Abstract
Background
We compared effects of prior vaccination and added or lost protection from current season vaccination among those previously vaccinated.
Methods
Our analysis included data from the US Flu Vaccine Effectiveness Network among participants ≥9 years old with acute respiratory illness from 2012–2013 through 2017–2018. Vaccine protection was estimated using multivariate logistic regression with an interaction term for effect of prior season vaccination on current season vaccine effectiveness. Models were adjusted for age, calendar time, high-risk status, site, and season for combined estimates. We estimated protection by combinations of current and prior vaccination compared to unvaccinated in both seasons or current vaccination among prior vaccinated.
Results
A total of 31 819 participants were included. Vaccine protection against any influenza averaged 42% (95% confidence interval [CI], 38%–47%) among those vaccinated only the current season, 37% (95% CI, 33–40) among those vaccinated both seasons, and 26% (95% CI, 18%–32%) among those vaccinated only the prior season, compared with participants vaccinated neither season. Current season vaccination reduced the odds of any influenza among patients unvaccinated the prior season by 42% (95% CI, 37%–46%), including 57%, 27%, and 55% against A(H1N1), A(H3N2), and influenza B, respectively. Among participants vaccinated the prior season, current season vaccination further reduced the odds of any influenza by 15% (95% CI, 7%–23%), including 29% against A(H1N1) and 26% against B viruses, but not against A(H3N2).
Conclusions
Our findings support Advisory Committee on Immunization Practices recommendations for annual influenza vaccination. Benefits of current season vaccination varied among participants with and without prior season vaccination, by virus type/subtype and season.
Keywords: influenza, vaccines, prior vaccination, repeat vaccination, vaccine effectiveness
We compared 2 methods for evaluating prior vaccination effects. Our findings support current Advisory Committee on Immunization Practices recommendations for annual influenza vaccination. We documented added protection of current season vaccination among those with prior season vaccination, which varied by virus type/subtype and season.
In the United States (US), the Advisory Committee on Immunization Practices (ACIP) recommends routine annual influenza vaccination for all persons aged ≥6 months without contraindications [1]. The Centers for Disease Control and Prevention estimated that vaccination coverage was 63% for ≥1 dose among children and 45% among adults during the 2018–2019 influenza season [2]. With a maturing annual vaccination program, most vaccinated individuals are likely to be repeatedly vaccinated [3]. However, published vaccine effectiveness (VE) estimates among those with prior influenza vaccination have been inconsistent between seasons and subtypes [3].
The US Influenza Vaccine Effectiveness Network (US Flu VE Network) has published prior vaccination effects using combinations of current and prior season vaccination compared to a single referent group of participants unvaccinated in both seasons [4–9]. A meta-analysis of influenza VE studies using the test-negative design demonstrated that protection from seasonal vaccination in the current season averaged 33% against A(H3N2), 54% against influenza B, and 61% against A(H1N1)pdm09 illnesses in the outpatient setting [10]. Because current season VE encompasses both participants with and without prior season vaccination, vaccination history may confound VE estimates [11]. Foppa et al found that confounding bias can be substantially reduced by accounting for vaccination in 1 prior season [11]. Several studies have considered the impact of prior season vaccination on current season VE [4–8, 12–15]. For the 2016–2017 season, the US Flu VE Network published prior season vaccination effects using 2 different referent groups of participants with and without prior season vaccination [8], analogous to the “patient perspective,” which considers the patient’s decision on whether or not to get vaccinated given prior season vaccination [12]. In this analysis, we compared the effects of prior vaccination and added or lost protection from current season vaccination among those vaccinated in the prior year.
METHODS
Study Setting and Enrollment
We analyzed data from the US Flu VE Network collected over 6 influenza seasons from 2012–2013 through 2017–2018 using the test-negative design. Methods of the US Flu VE Network have been described previously [4–8, 16]. In brief, network sites in 5 states (Michigan, Pennsylvania, Texas, Washington, and Wisconsin) enroll ambulatory patients aged ≥6 months presenting within 7 days of onset of acute respiratory illness with cough. Study staff obtained informed consent from patients (parent/guardian for minors) and interviewed patients regarding demographics, influenza risk factors, self-reported health status (before illness), receipt of the current season’s influenza vaccine, and symptoms of the presenting illness. Respiratory specimens were collected from all enrolled patients for influenza testing by reverse-transcription polymerase chain reaction (RT-PCR) and influenza virus typing/subtyping for positive specimens.
Influenza Vaccination Status
Current season influenza vaccination status was determined based on documented doses in medical records and registries for the Wisconsin site and medical records and plausible self-report (reported vaccination timing and location without documented receipt) at sites in Michigan, Pennsylvania, Texas, and Washington. We considered patients to be vaccinated in the current season if they received an influenza vaccine at least 14 days before illness onset in the study enrollment season. Prior season vaccination status was determined based on documented immunization records alone [17]. We considered patients to be vaccinated in the prior season if they had documented vaccination in the influenza season immediately preceding the study enrollment season. Combinations of current and prior vaccination included vaccinated in the prior season only, current season only, both prior and current seasons, or unvaccinated in both seasons.
