Abstract
Background
Penicillin allergies are associated with inferior patient and antimicrobial stewardship outcomes. We implemented a whole-of-hospital program to assess the efficacy of inpatient delabeling for low-risk penicillin allergies in hospitalized inpatients.
Methods
Patients ≥ 18 years of age with a low-risk penicillin allergy were offered a single-dose oral penicillin challenge or direct label removal based on history (direct delabeling). The primary endpoint was the proportion of patients delabeled. Key secondary endpoints were antibiotic utilization pre- (index admission) and post-delabeling (index admission and 90 days).
Results
Between 21 January 2019 and 31 August 2019, we assessed 1791 patients reporting 2315 antibiotic allergies, 1225 with a penicillin allergy. Three hundred fifty-five patients were delabeled: 161 by direct delabeling and 194 via oral penicillin challenge. Ninety-seven percent (194/200) of patients were negative upon oral penicillin challenge. In the delabeled patients, we observed an increase in narrow-spectrum penicillin usage (adjusted odds ratio [OR], 10.51 [95% confidence interval {CI}, 5.39–20.48]), improved appropriate antibiotic prescribing (adjusted OR, 2.13 [95% CI, 1.45–3.13]), and a reduction in restricted antibiotic usage (adjusted OR, 0.38 [95% CI, .27–.54]). In the propensity score analysis, there was an increase in narrow-spectrum penicillins (OR, 10.89 [95% CI, 5.09–23.31]) and β-lactam/β-lactamase inhibitors (OR, 6.68 [95% CI, 3.94–11.35]) and a reduction in restricted antibiotic use (OR, 0.52 [95% CI, .36–.74]) and inappropriate prescriptions (relative risk ratio, 0.43 [95% CI, .26–.72]) in the delabeled group compared with the group who retained their allergy label.
Conclusions
This health services program using a combination of direct delabeling and oral penicillin challenge resulted in significant impacts on the use of preferred antibiotics and appropriate prescribing.
Keywords: antimicrobial stewardship, antibiotic allergy, penicillin allergy, oral challenge, direct provocation
Following penicillin allergy assessment, 355 inpatients were delabeled: 161 by direct delabeling and 194 via direct oral penicillin challenge. In the delabeled patients, there was a significant increase in narrow-spectrum penicillin use, appropriate prescribing, and reduced restricted antibiotic usage.
Patient-reported antibiotic allergies (so-called antibiotic allergy labels) are an international public health concern, due to the high population burden and associations with inappropriate prescribing and drug-resistant infections [1, 2]. Penicillin allergy is the most frequently encountered drug allergy, with an estimated 10% of the global population, including 30 million North Americans, affected [2]. It is associated with the acquisition of methicillin-resistant Staphylococcus aureus [3], increased hospital length of stay (LOS) and drug costs [4, 5], and excess patient mortality [6]. However, 90% of these patients can have their penicillin allergy removed or be delabeled by assessment and/or allergy testing [7, 8]. Delabeling has been a focus of global antimicrobial stewardship (AMS) programs [8, 9] due to improvements in antibiotic appropriateness posttesting [10]. However, there are few reported point-of-care programs that can be applied to a spectrum of care settings.
Recent observational data and a single-center randomized controlled trial have demonstrated the safety and efficacy of direct oral penicillin challenge to avoid traditional skin testing in patients identified as having low-risk penicillin allergy histories [11, 12]. However, such programs have primarily been studied in the outpatient setting, and some used allergist-delivered split-dose challenges. In a pilot study, AMS-led inpatient oral penicillin challenge was found to be safe and effective, using a penicillin allergy toolkit comprised of a validated antibiotic allergy assessment tool (AAAT) and a low-risk oral penicillin challenge protocol [13, 14]. We sought to prospectively assess all patient-reported antibiotic allergies at 2 health services and to implement this toolkit as a multicenter whole-of-hospital health services intervention.
METHODS
Study Design and Participants
The Penicillin Allergy Delabeling Program was implemented at Austin Health and the Peter MacCallum Cancer Centre (Victoria, Australia). Patients were recruited between 21 January 2019 and 31 August 2019, and data were collected over a 90-day follow-up period. Austin Health is a 400-inpatient-bed tertiary referral center and Peter MacCallum Cancer Centre is a 96-inpatient-bed tertiary cancer hospital. Both centers have established outpatient antibiotic allergy testing services overseen by infectious diseases physicians [10].
Acute inpatients aged ≥ 18 years with a reported antibiotic allergy or adverse drug reaction in the medical record at 8 am daily (Monday–Friday) were prospectively identified and assessed by trained nursing, pharmacy, and/or medical staff; those with a penicillin allergy were risk-stratified into low- or high-risk penicillin allergy, using the previously validated AAAT (Supplementary Materials) [13]. Use of the AAAT relied on obtaining a detailed allergy reaction history from the patient, together with corroborating information from the hospital and family physician medical records. Patients with a low-risk penicillin allergy meeting the eligibility criteria were either directly delabeled based on history or offered a direct oral penicillin challenge using a previously published protocol (Supplementary Materials) [14]. The criteria for direct delabeling and oral challenge are provided in the Procedures section below. In patients with an allergy to > 1 penicillin antibiotic, the most severe reaction was used to determine whether delabeling by history or direct oral challenge was performed. Multisite ethics approval was obtained from the Austin Health Research Ethics Committee (47585-Austin-2018).
Procedures
An antibiotic allergy label was defined as a patient-reported allergy or adverse drug reaction. A low-risk phenotype was defined by the AAAT to be a “white” or “green” and a high-risk phenotype as “orange” or “red” coded selection (Supplementary Materials).
Direct historical delabeling was performed in patients reporting a mild non-immune-mediated adverse drug reaction as per the AAAT or where subsequent tolerance to the implicated penicillin was ascertained from a medical record or pharmacy prescription record reconciliation.
The oral challenge eligibility criteria are outlined below (adapted from previously published definitions [15]):
Inclusion criteria for oral penicillin challenge:
A reported low-risk penicillin allergy (“green” or “white” using the AAAT);
Unknown reaction > 10 years previously;
Type A adverse drug reaction (pharmacologically predictable intolerance) where direct delabeling was not accepted by the patient;
History of an unspecified childhood rash, localized injection site reaction (only), or maculopapular exanthem > 10 years ago.
