Abstract
Xylooligosaccharide (XOS) has been considered to be an effective prebiotic, but its exact mechanisms remain unknown. This research was conducted to evaluate the effects of XOS on pig intestinal bacterial community and mucosal barrier using a lipopolysaccharide (LPS)-caused gut damage model. Twenty-four weaned pigs were assigned to 4 treatments in a 2 × 2 factorial design involving diet (with or without XOS) and immunological challenge (saline or LPS). After 21 d of feeding 0% or 0.02% commercial XOS product, piglets were treated with saline or LPS. After that, blood, small intestinal mucosa and cecal digesta were obtained. Dietary XOS enhanced intestinal mucosal integrity demonstrated by higher villus height, villus height-to-crypt depth ratio, disaccharidase activities and claudin-1 protein expression and lower crypt depth. XOS also caused down-regulation of the gene expression of toll-like receptor 4 and nucleotide-binding oligomerization domain protein signaling, accompanied with decreased pro-inflammatory cytokines and cyclooxygenase 2 contents or mRNA expression and increased heat shock protein 70 mRNA and protein expression. Additionally, increased Bacteroidetes and decreased Firmicutes relative abundance were observed in the piglets fed with XOS. At the genus level, XOS enriched the relative abundance of beneficial bacteria, e.g., Faecalibacterium, Lactobacillus, and Prevotella. Moreover, XOS enhanced short chain fatty acids contents and inhibited histone deacetylases. The correlation analysis of the combined datasets implied some potential connections between the intestinal microbiota and pro-inflammatory cytokines or cecal metabolites. These results suggest that XOS inhibits inflammatory response and beneficially modifies microbes and metabolites of the hindgut to protect the intestine from inflammation-related injury.
Keywords: Xylooligosaccharide, Piglet, Lipopolysaccharide, Inflammation, Intestinal microbiota
1. Introduction
The intestine is an important organ associated with the health and survival of neonatal animals. It plays a vital role in nutrition absorption and utilization, and acts as a barrier to restrain the invasion of foreign antigens, microorganisms and toxins from the environment into the body. The functions of the intestinal epithelium are affected by many elements, e.g., bacterial infection, endotoxin challenge and oxidative stress, which lead to gut damage and dysfunction (Liu et al., 2012; Zhu et al., 2018). Though exact mechanism remains unknown, evidence from clinical and experimental studies have demonstrated intestinal epithelial barrier dysfunction is at least partly associated with the development of inflammatory conditions in the gastrointestinal tract (Liu et al., 2012; Zhu et al., 2018). Our earlier studies have found that active inflammatory signaling pathways [mediated by toll-like receptor (TLR) and nucleotide-binding oligomerization domain protein (NOD)] and excessive pro-inflammatory cytokines are implicated in endotoxin-stimulated gut damage (Liu et al., 2012; Zhu et al., 2018).
In addition, recent research has observed that gut microbes may participate in the underlying mechanism of gut damage and repair. When the epithelium is injured and repaired improperly, the gut microbes may become dysbiosis or misallocated, which activates innate immunity to cause inflammation via pattern recognition receptors, e.g., TLR, thereby resulting in inflammatory diseases such as inflammatory bowel disease and colorectal cancer (Grivennikov et al., 2012; Song et al., 2014). Therefore, an optimized gastrointestinal microbiome is crucial for maintaining normal gastrointestinal tract function. The microorganism can produce short chain fatty acids (SCFA) by fermentation of dietary fibers in the large intestine (Kobayashi et al., 2017). Locally, SCFA act as fuel for intestinal epithelial cells (Kobayashi et al., 2017). These fatty acids are also taken up from the intestine into the blood stream, then used as substrates or signal molecules in organs of the host (Kobayashi et al., 2017). Besides, SCFA have been recognized as the key players in maintenance of intestinal homeostasis and suppression of intestinal inflammation (Zhang et al., 2016).
Prebiotics are non-digestible food ingredients which are selectively fermented in the intestine and therefore nourish probiotics to function more efficiently and allow the gastrointestinal microbiota to stay within a healthy balance (Lin et al., 2016). Prebiotics also achieve their therapeutic effect in the prevention of disease such as diabetes (Hansen et al., 2013), inflammatory bowel diseases (Orel and Kamhi Trop, 2014), and colorectal cancers (Clark et al., 2012). Xylooligosaccharide (XOS), made up of xylose units linked with β-(1-4) linkages, is an emerging prebiotic. Currently, XOS can be used as functional ingredients in foods to confer benefits upon host health. XOS feeding increases the number of beneficial microbes (Gobinath et al., 2010), regulates the markers of immune function (Childs et al., 2014), and ameliorates systemic inflammation (Hansen et al., 2013). However, despite certain biologic functions of XOS have been determined, its detailed mechanism on intestinal health remains incomplete.
Accordingly, we speculated that nutritional intervention of inflammation and gut microbiota may exert beneficial effects in alleviating gut damage. Lipopolysaccharide (LPS) is a potent endotoxin used to induce inflammation and tissue injury and a common tool for exploring the effects of dietary regimes (Liu et al., 2008, 2012; Zhu et al., 2017). Previous research has discovered the LPS-induced adverse changes in intestinal morphology, barrier function, mucosal permeability and bacterial translocation (Liu et al., 2008, 2012; Zhu et al., 2017). In this study, a model of intestinal injury induced by LPS has been used to investigate whether XOS could attenuate pig gut damage via modulating intestinal bacterial community and metabolites, and subsequently reducing inflammation.
2. Materials and methods
2.1. Animal care and experimental design
This study was performed according to the recommendations confirmed by the Animal Care and Use Committee of Wuhan Polytechnic University. The animal research was carried out on the basis of the protocols of “Laboratory animals-Guideline for ethical review of animal welfare” (GB/T 35892-2018, China), and “Animal research: reporting in vivo experiments: the ARRIVE guidelines” (Kilkenny et al., 2010). Twenty-four male piglets (Duroc × Large White × Landrace, weaned at 21 ± 1 d of age, 7.9 ± 0.2 kg initial body weight) were randomly assigned to 2 treatments and offered feed either without (control group) or with 0.02% commercial XOS product (containing 35% XOS with 65% maltodextrin as carrier; REF: a002; Shandong Longlive Biotechnology Co., Ltd, Shandong, China) (XOS treatment group). The basal diet which was formulated to meet the requirements of weaned pigs (NRC, 2012), was the same as our previous studies (Wang et al., 2019). The dose of XOS was chosen in accordance with the manufacturer's instructions. The piglets were kept in the environmental-controlled nursery pens (1.80 m × 1.10 m; 1 pig per pen), and all had free access to water and feed for 3 weeks before the LPS challenge. The feed intake for all piglets was recorded every day. On d 21, piglets were weighed, and then half of the piglets in each dietary treatment were injected i.p. with either Escherichia coli LPS (E. coli serotype 055: B5; purity > 99%; REF: L2880; Sigma–Aldrich, St Louis, MO, USA) at 100 μg/kg body weight (Liu et al., 2012) or the same amount of sterile saline. After LPS or saline injection, the pigs were fasted with free access to water before blood and intestinal samples collection.
2.2. Blood and intestinal sample collection
Two and then four hours after the LPS or sterile saline treatment, blood samples were obtained from the anterior vena cava using heparinized vacuum tubes. The samples were centrifuged at 3,500 × g for 10 min at 4 °C to separate plasma. Following blood collection, piglets were humanely killed by intramuscular injection of pentobarbital sodium (80 mg/kg BW). From the jejunum and ileum, a 3-cm-long fragment was placed in 4% paraformaldehyde in PBS for histological evaluation, and a 10-cm-long fragment was excised to separate mucosa. The digesta was obtained from the cecum. The plasma, intestinal mucosa and cecal digesta were stored at −80 °C until detection.
2.3. Histological evaluation
Histological evaluation was performed on the basis of the method described previously (Liu et al., 2012). In brief, after a 24-h fixation, the jejunal and ileal samples were dehydrated, embedded in paraffin, sectioned and stained with hematoxylin-eosin. Measurements for villus height and crypt depth were taken by using a microscope (Olympus, Japan) at 10× magnification using Image-Pro Plus software.