Statistical Analysis
We estimated vaccine protection as the ratio of odds of influenza among vaccination groups compared to the referent group [(1 – adjusted odds ratio) × 100] using logistic regression models in combined and individual seasons. We used all weeks with influenza-positive cases for models by type and subtype. Each model was adjusted for site, patient age (natural cubic spline with 4 knots), presence of any high-risk medical condition, calendar time (month), and season for combined estimates. We limited analyses to patients ≥9 years old with at least 1 year of medical records available. Participants were also excluded if they were enrolled outside of the local influenza circulation period, were enrolled >7 days after illness onset, had inconclusive RT-PCR results, or were vaccinated <14 days prior to illness onset.
Each model included an interaction term for the effect of prior season vaccination on current season VE. Using contrast statements to generate specific vaccination and referent group combinations in the model, we estimated vaccine protection against any influenza virus or virus type/subtype for seasons with sufficient numbers of cases. The vaccine protection endpoints included protection of vaccination in (1) only the current season, (2) both prior and current season, and (3) only the prior season compared to unvaccinated in both seasons. In addition, we estimated effectiveness of current season vaccination among persons vaccinated in the prior season. We also explored whether excluding those with plausible self-report but no documentation of current season vaccination would affect our results.
Statistical significance for point estimates of vaccine protection was evaluated based on 95% confidence limits excluding the null value. Analyses were performed using SAS statistical software (version 9.4).
RESULTS
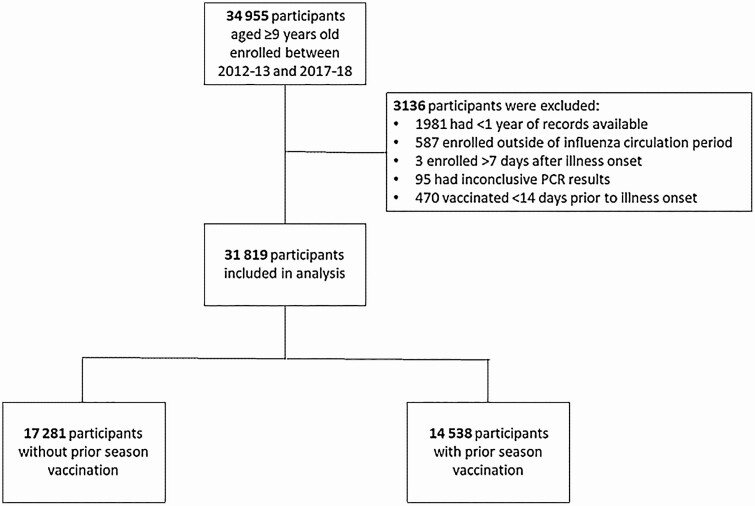
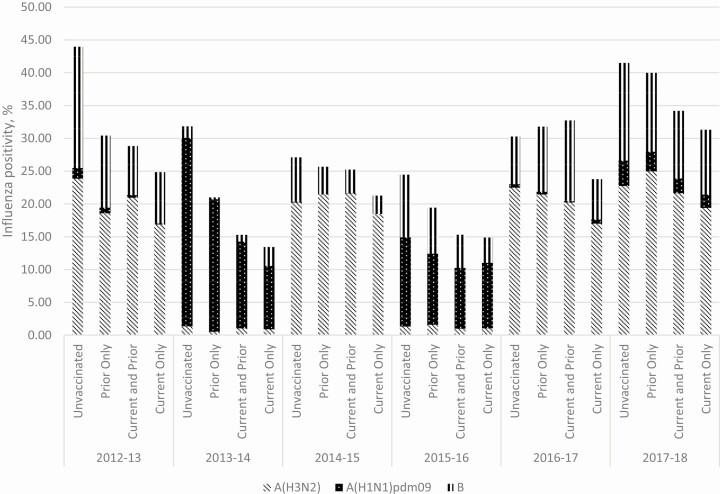
This analysis included 31 819 participants aged ≥9 years enrolled in the US Flu VE Network over 6 influenza seasons (Figure 1). In total, 12 517 (39%) participants were unvaccinated in both the current and prior seasons, 2839 (9%) were vaccinated in the preceding but not in the current season, and 16 463 (52%) were vaccinated in the current season. Of participants vaccinated in the current season, most were vaccinated in both the current and prior season (71%) while a smaller proportion were vaccinated in the current season alone (29%). Characteristics of participants vaccinated or unvaccinated in the preceding season differed (Table 1). Participants with prior season vaccination were more likely to be ≥65 years old and have a high-risk medical condition present. Participants unvaccinated in both seasons had the highest influenza positivity in each of the 6 seasons, ranging from 27% to 44% (Figure 2). Among those vaccinated, participants vaccinated in the current season alone had the lowest percentage positivity across all seasons (13%–32%). Influenza positivity was also lower among patients vaccinated only in the prior season (22%–41%) and those vaccinated in both seasons (16%–34%), compared to the unvaccinated, indicating vaccine protection in all groups with vaccination (13%–32%).
