Abstract
Background
Antiretroviral therapy (ART) regimens that contain dolutegravir (DTG) have been associated with increases in body mass index (BMI) in adults. However, this relationship has not been well described in adolescents.
Methods
In a retrospective observational cohort of 460 virally suppressed (<200 copies/mL) adolescents living with human immunodeficiency virus at a clinical site in Eswatini, body mass index (BMI) measurements were analyzed between 1 year prior to the transition to DTG and up to 1 year after DTG transition. Random-effects linear spline models were used to describe the rate of change in BMI before and after the transition to DTG.
Results
In adolescents, BMI increased at a rate of 0.3 kg/m2 per year before DTG transition and increased to a rate of 1.2 kg/m2 per year after DTG transition. Sex of the adolescent modified the relationship between DTG and rate of BMI change: BMI rate of change after DTG transition was increased by 1.1 kg/m2 in females and 0.6 kg/m2 per year in males.
Conclusions
Transition to DTG in virally suppressed adolescents (aged 10–19 years) is associated with an increase in the rate of BMI change. Female adolescents may experience a larger change than males. Further investigation is required to elucidate the mechanism that underlies these observations and to assess how DTG impacts BMI in adolescents following longer durations of treatment.
Keywords: HIV, adolescent, body mass index, adverse events
Dolutegravir has been associated with weight gain in adults. In this cohort of virally suppressed adolescents living with Human Immunodeficiency Virus, transition to dolutegravir was associated with an accelerated rate of body mass index increase. The effect was most pronounced in female adolescents.
Antiretroviral therapy (ART) reduces human immunodeficiency virus (HIV)–associated morbidity and mortality. Adolescents struggle with poor adherence and ART-related adverse events that can reduce the efficacy of treatment [1–3]. Dolutegravir (DTG)–based ART results in similar rates of viral suppression as efavirenz (EFV)–based ART but has lower rates of treatment discontinuation due to medication-related side effects [4]. The World Health Organization (WHO) now recommends DTG-containing regimens as the preferred ART for adolescents living with HIV (ALHIV) [5].
Initiation of ART is associated with an improved growth trajectory in children, which reflects the beneficial effects of ART on the immune system and metabolism [6]. Data on how ART initiation in adolescence, among perinatally infected children, impacts growth trajectory is more inconclusive [7]. Integrase inhibitors (INSTIs) are associated with more weight gain in adults following initiation of ART than other HIV drug classes [8]. Several studies suggest that this effect is increased by DTG when compared to other INSTI drugs [8–10]. Women, black people, and people on tenofovir alafenamide fumarate as a companion drug to DTG are at risk for greater weight gain [4, 10, 11]. Some literature indicates that virally suppressed adult patients who transition to DTG-based ART may also experience weight gain in excess of patients who remain on EFV-based regimens and compared with patients who transition to protease inhibitor (PI)–based regimens [11, 12]; however, there are conflicting data [13]. There is 1 case report of a 10-year-old girl who experienced excessive weight gain following transition to DTG [14], but otherwise there are no data on the impact of DTG-containing regimens on weight or body mass index (BMI) in ALHIV, and limited safety and efficacy data in treatment-experienced ALHIV [15].
Regardless of the specific antiretroviral regimen, children and young adolescents living with HIV (C/ALHIV) have improvements in weight-for-age z score (WAZ), height-for-age z score (HAZ), and BMI-for-age z score (BAZ) in the first 2 years after ART initiation [16]. Despite improvements, 30% of adolescent males and 15% of adolescent females living with HIV in sub-Saharan Africa are stunted toward the end of adolescence [17, 18]. Female ALHIV may also experience increases in percentage body fat compared with male ALHIV and female HIV-negative adolescents [19].
Therefore, ART initiated in childhood certainly improves growth trajectory for ALHIV, but ART may also be associated with increases in adiposity, especially in females. ALHIV throughout the world are transitioning to DTG-based ART because it is tolerable and highly effective. However, there is a growing body of literature suggesting that DTG is associated with increases in weight gain among adult patients. The effect of DTG-based regimens on growth trajectories and weight gain in adolescents needs to be explored as we transition children and adolescents to this medication.
In Eswatini, virally suppressed adolescent patients were transitioned to DTG during 2019 in accordance with guidance from the Ministry of Health [20]. Here we describe modifications in BMI, WAZ, HAZ, and BAZ trajectory among virally suppressed adolescents transitioned to DTG.
METHODS
We conducted a retrospective observational cohort analysis of BMI measurements in adolescents (10–19 years of age) living with HIV using data abstracted from the electronic medical record at the Baylor Children’s Foundation–Eswatini HIV Center of Excellence (COE). This study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines [21]. The COE, located in Mbabane, Eswatini, provides comprehensive HIV care for children, adolescents, and their families. All services are supported by the Eswatini Ministry of Health and are free of charge. Supportive programs for adolescents include adolescent-friendly services, a dedicated teen waiting area, a teen club, and teen camp. In early 2019, virally suppressed adolescent patients were transitioned to DTG-containing regimens in response to Ministry of Health guidance [20]. In June 2020, the electronic medical record of the COE was queried to identify individuals who had been transitioned to DTG.