Exclusion criteria for oral penicillin challenge:
Moderate or severe allergy history using the AAAT (“orange” or “red”);
Concurrent history of any anaphylaxis or idiopathic urticaria/anaphylaxis;
Hemodynamic instability (medical emergency team call criteria within the last 24 hours);
Concurrent > 10-mg prednisolone or corticosteroid equivalent or antihistamine therapy.
Patients with a high-risk penicillin allergy who were unable to be directly delabeled via medical reconciliation were recommended for referral to the outpatient antibiotic allergy services for skin testing upon discharge if they met a predefined referral criteria: (1) immunocompromised or (2) recent and future predicted antibiotic usage.
Following written informed consent, eligible low-risk participants underwent a supervised oral challenge with either a single dose of oral penicillin VK 250 mg or amoxicillin 250 mg and were observed for 2 hours by ward nursing staff. In patients with a documented history of a delayed hypersensitivity and where acute antibiotic therapy was not currently required, a prolonged 5-day challenge (500 mg twice daily) was offered. All patients were reviewed at challenge completion. Following antibiotic challenge, patient outcome was defined as having resulted in either (1) negative oral challenge with no reaction, or (2) positive oral challenge with either immune-mediated or non-immune-mediated adverse reactions. Patients with a negative single- or multiple-dose oral challenge were considered delabeled from their penicillin allergy. The choice of penicillin VK or amoxicillin was based upon the known or presumed implicated drug as outlined in the Supplementary Materials.
Overall, patients who had their penicillin allergy removed either by direct oral challenge or direct delabeling were termed the “delabeled cohort.” Patients with a penicillin allergy who remained labeled as penicillin allergic were termed the “nondelabeled cohort.”
The pretesting period was defined as the start of the index admission to the time of allergy assessment in the nondelabeled cohort or delabeling event in the delabeled cohort. The 90 day posttesting period was defined as 90 days from the date of allergy assessment in the nondelabeled cohort or 90 days from the date of the delabeling event in the delabeled cohort.
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Melbourne [16].
Outcomes
The primary endpoint was the proportion of patients delabeled, with a (1) penicillin allergy, (2) low-risk penicillin allergy, or (3) high-risk penicillin allergy. Secondary endpoints were the use of (1) narrow-spectrum penicillin, (2) restricted antibiotic, and (3) appropriate antibiotic in delabeled patients compared to (a) pretesting period and (b) nondelabeled patients. Readmission rate (90 days), LOS, and inpatient/90-day mortality data were also examined.
Statistical Analysis
Statistical analyses were performed in Stata version 15.1 software (StataCorp). Descriptive statistics were presented as frequency (percentage) and median (interquartile range [IQR]). Baseline characteristics between delabeled and nondelabeled groups were compared using Fisher exact test (categorical variables) and rank-sum test (continuous variables).
Antibiotic utilization pre- and posttesting in the delabeled group was examined using logistic regression (unadjusted and adjusted for sex, age-adjusted Charlson comorbidity index [CCI], microbial diagnosis, and sepsis). Odds ratios with 95% confidence intervals are presented.
All secondary outcomes were compared between the delabeled and nondelabeled groups using logistic regression (any antibiotic utilization, mortality, readmission), multinomial logistic regression (appropriateness of antibiotic), negative binomial regression (LOS), and zero-inflated negative binomial regression (duration of intravenous antibiotic use). Results are reported with 95% confidence intervals. Additionally, to control for imbalances between the delabeled and nondelabeled groups, the propensity score (PS) method using inverse probability of treatment weighting (IPTW) was applied. Details on the statistical analyses are provided in the Supplementary Materials.
Cost Analysis
A cost analysis (Supplementary Materials) of the whole-of-hospital penicillin allergy delabeling program was performed to report the cost of performing delabeling in the inpatient hospital setting (current intervention model of care) compared to an outpatient clinic setting (previous control model of care).
RESULTS
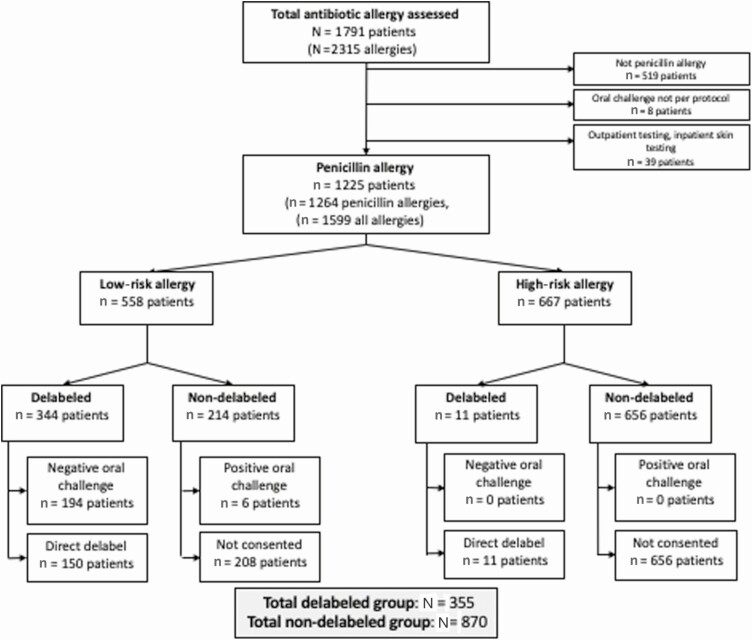
During the study period, 1791 patients reporting an antibiotic allergy were prospectively assessed, with 2315 individual antibiotic allergies reported (Supplementary Tables 1 and 2; Supplementary Figure 3). There were 1272 patients with a penicillin allergy. Additionally, 8 patients were excluded because they were administered an intravenous or oral penicillin prior to the AAAT being performed by the program team, and 39 patients were excluded due to inpatient skin testing or subsequent outpatient testing (Supplementary Table 2; Figure 1).
Figure 1.
Study profile. Positive oral challenge is defined as an immune or non-immune-mediated reaction following oral penicillin challenge. Negative oral challenge is defined as no reaction following oral penicillin challenge.