Figure 1.
Flowchart of participants included in analysis. Abbreviation: PCR, polymerase chain reaction.
Table 1.
Characteristics of Study Participants by Prior Season Vaccination Status Among Enrollees ≥9 Years Old in the United States Flu Vaccine Effectiveness Network During 6 Seasons, 2012–2013 Through 2017–2018
| Without Prior Season Vaccination | With Prior Season Vaccination | ||||
|---|---|---|---|---|---|
| Characteristic | No. | (Row %) | No. | (Row %) | P Valuea |
| Total | 17 281 | (54) | 14 538 | (46) | |
| Site | <.001 | ||||
| Michigan | 2470 | (52) | 2282 | (48) | |
| Pennsylvania | 3742 | (63) | 2153 | (37) | |
| Texas | 4192 | (67) | 2100 | (33) | |
| Washington | 3641 | (44) | 4610 | (56) | |
| Wisconsin | 3236 | (49) | 3393 | (51) | |
| Age group, y | <.001 | ||||
| 9–17 | 3365 | (60) | 2206 | (40) | |
| 18–49 | 8903 | (66) | 4566 | (34) | |
| 50–64 | 3619 | (49) | 3782 | (51) | |
| ≥65 | 1394 | (26) | 3984 | (74) | |
| Sex | <.001 | ||||
| Male | 6996 | (58) | 5092 | (42) | |
| Female | 10 285 | (52) | 9446 | (48) | |
| Race/ethnicityb | <.001 | ||||
| White, non-Hispanic | 13 111 | (53) | 11 732 | (47) | |
| Black, non-Hispanic | 1354 | (65) | 727 | (35) | |
| Hispanic | 1293 | (53) | 1157 | (47) | |
| Other, non-Hispanic | 1480 | (63) | 884 | (37) | |
| Any high-risk medical conditionc | <.001 | ||||
| No | 11 092 | (65) | 5846 | (34) | |
| Yes | 6189 | (42) | 8692 | (58) | |
| Illness onset to specimen collection, interval | <.001 | ||||
| ≤2 d | 5771 | (58) | 4210 | (42) | |
| 3–4 d | 6873 | (55) | 5546 | (45) | |
| 5–7 d | 4637 | (49) | 4779 | (51) | |
| Seasond | <.001 | ||||
| 2012–2013 | 2825 | (60) | 1853 | (40) | |
| 2013–2014 | 2219 | (53) | 1961 | (47) | |
| 2014–2015 | 3187 | (49) | 3278 | (51) | |
| 2015–2016 | 2765 | (56) | 2185 | (44) | |
| 2016–2017 | 2841 | (54) | 2464 | (46) | |
| 2017–2018 | 3444 | (55) | 2797 | (45) | |
a P values were calculated with a χ 2 test.
bEighty-one participants were missing race/ethnicity.
cPresence of ≥1 documented high-risk code in prior year that may increase risk for complications attributable to severe influenza as defined by the Advisory Committee on Immunization Practices, including chronic pulmonary (including asthma); cardiovascular (excluding isolated hypertension); renal, hepatic, neurologic, hematologic, or metabolic disorders (including diabetes mellitus); or persons who are immunocompromised due to any cause (including but not limited to immunosuppression caused by medications or human immunodeficiency virus infection) [1].
dDistribution of participants by enrollment season.
Figure 2.
Influenza positivity by current and prior season vaccination status and season among enrollees ≥9 years old in the United States Flu Vaccine Effectiveness Network.
Estimates of vaccine protection against any influenza were similar but with overlapping confidence limits among patients vaccinated only in the current season compared with those vaccinated in both the current and prior seasons with an unvaccinated referent group. Among those vaccinated only in the current season, vaccine protection against any influenza ranged from 26% (95% confidence interval [CI], 10%–39%) in 2014–2015 to 65% (95% CI, 54%–74%) in 2013–2014, with a combined estimate across all seasons of 42% (95% CI, 37%–46%) (Table 2). Compared to the unvaccinated, effectiveness of current and prior vaccination against any influenza ranged from 15% (95% CI, 2%–27%) in 2014–2015 to 56% (95% CI, 47%–64%) in 2013–2014, with a combined estimate across all seasons of 36% (95% CI, 32%–40%). All estimates were lower in 2014–2015 when antigenically drifted A(H3N2) viruses circulated and during other A(H3N2)-predominant seasons in 2012–2013, 2016–2017, and 2017–2018.
Table 2.