2.4. Disaccharidases activities
Intestinal mucosal disaccharidase activities was assayed by use of commercial kits (lactase, REF: A082-1; sucrase, REF: A082-2; maltase, REF: A082-3; Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.5. Western blotting measurement
The method for quantification of intestinal protein expression was the same as our previously described methods (Liu et al., 2012). In brief, the jejunal and ileal mucosa samples were homogenized and centrifuged to prepare tissue supernatants. An equal amount of intestinal proteins were separated on the polyacrylamide gel, and moved to polyvinylidene difluoride membranes. The membranes were blocked for at least 1 h and then incubated overnight with primary antibodies. The primary antibodies were as follows: claudin-1 (1:1,000; REF: 51-9000; Invitrogen, Carlsbad, CA, USA), heat shock protein 70 (HSP70; 1:1,000; REF: ADI-SPA-810; Enzo Life Sciences, Raamsdonksveer, The Netherlands), β-actin (1:1,000; REF: A2228; Sigma–Aldrich, St Louis, MO, USA), acetyl-histone H3 (1:1,000; REF: 27683; Cell Signaling Technology, Danvers, MA, USA), and total histone H3 (1:1,000; REF: 9717; Cell Signaling). After that, the membranes were incubated with secondary antibody for 2 h. Blots were then visualized with the chemiluminescent detection method.
2.6. Pro-inflammatory cytokine content in plasma and intestinal mucosa
The contents of pro-inflammatory cytokines in plasma and intestinal mucosa were measured by use of porcine ELISA kits (tumor necrosis factor-α [TNF-α], REF: PTA00; interleukin-6 [IL-6], REF: P6000B; R&D Systems, Minneapolis, MN, USA).
2.7. mRNA expression analysis by real-time PCR
Isolation and quantification of total RNA, cDNA synthesis, and real-time PCR were performed as recommended by Liu et al. (2012). The gene-specific primer sequences were used according to the previous studies (Liu et al., 2012; Wang et al., 2019). All primers were validated to ensure efficient amplification of a single product before being used in assays. The levels of gene expression were analyzed according to the method of Livak and Schmittgen (2001), and then normalized to the group fed basal diet and treated with saline.
2.8. Microbial diversity analysis
Microbial diversity of cecal digesta was analyzed according to our previously described methods (Wang et al., 2019) to get the high-quality 16S rRNA gene sequences. The sequences with a similarity level of more than 97% were clustered into operational taxonomic units (OTU) using Usearch (version 7.1; http://drive5.com/uparse/). OTU were used for the analysis of alpha diversity and richness, Good's coverage and rarefaction curves via Mothur (version v.1.30.1; http://www.mothur.org/wiki/Schloss_SOP#Alpha_diversity). Taxonomy-based analysis were performed by classifying each sequence using RDP Classifier (version 2.2; http://sourceforge.net/projects/rdp-classifier/) against the Silva (Release 128; http://www.arb-silva.de) 16S rRNA database at an 70% confidence level. The partial least squares discriminant analysis was conducted to graphically visualize the differences in bacterial composition among groups using the R language package “mixOmics”.
2.9. Microbial metabolites analysis
Cecal digesta was prepared for SCFA analysis as Han et al. (2016) described. The SCFA contents were measured with a HP 6890 Series Gas Chromatography (Hewlett Packard, Palo Alto, CA, USA) equipped with a HP 19091N-213 column with 30.0 m × 0.32 mm i.d. (Agilent, Santa Clara, CA, USA).
2.10. Statistical analysis
Data were analyzed by use of the ANOVA, a statistical model of the general linear model procedures (SAS Inst. Inc., Cary, NC, USA), for a 2 × 2 factorial design with diet (0 or 0.02% commercial XOS product), immunological challenge (saline or LPS) and their interactions as sources of variation. When there was a significant/trend for interaction, data were further analyzed by use of one-way ANOVA with Duncan's multiple range tests. Differences at P < 0.05 were identified significant, whereas 0.05 < P < 0.1 was considered a tendency. Correlations between bacterial abundance (at the genera level) and pro-inflammatory cytokine contents in plasma and intestinal mucosa or cecal SCFA contents were evaluated by Spearman's correlation test by use of the R language package “Pheatmap”.
3. Results
3.1. Growth performance
Throughout the entire 3 weeks experiment (before LPS challenge), no difference was observed in growth performance between control group and XOS treatment group (Appendix Table 1).
3.2. Small intestinal morphology
No interaction between dietary treatment and immunological challenge was discovered for intestinal morphology (Table 1). Compared with saline group, LPS challenge resulted in a decrease in jejunal villus height (P = 0.030) and villus height-to-crypt depth ratio (P = 0.034). XOS administration enhanced jejunal villus height-to-crypt depth ratio (P < 0.001), ileal villus height (P = 0.006) and villus height-to-crypt depth ratio (P = 0.023), and decreased jejunal crypt depth (P = 0.002) than the basal diet treatment.
Table 1.
Effects of XOS on intestinal morphology of piglets after LPS challenge at 4 h postinjection.1
| Item | Saline |
LPS |
SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| CON | XOS | CON | XOS | Diet | LPS | Interaction | ||
| Jejunum | ||||||||
| Villus height, μm | 265 | 264 | 249 | 253 | 6 | 0.852 | 0.030 | 0.657 |
| Crypt depth, μm | 73 | 65 | 71 | 66 | 2 | 0.002 | 0.829 | 0.467 |
| Villus height:crypt depth ratio | 3.64 | 4.09 | 3.52 | 3.86 | 0.08 | <0.001 | 0.034 | 0.483 |
| Ileum | ||||||||
| Villus height, μm | 232 | 278 | 246 | 260 | 10 | 0.006 | 0.837 | 0.115 |
| Crypt depth, μm | 76 | 82 | 76 | 73 | 3 | 0.492 | 0.192 | 0.177 |
| Villus height:crypt depth rato | 3.11 | 3.38 | 3.26 | 3.55 | 0.11 | 0.023 | 0.176 | 0.954 |
CON = control; XOS = xylooligosaccharide; LPS = lipopolysaccharide.
Values are means and pooled SEM, n = 6 (1 pig/pen).
3.3. Small intestinal disaccharidases activities
LPS challenge reduced jejunal maltase (P = 0.016), sucrase (P = 0.013) and lactase (P = 0.049) activities, and ileal sucrase activity (P = 0.030) (Table 2). A trend for the interaction between dietary treatment and immunological challenge was discovered for jejunal sucrase activity (P = 0.087), with higher value in the XOS_LPS group than the CON_LPS group. There was no interaction between dietary treatment and immunological challenge for other disaccharidase activities. XOS increased maltase activity in jejunum (P = 0.057) and ileum (P = 0.037).
Table 2.
Effects of XOS on intestinal disaccharidase activities of piglets after LPS challenge at 4 h postinjection (U/mg protein).1
| Item | Saline |
LPS |
SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| CON | XOS | CON | XOS | Diet | LPS | Interaction | ||
| Jejunum | ||||||||
| Maltase | 167 | 194 | 86 | 153 | 23 | 0.057 | 0.016 | 0.403 |
| Sucrase | 26.0b | 28.6b | 9.9a | 25.3b | 3.6 | 0.020 | 0.013 | 0.087 |
| Lactase | 32.6 | 28.0 | 16.2 | 25.7 | 4.5 | 0.577 | 0.049 | 0.129 |
| Ileum | ||||||||
| Maltase | 33 | 54 | 26 | 43 | 9 | 0.037 | 0.290 | 0.788 |
| Sucrase | 14.2 | 16.4 | 9.9 | 9.7 | 2.4 | 0.670 | 0.030 | 0.603 |
| Lactase | 1.8 | 1.7 | 1.4 | 2.3 | 0.6 | 0.483 | 0.887 | 0.402 |
CON = control; XOS = xylooligosaccharide; LPS = lipopolysaccharide.
a, b Labeled means in a row without a common letter differ at P < 0.05.
Values are means and pooled SEM, n = 6 (1 pig/pen).
3.4. Claudin-1 protein expression in the small intestine
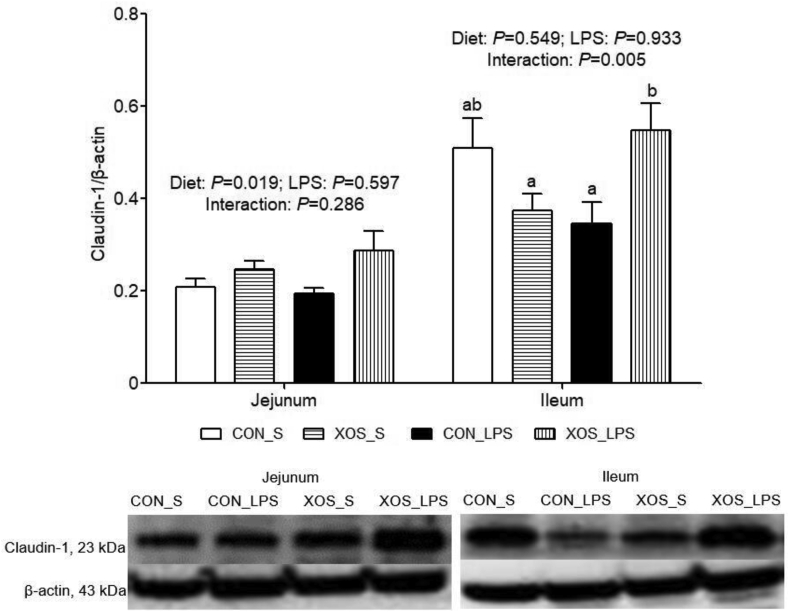
No interaction between dietary treatment and immunological challenge was discovered for jejunal claudin-1 protein expression (Fig. 1). XOS supplementation increased claudin-1 protein expression in jejunum than the basal diet group (P = 0.019). An interaction between dietary treatment and immunological challenge was found for ileal claudin-1 protein expression (P = 0.005), with higher value in the XOS_LPS group than the CON_LPS group.