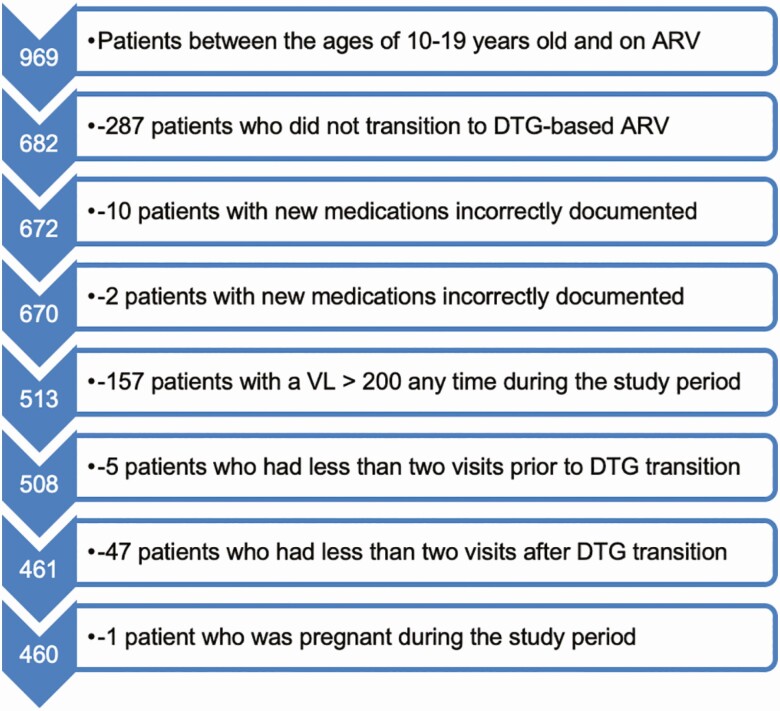
Participants were included if they were between the ages of 10 and 19 years at DTG transition. Visits were restricted to within 365 days before or after transition to DTG. Patients were excluded if their antiretroviral medication regimen was not accurately captured prior to or after transition to DTG, if during any of their visits they had a documented viral load of >200 copies/mL, if they had <2 visits recorded before or after DTG transition, or if they were pregnant during the study period (Figure 1). To minimize the influence of mismeasurements in BMI, visits whose rate of change in BMI between sequential visits was >0.05 kg/m2 per day were dropped. Laboratory values were extracted from clinic visits and were matched by chart number and visit date to clinic visits that also measured weight and height. Rate of BMI change was captured before and after DTG transition because BMI increases naturally throughout adolescence from a normal BMI of 16.25 kg/m2 at 10 years of age in boys and girls to a normal BMI of 21.5 kg/m2 in girls and a BMI of 22 kg/m2 in boys by 19 years of age [20]. This results in an average yearly increase of 0.525 kg/m2 in girls and 0.575 kg/m2 in boys to maintain a normal BMI. The z scores were calculated using the WHO growth charts, and the BMI categories of thinness (grades I–III), normal weight, overweight, and obese were calculated using the WHO BMI criteria [22, 23].
Figure 1.
Flowchart of patients included in the sample. Abbreviations: ARV, antiretroviral; DTG, dolutegravir; VL, viral load.
The descriptive statistics in Table 1 demonstrate the distribution of the variables for DTG companion drugs, prior ART regimens and BMI categories prior to and after DTG transmission for the full cohort and by sex. The average of anthropometric and clinical variables was computed for each patient among visits before and after DTG transition.
Table 1.
Demographic and Clinical Characteristics of the Full Cohort and Stratified by Sex
| Characteristic | Total (N = 460) | Female (n = 211) | Male (n = 249) |
|---|---|---|---|
| ART regimen post-DTG | |||
| ABC/3TC/DTG | 50 (10.9) | 16 (7.6) | 34 (13.7) |
| TDF/3TC/DTG | 410 (89.1) | 195 (92.4) | 215 (86.3) |
| Age at transition to DTG, y | |||
| 10–12 | 135 (29.3) | 56 (26.5) | 79 (31.7) |
| 13–15 | 176 (38.3) | 80 (37.9) | 96 (38.6) |
| 16–19 | 149 (32.4) | 75 (35.5) | 74 (29.7) |
| Median (IQR) | 14.0 (12.0–16.0) | 14.0 (12.0–17.0) | 14.0 (12.0–16.0) |
| Previous ARV regimen | |||
| PI | 4 (0.9) | 2 (0.9) | 2 (0.8) |
| EFV | 111 (24.1) | 58 (27.5) | 53 (21.3) |
| NVP | 345 (75.0) | 151 (71.6) | 194 (77.9) |
| Highest BMI category before DTG | |||
| Thin | 40 (8.7) | 10 (4.7) | 30 (12.0) |
| Normal | 393 (85.4) | 182 (86.3) | 211 (84.7) |
| Overweight/obese | 27 (5.9) | 19 (9.0) | 8 (3.2) |
| Highest BMI category after DTG | |||
| Thin | 33 (7.2) | 10 (4.7) | 23 (9.2) |
| Normal | 396 (86.1) | 177 (83.9) | 219 (88.0) |
| Overweight/obese | 31 (6.7) | 24 (11.4) | 7 (2.8) |
| Mean (SD) BMI before DTG | 18.5 (2.6) | 19.2 (3.0) | 17.8 (2.1) |
| Mean (SD) BMI after DTG | 19.1 (2.7) | 20.1 (3.0) | 18.4 (2.2) |
| Mean (SD) weight before DTG | 41.2 (10.7) | 42.9 (10.3) | 39.7 (10.8) |
| Mean (SD) weight after DTG | 44.4 (10.6) | 46.1 (10.0) | 42.9 (10.9) |
| Mean (SD) height before DTG | 148.0 (12.1) | 148.3 (10.0) | 147.8 (13.6) |
| Mean (SD) height after DTG | 151.2 (11.2) | 150.7 (8.8) | 151.5 (13.0) |
| Mean (SD) CrCl before DTG | 135.6 (28.0) | 134.4 (31.4) | 136.7 (24.8) |
| Mean (SD) CrCl after DTG | 112.2 (21.1) | 109.6 (22.5) | 114.3 (19.7) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; ARV, antiretroviral; BMI, body mass index; CrCl, creatinine clearance; DTG, dolutegravir; EFV, efavirenz; IQR, interquartile range; NVP, nevirapine; PI, protease inhibitor; SD, standard deviation; TDF, tenofovir disoproxil fumarate.