Adjusted Vaccine Protectiona Among United States Flu Vaccine Effectiveness Network Enrollees ≥9 Years Old by Prior Season Vaccination Status, Virus Type/Subtype, and Season
| Influenza Type and Season | Protection of Only Current Season, % (95% CI) | Protection of Current and Prior Season, % (95% CI) | Protection of Only Prior Season, % (95% CI) | Added/Lost Protection, % (95% CI) |
|---|---|---|---|---|
| Defined as Current Only Vaccination vs Unvaccinated Both Seasons | Defined as Prior and Current Season Vaccination vs Unvaccinated Both Seasons | Defined as Prior Only Vaccination vs Unvaccinated Both Seasons | Defined as Prior and Current Season Vaccination vs Prior Only Vaccination | |
| Any influenza | ||||
| 2012–2013 | 54 (45–62) | 49 (41–57) | 46 (31–58) | 6 (−22 to 28) |
| 2013–2014 | 65 (54–74) | 56 (47–64) | 44 (26–57) | 23 (−4 to 42) |
| 2014–2015 | 26 (10–39) | 15 (2–27) | 14 (−6 to 31) | 1 (−23 to 20) |
| 2015–2016 | 48 (35–59) | 43 (32–53) | 17 (−9 to 37) | 32 (9–49) |
| 2016–2017 | 39 (27–50) | 30 (19–40) | 17 (−3 to 33) | 16 (−4 to 33) |
| 2017–2018 | 31 (19–42) | 26 (16–35) | 3 (−18 to 20) | 24 (7–38) |
| Combined | 42 (37–46) | 36 (32–40) | 26 (18–32) | 15 (6–22) |
| A(H3N2)b | ||||
| 2012–2013 | 45 (31–56) | 39 (26–49) | 43 (23–58) | −9 (−49 to 21) |
| 2014–2015 | 11 (−11 to 28) | −2 (−20 to 14) | 4 (−21 to 25) | −6 (−35 to 16) |
| 2016–2017 | 35 (18–48) | 27 (13–38) | 15 (−9 to 34) | 14 (−11 to 33) |
| 2017–2018 | 17 (−2 to 32) | 5 (−12 to 19) | −13 (−43 to 11) | 16 (−8 to 34) |
| Combined | 27 (18–34) | 17 (10–24) | 13 (2–23) | 5 (−8 to 16) |
| A(H1N1)pdm09c | ||||
| 2013–2014 | 70 (59–78) | 58 (48–66) | 39 (18–54) | 31 (6–50) |
| 2015–2016 | 39 (19–54) | 40 (24–52) | 7 (−32 to 35) | 35 (7–55) |
| Combined | 57 (47–65) | 51 (43–58) | 31 (15–44) | 29 (11–44) |
| Influenza Bd | ||||
| 2012–2013 | 63 (50–73) | 63 (53–71) | 50 (28–66) | 26 (−11 to 51) |
| 2014–2015 | 60 (37–74) | 53 (37–65) | 37 (2–60) | 25 (−19 to 53) |
| 2015–2016 | 62 (43–75) | 48 (30–61) | 28 (−10 to 53) | 28 (−13 to 54) |
| 2016–2017 | 54 (34–67) | 42 (25–54) | 23 (−11 to 46) | 24 (−11 to 48) |
| 2017–2018 | 46 (30–58) | 47 (34–56) | 18 (−11 to 39) | 35 (10–53) |
| Combined | 55 (48–61) | 52 (47–57) | 35 (23–44) | 27 (13–38) |
Abbreviation: CI, confidence interval.
aAdjusted for study site, patient age, presence of ≥1 high-risk medical condition, calendar time, and season for combined estimates.
b2013–2014 and 2015–2016 were excluded due to insufficient A(H3N2) circulation with 45 and 58 cases, respectively.
c2012–2013, 2014–2015, 2016–2017, and 2017–2018 were excluded due to insufficient A(H1N1)pdm09 circulation with 45, 3, 21, and 183 cases, respectively.
d2013–2014 was excluded due to insufficient influenza B circulation with 58 cases.
Vaccination in only the prior season was associated with lower odds of any influenza with a combined estimate of 26% (95% CI, 18%–32%; Table 2). In 2 seasons, 2012–2013 and 2013–2014, vaccination in the prior season only provided statistically significant protection, or residual protection, against any influenza (46% [95% CI, 31%–58%] and 44% [95% CI, 26%–57%], respectively). We did not observe a consistent pattern of residual protection against the current circulating viruses during seasons either when vaccine strains remained unchanged or when updated from the previous season (Table 3). Protection from prior season only vaccination was observed both when the vaccine strain was not changed for A(H1N1)pdm09 and when the strain was changed for A(H3N2) in 2012–2013. Residual protection against B viruses in 2012–2013 was evident following a B lineage change in the trivalent vaccine from a B/Victoria to B/Yamagata, while in 2014–2015, vaccine strains for both B virus lineages and the lineage in the trivalent vaccine did not change from the preceding season.
Table 3.