Fig. 1.
Effects of XOS supplementation on intestinal claudin-1 protein expression of piglets after LPS challenge at 4 h postinjection. The bands were the representative Western blot image. Values are means and SE, n = 6 (1 pig/pen). CON_S, basal diet group treated with saline; XOS_S, XOS diet group treated with saline; CON_LPS, basal diet group treated with LPS; XOS_LPS, XOS diet group treated with LPS. XOS = xylooligosaccharide; LPS = lipopolysaccharide.
3.5. mRNA expression of TLR4 and NOD and their downstream signals in small intestine
LPS up-regulated the mRNA expression of jejunal TLR4 (P < 0.001), TNF receptor associated factor 6 (TRAF6) (P = 0.005), NOD1 (P = 0.044), NOD2 (P = 0.001), receptor-interacting serine/threonine-protein kinase 2 (RIP2) (P < 0.001), nuclear factor-κB (NF-кB) (P = 0.044), and ileal TLR4 (P = 0.015), NOD2 (P = 0.015) and RIP2 (P = 0.018) than the saline group (Table 3). No diet × LPS interaction was observed for the mRNA expression of jejunal TLR4, myeloid differentiation factor 88 (MyD88), IL-1 receptor-associated kinase 1 (IRAK1), NOD1, NOD2, and ileal TLR4, MyD88, IRAK1, TRAF6, NOD1, NOD2 and RIP2. XOS decreased the mRNA expression of TLR4 (P = 0.031) and NOD1 (P = 0.032) in jejunum, and TLR4 (P = 0.032), MyD88 (P = 0.037), NOD1 (P = 0.003), NOD2 (P = 0.048) in ileum. An interaction between dietary treatment and immunological challenge was discovered for jejunal TRAF6 (P = 0.012) and RIP2 (P = 0.001) and ileal NF-кB (P = 0.019), and a trend for the interaction was found for jejunal NF-кB (P = 0.054). Piglets fed with XOS had lower mRNA expression of jejunal TRAF6, RIP2 and NF-кB compared with piglets fed with basal diet among saline-treated piglets, and had lower ileal NF-кB mRNA expression compared with piglets fed with basal diet among LPS-stimulated piglets.
Table 3.
Effects of XOS on mRNA expression of key genes of inflammatory signaling pathways in the small intestine of piglets after LPS challenge at 4 h postinjection.1
| Item | Saline |
LPS |
SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| CON | XOS | CON | XOS | Diet | LPS | Interaction | ||
| Jejunum | ||||||||
| TLR4 | 1.00 | 0.67 | 1.36 | 1.24 | 0.10 | 0.031 | <0.001 | 0.303 |
| MyD88 | 1.00 | 0.81 | 0.93 | 0.91 | 0.07 | 0.173 | 0.860 | 0.249 |
| IRAK1 | 1.00 | 0.81 | 0.94 | 0.95 | 0.07 | 0.256 | 0.596 | 0.169 |
| TRAF6 | 1.00b | 0.69a | 1.02b | 1.01b | 0.05 | 0.009 | 0.005 | 0.012 |
| NOD1 | 1.00 | 0.72 | 1.26 | 0.98 | 0.12 | 0.032 | 0.044 | 0.995 |
| NOD2 | 1.00 | 0.86 | 2.74 | 1.36 | 0.61 | 0.251 | 0.001 | 0.857 |
| RIP2 | 1.00b | 0.62a | 1.45c | 1.59c | 0.07 | 0.099 | <0.001 | 0.001 |
| NF-кB | 1.00bc | 0.86a | 1.01bc | 1.25c | 0.09 | 0.572 | 0.044 | 0.054 |
| TNF-α | 1.00 | 0.73 | 0.95 | 0.52 | 0.15 | 0.034 | 0.384 | 0.614 |
| IL-6 | 1.00 | 1.06 | 1.35 | 1.60 | 0.13 | 0.235 | 0.002 | 0.454 |
| COX2 | 1.00 | 0.65 | 1.48 | 1.44 | 0.15 | 0.197 | <0.001 | 0.306 |
| HSP70 | 1.00a | 0.46a | 4.01a | 18.93b | 1.00 | 0.006 | 0.016 | 0.004 |
| Ileum | ||||||||
| TLR4 | 1.00 | 0.93 | 1.39 | 1.03 | 0.09 | 0.032 | 0.015 | 0.127 |
| MyD88 | 1.00 | 0.82 | 0.89 | 0.80 | 0.06 | 0.037 | 0.256 | 0.447 |
| IRAK1 | 1.00 | 0.97 | 0.97 | 0.84 | 0.06 | 0.231 | 0.184 | 0.422 |
| TRAF6 | 1.00 | 0.88 | 0.90 | 0.83 | 0.07 | 0.182 | 0.302 | 0.757 |
| NOD1 | 1.00 | 0.52 | 0.91 | 0.55 | 0.12 | 0.003 | 0.817 | 0.640 |
| NOD2 | 1.00 | 0.76 | 1.20 | 1.05 | 0.09 | 0.048 | 0.015 | 0.632 |
| RIP2 | 1.00 | 0.99 | 1.32 | 1.16 | 0.09 | 0.402 | 0.018 | 0.442 |
| NF-кB | 1.00b | 0.96b | 1.04b | 0.82a | 0.03 | 0.001 | 0.150 | 0.019 |
| TNF-α | 1.00 | 0.95 | 1.04 | 0.72 | 0.09 | 0.046 | 0.283 | 0.129 |
| IL-6 | 1.00b | 0.69a | 0.76a | 0.72a | 0.07 | 0.020 | 0.143 | 0.068 |
| COX2 | 1.00 | 0.65 | 0.90 | 0.75 | 0.10 | 0.024 | 0.981 | 0.359 |
| HSP70 | 1.00a | 0.93a | 12.53b | 31.07c | 3.00 | 0.006 | <0.001 | 0.006 |
CON = control; XOS = xylooligosaccharide; LPS = lipopolysaccharide; TLR4 = Toll-like receptor 4; MyD88 = myeloid differentiation factor 88; IRAK1 = IL-1 receptor-associated kinase 1; TRAF6 = TNF receptor associated factor 6; NOD = nucleotide binding oligomerization domain protein; RIP2 = receptor-interacting serine/threonine-protein kinase 2; NF-кB = nuclear factor-κB; TNF-α = tumor necrosis factor-α; IL-6 = interleukin-6; COX2 = cyclooxygenase 2; HSP70 = heat shock protein 70.
a, b, c Labeled means in a row without a common letter differ at P < 0.05.
Values are means and pooled SEM, n = 6 (1 pig/pen).
3.6. Plasma TNF-α and IL-6, and small intestinal TNF-α, IL-6, cyclooxygenase 2 (COX2), HSP70
Compared with piglets injected with saline, piglets injected with LPS displayed an increase in plasma TNF-α and IL-6 contents at 2 and 4 h post-injection (P < 0.001) (Table 4). An interaction between dietary treatment and immunological challenge was found for plasma IL-6 content at 2 h post-injection (P = 0.001), with lower value in the XOS_LPS group than the CON_LPS group.
Table 4.
Effects of XOS on pro-inflammatory cytokines contents in plasma and the small intestine of piglets after LPS challenge.1
| Item | Saline |
LPS |
SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| CON | XOS | CON | XOS | Diet | LPS | Interaction | ||
| 2 h postinjection | ||||||||
| Plasma | ||||||||
| TNF-α, pg/mL | ND | ND | 7,161 | 8,702 | 586 | 0.185 | <0.001 | 0.223 |
| IL-6, pg/mL | NDa | NDa | 6,012c | 3,681b | 305 | 0.001 | <0.001 | 0.001 |
| 4 h postinjection | ||||||||
| Plasma | ||||||||
| TNF-α, pg/mL | ND | ND | 699 | 414 | 142 | 0.633 | <0.001 | 0.142 |
| IL-6, pg/mL | ND | ND | 1,331 | 1,251 | 165 | 0.758 | <0.001 | 0.867 |
| Jejunum | ||||||||
| TNF-α, pg/mg protein | 19 | 5 | 43 | 5 | 12 | 0.046 | 0.341 | 0.336 |
| IL-6, pg/mg protein | 66 | 98 | 109 | 125 | 16 | 0.166 | 0.044 | 0.628 |
| Ileum | ||||||||
| TNF-α, pg/mg protein | 16a | 21ab | 32b | 23ab | 4 | 0.499 | 0.031 | 0.082 |
| IL-6, pg/mg protein | 24 | 34 | 60 | 58 | 13 | 0.755 | 0.030 | 0.638 |
CON = control; XOS = xylooligosaccharide; LPS = lipopolysaccharide; TNF-α = tumor necrosis factor-α; ND = not detectable, low than the minimum detectable dose; IL-6 = interleukin-6.
a, b, c Labeled means in a row without a common letter differ at P < 0.05.