Analysis was performed on a total of 1225 unique patients reporting 1264 penicillin allergies (Figure 1); allergy phenotypes and characteristics are provided in Supplementary Table 3. Patient-reported penicillin allergies were stratified into low and high risk as per the AAAT (Supplementary Figure 4), with 46% of patients reporting low-risk penicillin allergies (n = 558) and 54% (n = 667) reporting at least 1 high-risk allergy.
Penicillin Allergy Delabeling
Of the 1225 patients, 29% (355/1225) were delabeled (45% via direct delabeling and 55% via oral challenge). Of the patients with a penicillin allergy who were delabeled by oral challenge, 194 of these had a low-risk allergy and none had a high-risk allergy. Of the patients who were directly delabeled, 150 of these had a low-risk allergy and 11 had a high-risk allergy (Figure 1). Of patients reporting a low-risk penicillin allergy, 62% (344/558) were delabeled. The baseline demographics of the final delabeled and nondelabeled cohorts are provided in Table 1. The reasons patients were not consented (n = 208; Figure 1) are outlined in Supplementary Table 4.
Table 1.
Baseline Characteristics of Patients Assessed With a Penicillin Allergy
| Characteristic | Overall (N = 1225) |
Non-delabeled (n = 870) |
Delabeled (n = 355) |
P Value |
|---|---|---|---|---|
| Male sex | 487 (39.8) | 332 (38.2) | 155 (43.7) | .082 |
| Age, y, median (IQR) | 66 (52–78) | 66 (51–77) | 68 (55–80) | .022 |
| White ethnicity | 1125 (91.8) | 793 (91.1) | 332 (93.5) | .21 |
| Study site | ||||
| Austin | 1029 (84.0) | 732 (84.1) | 297 (83.7) | .86 |
| PMCC | 196 (16.0) | 138 (15.9) | 58 (16.3) | |
| Admitting unit | ||||
| Medical | 708 (57.8) | 491 (56.4) | 217 (61.1) | .32 |
| Surgical | 400 (32.7) | 294 (33.8) | 106 (29.9) | |
| Other | 117 (9.6) | 85 (9.8) | 32 (9.0) | |
| Resident of aged care facility | 44 (3.6) | 32 (3.7) | 12 (3.4) | 1 |
| ICU admission | 74 (6.0) | 59 (6.8) | 15 (4.2) | .11 |
| CCI, median (IQR) | 4 (2–6) | 4 (2–6) | 4 (2–7) | .015 |
| LOS, d, from admission to oral challenge (or assessment if no oral challenge), median (IQR) | 1 (1–2) | 1 (1–2) | 1 (1–3) | < .001 |
| Immunocompromised | 510 (41.6) | 355 (40.8) | 155 (43.7) | .37 |
| Mental health history | 299 (24.4) | 216 (24.8) | 83 (23.4) | .61 |
| Microbiological diagnosis (index admission) | 135 (11.0) | 80 (9.2) | 55 (15.5) | .024 |
| Admission diagnosis | ||||
| Noninfective diagnosis | 523 (42.7) | 395 (45.4) | 128 (36.1) | .003 |
| Infective diagnosis | 702 (57.3) | 475 (54.6) | 227 (63.9) | |
| Bone and joint | 17 (1.4) | 8 (0.9) | 9 (2.5) | .028 |
| Bacteremia | 4 (0.3) | 3 (0.3) | 1 (0.3) | .86 |
| Central nervous system | 4 (0.3) | 3 (0.3) | 1 (0.3) | .86 |
| Pneumonia | 81 (6.6) | 51 (5.9) | 30 (8.5) | .098 |
| Intra-abdominal | 84 (6.9) | 54 (6.2) | 30 (8.5) | .16 |
| Intravascular infection | 4 (0.3) | 2 (0.2) | 2 (0.6) | .35 |
| Gastroenteritis | 11 (0.9) | 8 (0.9) | 3 (0.8) | .90 |
| Infection from unknown source | 19 (1.6) | 16 (1.8) | 3 (0.8) | .20 |
| Skin and soft tissue | 96 (7.8) | 65 (7.5) | 31 (8.7) | .46 |
| Surgical prophylaxis | 250 (20.4) | 184 (21.1) | 66 (18.6) | .31 |
| Urogenital | 48 (3.9) | 32 (3.7) | 16 (4.5) | .50 |
| Upper respiratory tract | 17 (1.4) | 9 (1.0) | 8 (2.3) | .098 |
| Ear, nose, and throat | 5 (0.4) | 3 (0.3) | 2 (0.6) | .59 |
| Other | 139 (11.3) | 87 (10.0) | 52 (14.6) | .020 |
| Bacterial infection | 107 (8.7) | 65 (7.5) | 42 (11.8) | .52 |
| Sepsis | 58 (4.7) | 36 (4.1) | 22 (6.2) | .38 |
| Nonantibiotic drug allergy | 396 (32.3) | 287 (33.0) | 109 (30.7) | .44 |
| No. of different antibiotic allergies, median (IQR) | 1 (1–1) | 1 (1–1) | 1 (1–1) | .003 |
| Nonpenicillin β-lactam allergy | 96 (7.8) | 79 (9.1) | 17 (4.8) | .011 |
| Antibiotic sulfonamide allergy (or sulfonamide unclassified) | 75 (6.1) | 53 (6.1) | 22 (6.2) | .94 |
| Previously reviewed in antibiotic allergy clinic | 30 (2.4) | 29 (3.3) | 1 (0.3) | .002 |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: CCI, age-adjusted Charlson comorbidity index; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; PMCC, Peter MacCallum Cancer Centre.
Direct Delabeling
One hundred sixty-one patients with a penicillin allergy underwent direct delabeling. Of these, 113 patients were delabeled because they reported only type A adverse reactions to penicillins (gastrointestinal side effects were the most common type A reaction, 88/113); 48 were delabeled by patient history, pharmacy dispensing, and/or medical reconciliation as they had subsequently tolerated the implicated penicillin but retained a penicillin allergy label on their medical record.
