Abstract
Background
The cell-propagated inactivated quadrivalent influenza vaccine (ccIIV4) may offer improved protection in seasons where egg-derived influenza viruses undergo mutations that affect antigenicity. This study estimated the relative vaccine effectiveness (rVE) of ccIIV4 versus egg-derived inactivated quadrivalent influenza vaccine (eIIV4) in preventing influenza-related medical encounters in the 2018–2019 US season.
Methods
A dataset linking primary care electronic medical records with medical claims data was used to conduct a retrospective cohort study among individuals ≥ 4 years old vaccinated with ccIIV4 or eIIV4 during the 2018–2019 season. Adjusted odds ratios (ORs) were derived from a doubly robust inverse probability of treatment-weighted approach adjusting for age, sex, race, ethnicity, geographic region, vaccination week, and health status. rVE was estimated by (1 – OR) × 100 and presented with 95% confidence intervals (CI).
Results
Following the application of inclusion/exclusion criteria, the study cohort included 2 125 430 ccIIV4 and 8 000 903 eIIV4 recipients. Adjusted analyses demonstrated a greater reduction in influenza-related medical encounters with ccIIV4 versus eIIV4, with the following rVE: overall, 7.6% (95% CI, 6.5–8.6); age 4–17 years, 3.9% (95% CI, .9–7.0); 18–64 years, 6.5% (95% CI, 5.2–7.9); 18–49 years, 7.5% (95% CI, 5.7–9.3); 50–64 years, 5.6% (95% CI, 3.6–7.6); and ≥65 years, –2.2% (95% CI, –5.4 to .9).
Conclusions
Adjusted analyses demonstrated statistically significantly greater reduction in influenza-related medical encounters in individuals vaccinated with ccIIV4 versus eIIV4 in the 2018–2019 US influenza season. These results support ccIIV4 as a potentially more effective public health measure against influenza than an egg-based equivalent.
Keywords: influenza, cell-derived influenza vaccine, egg-derived influenza vaccine, quadrivalent inactivated influenza vaccine, relative vaccine effectiveness
During the 2018–2019 influenza season in the U.S., the cell-derived quadrivalent influenza vaccine demonstrated statistically significantly greater effectiveness in reducing influenza-related medical encounters in individuals ≥4 years of age compared to egg-derived quadrivalent vaccines.
Seasonal influenza is associated with considerable morbidity and mortality each year in the United States [1, 2]. Annual influenza vaccination is recommended by the Advisory Committee on Immunization Practices for all individuals aged 6 months and older without a contraindication to vaccination to help contain the impact of influenza on public health [3]. However, although influenza vaccines have an established safety record, their effectiveness varies each season. During traditional egg-based manufacturing of influenza vaccines, mutations can accumulate in the viral hemagglutinin protein in response to selective pressures in the egg environment [4]. These mutations can alter antigenicity and can contribute to reduced effectiveness of egg-derived influenza vaccines, occurring most frequently with the influenza A(H3N2) strain [2, 5–8]. In the US 2018–2019 season, the effectiveness of influenza vaccines was 44% (95% confidence interval [CI], 37–51) against A(H1N1)pdm09-related illnesses, but protection against A(H3N2)-related illnesses was limited (9% [95% CI, –4 to 20]) [9]. Emerging evidence suggests that egg-adapted mutations may have affected antigenicity of A(H3N2) viruses, which may explain the potential for lower vaccine effectiveness against A(H3N2) observed in the 2018–2019 season in the United States [10].
Replication of influenza viruses in cell culture prevents egg-adaptive mutations, resulting in a vaccine inclusive of influenza strains that are more antigenically faithful to the starting candidate virus [11–13]. The cell culture–derived, inactivated quadrivalent influenza vaccine (ccIIV4) (Flucelvax Quadrivalent, Seqirus USA Inc., Summit, NJ) was approved in the United States in May 2016. Clinical studies have demonstrated that ccIIV4 has comparable immunogenicity to egg-derived vaccines [14], and several observational studies have demonstrated a trend toward increased effectiveness of cell culture–derived vaccines compared with egg-derived vaccines [15–19].