Values are means and pooled SEM, n = 6 (1 pig/pen).
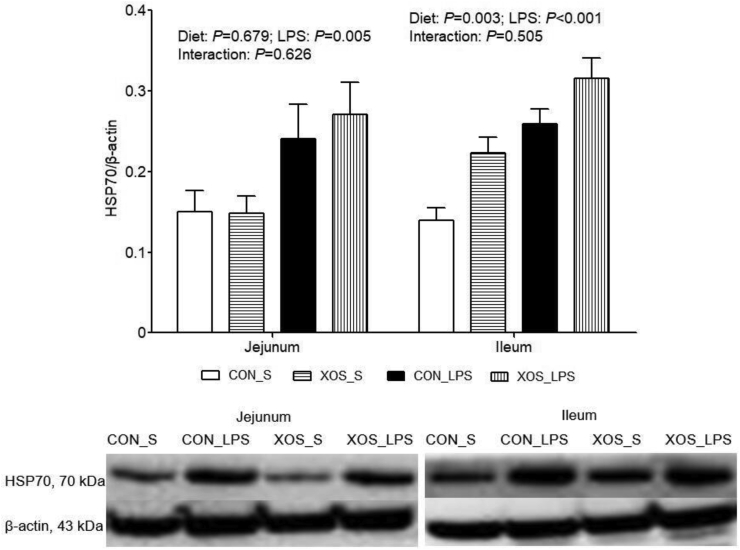
Higher TNF-α (P = 0.031) and IL-6 (P = 0.030) contents and HSP70 mRNA and protein expression (P < 0.001) in ileum, IL-6 content (P = 0.044) and mRNA expression (P = 0.002),COX2 (P < 0.001) mRNA expression and HSP70 mRNA (P = 0.016) and protein (P = 0.005) expression in jejunum were observed due to LPS challenge (Table 3, Table 4 and Fig. 2). An interaction between dietary treatment and immunological challenge was found for HSP70 mRNA expression in jejunum (P = 0.004) and ileum (P = 0.006), and a trend for the interaction was shown for IL-6 mRNA expression (P = 0.068) and TNF-α content (P = 0.082) in ileum. Piglets fed with XOS displayed higher HSP70 mRNA expression in jejunum and ileum compared with piglets fed with basal diet among LPS-challenged pigs, and had lower ileal IL-6 mRNA expression compared with piglets fed with basal diet among saline-treated piglets. No interaction between dietary treatment and immunological challenge was discovered for the other indexes. XOS suppressed jejunal TNF-α mRNA expression (P = 0.034) and content (P = 0.046), and ileal TNF-α (P = 0.046) and COX2 (P = 0.024) mRNA expression, and increased ileal HSP70 protein expression (P = 0.003).
Fig. 2.
Effects of XOS supplementation on intestinal HSP70 protein expression of piglets after LPS challenge at 4 h postinjection. The bands were the representative Western blot image. Values are means and SE, n = 6 (1 pig/pen). CON_S, basal diet group treated with saline; XOS_S, XOS diet group treated with saline; CON_LPS, basal diet group treated with LPS; XOS_LPS, XOS diet group treated with LPS. XOS = xylooligosaccharide; HSP70 = heat shock protein 70; LPS = lipopolysaccharide.
3.7. Bacterial composition in cecal digesta
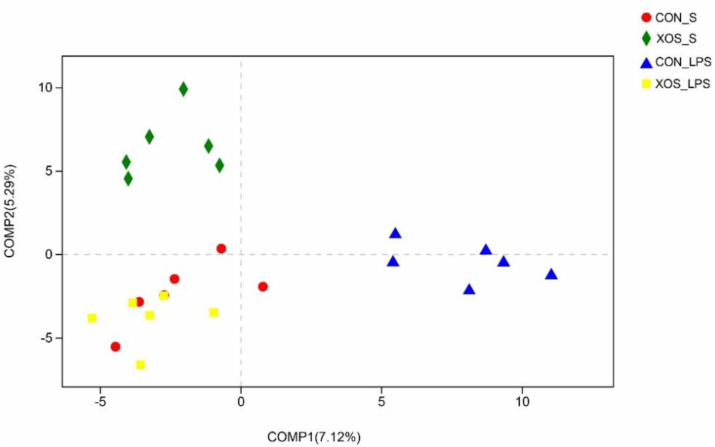
Across all of the samples, 882,631 high-quality sequence reads were generated. Rarefaction curves approximately trended to a plateau, and the Good's coverage of each treatment was over 99% (Appendix Fig. 1). No significant changes in community richness estimators (ACE and Chao) and the diversity estimators (Shannon and Simpson) were observed (Appendix Table 2). The results of partial least squares discriminant analysis revealed clear segregation and dissimilarities of microbiota composition among four groups (Fig. 3).
Fig. 3.
Partial least squares discriminant analysis on the OTU level. CON_S, basal diet group treated with saline; XOS_S, XOS diet group treated with saline; CON_LPS, basal diet group treated with LPS; XOS_LPS, XOS diet group treated with LPS. OUT = operational taxonomic units; XOS = xylooligosaccharide; LPS = lipopolysaccharide.
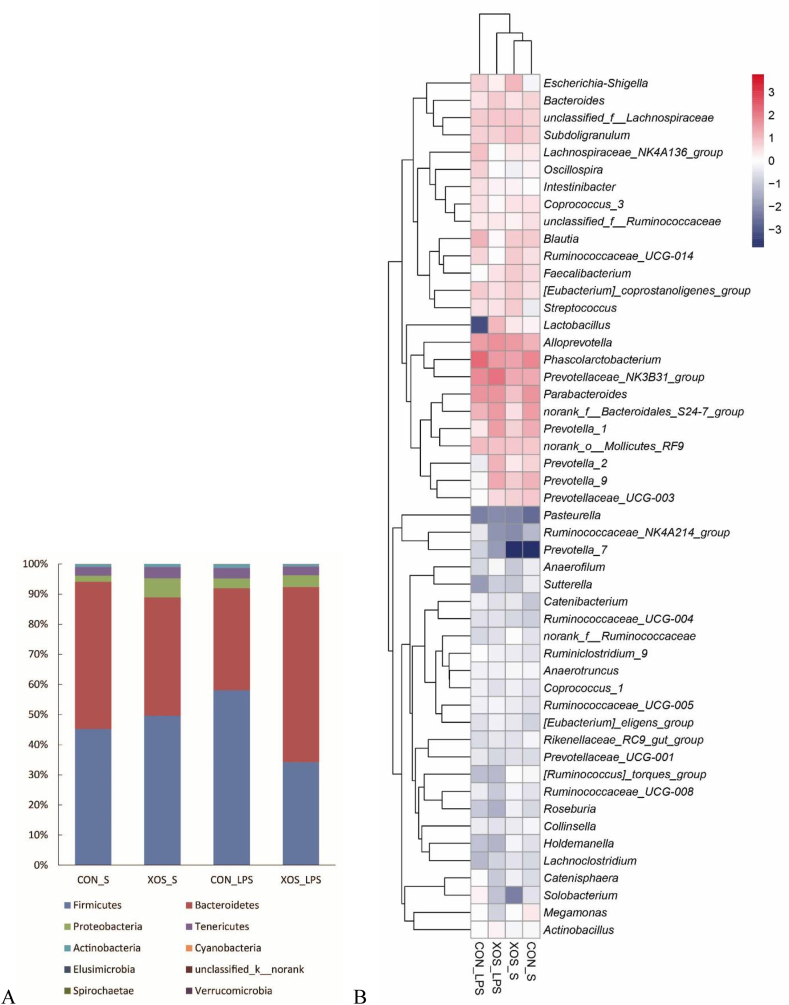
Relative abundance of bacterial communities at the phylum and genus levels were analyzed (Appendix Table 3 and Fig. 4). At the phylum level, an interaction between dietary treatment and immunological challenge was found for the abundance of Bacteroidetes (P = 0.001) and Firmicutes (P = 0.008). Piglets fed with XOS exhibited higher Bacteroidetes abundance and lower Firmicutes abundance compared with piglets fed with basal diet among LPS-stimulated piglets. There was no interaction between dietary treatment and immunological challenge for the abundance of Cyanobacteria and unclassified_k__norank. Compared with saline group, LPS reduced unclassified_k__norank abundance (P = 0.043). XOS supplementation decreased Cyanobacteria abundance than the basal diet group (P = 0.011).