The primary outcome was BMI and the covariates that were included in all analyses were time in years, sex, DTG companion drugs (abacavir [ABC]/lamivudine [3TC]/DTG vs tenofovir disoproxil fumarate [TDF]/3TC/DTG), prior ART regimens (EFV vs nevirapine [NVP] vs PI) and age at DTG transition, treated continuously. The rate of change in BMI was assessed before and after DTG transition. A subgroup analysis was performed to evaluate the impact of sex on the effect of DTG on the rate of change in BMI. The secondary outcomes examined were WAZ, HAZ, and BAZ.
For the primary outcome, a random-effects piecewise linear spline model was fit to model average BMI in the full cohort and in subgroups of patients stratified by normal, thin, or overweight/obese BMI classifications prior to DTG transition. In the subgroup analysis of sex, the same model was fit in males or females in the full cohort and in each of the categories of BMI prior to DTG transition. For the secondary analysis, a random-effects piecewise linear spline model was fit to model average BAZ, WAZ, or HAZ in the full cohort of patients and adjusted for all covariates. Supplemental analysis was conducted to model BAZ in subgroups stratified by BMI category and sex, as well as BMI in similar subgroups within all individuals who transitioned to DTG regardless of viral suppression. Graphs of predicted outcomes were constructed using the most prevalent covariate values, with 95% predictive intervals using the associated standard error of prediction from the fully adjusted model fitted to the relevant population.
All clinical investigation supporting the data handling, analysis, and reporting of these findings was conducted according to the principles in the Declaration of Helsinki. Approval was obtained from the Baylor College of Medicine Children’s Foundation Institutional Review Board (IORG number 0006978), the Eswatini National Health Research Review Board, and the Baylor College of Medicine Institutional Review Board (FWA 00000286).
RESULTS
The final cohort consisted of 460 patients (Table 1), of whom 211 (45.9%) were female and 249 (54.1%) were male. The cohort was exclusively black from sub-Saharan Africa. Fifty (10.9%) patients transitioned to ABC/3TC/DTG and 410 (89.1%) transitioned to TDF/3TC/DTG. The majority of patients (345 [75.0%]) in the cohort were transitioned to DTG from NVP-based regimens, whereas 111 (24.1%) transitioned from EFV-based regimens and 4 (0.9%) transitioned from PIs. The cohort was diverse in age range and well represented among the ages 10–19 years at DTG transition. BMI strata were different in females and males, with a greater percentage of females being classified as overweight or obese and a greater percentage of males being classified as thin before and after DTG transition.
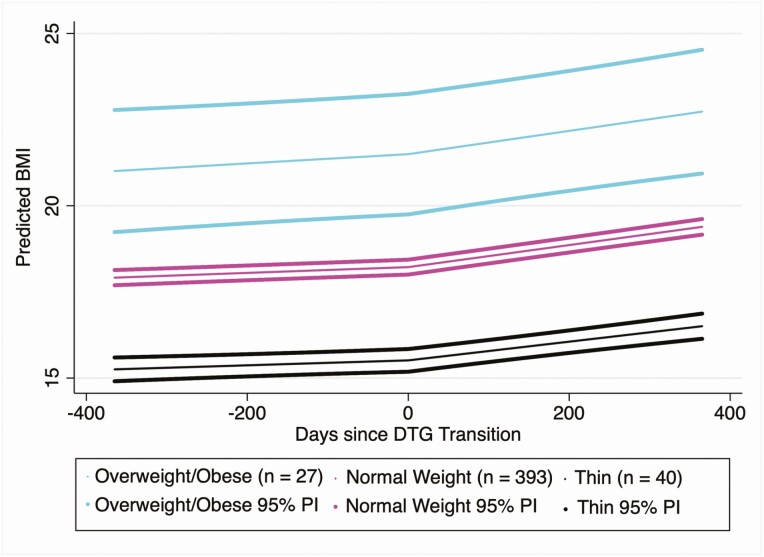
Table 2 demonstrates the results of the piecewise-linear regression approach used to model average BMI rate of change (kg/m2 per year) in the full cohort, and stratified by BMI category prior to DTG transition (Figure 2). Among the full cohort, the rate of increase in BMI prior to DTG was approximately 0.3 kg/m2 per year (95% confidence interval [CI], .2–.4). After DTG, the rate of change in BMI was approximately 1.2 kg/m2 per year (95% CI, 1.1–1.3), a difference of 0.8 kg/m2 per year (95% CI, .7–1.0). Similar effect sizes were observed in the normal and thin BMI populations. No measurable difference in the rates of change in BMI before and after DTG transition were observed in those who were overweight or obese prior to DTG, though the post-DTG BMI rate in this subgroup was determined to be significantly greater than zero (1.2 kg/m2 [95% CI, .5–1.9] per year,) while the pre-DTG BMI rate was not.