Oral Challenge
Over the study period, 200 patients underwent direct oral penicillin challenge, with the median time to oral challenge from time of assessment being 2 days (IQR, 1–3 days). The number of oral challenges performed per week of the study period is demonstrated in Supplementary Figure 1. The agents used for oral challenge were as follows: amoxicillin (115 [57.5%]), amoxicillin-clavulanate (5 [2.5%]), and penicillin VK (87 [43.5%]). Overall, 47 patients received a prolonged challenge (at least 5 days), and 60 patients received at least 1 further dose of any penicillin immediately following oral challenge (within 24 hours). The majority had a negative oral challenge (194/200 [97%]). Six patients had a positive oral challenge, and 3 patients reported a non-immune-mediated reaction to oral challenge (1 fever [38.1°C, concurrent urosepsis], 1 vomiting, and 1 pruritis without rash). No patient had an immune-mediated reaction during the 2-hour oral challenge period. Following completion of the oral challenge, 3 patients (1.5%) reported a positive oral challenge representing a presumed immune-mediated reaction; none of these were immunoglobulin E mediated and were probable T-cell–mediated reactions occurring between 5 and 7 days after oral challenge, which did not require any specific treatment (Supplementary Materials). At 90 days of follow-up, an additional 2 patients were relabeled (Supplementary Materials).
Antibiotic Utilization: Delabeled Patients Pre- and Posttesting
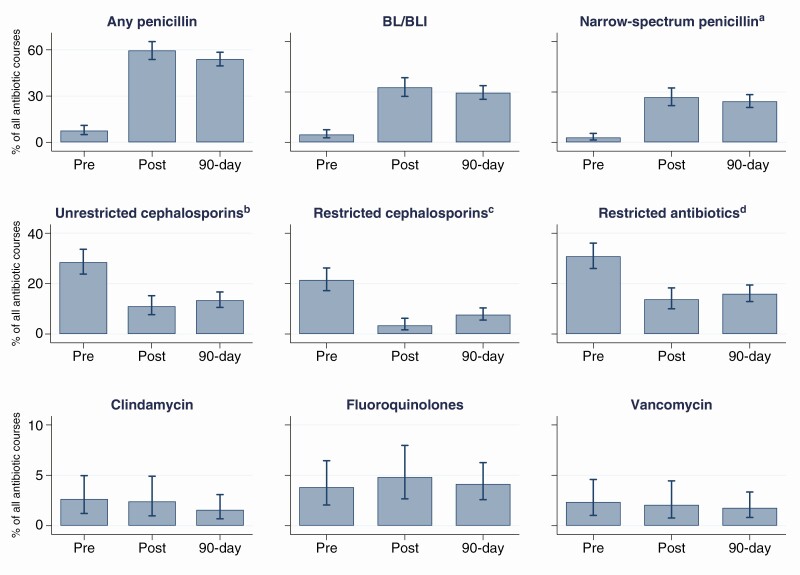
There were a total of 847 antibiotic courses prescribed in the cohort of delabeled patients (n = 355) over the whole study period. Three hundred forty courses were prescribed during the index admission prior to testing, and 507 within the 90-day period posttesting; of those posttesting courses, 290 (57.2%) were prescribed during the index admission, after completion of delabeling. Antibiotic utilization pre- and posttesting is demonstrated in Figure 2. After adjusting for sex, CCI, microbiological diagnosis, and sepsis, there was increased use of penicillins and reduced prescribing of restricted antibiotics and cephalosporins during both the index admission and 90 days posttesting. The use of other antibiotics did not change (Table 2).
Figure 2.
Antibiotic utilization in the delabeled cohort (n = 355). Errors bars represent 95% confidence intervals. aA narrow-spectrum penicillin was defined as 1 of penicillin VK, penicillin G, flucloxacillin, dicloxacillin, ampicillin, or amoxicillin. bAn unrestricted cephalosporin included first- or second-generation cephalosporins. cA restricted cephalosporin included third-generation or later cephalosporins. dA restricted antibiotic included lincosamides (ie, clindamycin, lincomycin), fluoroquinolones (ie, norfloxacin, ciprofloxacin, moxifloxacin), vancomycin, carbapenems (ie, ertapenem, meropenem), and third-generation or later cephalosporins (ie, cefepime, ceftazidime, ceftriaxone). Abbreviations: 90-day, 90-day posttesting admission; BL/BLI, β-lactam/β-lactamase inhibitor; pre, pretesting index admission; post, posttesting index admission.
Table 2.
Antibiotic Utilization in the Delabeled Group (n = 355)
| Antibiotic Courses | Index Admission: Posttesting vs Pretesting | 90-d Posttesting vs Index Admission Pretesting | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic | Index Admission Pretesting (n = 340) |
Index Admission Posttesting (n = 290) |
90 d Posttesting (n = 507) |
Unadjusted Analysis | Adjusted Analysisa | Unadjusted Analysis | Adjusted Analysisa | ||||
| OR (95% CI) |
P Value | OR (95% CI) |
P Value | OR (95% CI) |
P Value | OR (95% CI) |
P Value | ||||
| Narrow-spectrum penicillinb | 10 (2.9) | 78 (26.9) | 124 (24.5) | 12.14 (6.22–23.70) | < .001 | 12.39 (6.19–24.79) | < .001 | 10.68 (5.54–20.62) | < .001 | 10.51 (5.39–20.48) | < .001 |
| β-lactam/β-lactamase inhibitor | 16 (4.7) | 95 (32.8) | 150 (29.6) | 9.87 (5.04–19.31) | < .001 | 11.17 (5.76–21.65) | < .001 | 8.51 (4.40–16.44) | < .001 | 9.34 (4.83–18.07) | < .001 |
| Any penicillin | 26 (7.6) | 173 (59.7) | 274 (54.0) | 17.86 (10.50–30.37) | < .001 | 20.64 (12.09–35.22) | < .001 | 14.20 (8.63–23.36) | < .001 | 15.19 (9.19–25.10) | < .001 |
| Unrestricted cephalosporinc | 97 (28.5) | 32 (11.0) | 68 (13.4) | 0.31 (.20–.49) | < .001 | 0.31 (.20–.49) | < .001 | 0.39 (.27–.56) | < .001 | 0.40 (.28–.57) | < .001 |
| Restricted cephalosporind | 73 (21.5) | 10 (3.4) | 39 (7.7) | 0.13 (.07–.26) | < .001 | 0.12 (.06–.23) | < .001 | 0.30 (.21–.44) | < .001 | 0.29 (.19–.42) | < .001 |
| Restricted antibiotice | 105 (30.9) | 40 (13.8) | 81 (16.0) | 0.36 (.21–.60) | < .001 | 0.31 (.20–.50) | < .001 | 0.43 (.29–.62) | < .001 | 0.38 (.27–.54) | < .001 |
| Fluoroquinolones | 13 (3.8) | 14 (4.8) | 21 (4.1) | 1.28 (.62–2.62) | .508 | 1.20 (.61–2.36) | .594 | 1.09 (.52–2.26) | .824 | 0.94 (.47–1.87) | .86 |
| Vancomycin | 8 (2.4) | 6 (2.1) | 9 (1.8) | 0.88 (.32–2.44) | .801 | 0.75 (.26–2.16) | .598 | 0.75 (.28–1.99) | .563 | 0.62 (.25–1.58) | .318 |
| Clindamycin | 9 (2.6) | 7 (2.4) | 8 (1.6) | 0.91 (.25–3.32) | .886 | 0.80 (.23–2.82) | .73 | 0.59 (.16–2.12) | .418 | 0.53 (.14–1.95) | .337 |
| Carbapenems | 2 (0.6) | 3 (1.0) | 4 (0.8) | 1.77 (.30–10.27) | .526 | 2.17 (.44–10.79) | .345 | 1.34 (.25–7.18) | .729 | 1.42 (.31–6.64) | .652 |
| Antibiotic appropriatenessf | 229 (67.4) | 227 (78.3) | 404 (79.7) | 2.16 (1.41–3.31) | < .001 | 2.07 (1.35–3.18) | .001 | 2.19 (1.50–3.21) | < .001 | 2.13 (1.45–3.13) | < .001 |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: CI, confidence interval; OR, odds ratio.
