Abstract
Background. Intestinal parasites are still a serious public health problem and important cause of morbidity and mortality all over the world, particularly in developing countries. Unfortunately, pre-school children are more susceptible to infection. However, information is scarce in the study area. Thus, this study aimed to assess the prevalence of intestinal parasitosis and associated factors among children aged 6 to 59 months in Northcentral Ethiopia. Methods. An institutional-based cross-sectional study was carried out at Mekane Eyesus primary hospital from June 10 to November 30, 2020. Stool samples were collected from 322 children and examined by using direct wet mount and formal ether concentration techniques. The data were entered and analyzed using EPI Info v7 and SPSS v23 statistical software, respectively. Both bivariable and multivariable logistic analysis was carried out and potential associated factors were identified based on adjusted odds ratio with 95% confidence interval and P-value <.05. Results. The prevalence of intestinal parasitosis was 18.0% (95% CI: 14.0%-22.0%). A total of 4 parasites were examined and the dominant parasite was E. histolytica/dispar (8.1%) followed by A. limbricoide (4.7%). Children with irregular trimming of fingernails (AOR = 3.14, 95% CI: 1.59-6.21), and child who have habit of eating unwashed fruits/vegetables (AOR = 3.80, 95% CI: 1.14-12.82) were strongly associated with IPIs. Conclusions. Protozoa parasites are most common cause of diseases in children. The study identified some preventable and modifiable factors to address the prevalence of IPIs. Additionally, improving mothers/guardians awareness about source of infection and mode of transmission is necessary.
Keywords: intestinal parasitic infections, soil-transmitted helminths, protozoa parasites magnitude, children aged 6 to 59 months
Background
Intestinal parasitic infections (IPIs) are still a serious public health problem and distributed almost throughout the world. 1 They are an important cause of morbidity and mortality all over the world, particularly in developing countries. They affect the poorest and most deprived communities, where poor environmental sanitation, and personal hygiene are common. 2 In tropical and sub-tropical regions, where the climate is suitable for the spread of intestinal parasites (IPs) and 100s of 1000s of preventable deaths are happened each year. 3
Worldwide estimation showed that around 4.5 billion people are live in IPI endemic areas and more than 2 billion people are affected. Of these 450 million people develop clinical morbidity. Many of these are children from developing countries.4,5 IPIs are associated with 39 million disability-adjusted life year loss globally. 6 According to the World Health Organization (WHO) 7 fact sheet on Soil-transmitted helminths (STHs), universally over 267 million preschool-age children and over 568 million school-age children live in areas where IPs are intensively transmitted.
Every year 1.4 billion children were infected globally with an IP throughout the world. Preschool children were attributable to the prevalence between 10% and 20% of the total people with helminthiasis. 8 Intestinal parasites infections caused by helminths like Ascaris lumbricoides, Trichuris trichiura, and Hookworm accounts 122 million, 86 million, and 21 million preschool children infection respectively. And also, protozoan parasites like Entamoeba histolytica, Giardia lamblia, and Cryptosporidium species are the most common parasite species that affect children in the world.8-12 According to a WHO report, STHs are the second leading cause of mortality in <6 years old children of Africa. 13
There is a high burden of IPs in Ethiopia. The overall national prevalence of any helminth infection was 29.8% with variable degree of prevalence and species distribution among regions because of some geographical, environmental, and social factors. Amhara and Southern regional states account for the highest-burden of the disease.14,15 Different researches conducted in our country showed that the prevalence of IPIs among under-5 children were ranging from 15.5% to 85.1% and has been associated with adverse outcomes including growth deficits malnutrition and anemia.9,16-18
Children below the age of 5 years were more susceptible to IPIs. This is because of low immunity and children’s behavior like playing with soil, suck their fingernails, and soil eating habit. Specifically, children living in developing countries with limited or no access to safe drinking water, poor personal hygiene, inadequate sanitation, open field defecation, and substandard housing are the most affected peoples.5,15
Different studies have been conducted on the prevalence of IPIs among under-5 children in Ethiopia. However, information on the prevalence and distributions of IPs are incomplete and not updated periodically. On the other hand, there is no epidemiological information in many parts of the country on the prevalence of IPIs among more prone groups like children aged 6 to 59 months. Among those areas, Este wereda is the one which is located in the South Gondar zone, Northcentral Ethiopia. Therefore, this study is designed to assess the magnitude and associated risk factors of intestinal parasitosis among children aged 6 to 59 months attending Mekane Eyesus Primary Hospital.
Methods and Materials
Study Area
This study was conducted in Mekane Eyesus primary hospital. The hospital is found in Mekane Eyesus town which is the capital of Este wereda located in the South Gondar zone, Amhara regional state. It is located 114 km far from Bahir Dar (the capital city of Amhara Regional State) and 678 km far from Addis Ababa in the north direction. Mekane Eyesus has a latitude of 11°37′60″N and a longitude of 38°4′0″E with an elevation of 2616 m above sea level. The mean annual rainfall is 1301.29 mm and ranges from 759.8 to 1675.80 mm with the mean annual temperature ranges between 15.6°C and 18.7°C 19
According to the population projection of Ethiopia in 2017, an estimated total population of Mekane Eyesus town is 20 601 of whom 10 719 were males and 9882 were females. 20 The town has 1 primary hospital, 1 health center, and 6 private clinics. Mekane Eyesus primary hospital was established in 2015 with a total of 47 staff and currently, the hospital has a total of 168 staff.