Table 2.
Body Mass Index (BMI) Change Over Time in the Full Cohort and Stratified by BMI Category
| Rate of BMI Increase Before and After DTG | Full Cohort (N = 460) |
Thin (n = 40) |
Normal (n = 393) |
Obese/Overweight (n = 27) |
|---|---|---|---|---|
| Before | 0.3 (.2–.4)*** | 0.3 (.1–.5)* | 0.3 (.2–.4)*** | 0.5 (.0 to 1.1) |
| After | 1.2 (1.1–1.3)*** | 1.0 (.7–1.2)*** | 1.2 (1.1–1.3)*** | 1.2 (.5–1.9)** |
| Difference | 0.8 (.7–1.0)*** | 0.7 (.3–1.1)*** | 0.9 (.7–1.0)*** | 0.7 (–.5 to 1.8) |
Data are presented as kg/m2 per year (95% confidence interval). Selected coefficients relating to time on or off DTG are shown after adjusting for age at DTG transition, previous medications, DTG companion drugs, and sex.
Abbreviations: BMI, body mass index; DTG, dolutegravir.
*P < .05; **P < .01; ***P < .001.
Figure 2.
Dolutegravir (DTG) is associated with an acceleration in body mass index (BMI) increase. Predictions were constructed using the following covariate values: male, 14 years old at DTG transition, previously on nevirapine-based antiretroviral therapy, and transitioned to tenofovir disoproxil fumarate/lamivudine/DTG. Predicted BMI and 95% predictive intervals (PIs) were constructed using estimates and the associated standard error of prediction from the fully adjusted model fitted in those who were thin, normal weight, or obese/overweight prior to DTG transition.
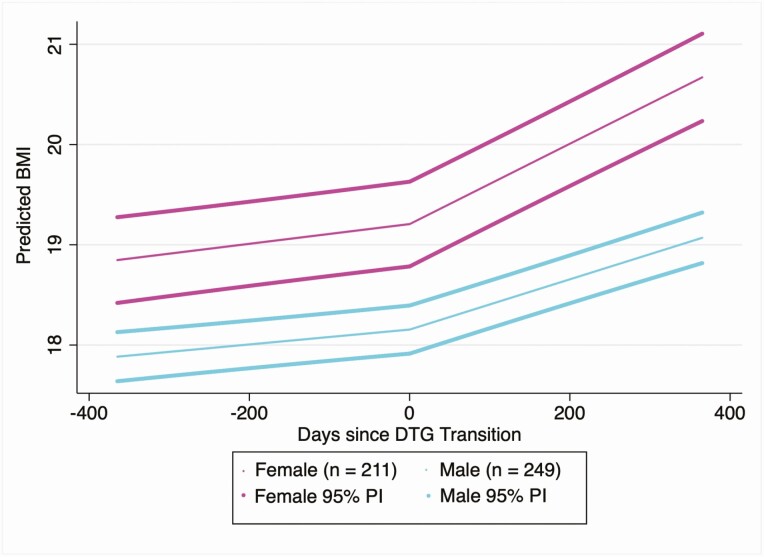
Table 3 and Figure 3 demonstrate the results of the subgroup analysis completed to determine whether sex modifies the effect of DTG on rate of BMI change. Among the full cohort, females increased their rate of change in BMI by approximately 1.1 kg/m2 per year after DTG (95% CI, .8–1.4) and males increased their rate of change in BMI by approximately 0.6 kg/m2 per year after DTG (95% CI, .4–.8). Among those who were normal weight prior to DTG transition, females increased their rate of change in BMI by 1.2 kg/m2 per year (95% CI, .9–1.5) and males increased their rate of change in BMI by 0.6 kg/m2 per year (95% CI, .4–.8) (Table 4). Similar results were observed in those who were thin prior to DTG transition but not in those who were overweight/obese. In this small group of overweight patients, males increased their BMI at a greater rate than females following DTG transition. Supplementary Figure 1 demonstrates individual plots of observed longitudinal BAZ measurements for 9 females in the cohort who transitioned from a normal BMI to an overweight/obese BMI after DTG transition.