However, the cyclical nature of influenza virus circulation necessitates the estimation of actionable vaccine effectiveness each influenza season. Assessment of vaccine performance under real-world conditions provides critical information that may be used to inform vaccine regulation, policy, and product development. The objective of this analysis was, therefore, to conduct a large retrospective cohort study to assess the real-world effectiveness of ccIIV4 relative to egg-derived inactivated quadrivalent influenza vaccine (eIIV4) in preventing influenza-related medical encounters during the 2018–2019 US influenza season.
METHODS
Study Design
A retrospective cohort study was conducted during the 2018–2019 influenza season using deidentified patient-level electronic medical records (EMRs) from primary care and specialty clinics linked with pharmacy and medical claims data. Data were evaluated for subjects ≥ 4 years of age who had a record of receiving either ccIIV4 or eIIV4 either in their EMRs or medical claims. The observation period was between 1 August 2018, and 18 May 2019. This study was designed, implemented, and reported in accordance with Good Pharmacoepidemiological Practice, applicable local regulations, and the ethical principles laid down in the Declaration of Helsinki. Study findings are reported in accordance with the Reporting of Studies Conducted using Observational Routinely Collected Health Data recommendations.
Data Sources and Linkage
A dataset integrating patient-level EMRs from Veradigm Health Insights (Allscripts Touchworks & Allscripts PRO, Chicago, IL; and Practice Fusion, Inc., San Francisco, CA) with pharmacy and medical claims data, where available (Komodo Health Inc., New York, NY), was used for the analysis. Each individual dataset was first required to meet the minimum Protected Health Information (PHI) data requirements. Research staff were not involved in preparation of datasets containing PHI or the actual running of the linkage algorithm. A third party (Datavant, San Francisco, CA) performed deidentification and linkage. The dataset underwent privacy certification to verify it contained no PHI and was evaluated and certified for Health Insurance Portability and Accountability Act compliance by a third-party statistician.
Study Population
The study population included US residents ≥ 4 years of age with a record of receiving either eIIV4 or ccIIV4 during the 2018–2019 Northern Hemisphere influenza season in the EMRs or claims datasets and who had at least 1 record in their primary care EMR in the year before the recorded influenza immunization. Subjects were considered fully protected against influenza 14 days after recorded receipt of the seasonal influenza vaccine to allow for development of vaccine-specific immunity. Subjects were excluded from the cohort if they were ≥ 9 years of age and had received > 1 influenza vaccination during the study season, were < 9 years of age and had received > 2 influenza vaccinations during the study season, or had an influenza-related medical encounter during the study season before the vaccination date. This study was a noninterventional, retrospective study using a certified Health Insurance Portability and Accountability Act–compliant database; as such, approval for this analysis by an institutional review board was not necessary.
Exposure Ascertainment
Current Procedural Terminology codes, codes for vaccines administered, and national drug codes (Supplementary Table 1) were used to identify potential study subjects if they had a record of immunization with either ccIIV4 or eIIV4 between 1 August 2018, and 28 February 2019, in either the EMR and/or claims components of the integrated dataset. The date of recorded immunization was considered the index date.
Outcome Ascertainment
The outcome of interest was the occurrence of an influenza-related medical encounter (hospital or primary care) ascertained using International Classification of Diseases codes for “influenza diagnosis” (as reported by the Armed Forces Health Surveillance Center [AFHSC] Code Set B case definition, listed in Supplementary Table 2) [20]. Of note, a second, broader case definition was also used (AFHSC Code Set A for “influenza like illness”). This broader definition of influenza like illness had a lower positive predictive value (63%) compared with Code Set B (96%) in a validation study among a population of Armed Forces members and their dependents with laboratory-confirmed influenza as “gold standard” (Supplementary Table 2) [21] and thus was not used as the primary case definition; results from this analysis are reported as part of the Supplementary Data.
Covariates
Covariates of interest were identified a priori based on subject-matter expertise, biological plausibility, and published literature on influenza vaccination and were identified in the 12 months before the recorded date of immunization with ccIIV4 or eIIV4 (termed the “pre-index period”). Data were ascertained from each subject’s EMR on age, sex, race, ethnicity, health status (quantified using the Charlson Comorbidity Index [CCI]) [22, 23], index date, and US geographic region (based on mutually exclusive US census regions: South, West, Northeast, Midwest, Other). Although all covariates were adjusted for as confounders, covariate balance between the exposure groups following per-protocol adjustment was assessed using standardized mean differences (SMDs) [24].