aAdjusted for sex, age-adjusted Charlson comorbidity index, microbiological diagnosis, and sepsis.
bA narrow-spectrum penicillin was defined as 1 of penicillin VK, penicillin G, flucloxacillin, dicloxacillin, ampicillin, or amoxicillin.
cAn unrestricted cephalosporin included first- or second-generation cephalosporins.
dA restricted cephalosporin included third-generation or later cephalosporins.
eA restricted antibiotic included lincosamides (ie, clindamycin, lincomycin), fluoroquinolones (ie, norfloxacin, ciprofloxacin, moxifloxacin), vancomycin, carbapenems (ie, ertapenem, meropenem), and third-generation or later cephalosporins (ie, cefepime, ceftazidime, ceftriaxone).
fA National Antimicrobial Prescribing Survey score of 1 or 2 (appropriate).
Antibiotic Utilization: Delabeled Versus Non-delabeled Patients
The delabeled cohort compared to the non-delabeled cohort showed higher use of penicillin antibiotics and lower use of cephalosporins, clindamycin, and restricted antibiotics during index admission posttesting and within the 90-day follow-up period (unadjusted results) (Table 3). Due to large differences in characteristics of both cohorts (Table 1), a PS analysis using IPTW as outlined in the methods was undertaken. The distribution of propensity scores was similar between the 2 groups (Supplementary Figure 2). A small proportion of observations (1.9%) was off-support (PS in 1 group being lower/higher than the lowest/highest in the other group); therefore, all observations were included in the analysis [17]. The weighted sample was balanced on all characteristics included in the PS model (Supplementary Table 5). Assigned weights ranged from 0.07 to 9.0, with a mean weight of 1 and standard deviation of 0.7. In the delabeled group, there was increased utilization posttesting (index admission and 90 days) of penicillins (P < .001) and a reduction in cephalosporin (unrestricted and restricted), clindamycin, and restricted antibiotics usage (P < .01) with increased appropriateness of prescribed antibiotics (Table 3). Truncating the weights at the 99th percentile (weight of 4.44) did not affect the results (Supplementary Table 6).
Table 3.
Antibiotic Utilization in Delabeled Versus Non-delabeled Patients (Unadjusted Results and Using Propensity Score)
| Antibiotic | Delabeled (n = 355) |
Non-delabeled (n = 870) | Unadjusted Delabeled vs Non-delabeled | Propensity Score IPTW Delabeled vs Non-delabeled | ||
|---|---|---|---|---|---|---|
| No. (%) | No. (%) | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Antibiotic utilization | ||||||
| Index admission pre- vs posttesting | ||||||
| Narrow-spectrum penicillina | 49 (13.8) | 6 (0.7) | 23.06 (9.78–54.37) | < .001 | 13.90 (4.36–44.30) | < .001 |
| β-lactam/β-lactamase inhibitor | 61 (17.2) | 16 (1.8) | 11.07 (6.29–19.51) | < .001 | 5.95 (3.21–11.03) | < .001 |
| Any penicillin | 106 (29.9) | 22 (2.5) | 16.41 (10.15–26.53) | < .001 | 9.02 (5.19–15.66) | < .001 |
| Unrestricted cephalosporinb | 28 (7.9) | 168 (19.3) | 0.36 (.23–.55) | < .001 | 0.45 (.29–.69) | < .001 |
| Restricted cephalosporinc | 9 (2.5) | 85 (9.8) | 0.24 (.12–.48) | < .001 | 0.30 (.15–.62) | .001 |
| Restricted antibioticd | 24 (6.8) | 164 (18.9) | 0.31 (.20–.49) | < .001 | 0.38 (.24–.61) | < .001 |
| Fluoroquinolones | 10 (2.8) | 43 (4.9) | 0.56 (.28–1.12) | .102 | 0.52 (.25–1.09) | .083 |
| Vancomycin | 6 (1.7) | 28 (3.2) | 0.52 (.21–1.26) | .146 | 1.18 (.46–3.06) | .726 |
| Clindamycin | 4 (1.1) | 48 (5.5) | 0.20 (.07–.55) | .002 | 0.24 (.09–.70) | .009 |
| Carbapenems | 3 (0.8) | 9 (1.0) | 0.82 (.22–3.03) | .76 | 0.78 (.19–3.19) | .729 |
| Any antibiotic | 143 (40.3) | 323 (37.1) | 1.14 (.89–1.47) | .302 | 0.86 (.65–1.13) | .275 |
| Appropriatenesse | ||||||
| All inappropriate | 24 (6.8) | 94 (10.8) | 0.66 (.41–1.06) | .085 | 0.47 (.28–.79) | .004 |
| Some appropriate | 15 (4.2) | 108 (12.4) | 0.36 (.20–.63) | < .001 | 0.36 (.20–.66) | .001 |
| All appropriate | 104 (29.3) | 121 (13.9) | 2.22 (1.63–3.01) | < .001 | 1.40 (.99–1.97) | .055 |
| Antibiotic not required | 212 (59.7) | 547 (62.9) | Reference | Reference | ||