Table 3.
Body Mass Index Change in Males Versus Females in Full Cohort (N = 460)
| Rate of BMI Increase Before and After DTG | Females (n = 211) | Males (n = 249) |
|---|---|---|
| Before | 0.4 (.2–.5)*** | 0.3 (.2–.4)*** |
| After | 1.5 (1.3–1.6)*** | 0.9 (.8–1.0)*** |
| Difference | 1.1 (.8–1.4)*** | 0.6 (.4–.8)*** |
Data are presented as kg/m2 per year (95% confidence interval). Selected coefficients relating to time on or off DTG are shown after adjusting for age at DTG transition, previous medications, DTG companion drugs, and 2 interaction terms between sex and the before and after rate coefficients.
Abbreviations: BMI, body mass index; DTG, dolutegravir.
***P < .001.
Figure 3.
Dolutegravir (DTG) is associated with a greater acceleration in body mass index (BMI) increase among females compared with males. Predictions were constructed using the following covariate values: 14 years old at DTG transition, previously on nevirapine-based antiretroviral therapy, and transitioned to tenofovir disoproxil fumarate/lamivudine/DTG. Predicted BMI and 95% predictive intervals (PIs) were constructed using estimates and the associated standard error of prediction from the fully adjusted model fitted in either male or female adolescents.
Table 4.
Body Mass Index (BMI) Change in Males Versus Females by BMI Category
| Rate of BMI Increase Before and After DTG | Thin | Normal Weight | Overweight/Obese | |||
|---|---|---|---|---|---|---|
| Females (n = 10) |
Males (n = 30) |
Females (n = 182) |
Males (n = 211) |
Females (n = 19) |
Males (n = 8) |
|
| Before | 0.3 (–.2 to .7) | 0.2 (.0–.5)* | 0.3 (.2–.5)*** | 0.3 (.2–.4)*** | 0.9 (.2–1.6)** | –0.6 (–1.7 to .5) |
| After | 1.5 (.9–2.2)*** | 0.9 (.6–1.1)*** | 1.5 (1.3–1.7)*** | 0.9 (.7–1.0)*** | 0.8 (–.1 to 1.7) | 2.0 (.9–3.2)*** |
| Difference | 1.3 (.3–2.2)** | 0.6 (.2–1.1)** | 1.2 (.9–1.5)*** | 0.6 (.4–.8)*** | –0.1 (–1.4 to 1.3) | 2.6 (.6–4.6)** |
Data are presented as kg/m2 per year (95% confidence interval). Selected coefficients relating to time on or off DTG are shown after adjusting for age at DTG transition, previous medications, DTG companion drugs, and 2 interaction terms between sex and the before and after rate coefficients, fitted in the 3 subgroups of BMI categories.
Abbreviations: BMI, body mass index; DTG, dolutegravir.
*P < .05; **P < .01; ***P < .001.
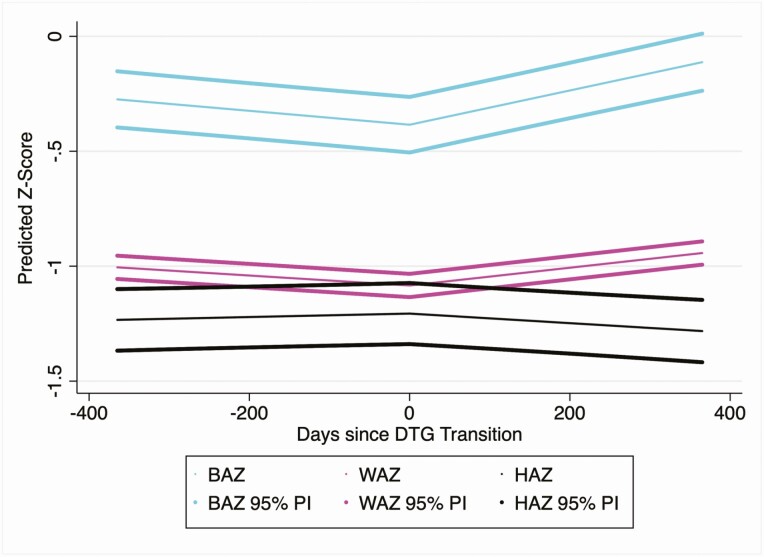
The secondary outcomes of BAZ, HAZ, and WAZ were modeled with the same methods in the full cohort (Table 5, Figure 4, and Supplementary Table 1). After adjusting for all covariates, individuals in the cohort were decreasing their BAZ prior to DTG transition at a rate of –0.1 per year (95% CI, –.1 to –.1) and subsequently increased their BAZ at a rate of 0.3 per year (95% CI, .2–.3) after DTG transition. A similar pattern was observed for the outcome of WAZ, in which the cohort was decreasing their WAZ at a rate of –0.1 per year (95% CI, –.1 to .0) prior to DTG transition and then began increasing their WAZ at a rate of 0.1 per year (95% CI, .1–.2) after DTG transition. The model for HAZ indicated that individuals were not changing their HAZ prior to DTG transition and began decreasing in HAZ at a rate of 0.1 per year (95% CI, –.2 to .0) after DTG transition.