Statistical Methods
Per-protocol Analyses
A descriptive analysis was conducted to first evaluate patient characteristics at the time of immunization. Continuous and categorical variables were reported as mean ± standard deviation and proportional values, respectively. Unadjusted odd ratios (ORs) for the outcome of influenza-related medical encounters were estimated from a univariable model with exposure status as the only predictor variable.
Adjusted ORs were derived from a weighted sample using inverse probability of treatment weighting (IPTW) [24]. First, propensity scores were calculated for each subject using a multivariable logit model adjusted for age, sex, race, ethnicity, geographic region, week of vaccination, and CCI. Propensity scores were then used to create stabilized inverse probability of treatment weights. Weights were truncated at the third and 97th percentile weight to attenuate any extreme variability from outlier patients. Adjusted ORs were then estimated for the full study sample using a logistic regression model in the IPTW-weighted cohort. The rVE was calculated as 100 × (1 – ORadjusted) and is reported with 95% CIs. Analyses were conducted using SQL and SAS (version 9.4).
Missing Data
Categorical variables with missing or null values in the EMR were classified as “not reported” or “unknown,” whereas continuous counts of comorbidities or CCI were recoded as 0. Missing or out-of-range values were not imputed.
Additional Analyses
Subgroup Analyses
The rVE of ccIIV4 versus eIIV4 was reestimated in subgroups defined by age (≥4 years, ≥4 to ≤17 years, ≥18 to ≤49 years, ≥18 to ≤64 years, ≥50 to ≤64 years, and ≥65 years). Specifically, propensity scores were recalculated for each subject using a multivariable logit model with study covariates as predictor variables. As with the main analysis, the propensity scores were truncated at the third and 97th percentile and were then used to generate stabilized weights. Adjusted ORs for each age subgroup were subsequently estimated using a logistic regression model with vaccine type as the sole independent variable in the IPTW-weighted sample.
Sensitivity Analysis: Restricted Influenza Season
As a sensitivity analysis, the adjusted rVE was reestimated in a restricted observation window corresponding to adjacent calendar weeks with peak laboratory-confirmed influenza activity [25]. This alternative observation window was defined as 17 December 2018, to 7 April 2019 [26].
Post hoc Analyses
A post hoc analysis was conducted evaluating the effect of adjusting for health status as a summary measure using the CCI versus adjusting for each health condition individually. As such, adjusted rVEs were reestimated using propensity scores derived from the same set of covariates as in the main analysis, except that the CCI score as a single variable was replaced by 17 binary variables for the presence or absence of the following comorbidities: acquired immunodeficiency syndrome/human immunodeficiency virus, cerebrovascular disease, chronic pulmonary disease, congestive heart failure, dementia, diabetes with chronic complications, diabetes without chronic complications, hemiplegia or paraplegia, liver disease, any malignancy, metastatic tumor, mild liver disease, myocardial infarction, peptic ulcer disease, peripheral vascular disease, renal disease, and rheumatic disease.
Last, following assessment of the per protocol IPTW approach using SMD graphs, a decision was made to re-run all analyses (both per protocol and post hoc) using a doubly robust adjustment methodology to account for any residual confounding from measured covariates [27]. Specifically, adjusted ORs for the overall population, and for subcohorts defined by age, were reestimated in an IPTW-weighted sample using a multivariable model that included all variables from the propensity score–generation model as covariates.
RESULTS
Study Subjects
Overall, 10 126 333 individuals were included in the study cohort; 2 125 430 (20.1%) had a record of receiving ccIIV4 and 8 000 903 (79.9%) received eIIV4 (Table 1). Subjects receiving ccIIV4 were, on average, 10 years older than eIIV4 recipients, whereas for both groups the majority of subjects were female and had a recorded race of white. A large proportion of individuals from both exposure groups were from the Southern geographic region, although the proportion of ccIIV4 recipients was greater (48.2% vs 36.0% of eIIV4 recipients). Nearly twice as many participants from the Midwest had a record of receiving eIIV4 (22.6%) as ccIIV4 (12.8%). Similar proportions of participants came from the Northeast and West (Table 2). Diabetes, chronic pulmonary disease, peripheral vascular disease, and cancer were the most common medical conditions in both exposure groups (Supplementary Table 3).