| Index admission pre vs 90 days posttesting | ||||||
| Narrow-spectrum penicillina | 67 (18.9) | 13 (1.5) | 15.34 (8.34–28.19) | < .001 | 10.89 (5.09–23.31) | < .001 |
| β-lactam/β-lactamase inhibitor | 81 (22.8) | 25 (2.9) | 9.99 (6.25–15.97) | < .001 | 6.68 (3.94–11.35) | < .001 |
| Any penicillin | 131 (36.9) | 36 (4.1) | 13.55 (9.11–20.16) | < .001 | 9.13 (5.75–14.50) | < .001 |
| Unrestricted cephalosporinb | 53 (14.9) | 226 (26.0) | 0.50 (.36–.69) | < .001 | 0.60 (.42–.84) | .003 |
| Restricted cephalosporinc | 34 (9.6) | 119 (13.7) | 0.67 (.45–1.00) | .05 | 0.75 (.48–1.15) | .188 |
| Restricted antibioticd | 48 (13.5) | 217 (24.9) | 0.47 (.33–.66) | < .001 | 0.52 (.36–.74) | < .001 |
| Fluoroquinolones | 13 (3.7) | 65 (7.5) | 0.47 (.26–.87) | .015 | 0.41 (.22–.77) | .006 |
| Vancomycin | 8 (2.3) | 48 (5.5) | 0.39 (.18–.84) | .016 | 0.63 (.28–1.41) | .26 |
| Clindamycin | 4 (1.1) | 62 (7.1) | 0.15 (.05–.41) | < .001 | 0.17 (.06–.49) | .001 |
| Carbapenems | 4 (1.1) | 18 (2.1) | 0.54 (.18–1.61) | .267 | 0.40 (.13–1.27) | .122 |
| Any antibiotic | 181 (51.0) | 399 (45.9) | 1.23 (.96–1.57) | .103 | 0.97 (.74–1.28) | .849 |
| Appropriatenesse | ||||||
| All inappropriate | 24 (6.8) | 109 (12.5) | 0.60 (.37–.96) | .033 | 0.43 (.26–.72) | .001 |
| Some appropriate | 37 (10.4) | 161 (18.5) | 0.62 (.42–.93) | .019 | 0.59 (.39–.90) | .015 |
| All appropriate | 120 (33.8) | 129 (14.8) | 2.52 (1.86–3.41) | < .001 | 1.78 (1.26–2.50) | .001 |
| Antibiotic not required | 174 (49.0) | 471 (54.1) | Reference | Reference | ||
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: CI, confidence interval; IPTW, inverse probability of treatment weighting; OR, odds ratio.
aA narrow-spectrum penicillin was defined as 1 of penicillin VK, penicillin G, flucloxacillin, dicloxacillin, ampicillin, or amoxicillin.
bAn unrestricted cephalosporin included first- or second-generation cephalosporins.
cA restricted cephalosporin included third-generation or later cephalosporins.
dA restricted antibiotic included lincosamides (ie, clindamycin, lincomycin), fluoroquinolones (ie, norfloxacin, ciprofloxacin, moxifloxacin), vancomycin, carbapenems (ie, ertapenem, meropenem), and third-generation or later cephalosporins (ie, cefepime, ceftazidime, ceftriaxone).
eMultinomial logistic regression used for analysis; results expressed as relative risk ratio.
Other Outcomes
There was no difference in the duration of intravenous antibiotic delivery, LOS, readmission rate, or mortality between the 2 groups (Table 4).
Table 4.
Other Outcomes in Delabeled Versus Non-delabeled Patients (Unadjusted Results and Using Propensity Score)
| Delabeled (n = 355) |
Non-delabeled (n = 870) |
Unadjusted, Not Propensity Score Delabeled vs Non-delabeled | Propensity Score IPTW Delabeled vs Non-delabeled | |||
|---|---|---|---|---|---|---|
| Outcome | Median (IQR) | Median (IQR) | IRR (95% CI) | P Value | IRR (95% CI) | P Value |
| Hospital admission | ||||||
| Length of stay, d | 3 (2–7) | 3 (1–6) | 1.13 (1.00–1.29) | .050 | 1.13 (.93–1.38) | .210 |
| Duration of IV antibiotics, d, index posttesting | 0 (0–1) | 0 (0–0) | 1.05 (.75–1.45) | .792 | 1.29 (.84–1.98) | .238 |
| Duration of IV antibiotics, d, 90 days posttesting | 0 (0–2) | 0 (0–1) | 1.13 (.86–1.48) | .384 | 1.32 (.90–1.92) | .152 |
| Mortality | No. (%) | No. (%) | OR (95% CI) | P Value | OR (95% CI) | P Value |
| In-hospital mortality | 7 (2.0) | 18 (2.1) | 0.95 (.39–2.30) | .911 | 0.85 (.31–2.31) | .754 |
| 90-d mortality | 13 (3.7) | 43 (4.9) | 0.73 (.39–1.38) | .330 | 0.70 (.35–1.37) | .298 |
| Readmissions within 90 d | ||||||
| Any readmissiona | 107 (30.1) | 270 (31) | 0.96 (.73–1.26) | .772 | 0.92 (.69–1.24) | .594 |
| Any readmission with infective diagnosis | 104 (12) | 46 (13) | 1.22 (.78–1.93) | .385 | 1.22 (.74–1.99) | .435 |
Abbreviations: CI, confidence interval; IPTW, inverse probability of treatment weighting; IQR, interquartile range; IRR, incidence rate ratio; IV, intravenous; OR, odds ratio.
aOverall, 10.6% (n = 129; 90 nondelabeled and 39 delabeled) had > 1 readmission.