Fig. 4.
Relative abundance of bacterial composition at the phylum (A) and genus (B) level. CON_S, basal diet group treated with saline; XOS_S, XOS diet group treated with saline; CON_LPS, basal diet group treated with LPS; XOS_LPS, XOS diet group treated with LPS. XOS = xylooligosaccharide; LPS = lipopolysaccharide.
Furthermore, top 50 genera were chosen for comparison. LPS challenge resulted in a decrease in the abundance of Prevotellaceae_UCG-003 (P = 0.055), Faecalibacterium (P < 0.001), norank_f__Ruminococcaceae (P = 0.028), [Ruminococcus]_torques_group (P = 0.085), Holdemanella (P = 0.054), Roseburia (P = 0.097), and an increase in the abundance of Ruminiclostridium_9 (P = 0.044), Ruminococcaceae_NK4A214_group (P = 0.050), Prevotella_7 (P = 0.037). There was an interaction between dietary treatment and immunological challenge for the abundance of norank_f__Bacteroidales_S24-7_group (P = 0.029), Prevotella_1 (P = 0.039), Prevotella_9 (P = 0.025), Blautia (P = 0.034), Escherichia-Shigella (P = 0.035), Prevotella_2 (P = 0.015), Sutterella (P = 0.049), and a trend for the interaction was found for the abundance of Ruminococcaceae_UCG-014 (P = 0.063), Streptococcus (P = 0.066), Lactobacillus (P = 0.090). Piglets fed with XOS showed lower Blautia abundance and higher abundance of Prevotella_1, Prevotella_9, Prevotella_2, Lactobacillus compared with piglets fed with basal diet among LPS-stimulated piglets, and had higher abundance of Escherichia-Shigella and Streptococcus compared with piglets fed with basal diet among saline-injected piglets. No interaction between dietary treatment and immunological challenge was observed for other genera. Relative to the control-diet group, XOS supplementation led to a decrease in the abundance of Phascolarctobacterium (P = 0.042), Oscillospira (P = 0.018), Solobacterium (P = 0.022), Ruminococcaceae_NK4A214_group (P = 0.005), an increase in the abundance of Faecalibacterium (P = 0.002), and tended to increase norank_f__Ruminococcaceae (P = 0.063).
3.8. SCFA content in cecal digesta
The LPS-stimulated piglets exhibited higher contents of propionate (P = 0.068), isobutyrate (P = 0.008), valerate (P = 0.088) and isovalerate (P < 0.001) in cecal digesta than piglets treated with saline (Table 5). An interaction between dietary treatment and immunological challenge was found for contents of propionate (P = 0.016), isobutyrate (P = 0.001), valerate (P = 0.012) and isovalerate (P = 0.001), and a trend for the interaction was found for acetate content (P = 0.084). Piglets fed with XOS showed higher contents of acetate, propionate, isobutyrate, valerate and isovalerate compared with piglets fed with basal diet among LPS-stimulated piglets. No interaction between dietary treatment and immunological challenge was found for butyrate content. XOS caused growing trend towards butyrate content (P = 0.080).
Table 5.
Effects of XOS on short chain fatty acids contents in cecal digesta of piglets after LPS challenge at 4 h postinjection (μg/g).1
| Item | Saline |
LPS |
SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| CON | XOS | CON | XOS | Diet | LPS | Interaction | ||
| Acetate | 2,080a | 2,164ab | 1,883a | 2,761b | 218 | 0.039 | 0.369 | 0.084 |
| Propionate | 1,204a | 1,287a | 1,117a | 1,856b | 125 | 0.004 | 0.068 | 0.016 |
| Butyrate | 408 | 423 | 409 | 589 | 53 | 0.080 | 0.128 | 0.132 |
| Isobutyrate | 135b | 107a | 125ab | 167c | 8 | 0.402 | 0.008 | 0.001 |
| Valerate | 148a | 134a | 130a | 217b | 18 | 0.059 | 0.088 | 0.012 |
| Isovalerate | 220a | 208a | 227a | 319b | 14 | 0.010 | <0.001 | 0.001 |
CON = control; XOS = xylooligosaccharide; LPS = lipopolysaccharide.
a, b, c Labeled means in a row without a common letter differ at P < 0.05.
Values are means and pooled SEM, n = 6 (1 pig/pen).
3.9. Correlations between bacterial abundance and pro-inflammatory cytokines content in plasma and intestinal mucosa or cecal SCFA content
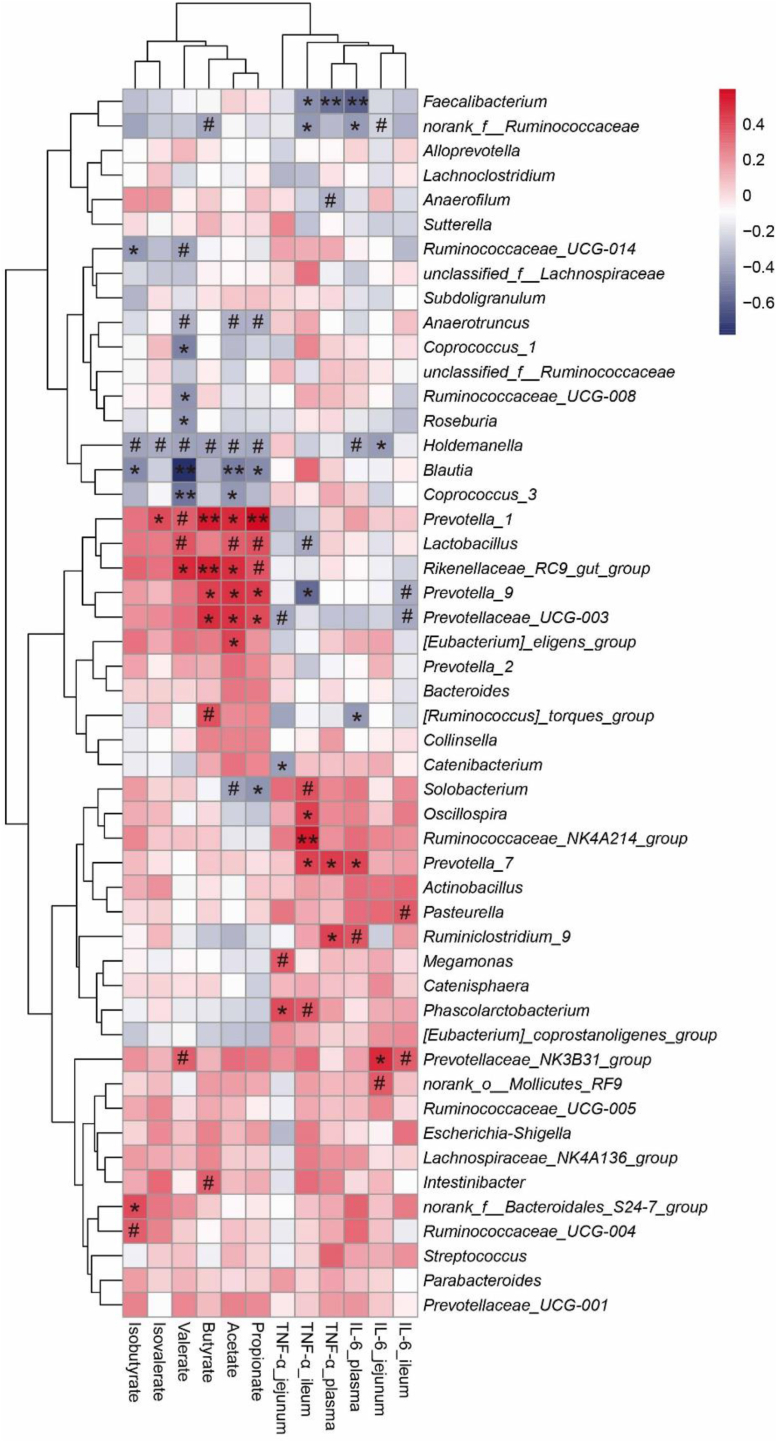
Acetate content was negatively correlated with Anaerotruncus (P < 0.1), Holdemanella (P < 0.1), Blautia (P < 0.01), Coprococcus_3 (P < 0.05), Solobacterium (P < 0.1), and positively correlated with Prevotella_1 (P < 0.05), Lactobacillus (P < 0.1), Rikenellaceae_RC9_gut_group (P < 0.05), Prevotella_9 (P < 0.05), Prevotellaceae_UCG-003 (P < 0.05), Eubacterium_eligens_group (P < 0.05) (Fig. 5). Propionate content was negatively correlated with Anaerotruncus (P < 0.1), Holdemanella (P < 0.1), Blautia (P < 0.05), Solobacterium (P < 0.05), and positively correlated with Prevotella_1 (P < 0.01), Lactobacillus (P < 0.1), Rikenellaceae_RC9_gut_group (P < 0.1), Prevotella_9 (P < 0.05), Prevotellaceae_UCG-003 (P < 0.05). Butyrate content was negatively correlated with norank_f__Ruminococcaceae (P < 0.1), Holdemanella (P < 0.1), and positively correlated with Prevotella_1 (P < 0.01), Rikenellaceae_RC9_gut_group (P < 0.01), Prevotella_9 (P < 0.05), Prevotellaceae_UCG-003 (P < 0.05), Ruminococcus_torques_group (P < 0.1), Intestinibacter (P < 0.1). Isobutyrate content was negatively correlated with Ruminococcaceae_UCG-014 (P < 0.05), Holdemanella (P < 0.1), Blautia (P < 0.05), and positively correlated with norank_f__Bacteroidales_S24-7_group (P < 0.05), Ruminococcaceae_UCG-004 (P < 0.1). Valerate content was negatively correlated with Ruminococcaceae_UCG-014 (P < 0.1), Anaerotruncus (P < 0.1), Coprococcus_1 (P < 0.05), Ruminococcaceae_UCG-008 (P < 0.05), Roseburia (P < 0.05), Holdemanella (P < 0.1), Blautia (P < 0.01), Coprococcus_3 (P < 0.01), and positively correlated with Prevotella_1 (P < 0.1), Lactobacillus (P < 0.1), Rikenellaceae_RC9_gut_group (P < 0.05), Prevotellaceae_NK3B31_group (P < 0.1). Isovalerate content was negatively correlated with Holdemanella (P < 0.1), and positively correlated with Prevotella_1 (P < 0.05).
