Abstract
Background
We aimed to determine if treatment of male sexual partners of women with recurrent bacterial vaginosis (BV) with oral metronidazole 2×/day for 7 days (ie, multidose metronidazole) significantly decreased BV recurrence rates in the female.
Methods
This was a multicenter, 2-arm, double-blind, placebo-controlled study. Women with recurrent BV and current diagnosis of BV by Amsel and Nugent were enrolled. Multidose metronidazole for 7 days was dispensed to women. Male partners were randomized to placebo versus multidose metronidazole for 7 days and asked to refrain from unprotected sex for 14 days. Female follow-up visits were conducted at day 21 and 8 and 16 weeks. Male follow-up visits occurred at days 14–21. BV cure was defined as 0–2 Amsel criteria and Nugent score 0–6 in the female partner with the primary endpoint at 16 weeks.
Results
214 couples were enrolled. In the intent-to-treat population, there was no significant difference between treatment arms for the primary outcome. BV treatment failure occurred in 81% and 80% of women in the metronidazole and placebo arms through the third follow-up visit, respectively (P > .999). However, women whose male partners adhered to study medication were less likely to fail treatment (adjusted relative risk, .85; 95% CI, .73–.99; P = .035). This finding persisted in post hoc comparisons in the metronidazole arm.
Conclusions
Overall, this study did not find that male partner treatment with multidose metronidazole significantly reduces BV recurrence in female partners, although women whose partners adhered to multidose metronidazole were less likely to fail treatment.
Clinical Trials Registration
(NCT02209519).
Keywords: bacterial vaginosis, male partners, treatment, metronidazole, sexually transmitted infection
Treatment of the male sexual partner with oral metronidazole 500 mg 2×/day for 7 days showed no effect on bacterial vaginosis recurrence in the female. However, women whose partners were adherent to multidose metronidazole were less likely to fail treatment.
(See the Editorial Commentary by Vodstrcil and Bradshaw on pages e680–2.)
Bacterial vaginosis (BV) is a common vaginal infection [1, 2]. It is associated with adverse birth outcomes, pelvic inflammatory disease, and increased risk of acquisition of human immunodeficiency virus (HIV) and sexually transmitted infections (STIs) [3–8]. Bacterial vaginosis is a vaginal dysbiosis with loss of H2O2 and lactic acid–producing Lactobacillus spp. and high concentrations of facultative and strict anaerobic bacteria [9–11]. Symptomatic BV is characterized by vaginal discharge and/or odor; however, many women are asymptomatic [1]. Metronidazole or clindamycin are currently recommended therapies; however, recurrence rates are 50–70% [12]. Although BV epidemiology strongly suggests sexual transmission [13–15], treatment of sexual partners is not recommended [16]. This is based on prior male partner treatment studies of women with recurrent BV [17–22]. A meta-analysis found these studies had randomization flaws, inadequate medication dosing, lack of adherence measures, and limited power [23]. Given inadequacies of prior partner treatment trials, we aimed to determine if the treatment of male partners of women with recurrent BV with 7 days of oral metronidazole twice daily (the current preferred regimen for BV treatment [16]) significantly decreased the BV recurrence rate in the female.
METHODS
Study Design
This was a multicenter, 2-arm, double-blind, placebo-controlled trial (clinicaltrials.gov; NCT02209519). Women were recruited from the Jefferson County Department of Health (JCDH), the University of Alabama at Birmingham (UAB) Personal Health Clinic, and the Vaginitis Clinic at Wayne State University (WSU). The study was approved by the Institutional Review Boards of UAB and WSU. Women with symptomatic BV by Amsel criteria [1] and no evidence of concurrent trichomoniasis were invited. Inclusion criteria were 18 years of age or older, self-identity of heterosexual, symptoms of vaginal odor and/or discharge, current BV diagnosis by Amsel and Nugent, history of 2 or more BV episodes in the previous year, and a current, regular (sexually active at least 1 month) male partner. Exclusion criteria were presence of trichomoniasis, allergy to metronidazole, pregnant/nursing, HIV, and inability to keep appointments. Women with a history of recurrent BV for more than 2 years or who failed previous BV treatment studies were excluded as they are difficult-to-treat women, often requiring therapy beyond a 7-day course of oral metronidazole.
After baseline assessment, male partners were randomized to metronidazole or placebo using a permuted, random, block randomization generated by study statisticians, stratified by site. Participants, investigators, and study staff were blinded to treatment assignments. The UAB Investigational Drug Research Pharmacy (IDRP) formulated the placebo pills (capsules filled with lactose). Active tablets of metronidazole were over-encapsulated in a gelatin capsule with lactose filler. The placebo had an identical shape and size to the over-encapsulated metronidazole pill. Pills were placed in amber medication vials, labeled with the study drug number, and allocated according to the randomization scheme. Medication vials were placed in plastic bags with an instruction sheet for metronidazole. The UAB IDRP prepared study medications for both sites where they were kept in a locked file cabinet at room temperature until administered. Each randomized male received a vial of medication and was instructed to take 1 tablet by mouth twice daily for 7 days. A drug accountability log was kept. Adverse events, grade of severity, and relationship to study medication were monitored.