Table 1.
Subject Selection in the 2018–2019 Influenza Season
| Criteria | Subjects, Overall N (%) | Stepwise Change (%) |
|---|---|---|
| 1. Patient received influenza vaccine between 1 August 2018, and 28 February 2019 | 14 734 352 (100.0) | - |
| 2. Patient is ≥4 years of age at time of immunization | 14 211 914 (96.5) | 96.5 |
| 3. Patient does not have >1 influenza immunization during the influenza season unless they are <9 years of age | 13 848 844 (94.0) | 97.4 |
| 4. Patient does not have an influenza-related medical encounter in the influenza season before immunization | 13 808 250 (93.7) | 99.7 |
| 5. Patient has a transcript record in the study EMR at least 1 year before immunization date | 10 126 333 (68.7) | 73.3 |
| ccIIV4 | 2 125 430 (14.4) | 21.0 |
| eIIV4 | 8 000 903 (54.3) | 79.0 |
Abbreviations: ccIIV3, cell culture–derived inactivated quadrivalent influenza vaccine; eIIV3, egg-derived inactivated quadrivalent influenza vaccine; EMR, electronic medical record.
Table 2.
Subject Demographics at Baseline
| Characteristic | ccIIV4 (n = 2 125 430) | eIIV4 (n = 8 000 903) |
|---|---|---|
| Mean age, y ± SD | 53.2 ± 18.2 | 42.8 ± 21.8 |
| 4–17 y, n (%) | 78 602 (3.7) | 1 628 038 (20.3) |
| 18–49 y, n (%) | 700 729 (33.0) | 2 641 268 (33.0) |
| 50–64 y, n (%) | 828 460 (39.0) | 2 743 654 (34.3) |
| ≥65 y, n (%) | 517 639 (24.4) | 987 943 (12.3) |
| Female, n (%) | 1 301 982 (61.3) | 4 714 325 (58.9) |
| Race and ethnicity, n (%) | ||
| White | 895 550 (42.1) | 3 557 664 (44.5) |
| Black or African American | 111 817 (5.3) | 417 032 (5.2) |
| Other | 219 028 (10.3) | 825 930 (10.3) |
| Race not reported | 899 035 (42.3) | 3 200 277 (40.0) |
| Hispanic ethnicity | 126 116 (5.9) | 574 828 (7.2) |
| Non-Hispanic ethnicity | 1 619 011 (76.2) | 5 887 982 (73.6) |
| Ethnicity not reported | 380 303 (17.9) | 1 538 093 (19.2) |
| Geographic region, n (%) | ||
| Northeast | 392 918 (18.5) | 1 455 385 (18.2) |
| Midwest | 271 470 (12.8) | 1 809 363 (22.6) |
| South | 1 024 956 (48.2) | 2 880 898 (36.0) |
| West | 325 562 (15.3) | 1 496 358 (18.7) |
| Not reported/other | 110 524 (5.2) | 358 899 (4.5) |
| CCI ± SD | .5 ± 1.1 | .4 ± 1.0 |
Abbreviations: ccIIV3, cell culture–derived inactivated quadrivalent influenza vaccine; CCI, Charlson comorbidity index; eIIV3, egg-derived inactivated quadrivalent influenza vaccine; SD, standard deviation.