Health Economics Modeling
The cost of de-labeling for the inpatient program was modeled against other inpatient and outpatient testing models (Supplementary Materials). The delabeling cost per patient was less in the inpatient setting compared to the outpatient setting for direct delabeling (no cost vs Australian $131.25, respectively) and for oral challenge ($35.18 vs $202.69, respectively) (Supplementary Table 7). For the 355 patients delabeled in the inpatient setting, the total cost was $6825, assuming implementation of the program into routine care and minimal additional physician time for consenting and documentation of oral challenges. Had this been performed in the outpatient clinic setting, the total cost would have been $60 447: a direct delabeling cost of $21 125 (n = 161 × $131.21) and an oral challenge cost of $39 322 (n = 194 × $202.69). Sensitivity analyses for the inpatient model and outpatient model, accounting for degrees of program implementation and potential physician billing rebates for care, are provided in the Supplementary Materials.
DISCUSSION
The dwindling antibiotic pipeline and association of patient-reported penicillin allergy with increased drug-resistant infections have brought the issue of antibiotic allergy into a sharper AMS and public health focus [2, 8]. In this whole-of-hospital program, led by the infectious diseases AMS program, we demonstrated that a significant proportion of patients can be delabeled at point of care safely, with significant positive effects on prescribing. Furthermore, we provide a unique prospective assessment of adult patients reporting an antibiotic allergy in the inpatient setting, allowing a stratification of allergy phenotypes and severity across healthcare networks.
The safety of performing a direct oral penicillin challenge, without prior skin testing, is increasingly recognized [11]. The implementation of oral penicillin challenge protocols has been hindered by the prior observational studies using complex test dose procedures (ie, 2–5 steps), being limited to the outpatient setting, pediatric predominant cohorts, and allergist-only delivery [12, 18–28]. To our knowledge, there has been no prospective multicenter study in adult inpatients examining single-dose penicillin challenge delivered by nonallergists. The low rate of immune-mediated positive oral challenges in this study of only 1.5% delayed-onset hypersensitivity reactions and no acute-onset hypersensitivity reactions, with an absence of serious adverse events, demonstrates the clear safety of the program—which is encouraging considering the complex inpatient population recruited (median CCI, 4; median age, 68 years; and 42% immunocompromised). Although a more expansive direct challenge inclusion criteria (eg, rash < 5 years previous or urticaria) would have yielded a larger testing cohort and could be the focus of future studies, an adverse event rate of 4.6%–10.2% as seen in some other studies with such criteria [18, 20, 21, 23, 26] may not be considered acceptable to treating clinicians of unwell inpatients. These results should also be considered against a baseline risk of a cutaneous reaction to a penicillin of approximately 2%–5% and that this could occur independent of the original reaction [2]. Our results demonstrate that inpatient oral penicillin challenge is feasible without dedicated allergy services.
Numerous studies have demonstrated the benefits of penicillin allergy testing to AMS practice [8, 29]. We previously demonstrated in an outpatient-predominant setting that an AMS-led testing service could improve both penicillin use and antibiotic appropriateness [10]. Pharmacist-led and allergist-supported programs have also been successful in the inpatient setting [23]. Our study in hospitalized inpatients demonstrated, at an individual and propensity-adjusted level, a dramatic increase in appropriate and narrow-spectrum penicillin use in those delabeled, without an increase in total antibiotic consumption. This was more notable as both hospitals had established AMS programs with mandatory preapproval systems for restricted antibiotics even prior to the intervention.
An antibiotic allergy label has been associated with additional direct drug costs, as well as additional total inpatient costs [29]. Although we demonstrated the clear benefits of inpatient direct oral challenge to AMS, cost-effectiveness is also a health services requirement. Overall, the proposed inpatient-embedded model of care, when compared to the outpatient clinic model of care, appears to have less cost for penicillin allergy testing, and this is without consideration of the potential savings to the inpatient services associated with reduced direct drug costs or reduced total inpatient costs [29]. It is noted that the inpatient setting provides a robust public health model that captures patients at the point of care where an immediate need for antibiotics is more likely to arise and avoids the uncertainty of relying on patient compliance with outpatient appointments and additional outpatient separations, which are associated with both real and opportunity costs for the patient and health service.
This study also has limitations, including the nonrandomized design, with both centers located in a single geographical region, and the exclusion of critically ill patients. Our AMS structure, which enforces significant restriction on antibiotic use, is still unusual by international standards and allowed us to document and capture differences between the delabeled and nondelabeled group. The conservative clinical criteria used in this study, in particular the exclusion of rash < 10 years, was consistent with the clinical criteria used in the pilot inpatient study [14]. Such criteria may be further adapted in the future to be more inclusive of other low-risk assessment tools reported in recent studies, including those associated with a PEN-FAST score of < 3 (penicillin allergy, five or fewer years ago, anaphylaxis/angioedema, severe cutaneous adverse reaction (SCAR), and treatment required for allergy episode) [30]. Nonetheless, our criteria and assessment tool are portable and can be used by a range of nonallergists, including nonmedical staff, and is potentially transferable to a range of healthcare systems. We identified that almost 50% of patients admitted with a penicillin allergy are low risk and amenable to delabeling efforts. The strategy provided here should enable clinicians globally to implement and incorporate similar antibiotic allergy delabeling programs into their health service as a novel medication safety and AMS intervention with minimal additional resources. Future work is required to assess the durability of allergy label removal.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the Infectious Diseases Departments of Austin Health and the Peter MacCallum Cancer Centre; the Honourable Justice Jennifer Coate, Ms Allyson Manley, and Ms Caroline Frankland; and the pharmacy and nursing departments of both participating hospitals.
Disclaimer. The funder played no role in study design, analysis, or manuscript preparation.