Fig. 5.
Correlations between bacterial abundance (at the genera level) and pro-inflammatory cytokines contents in plasma and intestinal mucosa or cecal SCFA content. The red represents a positive correlation, and the blue represents a negative correlation. #, P < 0.1; ∗, P < 0.05; ∗∗, P < 0.01. TNF-α = tumor necrosis factor-α; IL-6 = interleukin-6; SCFA = short chain fatty acids.
TNF-α_jejunum was negatively correlated with Prevotellaceae_UCG-003 (P < 0.1), Catenibacterium (P < 0.05), and positively correlated with Megamonas (P < 0.1), Phascolarctobacterium (P < 0.05). TNF-α_ileum was negatively correlated with Faecalibacterium (P < 0.05), norank_f__Ruminococcaceae (P < 0.05), Lactobacillus (P < 0.1), Prevotella_9 (P < 0.05), and positively correlated with Solobacterium (P < 0.1), Oscillospira (P < 0.05), Ruminococcaceae_NK4A214_group (P < 0.01), Prevotella_7 (P < 0.05), Phascolarctobacterium (P < 0.1). TNF-α_plasma was negatively correlated with Faecalibacterium (P < 0.01) and Anaerofilum (P < 0.1), and positively correlated with Prevotella_7 (P < 0.05) and Ruminiclostridium_9 (P < 0.05). IL-6_plasma was negatively correlated with Faecalibacterium (P < 0.01), norank_f__Ruminococcaceae (P < 0.05), Holdemanella (P < 0.1), [Ruminococcus]_torques_group (P < 0.05), and positively correlated with Prevotella_7 (P < 0.05) and Ruminiclostridium_9 (P < 0.1). IL-6_jejunum was negatively correlated with norank_f__Ruminococcaceae (P < 0.1) and Holdemanella (P < 0.05), and positively correlated with Prevotellaceae_NK3B31_group (P < 0.05) and norank_o__Mollicutes_RF9 (P < 0.1). IL-6_ileum was negatively correlated with Prevotella_9 (P < 0.1) and Prevotellaceae_UCG-003 (P < 0.1), and positively correlated with Pasteurella (P < 0.1) and Prevotellaceae_NK3B31_group (P < 0.1).
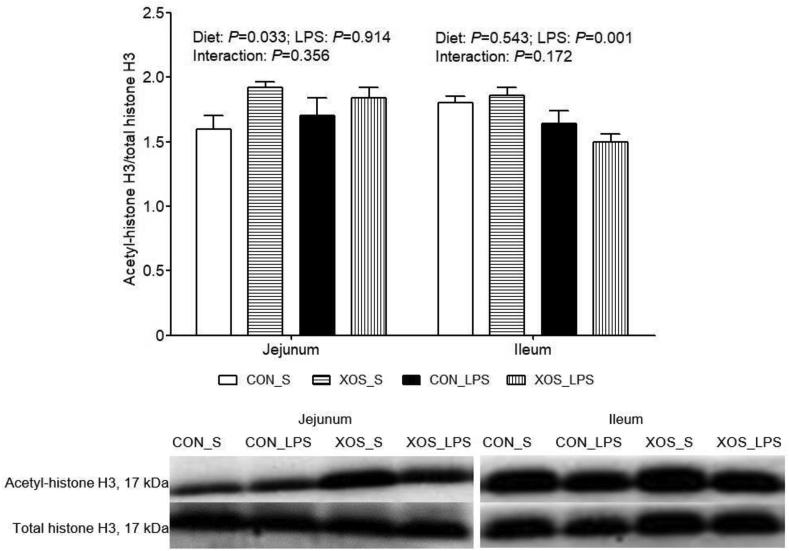
3.10. Protein expression of acetyl-histone H3 in the small intestine
No interaction between dietary treatment and immunological challenge was found for protein expression of acetyl-histone H3 in small intestine (Fig. 6). LPS administration led to a decrease in ileal acetyl-histone H3 protein expression than the saline group (P = 0.001). The piglets fed with XOS exhibited higher jejunal acetyl-histone H3 protein expression compared with piglets fed with basal diet (P = 0.033).
Fig. 6.
Effects of XOS supplementation on intestinal acetyl-histone H3 of piglets after LPS challenge at 4 h postinjection. The bands were the representative Western blot image. Values are means and SE, n = 6 (1 pig/pen). CON_S, basal diet group treated with saline; XOS_S, XOS diet group treated with saline; CON_LPS, basal diet group treated with LPS; XOS_LPS, XOS diet group treated with LPS. XOS = xylooligosaccharide; LPS = lipopolysaccharide.
4. Discussion
Integrity of the intestinal structure is required for nutrient digestion and absorption. Morphologic alterations in the digestive tract, including villus atrophy and crypt hyperplasia, reflect malabsorption and growth inhibition of the body (Liu et al., 2012; Zhu et al., 2018). Intestinal disaccharidases are useful criterion for assessing intestinal digestive capacity (Liu et al., 2012; Zhu et al., 2018). Tight junction proteins are responsible for restricting the entry of harmful substances, and the up-regulated levels of these proteins mean lower inflammatory disease risk in intestine (Chen et al., 2017). As shown by our results, LPS caused acute intestinal injury, but XOS showed positive impact on improving intestinal mucosa morphology and enhancing intestinal disaccharidase activities and claudin-1 protein expression. Consistently, XOS administration to weanling pigs elevated villus height-to-crypt depth ratio in jejunum (Liu et al., 2018), decreased the number of colonic aberrant crypt foci of 1,2-dimethylhydrazine-challenged rats (Hsu et al., 2004), and increased gene expression of tight junction protein occluding in rats (Christensen et al., 2014). These data indicate that administration with XOS may exert beneficial effects on intestine.
The development of inflammatory conditions have been proposed to affect intestinal integrity (Liu et al., 2012). Due to this property, the related indicators of inflammation were detected in this study. TLR and NOD, collectively referred to as pattern recognition receptors, are able to perceive conserved molecular motives including microbe-/pathogen-associated molecular patterns (Valentini et al., 2014). They are also key components of innate immunity, which provide immediate responses against pathogenic invasion or tissue injury, and are included in the pathogenesis of several infectious and inflammatory diseases (Liu et al., 2012). TLR signaling-related molecules included TLR4, MyD88, IRAK1, and TRAF6. NOD1, NOD2, and RIP2 are the members of NOD signaling pathway. These two signaling pathways can induce the activation of NF-κB, which can control the activities of multiple factors important for immunity, inflammation, and stress responses, e.g., pro-inflammatory cytokines, COX2, and HSP (Liu et al., 2012; Natarajan et al., 2018). The production of excessive proinflammatory cytokines can decrease the tight junction barrier of epithelium and result in mucosal disruption in intestine (Liu et al., 2012). The expression of COX2 and HSP is typically induced by inflammation (Liu et al., 2012).