Overall rVE
Among ccIIV4 recipients, 1.6% reported an influenza-related medical encounter compared with 2.4% in the eIIV4 cohort. The unadjusted rVE for ccIIV4 vs eIIV4 for the overall cohort was 33.2% (95% CI, 32.4–33.9), and the per-protocol IPTW-adjusted rVE was 22.2% (95% CI, 21.4–23.1) (Supplementary Figure 1). For age subcohorts, the IPTW adjusted rVE was 5.9% (95% CI, 2.9–8.8) for subjects ≥ 4 to ≤17 years, 6.5% (95% CI, 5.2–7.9) for ≥18 to ≤64 years, 7.5% (95% CI, 5.6–9.3) for ≥18 to ≤49, 5.5% (95% CI, 3.5–7.5) for ≥50 to ≤64, and –2.3% (95% CI, –5.5 to .9) for ≥65 years (Supplementary Figure 1). SMDs for all covariates in the IPTW-weighted exposure groups are presented in Supplementary Figure 2. Several variables had SMDs > 0.1, the accepted threshold for negligible imbalances between comparison groups using the IPTW methodology, necessitating a post hoc doubly robust IPTW analysis to account for remaining imbalances in observed confounders between the exposure groups [15, 28].
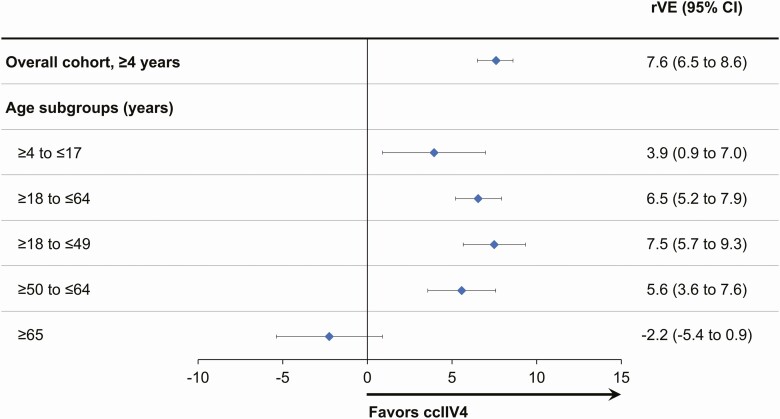
After post hoc doubly robust adjustment, the rVE for ccIIV4 versus eIIV4 was 7.6% (95% CI, 6.5–8.6) for the overall cohort, 3.9% (95% CI, .9–7.0) for subjects 4–17 years, 7.5% (95% CI, 5.7–9.3) for 18–49 years, 5.6% (95% CI, 3.6–7.6) for 50–64 years, 6.5% (95% CI, 5.2–7.9) for 18–64 years, and –2.2% (95% CI, –5.4 to .9) for ≥65 years (Figure 1). Results using the broader definition of influenza-related medical encounters (AFHSC Code Set A) are presented in Supplementary Figure 3.
Figure 1.
Relative vaccine effectiveness (rVE) of ccIIV4 compared with eIIV4 among individuals ≥4 years in the 2018–2019 influenza season using doubly robust IPTW adjustment methodology. Abbreviations: ccIIV4, cell culture–derived inactivated quadrivalent influenza vaccine; CI, confidence interval; eIIV4, egg-derived inactivated quadrivalent influenza vaccine; IPTW, inverse probability of treatment weighting.
Sensitivity Analyses
The per-protocol IPTW-adjusted rVE during peak influenza activity was 23.1% (95% CI, 22.2–24.1). rVE by age subcohorts is shown in Supplementary Figure 4A. In the doubly robust post hoc analysis conducted within the restricted influenza season, the overall rVE was 8.4% (95% CI, 7.3–9.5), and results favored ccIIV4 in all age subgroups except ≥65 years. However, the results in this subcohort were not statistically significant (Figure 2).
Figure 2.
rVE of ccIIV4 vs eIIV4 using doubly robust adjustment methodology during restricted season with peak influenza activity between 1 August 2018 and 7 April 2019. Abbreviations: ccIIV4, cell culture–derived inactivated quadrivalent influenza vaccine; CI, confidence interval; eIIV4, egg-derived inactivated quadrivalent influenza vaccine; rVE, relative vaccine effectiveness.
In the post hoc sensitivity analysis adjusting for health status using individual variables rather than the CCI (Supplementary Table 3), the per-protocol IPTW-adjusted rVE for ccIIV4 versus eIIV4 was 18.9% (95% CI, 18.0–19.8) (Supplementary Figure 5A); the rVE from the post hoc doubly robust IPTW was 7.1% (95% CI, 6.1–8.3) in the overall cohort of subjects ≥ 4 years of age (Supplementary Figure 5B). Results within subgroups defined by age were generally similar to those from the overall study population.