Female Procedures
Females were administered sexual history questionnaires as well as an exploratory couples’ verification tool [24]. A pelvic examination was conducted and findings noted including vaginal pH, whiff test, and wet-mount microscopy. A vaginal Gram stain was performed for Nugent score determination by research laboratory staff; if the baseline score was less than 7, the women were dropped. Nucleic acid amplification testing (NAAT) for Neisseria gonorrhoeae and Chlamydia trachomatis was performed as well as Trichomonas vaginalis InPouch culture (BioMed Diagnostics, White City, OR). Metronidazole 500 mg orally twice daily for 7 days was dispensed. Adherence counseling was verbally provided and participants were asked to avoid douching or other medication use as well as unprotected sex for 2 weeks. If a woman’s male partner did not accompany her she was asked to contact him at the time of the visit to schedule an appointment within 48 hours. Women testing positive for gonorrhea or chlamydia or if their partner tested positive were treated and continued in the study. If trichomoniasis was detected in either partner, they were both treated and discontinued.
Follow-up visits for women were conducted at day 21 and at 8 and 16 weeks. At each follow-up visit, an interval sexual history questionnaire was administered. Amsel and Nugent criteria were repeated. Participants were asked to return their metronidazole pill containers at the first follow-up visit. Adherence was defined as taking all medication in the container; otherwise, women were classified as nonadherent. Reimbursement was $50/visit at UAB and $60/visit at WSU.
Male Procedures
Male partners were seen within 48 hours of female enrollment. A sexual history questionnaire was administered as well as our couples’ verification tool [24]. Men were examined and screened for gonorrhea and chlamydia. They were randomized to placebo versus metronidazole 500 mg twice daily for 7 days. Adherence counseling was verbally provided and men were asked to bring their medication vial to follow-up. They were also asked to refrain from unprotected sex for 2 weeks.
Follow-up visits occurred at 14–21 days during which interval sexual history questionnaires and physical examinations were performed. Metronidazole pill containers were reviewed. Adherence was defined as taking all medication; otherwise, men were classified as nonadherent. Males found to have either gonorrhea or chlamydia or if their partner tested positive were treated according to standard protocol and continued. If their partner had a positive T. vaginalis culture, they were treated with metronidazole and the couple was discontinued. Reimbursement was $50/visit at UAB and $60/visit at WSU.
Statistical Methods
Based on Sobel et al [25], the recurrence rate for women with BV is 60% after 16 weeks. To detect a reduction in the recurrence rate to 40% at the 2-sided .05 significance level with a power of .90 required 154 couples per arm. To allow for a potential 16% drop-out rate [25], 368 couples were targeted for enrollment. It was estimated that 40% of the male partners would not keep appointments. Thus, 614 women were targeted for screening to enroll 368 couples.
The primary analysis population was the intent-to-treat (ITT) population, which included all couples randomized and for whom male partners were provided with study medication. A secondary analysis of efficacy was performed on the per-protocol (PP) population, which comprised all enrollees whose male partners completed the protocol-defined treatment and excluded pre-existing protocol violations of entry criteria. Cure was defined as 0–2 Amsel criteria and Nugent score 0–6 in the female partner. The primary endpoint of BV recurrence (treatment failure) was defined as the proportion of participants who did not meet the criteria for clinical cure on or before the 16-week follow-up.
Participants with BV recurrence through the third follow-up (days 112–119) were added to the cumulative failures for the study. Participants who did not return for their test-of-cure visit (third follow-up visit) or who met criteria for BV recurrence were considered treatment failures. Designating unevaluable or lost participants as treatment failures is consistent with ITT analyses for superiority studies and International Conference on Harmonization guidelines [26]. For the primary endpoint, the cumulative proportions of women failing treatment by the third follow-up visit were compared according to treatment arm using Fisher’s exact test. Time to recurrence was assessed using the Kaplan-Meier method, with participants without recurrence (including those lost to follow-up) censored at their last visit; treatment arms were compared with respect to time to recurrence using the log-rank test.
Univariate associations with BV treatment failure for female and male risk factors at baseline and treatment adherence were investigated using Fisher’s exact test or chi-square test. Treatment arm and factors that were significant at P < .15 in univariate analyses were considered in a multivariable relative risk log-binomial model. Backwards variable selection was used to reduce the number of variables further by maintaining variables that were significant at P < .15. Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). A data safety monitoring board (DSMB) monitored the progression and results annually.
