerland: WHO; 2014. [Google Scholar]
- 16. Kotloff KL, Platts-Mills JA, Nasrin D, Roose A, Blackwelder WC, Levine MM. Global burden of diarrheal diseases among children in developing countries: Incidence, etiology, and insights from new molecular diagnostic techniques. Vaccine 2017; 35:6783–9. [DOI] [PubMed] [Google Scholar]
- 17. Platts-Mills JA, Liu J, Rogawski ET, et al. ; MAL-ED Network Investigators . Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health 2018; 6:e1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sullivan LM, Massaro JM, D’Agostmo RB. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med 2004; 23:1631–60. [DOI] [PubMed] [Google Scholar]
- 19. Lamberti LM, Fischer Walker CL, Taneja S, Mazumder S, Black RE. The influence of episode severity on caregiver recall, care-seeking, and treatment of diarrhea among children 2–59 months of age in Bihar, Gujarat, and Uttar Pradesh, India. Am J Trop Med Hyg 2015; 93:250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lamberti LM, Fischer Walker CL, Black RE. Systematic review of diarrhea duration and severity in children and adults in low- and middle-income countries. BMC Public Health 2012; 12:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kotloff KL, Nasrin D, Blackwelder WC, et al. . The incidence, aetiology, and adverse clinical consequences of less severe diarrhoeal episodes among infants and children residing in low-income and middle-income countries: a 12-month case-control study as a follow-on to the Global Enteric Multicenter Study (GEMS). Lancet Glob Health 2019; 7:e568–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organization. The treatment of diarrhoea: a manual for physicians and other senior health workers. Geneva, Switzerland: WHO; 2007. [Google Scholar]
- 23. Huskins WC, Griffiths JK, Faruque AS, Bennish ML. Shigellosis in neonates and young infants. J Pediatr 1994; 125:14–22. [DOI] [PubMed] [Google Scholar]
- 24. Bennish ML, Harris JR, Wojtyniak BJ, Struelens M. Death in shigellosis: incidence and risk factors in hospitalized patients. J Infect Dis 1990; 161:500–6. [DOI] [PubMed] [Google Scholar]
- 25. Bennish ML, Wojtyniak BJ. Mortality due to shigellosis: community and hospital data. Rev Infect Dis 1991; 13 Suppl 4:S245–51. [DOI] [PubMed] [Google Scholar]
- 26. Levine MM, Nasrin D, Acácio S, et al. . Diarrhoeal disease and subsequent risk of death in infants and children residing in low-income and middle-income countries: analysis of the GEMS case-control study and 12-month GEMS-1A follow-on study. Lancet Glob Health 2020; 8:e204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Freedman SB, Eltorky M, Gorelick M; Pediatric Emergency Research Canada Gastroenteritis Study Group . Evaluation of a gastroenteritis severity score for use in outpatient settings. Pediatrics 2010; 125:e1278–85. [DOI] [PubMed] [Google Scholar]
- 28. Schnadower D, Tarr PI, Gorelick MH, et al. . Validation of the modified Vesikari score in children with gastroenteritis in 5 US emergency departments. J Pediatr Gastroenterol Nutr 2013; 57:514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.