DISCUSSION
In this analysis of more than 10 million vaccinated individuals, ccIIV4 was statistically significantly more effective than egg-derived eIIV4 in preventing influenza-related medical encounters in individuals ≥ 4 years in the 2018–2019 US influenza season. Results remained statistically significant and directionally similar in age subgroups except for the cohort of individuals ≥ 65 years of age, where nonstatistically significant results preclude definitive conclusions. An MF59-adjuvanted vaccine and a high-dose nonadjuvanted vaccine were developed specifically to address the impact of immunosenescence in adults ≥ 65 years of age by providing enhanced levels of protection against influenza.
Cell-based influenza vaccine technology may offer advantages over the standard influenza manufacturing process, including being more scalable and offering faster production [29]. Furthermore, the propagation of influenza vaccine viruses in mammalian cells rather than embryonated chicken eggs eliminates the opportunities for adaptive viral mutations to occur and maintains viral antigenicity, which supports the improved effectiveness of ccIIV4 observed in this study [19]. Results from the current analysis are not unexpected given the observed egg-adaptive amino acid changes in the hemagglutinin protein of egg-derived viruses identified within a subset of A(H3N2) viruses assessed in the 2018–2019 US season [10].
It is widely accepted that egg adaptation, particularly for A(H3N2) and B influenza viruses, could affect vaccine effectiveness [9, 30, 31]. The 2017–2018 season was generally dominated by A(H3N2) viruses and was the first season in which the seed virus for the A(H3N2) antigen for the cell-cultured vaccine did not undergo any egg passage. Alternatively, the 2018–2019 influenza season in the United States was dominated by 2 waves of influenza virus circulation: influenza A(H1N1) viruses from October 2018 to mid-February 2019 and influenza A(H3N2) viruses from February through May 2019. The absolute vaccine effectiveness during the 2018–2019 season was 29% (95% CI, 21–35) as reported by the US Centers for Disease Control and Prevention, likely because of an apparent mismatch between circulating A(H3N2) strains and vaccine virus strains [2, 9, 10, 31]. Furthermore, in the 2018–2019 season, the seed virus used to produce the A(H1N1)pdm09 antigens for the cell-cultured vaccine was egg grown, whereas the seed viruses for the A(H3N2) and B antigens were cell grown. Thus, the A(H3N2) vaccine-circulating strain mismatch coupled with the fact that the seed viruses for the cell-cultured and egg-based A(H1N1)pdm09 antigens were both produced using egg-based seed viruses may explain why we did not observe a higher rVE for the cell-cultured vaccine in the 2018–2019 season compared to the 2017–2018 season [19]. This trend is similar to that reported by Izurieta et al for these 2 US seasons [15, 32]. Overall, the general conclusions of this study support the trend in published literature that ccIIV4 may be more effective compared with eIIV4 in seasons affected by egg adaptation of egg-derived vaccine-strain viruses [15, 16, 19, 32–34].
The use of a large integrated dataset provided a more complete, accurate, and well-rounded picture of an individual’s health status and service utilization in comparison to the use of EMRs or claims data alone. Although validation of information in subjects’ EMRs and claims was not possible, exposure, outcome, and covariate information was ascertained retrospectively from the integrated database in the same manner for both vaccine cohorts, limiting the possibility of differential covariate misclassification between the exposure groups. Additionally, doubly robust adjustment methodology was implemented in all analyses, which further controlled for residual confounding. The conclusions from the main analysis were either confirmed or supported by planned sensitivity analyses and post hoc evaluations.