RESULTS
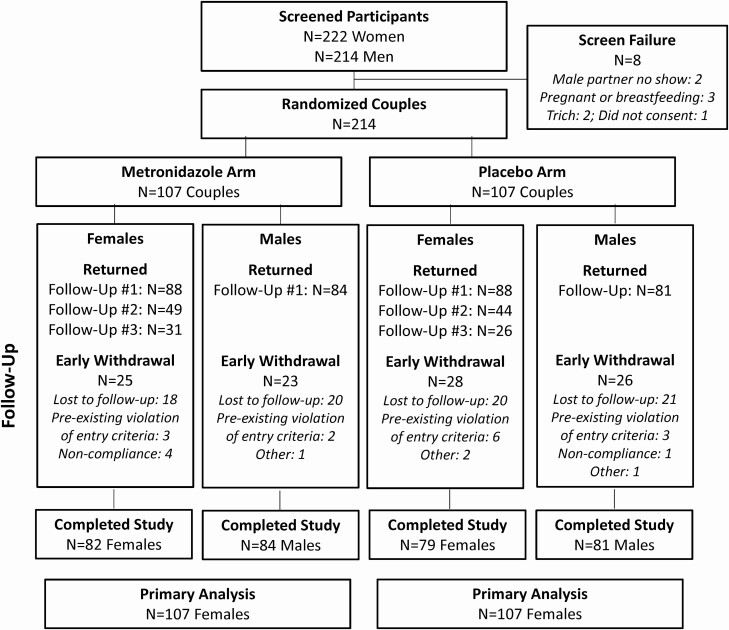
This study enrolled 214 couples between February 2015 and March 2019 (Figure 1) at which time it was stopped for futility by the DSMB. No couples were excluded based on our couples’ tool [24]. Visit adherence for females in the metronidazole arm ranged from 85% to 97% versus 87% to 92% for the placebo arm across the 3 follow-up visits. The follow-up return rate for males was 81% and 80% for the metronidazole and placebo arms, respectively. Across arms and gender, 74% of participants completed the study, 18% were lost to follow-up, 3% had pre-existing violation of entry criteria, and 5% discontinued early.
Figure 1.
Trial profile. Abbreviation: Trich, Trichomonas vaginalis.
Table 1 shows baseline characteristics of participants, stratified by treatment group. Treatment arms did not significantly differ across partners according to demographic or participant characteristics, with the exception of chlamydia positivity by NAAT in women at baseline. We did not control for chlamydia positivity in subsequent analyses, as the frequency of positive cases was so small.
Table 1.
Participant Characteristics at Baseline
| Total | Treated | Placebo | ||||
|---|---|---|---|---|---|---|
| Females (N = 214) | Males (N = 214) | Females (n = 107) | Males (n = 107) | Females (n = 107) | Males (n = 107) | |
| Age, mean (SD), years | 32.1 (7.5) | 34.3 (9.6) | 31.5 (6.9) | 33.9 (9.5) | 32.8 (8.1) | 34.8 (9.7) |
| Race, n (%) | ||||||
| Black or African American | 177 (83) | 181 (85) | 89 (83) | 93 (87) | 88 (82) | 88 (83) |
| White | 29 (14) | 21 (10) | 14 (13) | 9 (8) | 15 (14) | 12 (11) |
| Other/multiracial | 8 (4) | 11 (5) | 4 (4) | 5 (5) | 4 (4) | 6 (6) |
| Hispanic ethnicity, n (%) | 10 (5) | 5 (2) | 6 (6) | 3 (3) | 4 (4) | 2 (2) |
| Total partners, last 3 months, n (%) | ||||||
| ≥2 | 31 (14) | 39 (18) | 13 (12) | 19 (18) | 18 (17) | 20 (19) |
| 0–1a | 183 (86) | 175 (82) | 94 (88) | 88 (82) | 89 (83) | 87 (81) |
| Total partners, lifetime, n (%) | ||||||
| ≥11 | 60 (28) | 117 (55) | 30 (28) | 56 (52) | 30 (28) | 61 (57) |
| 6–10 | 77 (36) | 59 (28) | 46 (43) | 32 (30) | 31 (29) | 27 (25) |
| 1–5 | 76 (36) | 38 (18) | 30 (28) | 19 (18) | 46 (43) | 19 (18) |
| One or more new partners, last 3 months, n (%) | 13 (6) | 10 (5) | 6 (6) | 2 (2) | 7 (7) | 8 (7) |
| How often used a condom, last 3 months, n (%) | ||||||
| Always | 9 (4) | 8 (4) | 4 (4) | 3 (3) | 5 (5) | 5 (5) |
| Sometimes/never/don’t know | 204 (96) | 206 (96) | 103 (96) | 104 (97) | 101 (95) | 102 (95) |
| Used a condom at last sexual encounter, n (%) | 21 (10) | 23 (11) | 12 (11) | 13 (12) | 9 (8) | 10 (9) |
| Baseline STI-positive result, n (%) | ||||||
| Gonorrhea | 5 (2) | 0 (0) | 0 (0) | 0 (0) | 5 (5) | 0 (0) |
| Chlamydia | 6 (3) | 0 (0) | 0 (0) | 0 (0) | 6 (6) | 0 (0) |
| Lifetime STI history, n (%) | ||||||
| Any STI | 146 (68) | 107 (50) | 75 (70) | 58 (54) | 71 (66) | 49 (46) |
| Gonorrhea | 58 (27) | 55 (26) | 31 (29) | 28 (26) | 27 (25) | 27 (25) |
| Chlamydia | 93 (43) | 59 (28) | 45 (42) | 33 (31 | 48 (45) | 26 (24) |
| MPC | 2 (1) | … | 1 (1) | … | 1 (1) | … |
| NGU | … | 3 (1) | … | 1 (1) | … | 2 (2) |
| Trichomonas | 82 (38) | 20 (9) | 43 (40) | 10 (9) | 39 (36) | 10 (9) |
| Syphilis | 4 (2) | 2 (1) | 1 (1) | 1 (1) | 3 (3) | 1 (1) |
| Genital warts | 8 (4) | 4 (2) | 4 (4) | 2 (2) | 4 (4) | 2 (2) |
| Herpes | 14 (7) | 14 (7) | 7 (7) | 7 (7) | 7 (7) | 7 (7) |
Abbreviations: MPC, mucopurulent cervicitis; NGU, nongonococcal urethritis; SD, standard deviation; STI, sexually transmitted infection.