The present study displayed that plasma/intestinal TNF-α and IL-6, intestinal COX2, HSP70, and the key members of TLR4 and NOD signaling were up-regulated in LPS challenged-piglets, which corroborated the findings of previous study (Liu et al., 2012), suggesting LPS stimulation induced intestinal inflammation in piglets. Dietary addition of XOS attenuated the impact of LPS. A test showed similar results that both pre- and post-treated XOS suppressed the pro-inflammatory cytokine generation in LPS-treated RAW264.7 cells in a dose-dependent manner (Chen et al., 2012a). Lecerf et al. (2012) found that XOS in combination with inulin resulted in a lower pro-inflammatory IL-1β level together with a higher anti-inflammatory IL-13 level in blood incubated with LPS. Hansen et al. (2013) also reported that the expression of IL-1β and interferon γ was down-regulated systemically from XOS-fed mice. Interestingly, XOS addition to the LPS-stimulated piglets also enhanced the mRNA and protein expression of HSP70. HSP70 has been known to be a universal cytoprotective protein, and prevent toxic agents from disrupting barrier integrity in intestinal epithelial cells (Zhong et al., 2010). Higher HSP70 expression can promote ulcer healing via improvement of cell proliferation, suppression of cell apoptosis, and increase of protein synthesis (Zhong et al., 2010). Based on these observations, XOS may improve LPS-induced intestinal injury by increasing the synthesis of anti-inflammatory mediators, but decreasing the generation of pro-inflammatory mediators.
The correlation analysis demonstrated a significant connections between gut microbiota and pro-inflammatory cytokines. Iida et al. (2013) have reported that the intestinal microbiota influences immunity and inflammation not only locally at the mucosal level but also systemically. We speculated that XOS improves intestinal integrity and inflammatory response through modulating microbiota composition. The gastrointestinal tract hosts a complex microbial community dominated by two bacterial phyla, the Bacteroidetes and the Firmicutes. The commensal microbiota is easily modified by many factors containing health status, dietary change, genetics, etc. Analysis of cecal digesta by16S rRNA gene pyrosequencing demonstrated a clear alteration in the gut microbiota in pigs receiving XOS after LPS treatment. Similarly, studies have observed that XOS feeding modulated mice cecum microbiome (Long et al., 2019), reduced Firmicutes and increased Bacteroidetes of mice (Petersen et al., 2010; Long et al., 2019), and also decrease Firmicutes-to-Bacteroidetes ratio in rats treated with a high-fat diet (Thiennimitr et al., 2018). The contributions of Bacteroidetes to their host's health contain the degradation of resistant dietary polymers and the SCFA production (Thomas et al., 2011). Some research has also observed the implications of Bacteroidetes for immune system, normal development of gastrointestinal tract, and restriction of the gastrointestinal tract colonization by potential pathogenic bacteria (Thomas et al., 2011). However, some members of Bacteroidetes may display a strong pathogenic behavior toward the host (Thomas et al., 2011). We then presented a comparison at genus level of the gut microbiota.
Our result found a clear alteration in the gut microbiota in treatments at genus level. XOS enhanced the relative abundance of beneficial bacteria, e.g., Faecalibacterium, Lactobacillus, and Prevotella. Faecalibacterium is negatively correlated to colorectal cancer (Chen et al., 2012b). Its main specie, Faecalibacterium prausnitzii, plays a role in providing energy to intestinal epithelial cells, and exhibits a strong anti-inflammatory effect by inhibiting NF-κB activation and pro-inflammatory mediator secretion (Cao et al., 2014; Sokol et al., 2008). In addition, Lactobacillus also has a high correlation with good health, and in vitro and in vitro experimental results support the concept that it provides far more benefit than harm to its hosts. The mechanisms may include antioxidative capacity, modulation of immune responses, limit of harmful bacteria colonization, restoration of microbial homeostasis, and etc. (Lebeer et al., 2008; Valeriano et al., 2017). Prevotella is well known for cellulose and xylan hydrolysis, and formation of SCFA (Zhou et al., 2016). Moreover, XOS alters other bacterial composition. However, effects of the bacteria are unclear, e.g., some species of Streptococcus exist similar probiotic effects as Lactobacillus, but some are potential pathogens (Zhou et al., 2016). Therefore, the mechanism needs to be researched further.
The effects of gut microbiota are at least partly mediated by the SCFA. In this study, the close correlation between gut microbiota and SCFA content was observed. XOS in diet increased acetate, butyrate, propionate, isobutyrate, valerate and isovalerate contents in cecal digesta, which is in agreement with several in vitro and in vitro studies (Broekaert et al., 2011; De Maesschalck et al., 2015; Long et al., 2019). SCFA can supply energy for epithelial cells (Kobayashi et al., 2017), improve tight junction proteins expression (Han et al., 2016), and promote the beneficial bacteria growth (Kobayashi et al., 2017). Study has also found that acetate, propionate and butyrate attenuated LPS-stimulated TNF-α release, and suppressed downstream NF-κB signaling (Tedelind et al., 2007).
To date inhibition of histone deacetylase (HDAC), an epigenetic modifier, is currently deemed to underlie the anti-inflammatory effects of SCFA (Kobayashi et al., 2017). Our results found that XOS enhanced acetyl-histone H3 protein expression (reflecting inhibition of HDAC) in jejunum, which may relate to the increased SCFA production. Histone acetylations, performed by histone acetyltransferases and HDAC, play as a crucial part in gene expression under physiological and pathological processes to take part in the inflammatory and host defense responses (Ciarlo et al., 2013). In the study of colitis, there is a close link between HDAC and intestinal inflammation (Felice et al., 2015). Thus, XOS attenuated LPS-induced intestinal damage possibly via increasing SCFA content, which function as HDAC inhibitors to modulate inflammation.
In conclusion, XOS promotes the intestinal barrier integrity and reduces intestinal injury. These functions of XOS are associated, in part, with inhibition of inflammation and modulation of intestinal bacterial community and metabolites.
Author contributions
Y. Liu: conceptualization; X. Wang, C. Yu, L. Wang and Y. Liu: formal analysis; X. Wang, K. Xiao, C. Yu, L. Wang, T. Liang, H. Zhu, X. Xu and Y. Liu: investigation; X. Wang: writing – original draft; X. Wang and Y. Liu: writing – review & editing.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This research was funded by the Projects of Innovative Research Groups of the Natural Science Foundation of Hubei Province (grant number 2019CFA015), National Natural Science Foundation of China (grant number 31802070 and 31772615), and Wuhan Science and Technology Bureau (grant number 2018020401011304).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Appendix
Appendix to this article can be found online at https://doi.org/10.1016/j.aninu.2020.11.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Broekaert W.F., Courtin C.M., Verbeke K., Van de Wiele T., Verstraete W., Delcour J.A. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit Rev Food Sci Nutr. 2011;51:178–194. doi: 10.1080/10408390903044768. [DOI] [PubMed] [Google Scholar]
- Cao Y., Shen J., Ran Z.H. Association between Faecalibacterium prausnitzii reduction and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Gastroenterol Res Pract. 2014;2014:872725. doi: 10.1155/2014/872725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.H., Chen Y.K., Chang H.C., Lin S.Y. Immunomodulatory effects of xylooligosaccharides. Food Sci Technol Res. 2012;18:195–199. [Google Scholar]
- Chen W., Liu F., Ling Z., Tong X., Xiang C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PloS One. 2012;7 doi: 10.1371/journal.pone.0039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Mou D., Hu L., Zhen J., Che L., Fang Z. Effects of maternal low-energy diet during gestation on intestinal morphology, disaccharidase activity, and immune response to lipopolysaccharide challenge in pig offspring. Nutrients. 2017;9 doi: 10.3390/nu9101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs C.E., Röytiö H., Alhoniemi E., Fekete A.A., Forssten S.D., Hudjec N. Xylo-oligosaccharides alone or in synbiotic combination with Bifidobacterium animalis subsp. lactis induce bifidogenesis and modulate markers of immune function in healthy adults: a double-blind, placebo-controlled, randomised, factorial cross-over study. Br J Nutr. 2014;111:1945–1956. doi: 10.1017/S0007114513004261. [DOI] [PubMed] [Google Scholar]