Northern Hemisphere Vaccine Strain Change by Virus Type/Subtype From 2011–2012 to 2017–2018
| Season | A(H1N1) | A(H3N2) | B Trivalent | B Quadrivalent |
|---|---|---|---|---|
| 2011–2012 | California/7/2009 | Perth/16/2009 | Brisbane/60/2008 (Vic) | … |
| 2012–2013 | California/7/2009 | Victoria/361/2011 | Wisconsin/1/2010 (Yam) | Brisbane/60/2008 (Vic) |
| 2013–2014 | California/7/2009 | Texas/50/2012 | Massachusetts/2/2012 (Yam) | Brisbane/60/2008 (Vic) |
| 2014–2015 | California/7/2009 | Texas/50/2012 | Massachusetts/2/2012 (Yam) | Brisbane/60/2008 (Vic) |
| 2015–2016 | California/7/2009 | Switzerland/9715293/2013 | Phuket/3073/2013 (Yam) | Brisbane/60/2008 (Vic) |
| 2016–2017 | California/7/2009 | Hong Kong/4801/2014 | Brisbane/60/2008 (Vic) | Phuket/3073/2013 (Yam) |
| 2017–2018 | Michigan/45/2015 | Hong Kong/4801/2014 | Brisbane/60/2008 (Vic) | Phuket/3073/2013 (Yam) |
Abbreviations: Vic, Victoria; Yam, Yamagata.
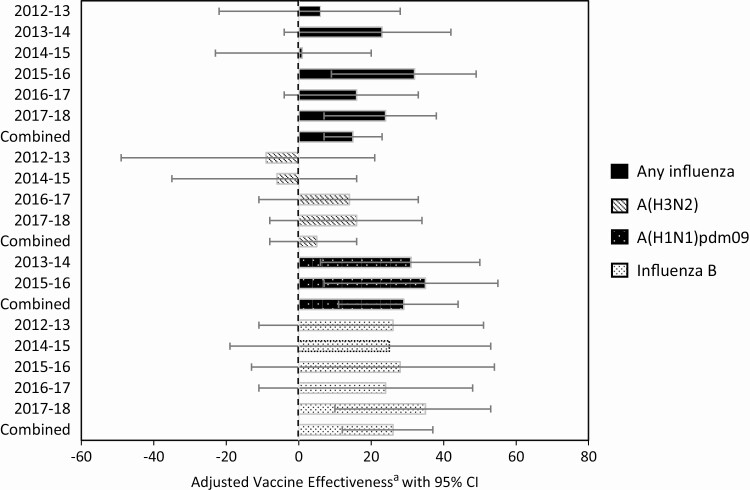
Among those vaccinated in the prior season, current season vaccination provided significant added protection against any influenza virus in only 2 of 6 seasons, 2015–2016 and 2017–2018 (Table 2). Combined across 6 seasons, current season vaccination further reduced the odds of influenza among patients vaccinated in the prior year by 15% (95% CI, 6%–22%) against any influenza, 29% (95% CI, 11%–44%) against A(H1N1)pdm09, and 27% (95% CI, 13%–38%) against influenza B viruses. Significant added protection was observed against A(H1N1)pdm09 in 2013–2014 and 2015–2016 and against influenza B viruses in 2017–2018. For each of these seasons, no vaccine strain change from the previous season occurred for the A(H1N1)pdm09 and B virus vaccine components. We did not observe any added or lost protection from current season vaccination against influenza A(H3N2) during any of the individual seasons (Figure 3). Across all virus types/subtypes, we observed added protection from current season vaccination among participants with prior vaccination for most seasons.
Figure 3.
Added or lost protection from current season vaccination in those with prior vaccination by influenza virus type/subtype and season. aAdjusted for study site, patient age, presence of ≥1 underlying medical condition, calendar time, and season for combined estimates. Abbreviation: CI, confidence interval.
Unadjusted vaccine protection estimates were overall similar to or at least had overlapping CIs with their corresponding adjusted estimates (Supplementary Table 1). Excluding participants with plausible self-reported but no documented current season vaccination did not change our results (Supplementary Table 2).
DISCUSSION
In this analysis of data from the US Flu VE Network collected over 6 influenza seasons, both current and prior season vaccinations decreased the likelihood of medically attended influenza. Among participants unvaccinated in the prior season, current season VE reduced the odds of influenza by an average of 42% (95% CI 37%–46%). In some seasons, we observed residual protection of prior vaccination only for all subtypes regardless of strain change. One large, multiyear US randomized trial found that efficacy of repeated vaccination was similar to or better than current season vaccination only, even when circulating strains had drifted antigenically from the vaccine strains [18]. Compared with protection offered by current season vaccination only, vaccination in both the current and prior seasons was not appreciably diminished. Importantly, we observed an average added protection of 15% (95% CI 6%–22%) for current season vaccination among participants vaccinated in the prior season.
Our analytic approach to estimate the added protection of current season vaccination among people with prior season vaccination is similar to that of Valenciano et al [13] and Ramsay et al from the “patient perspective” [12]. Some immunology and VE studies have suggested that repeated vaccination may increase influenza risk, especially against A(H3N2) viruses [14, 15, 19–23]. We demonstrated no evidence of loss of protection against A(H3N2) viruses, even in 2014–2015 when the circulating virus was antigenically distinct from the vaccine strain. Overall benefit against any influenza may be an important consideration from the patient perspective as vaccines may provide protection against influenza illness during a season even when no significant protection is observed against any one virus.