Table 5.
Body Mass Index-for-Age z Score, Height-for-Age z Score, and Weight-for-Age z Score Changes in the Full Cohort
| Rate of BMI Increase Before and After DTG | BMI-for-Age z Score (95% CI) |
Weight-for-Age z Score (95% CI) | Height-for-Age z Score (95% CI) |
|---|---|---|---|
| Before | –0.1 (–.1 to –.1)*** | –0.1 (–.1 to .0)*** | 0.0 (.0 to .1) |
| After | 0.3 (.2–.3)*** | 0.1 (.1–.2)*** | –0.1 (–.1 to .0)*** |
| Difference | 0.4 (.3–.5)*** | 0.2 (.2–.3)*** | –0.1 (–.2 to .0)** |
Selected coefficients relating to time on or off DTG are shown after adjusting for age at DTG transition, previous medications, DTG companion drugs, and sex.
Abbreviations: BMI, body mass index; CI, confidence interval; DTG, dolutegravir.
**P < .01; ***P < .001.
Figure 4.
Dolutegravir (DTG) transition is associated with changes in body mass index-for-age z score (BAZ), weight-for age z score (WAZ), and height-for-age z score (HAZ). Predictions were constructed using the following covariate values: male, 14 years old at DTG transition, previously on nevirapine-based antiretroviral therapy, and transitioned to tenofovir disoproxil fumarate/lamivudine/DTG. Predicted BAZ, WAZ, and HAZ and 95% predictive intervals (PIs) were constructed using estimates and the associated standard error of prediction from the fully adjusted models fitted in the whole cohort.
Discussion
In this observational longitudinal cohort of 460 virally suppressed adolescents transitioned to DTG, we demonstrate that adolescents increased their rate of BMI change by 0.8 kg/m2 per year (95% CI, .7–1.0) in the year after DTG start to a rate of 1.2 kg/m2 per year (95% CI, 1.1–1.3). These data are among the first, outside of a clinical trial, to evaluate DTG use in adolescents in sub-Saharan Africa, and the first to demonstrate that DTG is associated with an increase in BMI among ALHIV. The increase was similar in normal and thin patients, while no change was detected among those who were obese or overweight prior to DTG transition. These modeled rates of BMI increase per year are greater, by approximately 2-fold in the entire cohort and 2.8-fold in females, than rates required to maintain a normal BMI rate of increase on the WHO child and adolescent growth charts [22]. If this rate of increase in BMI per year remains constant throughout adolescence, this may result in increases in overweight or obesity among ALHIV treated with DTG.
The overall cohort was increasing BMI at a rate resulting in a slight decrease in the BAZ over time prior to DTG transition. The reversal in this trend associated with DTG transition may be beneficial for adolescents with low BMI on EFV-based regimens if the initial normalization of BMI increase does not ultimately result in obesity. Adolescents in this study who were thin prior to DTG transition did not have a higher increase in BMI rate of change than adolescents who started at a normal BMI, as has been seen in cohorts in adults [24, 25]. This may be due to the fact that our cohort was limited to patients already virally suppressed on ART and did not include ART-naive individuals. Adolescents who were overweight or obese prior to DTG transition also experienced increases in BMI rate of change associated with DTG transition. Though the change was not significant at an α = .05, it is possible that the small size of this population limited power to detect a significant change. Indeed, the consistency of this effect across BMI strata suggests that all adolescents on DTG may need to be monitored for accelerated BMI increases. This may have significant health implications as adolescents age into adulthood; increases in BMI among adults living with HIV has been linked to inflammation [26] and dyslipidemia [27], and is particularly concerning due to the increased risk of cardiovascular complications attributable to HIV infection [28].
Our data suggest that adolescent females are at particular risk for weight gain following the transition to DTG. Consistent with prior data on female C/ALHIV, females in this cohort had a BMI that was higher than that among males overall [19, 29]. Despite having a higher BMI on average, females also increased their rate of BMI change 3.8-fold following DTG transition as compared to 3.0-fold for males. This greater increase in BMI was even more dramatic in normal-weight and thin females compared with males in the same BMI strata. These data are consistent with data from adult studies, which have identified women as being at greater risk for BMI changes following DTG [10, 11]. Prepregnancy obesity also carries the additional risk for females of adverse reproductive and pregnancy outcomes [30]. Overall, it speaks to the need for counseling for female ALHIV on the risks of obesity and healthy diet and exercise, and points to the need for an approach to HIV medicine in high-burden settings that is differentiated across diverse patient populations.
There are high rates of stunting among ALHIV [17]. This cohort was marginally stunted on average and we also observed a slight decrease in the rate of HAZ change after DTG transition. This observed reduction in HAZ change is unlikely attributable to DTG. Notably, the effect size of DTG on WAZ was twice as large as the effect on HAZ. Chronic undernutrition and stunting in childhood have paradoxically been linked to a greater risk of becoming overweight in adulthood [29, 31]. Therefore, stunted ALHIV, such as those in this cohort, may be at greater risk for adult obesity, and further study is needed to determine whether DTG may additionally contribute to this risk.