aOnly 1 male partner in each arm reported 0 sexual partners in the last 3 months.
Table 2 shows results of the primary outcome. In the ITT population, there was no significant difference between treatment arms. Treatment failure occurred in 81% of female participants in the metronidazole arm and 80% of women in the placebo arm through the third follow-up visit (P > .999). This difference was also nonsignificant for the PP population. In returning, evaluable women, recurrent/persistent BV occurred in 76% (62/82) in the metronidazole arm versus 74% (60/81) in the placebo arm (P = .858). Kaplan-Meier estimates of the BV recurrence distribution by arm are shown in Supplementary Figure 1. By day 60, 55% (95% confidence interval [CI], 44–65%) of participants had recurrent BV in the treatment arm compared with 62% (52–72%) in the placebo arm.
Table 2.
Analysis of Primary Outcome
| BV Treatment Failure,a % (n/N) | ||
|---|---|---|
| Intent-to-Treat Population (n = 214) | Per-Protocol Populationb (n = 133) | |
| Arm | ||
| Metronidazole | 81 (87/107) | 72 (50/69) |
| Placebo | 80 (86/107) | 75 (48/64) |
| Two-sided P valuec | >.999 | .844 |
In the intent-to-treat population, 51 who did not attend the last visit were treated as failures; this includes 31 without any BV testing and 20 with negative BV tests at follow-up visits 1 and/or 2.
Abbreviation: BV, bacterial vaginosis.
aParticipants with recurrence/persistence through the third follow-up visit (day 112–119) were added to the cumulative failures for the study. Participants who did not return for their test-of-cure visit were also considered treatment failures.
bIncludes women whose partners completed treatment and excludes pre-existing protocol violations of entry criteria.
cFisher’s exact test.
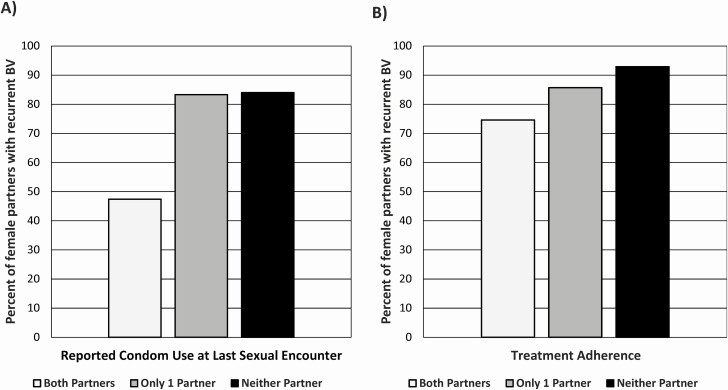
Table 3 shows univariate associations with treatment failure, stratified by gender. Baseline condom use at the last sexual encounter (LSE) reported by both females and males was highly protective against recurrent BV, with 52% of females failing treatment when women reported consistently using condoms compared with 84% when they were not. Among couples who both reported baseline condom use at the LSE, 47% of women had recurrent BV versus 83% and 84% of women when only 1 or neither partner reported using a condom (P < .001) (Figure 2). Male and female nonadherence was associated with treatment failure, with very high concordance in this behavior within couples (κ = 0.85 and 0.70, respectively). Both participants being adherent to medication had lower failure rates than when 1 or neither was adherent (Figure 2). There was a nonsignificant trend for STI history in the females as a risk factor for recurrent BV (P = .064). Treatment failure did not differ significantly by enrollment site (78% for UAB vs 85% for WSU; P = .185). Extra-couple sex was infrequent; only 10 women and 2 men admitted to new sexual partners at follow-up visits; of these 12 couples with extra-couple sex, 5 women had recurrent/persistent BV.
Table 3.