Similar to previous studies, we documented that current season vaccination provided significant benefit among those unvaccinated in the prior season [12]. This benefit may be attributable to the increased influenza risk in the current season among persons unvaccinated in the prior season compared to those vaccinated [12]. Several meta-analyses found that those vaccinated only in the current season were consistently protected against each virus type and subtype compared to the unvaccinated [3, 12, 24]. Our analysis yielded similar results where VE of current vaccination only was 57% (95% CI 47%–65%) against A(H1N1)pdm09, 55% (95% CI 48%–61%) against influenza B, and 27% (95% CI 18%–34%) against A(H3N2) viruses. However, of those vaccinated in the US Flu VE Network, a small percentage of participants was vaccinated in only the current season (Supplementary Table 3).
Studies on immune response and antibody duration among repeat vaccinees have also contributed to understanding the effects of annual vaccination with changing vaccine components. One study that investigated the association between number of vaccine doses and laboratory-confirmed infection found a significant reduction in postvaccination geometric mean titers (GMTs) against A(H3N2) with an increasing number of sequential vaccinations [18]. This study occurred in 1987–1988 when the A(H3N2) vaccine component had been updated and was antigenically similar to the prior season strain but mismatched with the circulating strain. Results were similar for a study in healthcare workers where antibody titers against A(H3N2) were highest in participants with 1 vaccine vs repeated vaccination [25]. In one clinical trial among adults, investigators found postvaccination GMTs in the repeated inactivated influenza vaccine (IIV) vaccination group were significantly lower than that in the single IIV vaccination group for up to 18 months postvaccination, suggesting prolonged blunted immune response beyond a single season [26]. Our results for A(H3N2) found that no significant protection is added from current vaccination among prior season vaccinees. However, we did not find any evidence of decrease in protection from current season vaccination against any virus type or subtype among participants vaccinated in the prior season compared with those not vaccinated in the prior season. In a study exploring antibody response to A(H1N1)pdm09, investigators observed blunted immune responses among healthcare personnel who received the trivalent inactivated vaccine after the monovalent inactivated vaccine in the prior season [27]. In this current analysis, we observed lower protection in some seasons and subtypes but overall found that current season vaccination provided added benefit to previously vaccinated persons in both seasons when A(H1N1)pdm09 circulated. Furthermore, antibody titers against A(H1N1)pdm09 were similar among participants with and without prior season vaccination in household settings with children during the 2013–2014 season [28]. These results highlight the need for VE studies considering prior vaccination across multiple seasons by virus type and subtype.
Three additional findings have been observed related to prior season vaccination. First, in some VE studies, vaccination in the prior season alone provided some residual protection in the current season [3–6, 29–33]. These VE studies are supported by serology studies demonstrating that antibody titers to influenza hemagglutinin and neuraminidase antigens may persist beyond the current season [26]. Second, although residual protection of prior vaccination may exist, current season vaccination could provide added protection by immune boosting or by eliciting a new immune response against an antigenically drifted circulating virus [34, 35]. Last, some evidence also suggests that prior season vaccination could negatively interact with current season vaccine protection [3, 14, 19–25, 29–39]. These effects from prior season vaccination could vary by influenza virus type/subtype, antigenic match between vaccine and circulating viruses, season, study, and age group of participants [3, 12, 24].
Separating effects of repeat vaccination from residual protection is challenging. Prior season vaccination contributes to current season VE in addition to immune boosting or new immune response to a specific circulating virus. Waning immunity with increasing time since vaccination also likely impacts residual protection and its role in repeat vaccination effects [36]. When there was evidence of residual protection, added protection from current season vaccination varied by subtype. Current season vaccination among those previously vaccinated provided added protection against A(H1N1)pdm09 in seasons when there was and was not residual protection from prior season vaccination. However, it provided no additional benefit against A(H3N2), regardless of observed residual protection. We observed that additional benefit of current season vaccination was greater against A(H1N1)pdm09 (29% [95% CI 11%–44%]) and influenza B (26% [95% CI 12%–37%]) and was not statistically significant against A(H3N2) viruses. However, estimates of protection in those vaccinated in both seasons and in the prior season only compared to the unvaccinated may be biased due to differences in influenza risk by prior vaccination status that are not controlled for when using an unvaccinated referent group [11]. Foppa et al have demonstrated that the group unvaccinated in both seasons may not be an appropriate comparison for those with prior vaccination history [11]. This analysis has implications for VE studies that may not consider prior season vaccination effects, such as diluting the effect of current season VE. However, this implication does not change the need to continue presenting an overall VE estimate for the benefit of vaccination in the overall population.
These results are subject to several limitations. Because of how we determined prior and current vaccination status, we may have missed doses received outside of the healthcare system. However, estimates excluding participants with self-reported but no documented current season vaccination were consistent with our primary results. Additionally, we were unable to control for the potential bias of influenza infection history which may have biased our results. Next, we considered the effect of repeat vaccination in only 1 season preceding enrollment rather than over several seasons due to lack of documented doses beyond the immediate prior season and reliability of self-reported prior vaccination status [17]. When exploring the impact of vaccine strain change on residual and added/lost protection, we did not investigate antigenic match of circulating viruses. Although effects of antigenic distance on subsequent VE is important to understanding prior vaccination effects [15], we sought to present data relevant to the patient perspective that could be used when deciding to get vaccinated given prior season vaccination. We did not assess the vaccine formulations received and how different formulations may influence any effect of prior vaccination because numbers in some groups when stratified by vaccine type were insufficient in the network. Finally, we did not explore age or birth cohort effects, though early-life influenza infections with specific viruses or epitopes may influence vaccine responses [22].