Nonetheless, results must be interpreted in light of several limitations. First, the study outcome was not laboratory confirmed. However, consistent results were observed when the observation window was limited to the weeks of peak influenza circulation with highest laboratory-confirmed influenza activity (Figure 2) [20]. A descriptive evaluation of the overlap between the incidence of Centers for Disease Control and Prevention–reported, laboratory-confirmed influenza and the incidence of influenza-related medical encounters (AFHSC Code Set B) in the integrated dataset was also conducted. Concordance between trends was observed (Supplementary Figure 6), supporting the use of the diagnostic AFHSC Code Set B in evaluations of influenza. Another limitation of this study was that the analyses did not specifically adjust for frailty. However, this limitation would most likely only affect a subset of the study population ≥ 65 years of age. Furthermore, the present study population included individuals for whom at least some pharmacy and medical claims data were available, thus limiting the study cohort to insured individuals but not requiring healthcare resource utilization beyond the index vaccination. Finally, despite the adoption of a doubly robust adjustment methodology to control for measured confounders, unmeasured confounding is a potential source of bias in all observational research. It is particularly prominent in studies using routinely collected data because these data are not specifically collected for research purposes.
CONCLUSION
The results of this study demonstrate that individuals ≥ 4 years of age vaccinated with ccIIV4 had statistically significantly fewer influenza-related medical encounters compared to individuals vaccinated with egg-derived eIIV4 in the 2018–2019 influenza season in the United States. Findings from this study provide further evidence supporting ccIIV4 as a potentially more effective public health measure against influenza than an egg-derived equivalent [15, 16, 19, 33, 34].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. C. B., J. A. M., and G. C. S. contributed to study conceptualization and design. C. B., J. A. M., G. C. S., L. F., D. O., and J. V. contributed to data collection, analysis, and interpretation.
Acknowledgments. The authors thank Mahrukh Imran (Seqirus Inc.) and VHN Consulting for their contribution to the manuscript. C. Gordon Beck and Amanda M. Justice provided editorial support (formatting, reference management) in the preparation of this manuscript, which was funded by Seqirus Inc.
Financial support. This work was supported by Seqirus.
Potential conflicts of interest. C. B. and J. A. M. are employees of Seqirus Inc. G. C. S. is an employee of Seqirus USA Inc. L. F., D. O., and J. V. work for Veradigm, a company that was contracted by Seqirus and received fees for data management and statistical analyses. J. V. reports funding from Astra-Zeneca and Pfizer, outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Centers for Disease Control and Prevention. Estimated influenza illnesses, medical visits, hospitalizations, and deaths averted by vaccination in the United States. Available at: https://www.cdc.gov/flu/about/disease/2015–16.htm. Accessed 22 October 2020.
- 2. Belongia EA, McLean HQ. Influenza vaccine effectiveness: defining the H3N2 problem. Clin Infect Dis 2019; 69:1817–23. [DOI] [PubMed] [Google Scholar]
- 3. Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rajaram S, Boikos C, Gelone DK, Gandhi A. Influenza vaccines: the potential benefits of cell-culture isolation and manufacturing. Ther Adv Vaccines Immunother 2020; 8:2515135520908121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Report prepared for the WHO annual consultation on the composition of influenza vaccine for the Northern Hemisphere 2017–2018. 2 March 2017. Available at: https://www.who.int/influenza/vaccines/virus/recommendations/2017_18_north/en/. Accessed 19 January 2021.
- 6. Wu NC, Zost SJ, Thompson AJ, et al. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog 2017; 13:e1006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zost SJ, Parkhouse K, Gumina ME, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A 2017; 114:12578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blanton L, Wentworth DE, Alabi N, et al. Update: influenza activity - United States and worldwide, May 21–September 23, 2017. MMWR Morb Mortal Wkly Rep 2017; 66:1043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flannery B, Kondor RJG, Chung JR, et al. Spread of antigenically drifted influenza A(H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season. J Infect Dis 2020; 221:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu X, Blanton L, Elal AIA, et al. Update: influenza activity in the United States during the 2018–19 season and composition of the 2019–20 influenza vaccine. MMWR Morb Mortal Wkly Rep 2019; 68:544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rajaram S, Suphaphiphat P, van Boxmeer J, et al. Retrospective assessment of the antigenic similarity of egg-propagated and cell culture-propagated reference influenza viruses as compared with circulating viruses across influenza seasons 2002-2003 to 2017-2018. Int J Environ Res Public Health, 2020; 17. [DOI] [PMC free article] [PubMed]
- 12. Katz JM, Naeve CW, Webster RG. Host cell-mediated variation in H3N2 influenza viruses. Virology 1987; 156:386–95. [DOI] [PubMed] [Google Scholar]
- 13. Rocha EP, Xu X, Hall HE, Allen JR, Regnery HL, Cox NJ. Comparison of 10 influenza A (H1N1 and H3N2) haemagglutinin sequences obtained directly from clinical specimens to those of MDCK cell- and egg-grown viruses. J Gen Virol 1993; 74(Pt 11):2513–8. [DOI] [PubMed] [Google Scholar]
- 14. Bart S, Cannon K, Herrington D, et al. Immunogenicity and safety of a cell culture-based quadrivalent influenza vaccine in adults: a phase III, double-blind, multicenter, randomized, non-inferiority study. Hum Vaccin Immunother 2016; 12:2278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Izurieta HS, Chillarige Y, Kelman J, et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017–2018. J Infect Dis 2019; 220:1255–64. [DOI] [PubMed] [Google Scholar]
- 16. Eick-Cost A, Hu Z. Relative effectiveness of cell-based influenza vaccines compared to egg-based influenza vaccines, active component U.S. Service members, 2017–18 season, abstract 129. International Conference on Emerging Infectious Diseases. Atlanta, GA, 2018:54.