Study Design, Period, and Study Subjects
An institutional-based cross-sectional study was carried out at Mekane Eyesus primary hospital from June 10 to November 30, 2020. All children who were attending a pediatric clinic at Mekane Eyesus primary hospital were considered as a source population while those children aged 6 to 59 months who visited the hospital with gastrointestinal symptoms and clinically suspected for IPIs during the data collection period were considered as a study population.
Sample Size Determination and Sampling Procedure
The required sample size was calculated using single population proportion formula; (n = [(Zα/2)2 × P (1-P)]/d2), where; n is the required sample size, Zα/2 is the level of confidence, P is the estimated prevalence of the IPI, d = tolerated margin of error. The following assumptions were used to determine sample size; 95% level of confidence, 5% margin of error, 25.4% prevalence of IPI among under-5 years’ children on the recent study in Ethiopia 21 and with a 10% non-response rate. Hence, a total of 322 study participants were included in this study. Study participants were selected using systematic random sampling and every second child was recruited to the study based on their order of arrival.
Inclusion and Exclusion Criteria
Children aged 6 to 59 months who attended a pediatric clinic with gastrointestinal symptoms and clinically suspected for IPIs during the study period, those who were willing to provide stool samples and whose parent/guardian can give consent were included in the study. However, children aged 6 to 59 months who were taking anti-helminths within the last 2 weeks and whose parents or guardians did not consent to participate in the study had to be excluded from the study.
Data Collection Procedure
Data were collected using a structured questionnaire through face-to-face interviews with the parents or guardians of the children. The questionnaire was developed after reviewing relevant literature. Socio-demographic characteristics of study participants and associated factors for IPIs were collected. During the interview, the cleanness, fingernail status, and shoe wearing habits of the child were collected through direct observations of the study participants.
Sample Collection and Laboratory Analysis
First clear orientation was given to each child’s parent or guardian on how to collect sufficient and appropriate specimens. After that, a clean, dry, leak-proof plastic container which is labeled with a unique ID number was given for each parent or guardian of the children to collect the sample. A 5 g of stool specimen was collected from each study participant and all specimens were examined by the direct wet mount method with in 30 minutes. The remaining sample was preserved with 10% formalin and processed by the formol-ether concentration method as per the standard operating procedures. All specimen processing and examination takes place at Mekane Eyesus primary hospital laboratory. Two medical laboratory technologists perform both wet mount and concentration techniques for the identification of IP through a light microscope. All standard procedures were strictly followed from specimen collection up to the reporting and recording of results.
Direct microscopic examination using normal saline and iodine preparation
About 1 to 2 mg of stool was emulsified in 1 to 2 drops of 0.85% normal saline or Lugol’s iodine solution. A cover-slip was then placed and the slide was scanned under 10× and 40× objective of a light microscope. Saline wet mount smear preparation was done to detect protozoal trophozoites and helminthic eggs or larvae. Iodine direct smear allows the examination of the characteristic features of the protozoa and the identification of the E. histolytica/dispar cyst from the commensal E. coli. In the iodine mount, the cytoplasm of the cyst will stain yellow or light brown and nuclei will stain dark brown.22,23
Formol–ether concentration technique
It is the most frequently used technique to concentrates a wide range of parasites with minimum damage to their morphology and it is important to increase the chance of detecting parasites. One gram of each sample was emulsified in 7 ml of 10% formalin solution into a centrifuge tube. Sieve the emulsified feces and collected them in to test tube. Add 3 to 4 ml of diethyl ether and mix for 1 minute. Then centrifuged immediately at 750 to 1000g (3000 rpm) for 1 minute. After centrifuging, the parasites were sedimented to the bottom of the tube. The centrifuged tube inverted to decant off the supernatant and allow a few drops of the deposit to remain. Then, after mixing the sediment was transferred to a slide and covered with a cover slip. Finally, the preparation was examined under 10× and 40× objective of a light microscope.22,23
Data Quality Control
Primarily, the data collection tool was prepared in the English language. Then, it was translated to the local (Amharic) language. Lastly, it was retranslated back to English to maintain its accuracy and consistency. It was pre-tested at a nearby hospital (Debre Tabor General Hospital) on 5% of calculated samples for the study to improve the quality of the data collection tool. After the pretest, the tool was modified for proper utilization. To ensure the quality of the investigation, appropriate training was given for data collectors. For each slide preparation, the 2 different laboratory technologists read the slides independently and their readings were compared. Discordant were immediately fixed by cross checking the slide by a senior medical parasitologist. Furthermore, all standard operating procedures were strictly followed during stool sample examination to ensure the quality and sensitivity of the test result.
Data Processing and Analysis
The data were checked for its completeness and coded manually before data entry. Coded data were entered using Epi-info version 7 statistical software and then exported to SPSS version 24 for analysis. Socio-demographic characteristics of the study participants and the overall magnitude of IPIs were analyzed using descriptive statistics of the sample. Based on the obtainable evidence, the least contributing factor to the dependent variable was used as a reference category. Associations between dependent and independent variables were analyzed by binary logistic regression. Firstly, bivariate analysis was done, and then variables with P-value <.25 in the binary regression were selected and analyzed by multivariable analysis to avoid the cofounding effect. Adjusted odds ratio (AOR) with 95% confidence interval (CI) was considered to ascertain the significance of the association between dependent and independent variables. In all cases, P-value <.05 was considered as a statistically significant association.