Univariate Associations with Bacterial Vaginosis Treatment Failure for Female and Male Risk Factors
| Females | Males | |||||
|---|---|---|---|---|---|---|
| na | Recurrent/Persistent BV, n (%) | P b | n | Recurrent/Persistent BV, n (%) | P b | |
| Age group | .109 | .240 | ||||
| <30 years | 91 | 69 (76) | 77 | 59 (77) | ||
| ≥30 years | 123 | 104 (85) | 137 | 114 (83) | ||
| Race, n (%) | .617 | .294 | ||||
| Black or African American | 177 | 142 (80) | 181 | 144 (80) | ||
| All others | 37 | 31 (84) | 28 (88) | |||
| Total partners, last 3 months | .643 | .492 | ||||
| ≥2 | 31 | 26 (84) | 39 | 30 (77) | ||
| 0–1 | 183 | 147 (80) | 175 | 143 (82) | ||
| Total partners, lifetime | ||||||
| ≥11 | 60 | 49 (82) | .977 | 117 | 96 (82) | .553 |
| 6–10 | 77 | 62 (81) | 59 | 45 (76) | ||
| 1–5 | 76 | 61 (80) | 38 | 32 (84) | ||
| Total new partners, last 3 months | .280 | .518 | ||||
| >1 | 13 | 9 (69) | 16 | 12 (75) | ||
| 0 | 201 | 164 (82) | 197 | 160 (81) | ||
| How often used a condom, last 3 months | .013 | |||||
| Always | 9 | 4 (44) | 18 | 6 (75) | .651 | |
| Sometimes/never/don’t know | 204 | 169 (83) | 206 | 167 (81) | ||
| Used a condom at last sexual encounter | .002 | .001 | ||||
| Yes | 21 | 11 (52) | 23 | 12 (52) | ||
| No | 192 | 161 (84) | 191 | 161 (84) | ||
| History of STI | .064 | .602 | ||||
| Yes | 146 | 123 (84) | 107 | 88 (82) | ||
| No | 68 | 50 (74) | 107 | 85 (79) | ||
| Circumcised | … | .665 | ||||
| Yes | … | … | 187 | 152 (81) | ||
| No | … | … | 27 | 21 (78) | ||
| Treatment adherence | .021 | .002 | ||||
| Adherent | 151 | 116 (77) | 137 | 102 (74) | ||
| Nonadherent | 63 | 57 (90) | 77 | 71 (92) | ||
Abbreviations: BV, bacterial vaginosis; SD, standard deviation; STI, sexually transmitted infection.
a“n” for some variables total to 193 instead of 194 due to missing responses.
bFisher’s exact or chi-square test.
Figure 2.
A, Percentage of female partners with recurrent BV, stratified by reported condom use at last sexual encounter. B, Percentage of female partners with recurrent BV, stratified by treatment adherence. Abbreviation: BV, bacterial vaginosis.
Table 4 shows the multivariable main-effects model. Treatment arm, which was nonsignificant, was retained in the backwards variable selection procedure. After adjusting for other variables, females whose male partners were adherent were less likely to fail treatment (adjusted relative risk [ARR], .85; 95% CI, .73–.99; P = .035). There was a trend for female partner STI history (ARR, 1.14; 95% CI, .99–1.33; P = .074) and male partner lack of condom use at LSE (ARR, 1.49; 95% CI, .99–2.23; P = .054) to be associated with treatment failure.
Table 4.
Multivariable Relative Risk Model for Bacterial Vaginosis Treatment Failure
| Effect | Adjusted Relative Risk | 95% Wald Confidence Limits | P a |
|---|---|---|---|
| Main-effects model | |||
| Female partner has history of STI | |||
| Yes | 1.14 | .99–1.33 | .074 |
| No | Ref | ||
| Male reports using condom last time had sex | |||
| No | 1.49 | .99–2.23 | .054 |
| Yes | Ref | ||
| Male adherent to medication | |||
| Yes | .85 | .73–.99 | .035 |
| No | Ref | ||
| Arm | |||
| Metronidazole | 1.06 | .90–1.24 | .480 |
| Placebo | Ref | ||
| Adding interaction to model above | |||
| Male medication adherence × arm interaction | … | … | .095 |
The following variables were considered in the multivariable model based on P < .15 in univariate analyses: female age, female and male report of condom use last time had sex, female and male medication adherence, treatment arm, female frequency of condom use, female history of STI. Variables that were significant at P < .15 were maintained. Treatment arm was forced to stay in the model.
Abbreviations: Ref, reference; STI, sexually transmitted infection.
aLikelihood ratio test.
Since the relationship between treatment failure and medication adherence was only relevant for the metronidazole arm, we explored the interaction between arm and male medication adherence by adding the interaction term to the final main-effects model (Table 4). Female STI history no longer met inclusion criteria (P = .162) and was dropped. The P value for the interaction between arm and male adherence was .095. Post hoc comparisons found that, in the metronidazole arm, females with adherent male partners were significantly less likely to fail than females with nonadherent partners (ARR, .78; 95% CI, .67–.91; P = .001). In the metronidazole arm, the failure rate was 73% (51/70) versus 97% (36/37) in women whose partners were adherent versus nonadherent for metronidazole use. In the placebo arm, this comparison was not significant; the failure rate was 76% (51/67) versus 88% (35/40) in women whose partners were adherent versus nonadherent for placebo use (ARR, .94; 95% CI, .80–1.12; P = .512). Comparisons of treatment arms within adherent and nonadherent groups were nonsignificant.
There were 82 adverse events reported in 45 female and 17 male participants (Supplementary Table 1). Three serious adverse events were unrelated to study participation. The most common adverse events were 19 occurrences of vaginal yeast infection in 19 females and 8 occurrences of STIs in 6 males. There was no significant difference in these adverse events according to randomization group.