- Christensen E.G., Licht T.R., Leser T.D., Bahl M.I. Dietary xylo-oligosaccharide stimulates intestinal bifidobacteria and lactobacilli but has limited effect on intestinal integrity in rats. BMC Res Notes. 2014;7:660. doi: 10.1186/1756-0500-7-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarlo E., Savva A., Roger T. Epigenetics in sepsis: targeting histone deacetylases. Int J Antimicrob Agents. 2013;42(Suppl):S8–S12. doi: 10.1016/j.ijantimicag.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Clark M.J., Robien K., Slavin J.L. Effect of prebiotics on biomarkers of colorectal cancer in humans: a systematic review. Nutr Rev. 2012;70:436–443. doi: 10.1111/j.1753-4887.2012.00495.x. [DOI] [PubMed] [Google Scholar]
- De Maesschalck C., Eeckhaut V., Maertens L., De Lange L., Marchal L., Nezer C. Effects of xylo-oligosaccharides on broiler chicken performance and microbiota. Appl Environ Microbiol. 2015;81:5880–5888. doi: 10.1128/AEM.01616-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felice C., Lewis A., Armuzzi A., Lindsay J.O., Silver A. Review article: selective histone deacetylase isoforms as potential therapeutic targets in inflammatory bowel diseases. Aliment Pharmacol Ther. 2015;41:26–38. doi: 10.1111/apt.13008. [DOI] [PubMed] [Google Scholar]
- Gobinath D., Madhu A.N., Prashant G., Srinivasan K., Prapulla S.G. Beneficial effect of xylo-oligosaccharides and fructo-oligosaccharides in streptozotocin-induced diabetic rats. Br J Nutr. 2010;104:40–47. doi: 10.1017/S0007114510000243. [DOI] [PubMed] [Google Scholar]
- Grivennikov S.I., Wang K., Mucida D., Stewart C.A., Schnabl B., Jauch D. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M., Song P., Huang C., Rezaei A., Farrar S., Brown M.A. Dietary grape seed proanthocyanidins (GSPs) improve weaned intestinal microbiota and mucosal barrier using a piglet model. Oncotarget. 2016;7:80313–80326. doi: 10.18632/oncotarget.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C.H.F., Frøkiær H., Christensen A.G., Bergström A., Licht T.R., Hansen A.K. Dietary xylooligosaccharide downregulates IFN-γ and the low-grade inflammatory cytokine IL-1β systemically in mice. J Nutr. 2013;143:533–540. doi: 10.3945/jn.112.172361. [DOI] [PubMed] [Google Scholar]
- Hsu C.K., Liao J.W., Chung Y.C., Hsieh C.P., Chan Y.C. Xylooligosaccharides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. J Nutr. 2004;134:1523–1528. doi: 10.1093/jn/134.6.1523. [DOI] [PubMed] [Google Scholar]
- Iida N., Dzutsev A., Stewart C.A., Smith L., Bouladoux N., Weingarten R.A. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C., Browne W., Cuthill I.C., Emerson M., Altman D.G. NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Mikami D., Kimura H., Kamiyama K., Morikawa Y., Yokoi S. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem Biophys Res Commun. 2017;486:499–505. doi: 10.1016/j.bbrc.2017.03.071. [DOI] [PubMed] [Google Scholar]
- Lebeer S., Vanderleyden J., De Keersmaecker S.C.J. Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev. 2008;72:728–764. doi: 10.1128/MMBR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecerf J.M., Dépeint F., Clerc E., Dugenet Y., Niamba C.N., Rhazi L. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br J Nutr. 2012;108:1847–1858. doi: 10.1017/S0007114511007252. [DOI] [PubMed] [Google Scholar]
- Lin S.H., Chou L.M., Chien Y.W., Chang J.S., Lin C.I. Prebiotic effects of xylooligosaccharides on the improvement of microbiota balance in human subjects. Gastroenterol Res Pract. 2016;2016:5789232. doi: 10.1155/2016/5789232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.B., Cao S.C., Liu J., Xie Y.N., Zhang H.F. Effect of probiotics and xylo-oligosaccharide supplementation on nutrient digestibility, intestinal health and noxious gas emission in weanling pigs. Asian-Australas J Anim Sci. 2018;31:1660–1669. doi: 10.5713/ajas.17.0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Chen F., Odle J., Lin X., Jacobi S.K., Zhu H. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J Nutr. 2012;142:2017–2024. doi: 10.3945/jn.112.164947. [DOI] [PubMed] [Google Scholar]
- Liu Y., Huang J., Hou Y., Zhu H., Zhao S., Ding B. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br J Nutr. 2008;100:552–560. doi: 10.1017/S0007114508911612. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Long J., Yang J., Henning S.M., Woo S.L., Hsu M., Chan B. Xylooligosaccharide supplementation decreases visceral fat accumulation and modulates cecum microbiome in mice. J Funct Foods. 2019;52:138–146. [Google Scholar]
- Natarajan K., Abraham P., Kota R., Isaac B. NF-κB-iNOS-COX2-TNF α inflammatory signaling pathway plays an important role in methotrexate induced small intestinal injury in rats. Food Chem Toxicol. 2018;118:766–783. doi: 10.1016/j.fct.2018.06.040. [DOI] [PubMed] [Google Scholar]
- NRC . 11th ed. National Academic Press; Washington, DC: 2012. Nutrient requirements of swine. [Google Scholar]
- Orel R., Kamhi Trop T. Intestinal microbiota, probiotics and prebiotics in inflammatory bowel disease. World J Gastroenterol. 2014;20:11505–11524. doi: 10.3748/wjg.v20.i33.11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A., Bergström A., Andersen J.B., Hansen M., Lahtinen S.J., Wilcks A. Analysis of the intestinal microbiota of oligosaccharide fed mice exhibiting reduced resistance to Salmonella infection. Benef Microbes. 2010;1:271–281. doi: 10.3920/BM2010.0016. [DOI] [PubMed] [Google Scholar]
- Sokol H., Pigneur B., Watterlot L., Lakhdari O., Bermúdez-Humarán L.G., Gratadoux J.J. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Gao H., Lin Y., Yao Y., Zhu S., Wang J. Alterations in the microbiota drive interleukin-17C production from intestinal epithelial cells to promote tumorigenesis. Immunity. 2014;40:140–152. doi: 10.1016/j.immuni.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Tedelind S., Westberg F., Kjerrulf M., Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13:2826–2832. doi: 10.3748/wjg.v13.i20.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiennimitr P., Yasom S., Tunapong W., Chunchai T., Wanchai K., Pongchaidecha A. Lactobacillus paracasei HII01, xylooligosaccharides, and synbiotics reduce gut disturbance in obese rats. Nutrition. 2018;54:40–47. doi: 10.1016/j.nut.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Thomas F., Hehemann J.H., Rebuffet E., Czjzek M., Michel G. Environmental and gut bacteroidetes: the food connection. Front Microbiol. 2011;2:93. doi: 10.3389/fmicb.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini M., Piermattei A., Di Sante G., Migliara G., Delogu G., Ria F. Immunomodulation by gut microbiota: role of Toll-like receptor expressed by T cells. J Immunol Res. 2014;2014:586939. doi: 10.1155/2014/586939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeriano V.D.V., Balolong M.P., Kang D.K. Probiotic roles of Lactobacillus sp. in swine: insights from gut microbiota. J Appl Microbiol. 2017;122:554–567. doi: 10.1111/jam.13364. [DOI] [PubMed] [Google Scholar]
- Wang X.Y., Wang W.J., Wang L.M., Yu C., Zhang G.L., Zhu H.L. Lentinan modulates intestinal microbiota and enhances barrier integrity in a piglet model challenged with lipopolysaccharide. Food Funct. 2019;10:479–489. doi: 10.1039/c8fo02438c. [DOI] [PubMed] [Google Scholar]
- Zhang H., Du M., Yang Q., Zhu M.J. Butyrate suppresses murine mast cell proliferation and cytokine production through inhibiting histone deacetylase. J Nutr Biochem. 2016;27:299–306. doi: 10.1016/j.jnutbio.2015.09.020. [DOI] [PubMed] [Google Scholar]
- Zhong X., Wang T., Zhang X., Li W. Heat shock protein 70 is upregulated in the intestine of intrauterine growth retardation piglets. Cell Stress Chaperones. 2010;15:335–342. doi: 10.1007/s12192-009-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Fang L., Sun Y., Su Y., Zhu W. Effects of the dietary protein level on the microbial composition and metabolomic profile in the hindgut of the pig. Anaerobe. 2016;38:61–69. doi: 10.1016/j.anaerobe.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Zhu H., Pi D., Leng W., Wang X., Hu C.A.A., Hou Y. Asparagine preserves intestinal barrier function from LPS-induced injury and regulates CRF/CRFR signaling pathway. Innate Immun. 2017;23:546–556. doi: 10.1177/1753425917721631. [DOI] [PubMed] [Google Scholar]
- Zhu H., Wang H., Wang S., Tu Z., Zhang L., Wang X. Flaxseed oil attenuates intestinal damage and inflammation by regulating necroptosis and TLR4/NOD signaling pathways following lipopolysaccharide challenge in a piglet model. Mol Nutr Food Res. 2018;62 doi: 10.1002/mnfr.201700814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.