Results
Socio-demographic Characteristics
A total of 322 children within the age of 6 to 59 months who fulfilled the inclusion criteria’s and visited Mekane Eyesus primary hospital within the study period was included in this study. Among the total study participants, 56.2% were females while the remaining 43.8% were males. The mean age of the study participants was 28.86 months with a standard deviation of 12.516 months. The age distribution of children showed that the majority (37.6%) were in the age group of 24 to 36 months.
Children from parents or guardians with illiterate, Able to read and writing, primary school, and secondary school and above education level were accounted 81 (25.2%), 85 (26.4%), 50 (15.5%), and 106 (32.9%), respectively. A majority of the children were rural dwellers, 64.6% and more than half of children (57.1%) had families with 3 up to 5 members (Table 1).
Table 1.
Intestinal Parasite Infections in Relation to Socio-Demographic Characteristics of Children Aged 6 to 59 Months and Their Parents/Guardians in Mekane Eyesus Primary Hospital, Northcentral Ethiopia, 2020 (n = 322).
| Variables | Category | IPI | χ2-test | P-value | |
|---|---|---|---|---|---|
| Number of positive (%) | Total number (%) | ||||
| Sex | Male | 25 (17.7) | 141 (43.8) | 0.014 | .908 |
| Female | 33 (18.2) | 181 (56.2) | |||
| Family residence | Urban | 11 (9.6) | 114 (35.4) | 8.359 | .004* |
| Rural | 47 (22.6) | 208 (64.6) | |||
| Age in months | <24 | 17 (15.2) | 112 (34.8) | 0.985 | .611 |
| 24-36 | 23 (19.0) | 121 (37.6) | |||
| >36 | 18 (20.2) | 89 (27.6) | |||
| Mother’s (guardian’s) educational status | Illiterate | 18 (22.2) | 81 (25.2) | 3.705 | .295 |
| Able to read and write | 17 (20.0) | 85 (26.4) | |||
| Primary school | 10 (20.0) | 50 (15.5) | |||
| Secondary school and above | 13 (12.3) | 106 (32.9) | |||
| Mother’s (guardian’s) occupational status | Housewife | 17 (18.3) | 93 (28.9) | 7.885 | .019* |
| Farmer | 26 (26.0) | 100 (31.0) | |||
| Other a | 15 (11.6) | 129 (40.1) | |||
| Religion of mother’s (guardian’s) | Orthodox | 50 (18.2) | 275 (85.4) | 0.037 | .848 |
| Muslim | 8 (17.0) | 47 (14.6) | |||
| Family size | ≤3 | 7 (12.1) | 58 (18.0) | 6.895 | .032* |
| 3-5 | 29 (15.8) | 184 (57.1) | |||
| >5 | 22 (27.5) | 80 (24.9) | |||
Government employee and merchant.
P < .05.
Bold: statistically significant association.
Prevalence of Intestinal Parasitic Infections
The overall prevalence of IPIs among children aged 6 to 59 months was 18.0% (95% CI: 14.0%-22.0%) which is detected in 58 suspected children. The prevalence of IPIs was significantly highest among children who were rural dwellers (22.6%), whose mothers (guardians) are farmers in occupation (26.0%) and had a family size of greater than 5 (27.5%). There was no significant difference of IPI with regard to sex (P = .908), Age group (P = .611), educational background of the parent (P = .295) and religion of the mothers (guardians) (P = .848) (Table 1).
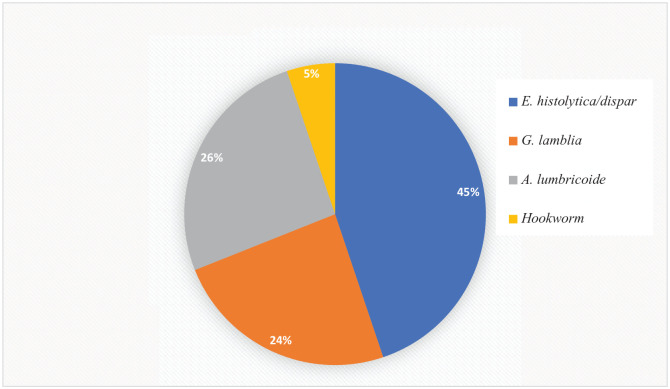
Among the observed parasites, protozoa parasites, 40 (12.4% [95% CI: 9.1%-16.1%]), were the most dominant parasitic infections among the children. Only E. histolytica and G. lamblia were the pathogenic protozoan parasites found with a prevalence of 26 (8.1% [95% CI: 5.3%-11.2%]) and 14 (4.3% [95% CI: 2.2%-6.8%]), respectively. On the other hand, 18 (5.6% [95% CI: 3.4%-8.4%]) children were infected with helminth parasites. All of the infected children had a single infection. The distribution of identified parasites among children was described in Figure 1.
Figure 1.
Types of intestinal parasites among children aged 6 to 59 months attending at Mekane Eyesus primary hospital, North central Ethiopia, 2020 (n = 58).
Analysis of Associated Factors for Intestinal Parasitic Infections
Univariate analysis showed that child who has farmer mothers or guardians, rural resident and family size of greater than 5 are among socio-demographic characteristics which were significantly associated with an increased risk for an intestinal parasitic infection (P-value <.05). Similarly, from the potential associated risk factors; regular trimming fingernails, the habit of geophage, and latrine availability at home were found to be statistically associated with IPI in univariate analysis (see Table 2).