DISCUSSION
Few studies have examined the effect of male partner treatment on women with recurrent BV, with controversial results. A literature review shows 6 prior male partner treatment trials for women with BV [17–22]. Five of these trials showed no benefit in reducing BV recurrence rates in the female [17–20, 22]. Two trials randomized men to a single 2-g dose of oral metronidazole versus placebo [21, 22], 1 randomized men to single-dose metronidazole twice over 2 days versus placebo [18], 1 to single-dose metronidazole or multidose metronidazole for 7 days [17], while the others randomized men to either oral clindamycin (150 mg 4 times daily for 7 days) versus placebo or a single 2-g dose of oral tinidazole versus placebo [19, 20]. Only 1 trial demonstrated a small benefit of male treatment with 2 g of oral metronidazole [21]; at 2 and 5 weeks, women whose male partners were treated had less BV by Nugent score. In addition, at 8 weeks, women whose male partners were treated were more likely to report resolution of BV symptoms. However, results were only presented graphically and effect sizes were not stated. All trials had multiple limitations, including insufficient power, deficient randomization methods, inadequate reporting of adherence, and/or use of ineffective male partner treatment [23].
Our current study aimed to minimize these limitations, including a sufficient power calculation, randomization of men face-to-face, monitoring of male adherence, and use of the currently recommended 7-day metronidazole regimen [16]. Despite these strengths, we found that male partner treatment with metronidazole did not reduce BV recurrence in female partners. This was surprising, as the large majority of epidemiological data suggests that BV is an STI [13–15]. There could be several reasons for this negative finding. First, in the ITT population, the BV cure rate in women in both arms was very low (treatment failure was high in both groups; interestingly, higher than in previous studies [25]). This suggests that women in both arms were heavily BV experienced and likely had persistent BV biofilm on their vaginal mucosa [27] that was insufficiently eradicated with oral metronidazole [28]. At this stage in their disease process, treatment of the male sexual partner may have been too late in the setting of a persistent, mature BV biofilm [29, 30]. Several studies have investigated the use of BV biofilm disrupters, including in women with recurrent BV, with some benefit [31–33]; however, there are no large trials to inform a change in treatment recommendations. Extra-couple sex was infrequent and unlikely to be a major contributing factor.
In addition, lack of female and male partner adherence to multidose metronidazole also contributed to our negative results. We used a proxy of medication adherence (ie, pill bottle review) instead of a biological measure of drug exposure (ie, serum metronidazole levels). Compliance with multidose metronidazole has been relatively low in some studies [34] and future BV partner treatment trials should use a currently approved single-dose treatment for BV to limit issues surrounding adherence. One option could be a single 2-g oral dose of secnidazole [35], which is Food and Drug Administration–approved for BV [36].
Another reason for our null result is that oral metronidazole may not have effectively cleared BV-associated bacteria from the penile microbiome and/or prostatic or seminal vesicle reservoirs. In light of studies isolating BV-associated bacteria from penile surfaces [15, 37] and linking BV to male hygiene factors [38], regular external penile cleansing may be beneficial in preventing recurrent BV in female partners. One study examined the use of ethyl alcohol gel as a topical penile microbicide immediately prior to sex for the prevention of recurrent BV in female partners and found an unexpected increase in recurrent BV. The authors speculated that the use of the microbicide may have only caused short-term alterations in the penile and vaginal microbiota [39]. Future studies should evaluate regular external penile cleansing with systemic antimicrobial therapy to prevent recurrent BV in female partners, considering use of antiseptic solutions such as boric acid or gentian violet. External penile cleansing alone is unlikely to be of benefit [40, 41].
Along these lines, optimal therapy to promote clearance of BV-associated bacteria in penile skin and the urethra, semen, and urine in men may alternatively require treatment with combination oral and topical antibiotics [42]. A phase 3 trial to evaluate the efficacy of oral metronidazole 400 mg twice daily and topical 2% clindamycin cream to the glans penis and upper shaft twice daily, both for 7 days, is underway (Australia/New Zealand Clinical Trials Registry #12619000196145).
Although our study was negative, we did find that male condom use was highly protective against recurrent BV, similar to a prior meta-analysis [43]. In addition, in the metronidazole arm, women with nonadherent male partners were significantly more likely to have recurrent BV than females with adherent partners. There was a strong correlation of these behaviors within couples, suggesting that the intervention may be more likely to work among a subset of adherent patients practicing consistent condom use.
Our study has several limitations. We did not collect data on the length of time that couples were together prior to enrollment, the date of their LSE, or data on noncondom contraceptive use (ie, intrauterine device use), which may be associated with increased risk of BV acquisition [44, 45]. Administration of multidose medication was not directly observed. Given concerns with adherence, future BV partner treatment trials should consider the use of single-dose oral secnidazole [36]. Although participants were counseled to refrain from unprotected sex during the first 2 weeks and to practice consistent condom use afterward, this may not have always occurred; we were unable to control for sexual activity in our analyses. Finally, these results may not be generalizable.