- 17. Klein NP, Fireman B, Goddard K, et al. LB15. Vaccine effectiveness of Flucelvax relative to inactivated influenza vaccine during the 2017–18 influenza season in Northern California. Open Forum Infect Dis 2018; 5(Suppl 1): S764-S. [Google Scholar]
- 18. Barr IG, Donis RO, Katz JM, et al. Cell culture-derived influenza vaccines in the severe 2017-2018 epidemic season: a step towards improved influenza vaccine effectiveness. NPJ Vaccines 2018; 3:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boikos C, Sylvester GC, Sampalis JS, Mansi JA. Relative effectiveness of the cell-cultured quadrivalent influenza vaccine compared to standard, egg-derived quadrivalent influenza vaccines in preventing influenza-like illness in 2017–2018. Clin Infect Dis 2020; 71:e665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Armed Forces Health Surveillance Center (AFHSC). AFHSC standard case definitions: influenza-like illness. Falls Church, VA: Defense Health Agency, 2015. [Google Scholar]
- 21. Eick-Cost AA, Hunt DJ. Assessment of ICD-9-based case definitions for influenza-like illness surveillance. MSMR 2015; 22:2–7. [PubMed] [Google Scholar]
- 22. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 23. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004; 57:1288–94. [DOI] [PubMed] [Google Scholar]
- 24. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015; 34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nordin J, Mullooly J, Poblete S, et al. Influenza vaccine effectiveness in preventing hospitalizations and deaths in persons 65 years or older in Minnesota, New York, and Oregon: data from 3 health plans. J Infect Dis 2001; 184:665–70. [DOI] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention. FluView interactive. Available at: https://gis.cdc.gov/grasp/fluview/fluportaldashboard.html. Accessed 22 October 2020.
- 27. Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol 2011; 173:761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Centers for Disease Control and Prevention. Cell-based flu vaccines. Available at: https://www.cdc.gov/flu/prevent/cell-based.htm. Accessed 10 December 2020.
- 30. Flannery B, Fry AM. Comparing influenza vaccine types: the path toward improved influenza vaccine strategies. J Infect Dis 2019; 220:1237–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Monto AS, Petrie JG. Improving influenza vaccine effectiveness: ways to begin solving the problem. Clin Infect Dis 2019; 69:1824–6. [DOI] [PubMed] [Google Scholar]
- 32. Izurieta HS, Chillarige Y, Kelman J, et al. Relative effectiveness of influenza vaccines among the United States elderly, 2018–2019. J Infect Dis 2020; 222:278–87. [DOI] [PubMed] [Google Scholar]
- 33. Bruxvoort KJ, Luo Y, Ackerson B, et al. Comparison of vaccine effectiveness against influenza hospitalization of cell-based and egg-based influenza vaccines, 2017–2018. Vaccine 2019; 37:5807–11. [DOI] [PubMed] [Google Scholar]
- 34. DeMarcus L, Shoubaki L, Federinko S. Comparing influenza vaccine effectiveness between cell-derived and egg-derived vaccines, 2017–2018 influenza season. Vaccine 2019; 37:4015–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.