Financial support. This work was supported by the Better Care Victoria Innovation Fund, Better Care Victoria, Victoria Department of Health, Australia. J. A. T. is supported by a National Health and Medical Research Council (NHMRC) Early Career Research Grant (GNT 1139902), Royal Australasian College of Physicians (RACP) Research Establishment Fellowship and postdoctoral scholarship from the National Centre for Infections in Cancer (NCIC). E. J. P. receives funding from the National Institutes of Health (grants 1P50GM115305-01, R21AI139021, R34AI136815, and 1 R01 HG010863-01) and the National Health and Medical Research Council of Australia.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Trubiano JA, Chen C, Cheng AC, Grayson ML, Slavin MA, Thursky KA; National Antimicrobial Prescribing Survey (NAPS) . Antimicrobial allergy “labels” drive inappropriate antimicrobial prescribing: lessons for stewardship. J Antimicrob Chemother 2016; 71:1715–22. [DOI] [PubMed] [Google Scholar]
- 2. Blumenthal KG, Peter JG, Trubiano JA, Phillips EJ. Antibiotic allergy. Lancet 2019; 393:183–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blumenthal KG, Lu N, Zhang Y, Li Y, Walensky RP, Choi HK. Risk of meticillin resistant Staphylococcus aureus and Clostridium difficile in patients with a documented penicillin allergy: population based matched cohort study. BMJ 2018; 361:k2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sousa-Pinto B, Cardoso-Fernandes A, Araujo L, Fonseca JA, Freitas A, Delgado L. Clinical and economic burden of hospitalizations with registration of penicillin allergy. Ann Allergy Asthma Immunol 2018; 120:190–4.e2. [DOI] [PubMed] [Google Scholar]
- 5. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol 2014; 133:790–6. [DOI] [PubMed] [Google Scholar]
- 6. Blumenthal KG, Lu N, Zhang Y, Walensky RP, Choi HK. Recorded penicillin allergy and risk of mortality: a population-based matched cohort study. J Gen Intern Med 2019; 34:1685–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trubiano JA, Adkinson NF, Phillips EJ. Penicillin allergy is not necessarily forever. JAMA 2017; 318:82–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trubiano J, Phillips E. Antimicrobial stewardship’s new weapon? A review of antibiotic allergy and pathways to “de-labeling.” Curr Opin Infect Dis 2013; 26:526–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barlam TF, Cosgrove SE, Abbo LM, et al. . Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trubiano JA, Thursky KA, Stewardson AJ, et al. . Impact of an integrated antibiotic allergy testing program on antimicrobial stewardship: a multicenter evaluation. Clin Infect Dis 2017; 65:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep 2019; 19:27. [DOI] [PubMed] [Google Scholar]
- 12. Mustafa SS, Conn K, Ramsey A. Comparing direct challenge to penicillin skin testing for the outpatient evaluation of penicillin allergy: a randomized controlled trial. J Allergy Clin Immunol Pract 2019; 7:2163–70. [DOI] [PubMed] [Google Scholar]
- 13. Devchand M, Urbancic KF, Khumra S, et al. . Pathways to improved antibiotic allergy and antimicrobial stewardship practice: the validation of a beta-lactam antibiotic allergy assessment tool. J Allergy Clin Immunol Pract 2019; 7:1063–5.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trubiano JA, Smibert O, Douglas A, et al. . The safety and efficacy of an oral penicillin challenge program in cancer patients: a multicenter pilot study. Open Forum Infect Dis 2018; 5:ofy306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trubiano JA, Pai Mangalore R, Baey YW, et al. . Old but not forgotten: antibiotic allergies in general medicine (the AGM Study). Med J Aust 2016; 204:273. [DOI] [PubMed] [Google Scholar]
- 16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods 2010; 15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mill C, Primeau MN, Medoff E, et al. . Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr 2016; 170:e160033. [DOI] [PubMed] [Google Scholar]
- 19. Vezir E, Dibek Misirlioglu E, Civelek E, et al. . Direct oral provocation tests in non-immediate mild cutaneous reactions related to beta-lactam antibiotics. Pediatr Allergy Immunol 2016; 27:50–4. [DOI] [PubMed] [Google Scholar]
- 20. Confino-Cohen R, Rosman Y, Meir-Shafrir K, et al. . Oral challenge without skin testing safely excludes clinically significant delayed-onset penicillin hypersensitivity. J Allergy Clin Immunol Pract 2017; 5:669–75. [DOI] [PubMed] [Google Scholar]
- 21. Labrosse R, Paradis L, Lacombe-Barrios J, et al. . Efficacy and safety of 5-day challenge for the evaluation of nonsevere amoxicillin allergy in children. J Allergy Clin Immunol Pract 2018; 6:1673–80. [DOI] [PubMed] [Google Scholar]
- 22. Iammatteo M, Alvarez Arango S, Ferastraoaru D, et al. . Safety and outcomes of oral graded challenges to amoxicillin without prior skin testing. J Allergy Clin Immunol Pract 2019; 7:236–43. [DOI] [PubMed] [Google Scholar]
- 23. du Plessis T, Walls G, Jordan A, Holland DJ. Implementation of a pharmacist-led penicillin allergy de-labelling service in a public hospital. J Antimicrob Chemother 2019; 74:1438–46. [DOI] [PubMed] [Google Scholar]
- 24. Li J, Shahabi-Sirjani A, Figtree M, Hoyle P, Fernando SL. Safety of direct drug provocation testing in adults with penicillin allergy and association with health and economic benefits. Ann Allergy Asthma Immunol 2019; 123:468–75. [DOI] [PubMed] [Google Scholar]
- 25. Stevenson B, Trevenen M, Klinken E, et al. . Multicenter Australian study to determine criteria for low- and high-risk penicillin testing in outpatients. J Allergy Clin Immunol Pract 2020; 8:681–9.e3. [DOI] [PubMed] [Google Scholar]
- 26. Ibanez MD, Rodriguez Del Rio P, Lasa EM, et al. . Prospective assessment of diagnostic tests for pediatric penicillin allergy: from clinical history to challenge tests. Anna Allergy Asthma Immunol 2018; 121:235–44.e3. [DOI] [PubMed] [Google Scholar]
- 27. Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract 2017; 5:813–5. [DOI] [PubMed] [Google Scholar]
- 28. Vyles D, Adams J, Chiu A, Simpson P, Nimmer M, Brousseau DC. Allergy testing in children with low-risk penicillin allergy symptoms. Pediatrics 2017; 140:e20170471. [DOI] [PubMed] [Google Scholar]
- 29. Mattingly TJ 2nd, Fulton A, Lumish RA, et al. . The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract 2018; 6:1649–54.e4. [DOI] [PubMed] [Google Scholar]
- 30. Trubiano J, Vogrin S, Holmes NE, et al. . Development and validation of a penicillin allergy clinical decision rule. JAMA Intern Med 2020; 180:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.