Current season vaccination among those repeatedly vaccinated generally reduced medically attended influenza in the US Flu VE Network. We did not observe significant loss in protection from current season vaccination among those vaccinated in the prior year. Importantly, we documented significant added protection of current season vaccination among those with prior vaccination in some seasons against A(H1N1)pdm09, influenza B viruses, and overall influenza illnesses. Our findings support current ACIP recommendations for annual influenza vaccination and demonstrate that participants vaccinated in the prior season overall benefit from choosing to be vaccinated again in the current season.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Financial support. This study was supported by the Centers for Disease Control and Prevention (cooperative agreement numbers U01IP000466, U01IP000467, U01IP000471, U01IP000473, U01IP000474, and U01IP001034-U01IP001039). At Pittsburgh, the project was also supported by the National Institutes of Health (grant numbers UL1TR001857, UL1RR024153, and UL1TR000005).
Potential conflicts of interest. M. G. reports an institutional Influenza Clinical Investigation in Children Live Attenuated Influenza Vaccine Effectiveness Contract from MedImmune/Astra Zeneca, outside the submitted work. L. A. J. reports grants from Novavax, outside the submitted work. E. T. M. reports personal fees from Pfizer, outside the submitted work. H. Q. M. reports research support from Seqirus, outside the submitted work. M. L. J. reports funding for respiratory syncytial virus research unrelated to this work from Sanofi Pasteur. A. S. M. reports consulting fees from Sanofi Pasteur and Seqirus, outside the submitted work. M. P. N. reports grants from Merck & Co and Pfizer, outside the submitted work. R. K. Z. reports grants from Sanofi Pasteur, Pfizer, and Merck & Co, outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67(3):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2018–19 influenza season. Available at: https://www.cdc.gov/flu/fluvaxview/index.htm. Accessed 13 January 2019.
- 3. Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16:1–14. [DOI] [PubMed] [Google Scholar]
- 4. McLean HQ, Thompson MG, Sundaram ME, et al. . Influenza vaccine effectiveness in the United States during 2012–2013: variable protection by age and virus type. J Infect Dis 2014; 2015:1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gaglani M, Pruszynski J, Murthy K, et al. . Influenza vaccine effectiveness against 2009 pandemic influenza A(H1N1) virus differed by vaccine type during 2013-2014 in the United States. J Infect Dis 2016; 213:1546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zimmerman RK, Nowalk MP, Chung JR, et al. . 2014–2015 influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis 2016; 63:1564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jackson ML, Chung JR, Jackson LA, et al. . Influenza vaccine effectiveness in the United States during the 2015–2016 season. New Engl J Med 2017; 377:534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flannery B, Chung JR, Monto AS, et al. . Influenza vaccine effectiveness in the United States during the 2016–2017 season. Clin Infect Dis 2019; 68:1798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sullivan SG, Kelly H. Stratified estimates of influenza vaccine effectiveness by prior vaccination: caution required. Clin Infect Dis 2013; 57:474–6. [DOI] [PubMed] [Google Scholar]
- 10. Belongia EA, Simpson MD, King JP, et al. . Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 2016; 16:942–51. [DOI] [PubMed] [Google Scholar]
- 11. Foppa IM, Ferdinands JM, Chung J, Flannery B, Fry AM. Vaccination history as a confounder of studies of influenza vaccine effectiveness. Vaccine X 2019; 1:100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramsay LC, Buchan SA, Stirling RG, et al. . The impact of repeated vaccination on influenza vaccine effectiveness: a systematic review and meta-analysis. BMC Med 2019; 17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Valenciano M, Kissling E, Larrauri A, et al. . Exploring the effect of previous inactivated influenza vaccination on seasonal influenza vaccine effectiveness against medically attended influenza: results of the European I-MOVE multicentre test-negative case-control study, 2011/2012–2016/2017. Influenza Other Respir Viruses 2018; 2018:567–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Skowronski DM, Chambers C, Sabaiduc S, et al. . A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 season. Clin Infect Dis 2016; 63:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skowronski DM, Chambers C, De Serres G, et al. . Serial vaccination and the antigenic distance hypothesis: effects on influenza vaccine effectiveness during A(H3N2) epidemics in Canada, 2010–2011 to 2014–2015. J Infects Dis 2017; 2015:1059–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rolfes MA, Flannery B, Chung JR, et al. . Effects of influenza vaccination in the United States during the 2017–2018 influenza season. Clin Infect Dis 2019; 69:1845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. King JP, McLean HQ, Belongia EA. Validation of self-reported influenza vaccination in the current and prior season. Influenza Other Respir Viruses 2018; 12:808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 1997; 15:1114–22. [DOI] [PubMed] [Google Scholar]