Table 2.
Bivariate and Multivariate Binary Logistic Regression Analysis of Factors Associated With IPIs Among Children Aged 6 to 59 Months Attending at Mekane Eyesus Primary Hospital, Northcentral Ethiopia, 2020 (n = 322).
| Variables | Infection status | COR (95% CI) | AOR (95% CI) | P-value | |
|---|---|---|---|---|---|
| Number positive (%) | Number negative (%) | ||||
| Sex | |||||
| Male | 25 (17.7) | 116 (82.3) | 1 | ||
| Female | 33 (18.2) | 148 (81.8) | 1.03 (0.58-1.83) | ||
| Family residence | |||||
| Urban | 11 (9.6) | 103 (90.4) | 1 | 1 | |
| Rural | 47 (22.6) | 161 (77.4) | 2.73 (1.36-5.52) | 1.86 (0.55-6.33) | .319 |
| Age in months | |||||
| <24 | 17 (15.2) | 95 (84.8) | 1 | ||
| 24-36 | 23 (19.0) | 98 (81) | 0.76 (0.38-1.52) | ||
| >36 | 18 (20.2) | 71 (79.8) | 0.71 (0.34-1.47) | ||
| Mother’s (guardian’s) educational status | |||||
| Illiterate | 18 (22.2) | 63 (77.8) | 2.04 (0.94-4.46) | 1.37 (0.43-4.39) | .597 |
| Able to read and write | 17 (20.0) | 68 (80.0) | 1.79 (0.81-3.92) | 1.68 (0.49-5.70) | .409 |
| Primary school | 10 (20.0) | 40 (80.0) | 1.79 (0.72-4.42) | 1.08 (0.29-3.93) | .911 |
| Secondary school and above | 13 (12.3) | 93 (87.7) | 1 | 1 | |
| Mother’s (guardian’s) occupational status | |||||
| Housewife | 17 (18.3) | 76 (81.7) | 1.70 (0.80-3.61) | 1.82 (0.76-4.39) | .181 |
| Farmer | 26 | 74 | 2.67 (1.33-5.43) | 2.60 (1.08-6.25) | .033 |
| Other a | 15 (11.6) | 114 (88.4) | 1 | 1 | |
| Religion of mother’s (guardian’s) | |||||
| Orthodox | 50 (18.2) | 225 (81.8) | 1.08 (0.48-2.46) | ||
| Muslim | 8 (17.0) | 39 (83) | 1 | ||
| Family size | |||||
| ≤3 | 7 (12.1) | 51 (87.9) | 1 | 1 | |
| 3-5 | 29 (15.8) | 155 (84.2) | 1.36 (0.56-3.30) | 1.04 (0.39-2.80) | .939 |
| >5 | 22 (27.5) | 58 (72.5) | 2.76 (1.09-6.99) | 2.88 (0.91-7.30) | .076 |
| Hand wash after toilet before touching your child | |||||
| Always | 25 (15.4) | 137 (84.6) | 1 | 1 | |
| Sometimes | 33 (20.6) | 127 (79.4) | 1.42 (0.80-2.53) | 1.33 (0.65-2.71) | .435 |
| Habit of eat unwashed fruits and vegetables | |||||
| Always | 6 (26.1) | 17 (73.9) | 1.87 (0.67-5.26) | 3.80 (1.14-12.82) | .030 |
| Sometimes | 29 (18.8) | 125 (81.2) | 1.23 (0.67-2.25) | 1.68 (0.78-3.61) | .183 |
| Nor at all | 23 (15.9) | 122 (84.1) | 1 | 1 | |
| Child meal | |||||
| Always fresh | 16 (15.5) | 87 (84.5) | 1 | ||
| Sometimes fresh | 35 (18.7) | 152 (81.3) | 1.25 (0.66-2.39) | ||
| Not fresh | 7 (21.9) | 25 (78.1) | 1.52 (0.56-4.12) | ||
| Trimming child’s nails when grown | |||||
| Regularly | 16 (10.2) | 141 (89.8) | 1 | 1 | |
| Irregularly | 42 (25.5) | 123 (74.5) | 3.01 (1.61-5.62) | 3.14 (1.59-6.21) | .001 |
| Child playing ground | |||||
| Clean | 37 (16.8) | 183 (83.2) | 1 | ||
| Not clean | 21 (20.6) | 81 (79.4) | 1.28 (0.71-2.33) | ||
| Habit of geophage | |||||
| Yes | 19 (27.1) | 51 (72.9) | 2.04 (1.09-3.83) | 2.71 (1.31-5.59) | .007 |
| No | 39 (15.5) | 213 (84.5) | 1 | 1 | |
| Source of drinking water | |||||
| Tap water | 34 (16.3) | 174 (83.7) | 1 | ||
| Well water | 18 (20.7) | 69 (79.3) | 1.34 (0.71-2.52) | ||
| Stream/river water | 6 (22.2) | 21 (77.8) | 1.46 (0.55-3.89) | ||
| Latrine availability at home | |||||
| Yes | 28 (13.3) | 183 (86.7) | 1 | 1 | |
| No | 30 (27.0) | 81 (73.0) | 2.42 (1.36-4.31) | 1.85 (0.93-3.66) | .079 |
| Shoes wearing habit | |||||
| Yes | 33 (17.3) | 158 (83.7) | 1 | ||
| No | 25 (19.1) | 106 (81.9) | 1.13 (0.64-2.01) | ||
Abbreviations: AOR, adjusted odds ratio; COR, crude odds ratio; CI, confidence interval.