In conclusion, this study did not find that male partner treatment with multidose metronidazole significantly reduced BV recurrence in female partners. Given that the etiology of BV remains controversial, advances in treatment remain difficult.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Joy Lewis and Sanquetta McClendon for their assistance in recruiting and enrolling couples in this study. The authors also thank Wendy Cook for her efforts related to data entry and data quality.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) (grant number U01AI108509; to J. R. S.).
Potential conflicts of interest . C. A. M. reports K23 and R01 awards from the National Institutes of Health/NIAID; research grants and consultant fees from Lupin Pharmaceuticals; speaker honoraria from Cepheid, BD, and Abbot; consultant fees from BioFire Diagnostics; scientific advisory board fees from PhagoMed and Roche, outside the submitted work. J. R. S. reports consultant fees from StarPharma, Talis, and Toltec; speaker fees from Hologic; and grants from StarPharma, Talis, Toltec, Hologic, and BD Diagnostics, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983; 74:14–22. [DOI] [PubMed] [Google Scholar]
- 2. Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex Transm Dis 2019; 46:304–11. [DOI] [PubMed] [Google Scholar]
- 3. Cohen CR, Duerr A, Pruithithada N, et al. . Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chiang Mai, Thailand. AIDS 1995; 9:1093–7. [DOI] [PubMed] [Google Scholar]
- 4. Martin HL, Richardson BA, Nyange PM, et al. . Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999; 180:1863–8. [DOI] [PubMed] [Google Scholar]
- 5. Taha TE, Gray RH, Kumwenda NI, et al. . HIV infection and disturbances of vaginal flora during pregnancy. J Acquir Immune Defic Syndr Hum Retrovirol 1999; 20:52–9. [DOI] [PubMed] [Google Scholar]
- 6. Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis 2003; 36:663–8. [DOI] [PubMed] [Google Scholar]
- 7. Saigh JH, Sanders CC, Sanders WE Jr. Inhibition of Neisseria gonorrhoeae by aerobic and facultatively anaerobic components of the endocervical flora: evidence for a protective effect against infection. Infect Immun 1978; 19:704–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eschenbach DA. Bacterial vaginosis and anaerobes in obstetric-gynecologic infection. Clin Infect Dis 1993; 16:S282–7. [DOI] [PubMed] [Google Scholar]
- 9. Hill GB, Eschenbach DA, Holmes KK. Bacteriology of the vagina. Scand J Urol Nephrol Suppl 1984; 86:23–39. [PubMed] [Google Scholar]
- 10. Eschenbach DA, Davick PR, Williams BL, et al. . Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol 1989; 27:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spiegel CA, Amsel R, Eschenbach D, Schoenknecht F, Holmes KK. Anaerobic bacteria in nonspecific vaginitis. N Engl J Med 1980; 303:601–7. [DOI] [PubMed] [Google Scholar]
- 12. Joesoef MR, Schmid GP, Hillier SL. Bacterial vaginosis: review of treatment options and potential clinical indications for therapy. Clin Infect Dis 1999; 28:S57–65. [DOI] [PubMed] [Google Scholar]
- 13. Muzny CA, Lensing SY, Aaron KJ, Schwebke JR. Incubation period and risk factors support sexual transmission of bacterial vaginosis in women who have sex with women. Sex Transm Infect 2019; 95:511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Plummer EL, Vodstrcil LA, Murray GL, et al. . Gardnerella vaginalis clade distribution is associated with behavioral practices and Nugent score in women who have sex with women. J Infect Dis 2020; 221:454–63. [DOI] [PubMed] [Google Scholar]
- 15. Mehta SD, Agingu W, Nordgren RK, et al. . Characteristics of women and their male sex partners predict bacterial vaginosis among a prospective cohort of Kenyan women with non-optimal vaginal microbiota. Sex Transm Dis 2020; 47:840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Workowski KA. Centers for disease control and prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis 2015; 61:S759–62. [DOI] [PubMed] [Google Scholar]
- 17. Swedberg J, Steiner JF, Deiss F, Steiner S, Driggers DA. Comparison of single-dose vs one-week course of metronidazole for symptomatic bacterial vaginosis. JAMA 1985; 254:1046–9. [PubMed] [Google Scholar]
- 18. Vejtorp M, Bollerup AC, Vejtorp L, et al. . Bacterial vaginosis: a double-blind randomized trial of the effect of treatment of the sexual partner. Br J Obstet Gynaecol 1988; 95:920–6. [DOI] [PubMed] [Google Scholar]
- 19. Vutyavanich T, Pongsuthirak P, Vannareumol P, Ruangsri RA, Luangsook P. A randomized double-blind trial of tinidazole treatment of the sexual partners of females with bacterial vaginosis. Obstet Gynecol 1993; 82:550–4. [PubMed] [Google Scholar]
- 20. Colli E, Landoni M, Parazzini F. Treatment of male partners and recurrence of bacterial vaginosis: a randomised trial. Genitourin Med 1997; 73:267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mengel MB, Berg AO, Weaver CH, et al. . The effectiveness of single-dose metronidazole therapy for patients and their partners with bacterial vaginosis. J Fam Pract 1989; 28:163–71. [PubMed] [Google Scholar]