The mechanism of weight gain attributable to DTG is unknown, although it has been postulated that nonnucleoside reverse transcriptase inhibitors, such as EFV and NVP, may result in weight suppression through subclinical toxicity [32]. This may explain the less than ideal increases in BMI that were observed before the transition to DTG within this cohort. The physiologic underpinnings for increased weight gain in females living with HIV, and in association with DTG use, as compared to males is unknown and needs additional research.
These data are the first to describe the impact of DTG on adolescent BMI trajectory, but there are some limitations: notably, that this was a retrospective observational study, with data derived from the clinical record and with associated risks of inaccurate data entry and confounding. We took steps to limit the influence of these factors on our results by eliminating biologically implausible BMI changes (4.4% of visits) and controlling for confounding variables. In addition, adolescents were transitioned to DTG in accordance with Eswatini Ministry of Health guidance. Thus, the population not transitioning to DTG was inherently different than those who did, precluding meaningful comparisons. The clinic provides services for a large catchment area of urban and periurban families surrounding Mbabane, the capital of Eswatini. These adolescents may have higher socioeconomic status, lower rates of food insecurity, and higher educational attainment than adolescents in more rural areas, limiting generalizability. We have also limited the analysis to patients with viral suppression. Although this approach isolated the effect of DTG, apart from weight gain associated with changes in viral suppression, our results may be less generalizable to all ALHIV. Using the same models in the full cohort of individuals transitioned to DTG, regardless of viral suppression, we found that the effect estimates and conclusions remain robust, if not more dramatic (Supplementary Table 2). A possible source of bias arises from the potential for residual confounding or effect measure modification of age on BMI rate of change. Finally, the electronic medical record does not capture data on pubertal staging. Data suggest that delayed ART can delay pubertal development [33], but it is not clear whether transitioning to DTG among virally suppressed adolescents will have any impact on pubertal staging.
It is important that adolescents benefit from DTG; however, when transitioning adolescents to DTG, monitoring is needed to identify DTG-associated adverse events for which ALHIV may be uniquely or disproportionally at risk. Female adolescents transitioning to DTG may need particular attention due to larger increases in BMI change. Longitudinal cohorts that follow adolescents into adulthood are needed to determine how DTG initiated in children and adolescents may impact health outcomes in adulthood.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the patients and the dedicated clinicians at Baylor Clinic–Eswatini; and Nkulungwane Mthethwa for his thoughtful contributions to the data abstraction.
Financial support. This work was supported by the National Institutes of Health (grant number 1K01TW011482-01A1 to A. W. K.).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Mark D, Armstrong A, Andrade C, . et al. HIV treatment and care services for adolescents: a situational analysis of 218 facilities in 23 sub-Saharan African countries. J Int AIDS Soc 2017; 20:21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van de Wijer L, Mchaile DN, de Mast Q, . et al. Neuropsychiatric symptoms in Tanzanian HIV-infected children receiving long-term efavirenz treatment: a multicentre, cross-sectional, observational study. Lancet HIV 2019; 6:e250–8. [DOI] [PubMed] [Google Scholar]
- 3. Kanters S, Vitoria M, Doherty M, . et al. Comparative efficacy and safety of first-line antiretroviral therapy for the treatment of HIV infection: a systematic review and network meta-analysis. Lancet HIV 2016; 3:e510–20. [DOI] [PubMed] [Google Scholar]
- 4. Venter WDF, Moorhouse M, Sokhela S, . et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–15. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. Update of recommendations on first- and second-line antiretroviral regimens.2019. Available at: https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.15-eng.pdf?ua=1. Accessed 10 August 2020.