- 19. Zhang A, Stacey HD, Mullarkey CE, Miller MS. Original antigenic sin: how first exposure shapes lifelong anti-influenza virus immune responses. J Immunol 2019; 202:335–40. [DOI] [PubMed] [Google Scholar]
- 20. Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci U S A 1999; 96:14001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim JH, Skountzou I, Compans R, Jacob J. Original antigenic sin responses to influenza viruses. J Immunol 2009; 183:3294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skowronski DM, Sabaiduc S, Leir S, et al. . Paradoxical clade- and age-specific vaccine effectiveness during the 2018/19 influenza A(H3N2) epidemic in Canada: potential imprint-regulated effect of vaccine (I-REV). Euro Surveill 2019; 24:1900585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kissling E, Pozo F, Buda S, et al. . Low 2018/19 vaccine effectiveness against influenza A(H3N2) among 15-64-year-olds in Europe: exploration by birth cohort. Euro Surveill 2019; 24:1900604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bartoszko JJ, McNamara IF, Aras OAZ, et al. . Does consecutive influenza vaccination reduce protection against influenza: a systematic review and meta-analysis. Vaccine 2018; 36:3434–44. [DOI] [PubMed] [Google Scholar]
- 25. Thompson MG, Naleway A, Fry AM, et al. . Effects of repeated annual inactivated influenza vaccination among healthcare personnel on serum hemagglutinin inhibition antibody response to A/Perth/16/2009 (H3N2)-like virus during 2010–11. Vaccine 2016; 34:981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petrie JG, Ohmit SE, Johnson E, Truscon R, Monto AS. Persistence of antibodies to influenza hemagglutinin and neuraminidase following one or two years of influenza vaccination. J Infect Dis 2015; 212:1914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaglani M, Spencer S, Ball S, et al. . Antibody response to influenza A(H1N1)pdm09 among healthcare personnel receiving trivalent inactivated vaccine: effect of prior monovalent inactivated vaccine. J Infect Dis 2014; 209:1705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ohmit SE, Petrie JG, Malosh RE, et al. . Substantial influenza vaccine effectiveness in households with children during the 2013-2014 influenza season, when 2009 pandemic influenza A(H1N1) virus predominated. J Infect Dis 2016; 213:1229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Skowronski DM, Janjua NZ, Sabaiduc S, et al. . Influenza A/subtype and B/lineage effectiveness estimates for the 2011-2012 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis 2014; 210:126–37. [DOI] [PubMed] [Google Scholar]
- 30. Skowronski DM, Janjua NZ, De Serres G, et al. . A sentinel platform to evaluate influenza vaccine effectiveness and new variant circulation, Canada 2010–2011 Season. Clin Infect Dis 2012; 55:332–42. [DOI] [PubMed] [Google Scholar]
- 31. Syrjänen RK, Jokinen J, Ziegler T, et al. . Effectiveness of pandemic and seasonal influenza vaccines in preventing laboratory-confirmed influenza in adults: a clinical cohort study during epidemic seasons 2009-2010 and 2010-2011 in Finland. PLoS One 2014; 9:e108538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Skowronski DM, Chambers C, Sabaiduc S, et al. . Integrated sentinel surveillance linking genetic, antigenic, and epidemiologic monitoring of influenza vaccine-virus relatedness and effectiveness during the 2013-2014 influenza season. J Infect Dis 2015; 212:726–39. [DOI] [PubMed] [Google Scholar]
- 33. McLean HQ, Thompson MG, Sundaram ME, et al. . Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis 2014; 59:1375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine 2007; 25:6852–62. [DOI] [PubMed] [Google Scholar]
- 35. Künzel W, Glathe H, Engelmann H, Van Hoecke C. Kinetics of humoral antibody response to trivalent inactivated split influenza vaccine in subjects previously vaccinated or vaccinated for the first time. Vaccine 1996; 14:1108–10. [DOI] [PubMed] [Google Scholar]
- 36. Ferdinands JM, Fry AM, Reynolds S, et al. . Intraseason waning of influenza vaccine protection: evidence from the US Influenza Vaccine Effectiveness Network, 2011–12 through 2014–15. Clin Infect Dis 2017; 64:544–50. [DOI] [PubMed] [Google Scholar]
- 37. Fu C, Xu J, Lin J, et al. . Concurrent and cross-season protection of inactivated influenza vaccine against A(H1N1)pdm09 illness among young children: 2012-2013 case-control evaluation of influenza vaccine effectiveness. Vaccine 2015; 33:2917–21. [DOI] [PubMed] [Google Scholar]
- 38. Ohmit SE, Thompson MG, Petrie JG, et al. . Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Valenciano M, Kissling E, Reuss A, et al. . Vaccine effectiveness in preventing laboratory-confirmed influenza in primary care patients in a season of co-circulation of influenza A(H1N1)pdm09, B and drifted A(H3N2), I-MOVE multicentre case–control study, Europe 2014/15. Euro Surveill 2016; 21. doi: 10.2807/1560-7917.ES.2016.21.7.30139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.