- 22. Moi H, Erkkola R, Jerve F, et al. . Should male consorts of women with bacterial vaginosis be treated? Genitourin Med 1989; 65:263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mehta SD. Systematic review of randomized trials of treatment of male sexual partners for improved bacteria vaginosis outcomes in women. Sex Transm Dis 2012; 39:822–30. [DOI] [PubMed] [Google Scholar]
- 24. Muzny CA, Pontius A, Woznicki N, et al. . Use of a novel couples’ verification tool in a male partner treatment study of women with recurrent bacterial vaginosis. Sex Transm Dis 2020; 47:e58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sobel JD, Ferris D, Schwebke J, et al. . Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol 2006; 194:1283–9. [DOI] [PubMed] [Google Scholar]
- 26. International Conference on Harmonisation E9 Expert Working Group. ICH Harmonised Tripartite Guideline. Statistical principles for clinical trials. Stat Med 1999; 18:1905–42. [PubMed] [Google Scholar]
- 27. Bradshaw CS, Sobel JD. Current treatment of bacterial vaginosis-limitations and need for innovation. J Infect Dis 2016; 214:S14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muzny CA, Schwebke JR. Biofilms: an underappreciated mechanism of treatment failure and recurrence in vaginal infections. Clin Infect Dis 2015; 61:601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Swidsinski A, Mendling W, Loening-Baucke V, et al. . An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol 2008; 198:97.e1–6. [DOI] [PubMed] [Google Scholar]
- 30. Muzny CA, Taylor CM, Swords WE, et al. . An updated conceptual model on the pathogenesis of bacterial vaginosis. J Infect Dis 2019; 220:1399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis 2009; 36:732–4. [DOI] [PubMed] [Google Scholar]
- 32. Hymes SR, Randis TM, Sun TY, Ratner AJ. DNase inhibits Gardnerella vaginalis biofilms in vitro and in vivo. J Infect Dis 2013; 207:1491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marrazzo JM, Dombrowski JC, Wierzbicki MR, et al. . Safety and efficacy of a novel vaginal anti-infective, TOL-463, in the treatment of bacterial vaginosis and vulvovaginal candidiasis: a randomized, single-blind, phase 2, controlled trial. Clin Infect Dis 2019; 68:803–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bartley JB, Ferris DG, Allmond LM, Dickman ED, Dias JK, Lambert J. Personal digital assistants used to document compliance of bacterial vaginosis treatment. Sex Transm Dis 2004; 31:488–91. [DOI] [PubMed] [Google Scholar]
- 35. Nyirjesy P, Schwebke JR. Secnidazole: next-generation antimicrobial agent for bacterial vaginosis treatment. Future Microbiol 2018; 13:507–24. [DOI] [PubMed] [Google Scholar]
- 36. Schwebke JR, Morgan FG Jr, Koltun W, Nyirjesy P. A phase-3, double-blind, placebo-controlled study of the effectiveness and safety of single oral doses of secnidazole 2 g for the treatment of women with bacterial vaginosis. Am J Obstet Gynecol 2017; 217:678.e1–9. [DOI] [PubMed] [Google Scholar]
- 37. Holst E. Reservoir of four organisms associated with bacterial vaginosis suggests lack of sexual transmission. J Clin Microbiol 1990; 28:2035–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gray RH, Wawer MJ, Serwadda D, Kigozi G. The role of male circumcision in the prevention of human papillomavirus and HIV infection. J Infect Dis 2009; 199:1–3. [DOI] [PubMed] [Google Scholar]
- 39. Bukusi E, Thomas KK, Nguti R, et al. . Topical penile microbicide use by men to prevent recurrent bacterial vaginosis in sex partners: a randomized clinical trial. Sex Transm Dis 2011; 38:483–9. [PMC free article] [PubMed] [Google Scholar]
- 40. Swidsinski A, Dörffel Y, Loening-Baucke V, et al. . Desquamated epithelial cells covered with a polymicrobial biofilm typical for bacterial vaginosis are present in randomly selected cryopreserved donor semen. FEMS Immunol Med Microbiol 2010; 59:399–404. [DOI] [PubMed] [Google Scholar]
- 41. Nelson DE, Dong Q, Van der Pol B, et al. . Bacterial communities of the coronal sulcus and distal urethra of adolescent males. PLoS One 2012; 7:e36298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Unemo M, Bradshaw CS, Hocking JS, et al. . Sexually transmitted infections: challenges ahead. Lancet Infect Dis 2017; 17:e235–79. [DOI] [PubMed] [Google Scholar]
- 43. Fethers KA, Fairley CK, Hocking JS, Gurrin LC, Bradshaw CS. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clin Infect Dis 2008; 47:1426–35. [DOI] [PubMed] [Google Scholar]
- 44. Achilles SL, Austin MN, Meyn LA, Mhlanga F, Chirenje ZM, Hillier SL. Impact of contraceptive initiation on vaginal microbiota. Am J Obstet Gynecol 2018; 218:622.e1–.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peebles K, Kiweewa FM, Palanee-Phillips T, et al. . Elevated risk of bacterial vaginosis among users of the copper intrauterine device: a prospective longitudinal cohort study. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.