Government employee and merchant.
Multivariable logistic regression was conducted after adjusting variables which were P-value <.25 in the univariate analysis. The multivariable logistic regression model estimated that children who had a farmer family were 2.6 times (AOR = 2.60, 95% CI: 1.08-6.25; P = .033) more likely to be infected as compared with a child whose family occupation was merchant and government employee. Those children who had always eat unwashed fruits or vegetables were 3.8 (AOR = 3.80, 95% CI: 1.14-12.82; P = .030) more likely to be infected with IPI than children who hadn’t the habit of eating unwashed fruits or vegetables. The odds of intestinal parasitic infection were 3 times higher (AOR = 3.14, 95% CI: 1.59-6.21; P = .001) for under-5 children who had irregular trimming their fingernail as compared to those who did. Besides, participants with a habit of geophage were 2.71 times (AOR = 2.71, 95% CI: 1.31-5.59; P = .007) more likely to become infected as compared to non-geophage (Table 2).
Discussion
In this study, the prevalence and associated risk factors of intestinal parasitic infections among children aged 6 to 59 months were determined in Mekane Eyesus Primary Hospital, Northcentral Ethiopia. Locally appropriate epidemiological data on the prevalence of IPIs and identification of possible associated risk factors is essential to make an evidence-based decisions for design appropriate intervention strategies. We report an overall prevalence of IPIs was 18% (95% CI: 14.0%-22.0%) and have identified that child whose family was farmer, the habit of eating unwashed fruits and vegetables, infrequently trim the fingernails and habit of geophage are a potentially associated factor for IPIs. Improvements in these areas could address both short and long-term consequences of these conditions in this vulnerable population.
The prevalence of IPI in the present study corroborated the findings of different studies that have been conducted in Woreta (18.7%), 17 Dessie (15.5%), 16 Debre Birhan (17.4%), 24 Gondar (17.3%), 25 Ghana (17.3%), 26 and Saudi Arabia (17.7%). 27
However, the current study showed a lower prevalence of IPI than the previous studies conducted in various areas of Ethiopia such as the findings from Dembia (25.4% and 25.8%),21,28 Chwahit (35.2%), 29 Sekota (29.9%), 1 Wonji Shoa Sugar Estate (24.3%), 30 Butajira (27.6%), 31 and Hawassa (26.6%). 32 The possible explanation for the low prevalence of IPI in our study might be due to differences in the study period, study population, level of environmental sanitation, and geographic locations of the study participants.
A much higher prevalence of IPIs was reported in different studies that have been conducted in Wondo Genet (85.1%), 18 Goncha Siso Enese District (65.1%), 33 Tigray Region (58%), 9 North Shoa (52.3%), 34 and Hawassa Zuria District (51.3%). 35 This might be due to differences in study area, environmental and socio-economic conditions of the study population. Most of the above studies are conducted in highly endemic areas for IPs. It is due to either the warmer temperature that increases the rates of parasite development, facilitates higher rates of infectivity and heavier burdens of disease 36 or occurrence of water bodies like river and lake around the study area also increases the chance of waterborne IPs infestation. The other possible reason for the variation might be due to diagnostic laboratory method differences. All studies with a high prevalence of IPIs have used more sensitive diagnostic techniques like the Kato-katz technique. The technique was highly sensitive as compared with wet mount and formol-ether concentration in the diagnosis of IPs was recognized. 37
Furthermore, the prevalence IPIs in this study was lower than the studies conducted out of Ethiopia, such as the studies conducted in Kenya (25.6%), 38 Thailand (22.7%), 39 North Maharashtra, India (37.66%), 40 Karachi, India (68.8%), 41 Pakistan (52.8%), 42 Bogota (26.4%), 43 Cuba (71.1%), 44 and Brazil (27.5%). 45 This difference in this study might be due to the difference in practices of personal hygiene, level of environmental sanitation, drinking water source, season, cultural difference, educational, and socio-economic status of the study participant’s parent as well as geographical distribution of the parasites. On the other hand, some of the above studies include only children with diarrhea, collection of 3 different samples from each individual and using more sensitive laboratory method may result high prevalence of IPIs as compared with this study.
Intestinal protozoa parasitic infections are the most common cause of diseases in children. Their transmission occurs by ingestion of cysts through the fecal-oral route, either via person to person contact or via contamination of surface water or food. 24 In the present study, the protozoan parasite accounts for 69% (40/58) of the total IPIs. Protozoan parasites were also the predominant parasites detected in studies conducted in Wereta, 17 Debre Birhan, 24 Tigray Region (58%), 9 Hawassa, 32 Saudi Arabia 17.7%, 27 North Maharashtra, India, 40 and Brazil. 45 But, in some other studies like the finding from Dembia,21,28 Chwahit (35.2%), 29 Butajira (27.6%), 31 Wondo Genet, 18 and Ghana 17.3% 26 the most encountered parasite was helminths.