- 6. Almeida FJ, Kochi C, Sáfadi MAP. Influence of the antiretroviral therapy on the growth pattern of children and adolescents living with HIV/AIDS. J Pediatr 2019; 95(Suppl 1):95–101. [DOI] [PubMed] [Google Scholar]
- 7. Schomaker M, Leroy V, Wolfs T, . et al. Optimal timing of antiretroviral treatment initiation in HIV-positive children and adolescents: a multiregional analysis from Southern Africa, West Africa and Europe. Int J Epidemiol 2017; 46:453–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bourgi K, Rebeiro PF, Turner M, . et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2020; 70:1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kouanfack C, Mpoudi-Etame M, Bassega PO, et al. NAMSAL ANRS 12313 Study Group . Dolutegravir-based or low-dose efavirenz-based regimen for the treatment of HIV-1. N Engl J Med 2019; 381:816–26. [DOI] [PubMed] [Google Scholar]
- 10. Sax PE, Erlandson KM, Lake JE, . et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020; 71:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lake JE, Wu K, Bares SH, . et al. Risk factors for weight gain following switch to integrase inhibitor-based antiretroviral therapy [manuscript published online ahead of print 26 February 2020]. Clin Infect Dis 2020; 71:e471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Norwood J, Turner M, Bofill C, . et al. Brief report: weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr 2017; 76:527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burns JE, Stirrup OT, Dunn D, . et al. No overall change in the rate of weight gain after switching to an integrase-inhibitor in virologically suppressed adults with HIV. AIDS 2020; 34:109–14. [DOI] [PubMed] [Google Scholar]
- 14. Li J, Yusuf EH, Agwu AL. Excessive weight gain associated with dolutegravir initiation in a 10-year-old female with perinatally acquired human immunodeficiency virus: a case report and review of the literature [manuscript published online ahead of print 25 May 2020]. J Pediatric Infect Dis Soc 2020. doi: 10.1093/jpids/piaa052 [DOI] [PubMed] [Google Scholar]
- 15. Viani RM, Ruel T, Alvero C, . et al. Long-term safety and efficacy of dolutegravir in treatment-experienced adolescents with human immunodeficiency virus infection: results of the IMPAACT P1093 study. J Pediatric Infect Dis Soc 2020; 9:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jesson J, Koumakpaï S, Diagne NR, . et al. Effect of age at antiretroviral therapy initiation on catch-up growth within the first 24 months among HIV-infected children in the IeDEA West African Pediatric Cohort. Pediatr Infect Dis J 2015; 34:e159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jesson J, Schomaker M, Malasteste K, . et al. Stunting and growth velocity of adolescents with perinatally acquired HIV: differential evolution for males and females. A multiregional analysis from the IeDEA global paediatric collaboration. J Int AIDS Soc 2019; 22:e25412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cames C, Pascal L, Diack A, . et al. Risk factors for growth retardation in HIV-infected Senegalese children on antiretroviral treatment: the ANRS 12279 MAGGSEN pediatric cohort study. Pediatr Infect Dis J 2017; 36:e87–92. [DOI] [PubMed] [Google Scholar]
- 19. Sharma TS, Somarriba G, Arheart KL, . et al. Longitudinal changes in body composition by dual-energy radiograph absorptiometry among perinatally HIV-infected and HIV-uninfected youth: increased risk of adiposity among HIV-infected female youth. Pediatr Infect Dis J 2018; 37:1002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eswatini Ministry of Health. Amendment to the Eswatini integrated HIV management guidelines: implementation guide. Mbabane, Eswatini: Ministry of Health, 2019.
- 21. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370:1453–7. [DOI] [PubMed] [Google Scholar]
- 22. World Health Organization. WHO child growth standards and the identification of severe acute malnutrition in infants and children.2009. Available at: https://apps.who.int/iris/bitstream/handle/10665/44129/9789241598163_eng.pdf. Accessed 10 August 2020.
- 23. Vidmar SI, Cole TJ, Pan H. Standardizing anthropometric measures in children and adolescents with functions for egen: update. Stata J 2013; 13:366–78. [Google Scholar]
- 24. Mccomsey GA, Eron JJ, Santiago S, . et al. Weight gain during treatment among 3468 treatment-experienced adults with HIV. In: Conference on Retroviruses and Opportunistic Infections, 2020. Available at: https://www.croiconference.org/abstract/weight-gain-during-treatment-among-3468-treatment-experienced-adults-hiv/. Accessed 10 August 2020.
- 25. Taramasso L, Bonfanti P, Ricci E, . et al. Factors associated with weight gain in people treated with dolutegravir. Open Forum Infect Dis 2020; 7:ofaa195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mave V, Erlandson KM, Gupte N, . et al. Inflammation and change in body weight with antiretroviral therapy initiation in a multinational cohort of HIV-infected adults. J Infect Dis 2016; 214:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maia Leite LH, De Mattos Marinho Sampaio AB. Progression to overweight, obesity and associated factors after antiretroviral therapy initiation among Brazilian persons with HIV/AIDS. Nutr Hosp 2010; 25:635–40. [PubMed] [Google Scholar]
- 28. Schouten J, Wit FW, Stolte IG, . et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV Cohort Study. Clin Infect Dis 2014; 59:1787–97. [DOI] [PubMed] [Google Scholar]
- 29. Kimani-Murage EW, Kahn K, Pettifor JM, . et al. The prevalence of stunting, overweight and obesity, and metabolic disease risk in rural South African children. BMC Public Health 2010; 10:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu P, Xu L, Wang Y, . et al. Association between perinatal outcomes and maternal pre-pregnancy body mass index. Obes Rev 2016; 17:1091–102. [DOI] [PubMed] [Google Scholar]
- 31. Popkin BM, Richards MK, Montiero CA. Stunting is associated with overweight in children of four nations that are undergoing the nutrition transition. J Nutr 1996; 126:3009–16. [DOI] [PubMed] [Google Scholar]
- 32. Leonard MA, Bourgi K, Koethe J, . et al. Pharmacogenetics of weight gain after switch from efavirenz to integrase inhibitors. In: Conference on Retroviruses and Opportunistic Infections, 2020. Available at: https://2jg4quetidw2blbbq2ixwziw-wpengine.netdna-ssl.com/wp-content/uploads/sites/2/posters/2019/1430_Leonard_0472.pdf. Accessed 10 August 2020.
- 33. Szubert AJ, Musiime V, Bwakura-Dangarembizi M, . et al. Pubertal development in HIV-infected African children on first-line antiretroviral therapy. AIDS 2015; 29:609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.