The most predominant parasite identified in this study is E. histolytica/dispar 26 (8.1%) with a 95% CI of (5.3%-11.2%) followed by A. lumbricoides 15 (26%) with 95% CI of (2.5%-7.1%). This is agreed with some studies conducted in our country and different parts of the world that indicated the major parasite among children was E. histolytica/dispar.1,9,16,32,38,45 However, in some other studies the most encountered parasite was A. lumbricoides.21,28,29,31,33
In this study, none of the IP infected children had double parasitic infections whereas studies conducted in different parts of Ethiopia reported the occurrence of double parasitic infections on children.16,24,25,32,34 Variations in the level of environmental contamination, socioeconomic factors and endemicity of parasites might be responsible for the difference in the prevalence of multiple parasitic infections. The above studies with multiple parasitic infections used either Kato-katz 34 or modified acid-fast staining16,32 techniques as additional laboratory methods that may be important for detecting double infection.
Children born and raised from farmer family increases the odds of intestinal parasitic infestation by 2.6 times (AOR = 2.60, 95% CI: 1.08-6.25) than children of merchant and government employee. Most farmers live in rural areas of the country. This area is characterized as poverty, shortages of clean potable water, a high rate of open defecation, poor environmental sanitation and personal hygiene. Additionally, most farmers use excreta as compost which is an absolute source of infection since children and their mothers often go to the farm may attribute to a high rate of infection. 3
Childs who have a habit of eating unwashed fruits or vegetables were 3.8 times more likely to be infected with IPI than children who haven’t the habit of eating unwashed fruits or vegetables (AOR = 3.80, 95% CI:1.14-12.82). This was in line with other studies conducted in Cuba (RR = 8.40; 95% CI [3.69-19.12]). 44 The possible explanation may be due to the fact that contamination of fruits and vegetables through open defecation, and using night soil as fertilizer. Proper washing of fruits and vegetables before eating is very important to remove the infective stages of different parasites like cysts of protozoan species and eggs of worms as a prevention and control mechanism. 46
This institutional-based cross-sectional survey de-scribed that childhood parasitic infection was statistically associated with irregular trimming of fingernails (AOR = 3.14, 95% CI: 1.59-6.21) which is congruent with the findings of other studies.17,25,31 This is due to the playing habits of children with soil, poor socioeconomic status and poor hygienic practice results accumulated dirt containing eggs of parasites under fingernails.17,26 It results in significant source and transmission of IPI.
This study demonstrated that there was a significant association between the habit of geophage and the rate of IP infestations. Children who have a habit of geophage were 2.71 times more likely to be infected with IPs (AOR = 2.71, 95% CI: 1.31-5.59) compared with children who do not have a habit of geophage. It is in agreement with other study conducted in India showed that eating mud were found to be the major cause of parasitic infections. 41 This finding might be due to the high rate of open defecation and poor environmental sanitation results contamination of soil and important source of infection.
Limitations
Due to budget shortage, this study did not focus on molecular assays and other techniques which are best to estimate the prevalence of IPs and differentiating different species of ameabiasis. Also, a modified acid-fast staining technique was not used to detect Cryptosporidium species, and it might cause to miss this species.
Conclusions
In conclusion, the overall prevalence of IPI among children aged 6 to 59 months was 18.0% and all study participants were affected by single IPs. Protozoa parasites are the most common cause of diseases in children. The dominant parasite species was E. histolytica/dispar followed by A. limbricoide. Child habit of eating unwashed fruits or vegetables, family occupation, regular trimming fingernails and habit of geophage were significantly associated with IPIs. Therefore, strengthening health education about the importance of washing fruits or vegetables before eating and regular trimming of fingernails is very crucial. Furthermore, protecting the children from eating soil and exposing for the farming environment is another way of preventing the occurrence of infection. As well, improving mothers/guardians awareness about the source of infection and mode of IP transmission is necessary.
Acknowledgments
The authors would like to express their sincere gratitude to all laboratory staff and the management of Mekane Eyesus primary hospital for the cooperation to carry out this study. The authors also like to thank data collectors and supervisors for their commitment. We also extend our warm gratitude to study participant children, parents and guardians for their valuable information.
Footnotes
List of abbreviations: AOR: Adjusted Odds Ratio, CI: Confidence Interval, COR: Crude Odds Ratio, IP: Intestinal Parasite, IPI: Intestinal Parasitic Infection, SSA: Sub-Saharan African, STH: Soil Transmitted Helminth, WHO: World Health Organization.
Authors’ Contributions: TE was the primary researcher who involved in conceptualization, data curation, formal analysis, methodology, laboratory investigation, results interpretation, involved in execution, acquisition of data, data statistical analysis, drafting the manuscript, and engaged in critically revising of the manuscript. AW participated in overall data collection including questioner and stool specimen, laboratory investigation, execution, acquisition of data, participated in statistical analysis, result interpretations, have participated in drafting and substantially revising the manuscript. TK, TT, SD, MS, AA, ESC, and LW were involved in execution, acquisition of data, data statistical analysis, results interpretation, drafting the manuscript, engaged in critically revising of the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Consideration: The study was approved by the Debre Tabor University, College of Health Sciences, Research and Ethical Review Committee (reference number: CHS/224/2012 in Ethiopian calendar). A letter of support was secured from a woreda health office and a permission letter was obtained from the medical director of the hospital. Written informed consent was obtained from the parents or guardians of the children. Those children who were positive for IPs were linked to the responsible body in Mekane Eyesus primary hospital for treatment. All results were kept confidential and used only for research purposes. This study was conducted in accordance with the declaration of Helsinki.
ORCID iD: Tahir Eyayu  https://orcid.org/0000-0002-2041-7183
https://orcid.org/0000-0002-2041-7183
Availability of Data and Materials: The authors confirmed that all the data used in this research article are presented within the manuscript.
References
- 1. Kassaw MW, Abebe AM, Tlaye KG, Zemariam AB, Abate BB. Prevalence and risk factors of intestinal parasitic infestations among preschool children in Sekota Town, Waghimra Zone, Ethiopia. BMC Pediatr. 2019;19(1):437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Working to Overcome the Global Impact of Neglected Tropical Diseases: First WHO Report on Neglected Tropical Diseases. World Health Organization; 2010. [Google Scholar]
- 3. Tyoalumun K, Abubakar S, Christopher N. Prevalence of intestinal parasitic infections and their association with nutritional status of rural and urban pre-school children in Benue State, Nigeria. Int J MCH AIDS. 2016;5(2):146. [PMC free article] [PubMed] [Google Scholar]
- 4. Ostan I, Kilimcioğlu AA, Girginkardeşler N, Ozyurt BC, Limoncu ME, Ok UZ. Health inequities: lower socio-economic conditions and higher incidences of intestinal parasites. BMC Public Health. 2007;7(1):342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harhay MO, Horton J, Olliaro PL. Epidemiology and control of human gastrointestinal parasites in children. Expert Rev Anti-Infect Ther. 2010;8(2):219-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramana K. Intestinal parasitic infections: an overview. Ann Trop Med Public Health. 2012;5(4):279. [Google Scholar]
- 7. World Health Organization. Soil-Transmitted Helminth Infections: Fact Sheet. World Health Organization; 2020. Accessed March 11, 2021. https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections [Google Scholar]
- 8. de Silva NR. Impact of mass chemotherapy on the morbidity due to soil-transmitted nematodes. Acta Trop. 2003;86(2-3):197-214. [DOI] [PubMed] [Google Scholar]
- 9. Wasihun AG, Teferi M, Negash L, et al. Intestinal parasitosis, anaemia and risk factors among pre-school children in Tigray region, Northern Ethiopia. BMC Infect Dis. 2020;20:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014;7(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farthing M, Salam MA, Lindberg G, et al. Acute diarrhea in adults and children: a global perspective. J Clin Gastroenterol. 2013;47(1):12-20. [DOI] [PubMed] [Google Scholar]
- 12. Sperber AD, Drossman DA, Quigley EMM. The global perspective on irritable bowel syndrome: a Rome Foundation–World Gastroenterology Organisation symposium. Am J Gastroenterol. 2012;107(11):1602-1609. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization. Soil-transmitted helminthiasis: number of children treated 2007-2008: update on the 2010 global target: background. Wkly Epidemiol Rec. 2010;85(16):141-147. [PubMed] [Google Scholar]
- 14. Health FDRoEMo. Second Edition of National Neglected Tropical Diseases Master Plan (2015/16-2019/20). Health FDRoEMo; 2016. [Google Scholar]
- 15. Chelkeba L, Mekonnen Z, Alemu Y, Emana D. Epidemiology of intestinal parasitic infections in preschool and school-aged Ethiopian children: a systematic review and meta-analysis. BMC Public Health. 2020;20(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gebretsadik D, Metaferia Y, Seid A, Fenta GM, Gedefie A. Prevalence of intestinal parasitic infection among children under 5 years of age at Dessie Referral Hospital: cross sectional study. BMC Res Notes. 2018;11:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mekonnen HS, Ekubagewargies DT. Prevalence and factors associated with intestinal parasites among under-five children attending Woreta Health Center, Northwest Ethiopia. BMC Infect Dis. 2019;19:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nyantekyi L, Legesse M, Belay M, et al. Intestinal parasitic infections among under-five children and maternal awareness about the infections in Shesha Kekele, Wondo Genet, Southern Ethiopia. Ethiop J Health Dev. 2011;24(3):185-190. [Google Scholar]
- 19. Getachew B. Trend analysis of temperature and rainfall in South Gonder zone, Anhara Ethiopia. J Degraded Min Lands Manag. 2018;5(2):1111. [Google Scholar]
- 20. Ababa A. Federal Democratic Republic of Ethiopia Central Statistical Agency Population Projection of Ethiopia for All Regions at Wereda Level From 2014-2017. Central Statistical Agency; 2014. [Google Scholar]
- 21. Gizaw Z, Addisu A, Gebrehiwot M. Socioeconomic predictors of intestinal parasitic infections among under-five children in rural Dembiya, Northwest Ethiopia: a community-based cross-sectional study. Environ Health Insights. 2019;13:1178630219896804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheesbrough M. District Laboratory Practice in Tropical Countries, Part I. Cambridge University Press; 2009. [Google Scholar]
- 23. World Health Organization. Basic Laboratory Methods in Medical Parasitology. World Health Organization; 1991. [Google Scholar]
- 24. Zemene T, Shiferaw MB. Prevalence of intestinal parasitic infections in children under the age of 5 years attending the Debre Birhan referral hospital, North Shoa, Ethiopia. BMC Res Notes. 2018;11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aleka Y, Tamir W, Birhane M, Alemu A. Prevalence and associated risk factors of intestinal parasitic infection among under five children in University of Gondar Hospital, Gondar, Northwest Ethiopia. Biomed Res Ther. 2015;2(8):347-353. [Google Scholar]
- 26. Mirisho R, Neizer ML, Sarfo B. Prevalence of intestinal helminths infestation in children attending Princess Marie Louise Children’s Hospital in Accra, Ghana. J Parasitol Res. 2017;2017:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. AL-Megrin WAI. Assessment the prevalence of intestinal parasites and associated risk factors among preschool children in Riyadh, Saudi Arabia. Res J Parasitol. 2015;10(1):31-41. [Google Scholar]
- 28. Gizaw Z, Adane T, Azanaw J, Addisu A, Haile D. Childhood intestinal parasitic infection and sanitation predictors in rural Dembiya, Northwest Ethiopia. Environ Health Prev Med. 2018;23:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alemu A, Tegegne Y, Damte D, Melku M. Schistosoma mansoni and soil-transmitted helminths among preschool-aged children in Chuahit, Dembia district, Northwest Ethiopia: prevalence, intensity of infection and associated risk factors. BMC Public Health. 2016;16:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Degarege A, Erko B. Prevalence of intestinal parasitic infections among children under five years of age with emphasis on Schistosoma mansoni in Wonji Shoa Sugar Estate, Ethiopia. PLoS One. 2014;9(10):e109793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shumbej T, Belay T, Mekonnen Z, Tefera T, Zemene E. Soil-transmitted helminths and associated factors among pre-school children in Butajira Town, South-Central Ethiopia: a community-based cross-sectional study. PLoS One. 2015;10(8):e0136342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mulatu G, Zeynudin A, Zemene E, Debalke S, Beyene G. Intestinal parasitic infections among children under five years of age presenting with diarrhoeal diseases to two public health facilities in Hawassa, South Ethiopia. Infect Dis Poverty. 2015;4:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aiemjoy K, Gebresillasie S, Stoller NE, et al. Epidemiology of soil-transmitted helminth and intestinal protozoan infections in preschool-aged children in the Amhara region of Ethiopia. Am J Trop Med Hyg. 2017;96(4):866-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lewetegn M, Getachew M, Kebede T, Tadesse G, Asfaw T. Prevalence of intestinal parasites among preschool children and maternal KAP on prevention and control in Senbete and Bete Towns, North Shoa, Ethiopia. Int J Biomed Mater Res. 2019;7:1-7. [Google Scholar]
- 35. Alemneh K, Sintayehu A, Dejene H, Getenesh B. Intestinal parasitic infections and nutritional status of pre-school children in Hawassa Zuria District, South Ethiopia. Afr J Microbiol Res. 2017;11(31):1243-1251. [Google Scholar]
- 36. Blum AJ, Hotez PJ. Global “Worming”: Climate Change and Its Projected General Impact on Human Helminth Infections. Public Library of Science; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yimer M, Hailu T, Mulu W, Abera B. Evaluation performance of diagnostic methods of intestinal parasitosis in school age children in Ethiopia. BMC Res Notes. 2015;8:820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mbae CK, Nokes DJ, Mulinge E, Nyambura J, Waruru A, Kariuki S. Intestinal parasitic infections in children presenting with diarrhoea in outpatient and inpatient settings in an informal settlement of Nairobi, Kenya. BMC Infect Dis. 2013;13:1471-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wongstitwilairoong B, Srijan A, Serichantalergs O, et al. Intestinal parasitic infections among pre-school children in Sangkhlaburi, Thailand. Am J Trop Med Hyg. 2007;76(2):345-350. [PubMed] [Google Scholar]
- 40. Mane M, Kadu A, Mumbre S, Deshpande M, Gangurde N. Prevalence of intestinal parasitic infections and associated risk factors among pre-school children in tribal villages of North Maharashtra, India. Int J Res Health Sci. 2014;2(1):133-139. [Google Scholar]
- 41. Mumtaz S, Siddiqui H, Ashfaq T. Frequency and risk factors for intestinal parasitic infection in children under five years age at a tertiary care hospital in Karachi. High Educ. 2009;87:32. [PubMed] [Google Scholar]
- 42. Mehraj V, Hatcher J, Akhtar S, Rafique G, Beg MA. Prevalence and factors associated with intestinal parasitic infection among children in an urban slum of Karachi. PLoS One. 2008;3(11):e3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. C H Bouwmans M, Gaona MA, Chenault MN, Zuluaga C, Pinzon Rondon AM. Prevalence of intestinal parasitic infections in preschool-children from vulnerable neighborhoods in Bogotá. Rev Univ Ind Santander Salud. 2016;48(2):178-187. [Google Scholar]
- 44. Cañete R, Díaz MM, Avalos García R, Laúd Martinez PM, Manuel Ponce F. Intestinal parasites in children from a day care centre in Matanzas City, Cuba. PLoS One. 2012;7(12):e51394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nobre LN, Silva RV, Macedo MS, Teixeira RA, Lamounier JA, Franceschini SC. Risk factors for intestinal parasitic infections in preschoolers in a low socio-economic area, Diamantina, Brazil. Pathog Glob Health. 2013;107(2):103-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koutsoumanis K, Allende A, Alvarez-Ordóñez A, et al. Public health risks associated with food-borne parasites. EFSA J. 2018;16(12):e05495. [DOI] [PMC free article] [PubMed] [Google Scholar]