Abstract
Introduction:
The US Preventative Services Task Force recommends shared decision-making (SDM) between women aged 40 and 49 years and their physician regarding timing of mammography screening. This preliminary study evaluates women’s and physician’s satisfaction using Breast Cancer Risk Estimator & Decision Aid (BCARE-DA), a shared decision aid utilized during the clinical encounter, and examines SDM quality for these encounters.
Methods:
Fifty-three women and their physician utilized BCARE-DA and completed surveys measuring satisfaction with Likert-type and open-ended items and women completed the Decision Conflict Scale. Clinic visit transcripts were evaluated for SDM quality using Observer OPTION-5 and Breast Cancer Screening Decision Core Components Checklist.
Results:
Women and physicians positively evaluated BCARE-DA. Women had low decision conflict. Physicians demonstrated moderate effort toward SDM, greatest in offering options, and lowest for team talk. Physicians demonstrated 2/3 of core SDM elements in 80% to 100% of encounters.
Conclusion:
Preliminary findings suggest specific promise for such Decision Aids to facilitate SDM through understanding of personal risks for breast cancer formulated within each screening option, while some SDM elements likely require additional facilitating.
Keywords: shared decision-making, breast cancer screening, mammography, decision aid, patient–physician communication, primary care, womens health
Introduction
Despite the important role of screening mammography in reducing breast cancer burden and mortality (1), in the United States, mammography guidelines for non-high-risk women aged 40 to 49 years remain controversial and confusing. The American Cancer Society recommends women aged 40 to 44 years receive annual mammograms “if they wish to do so” (2), while women aged 45 to 54 years should be screened annually (2). The US Preventive Services Task Force recommends women aged 40 to 49 years engage in a shared decision with their physician about mammography initiation and frequency (3,4), without evidence-base tools guiding this shared decision-making (SDM) (5 –7). Shared decision-making is “a process in which patients are involved as active partners with the physician in clarifying acceptable medical options and in choosing a preferred course of clinical care” (8, p. 56). Shared decision-making models highlight the importance of discussing alternatives including benefits, risks, and uncertainties (9 –11). However, in a national US survey of patients aged 40+ years who received breast, prostate, or colorectal cancer screening, those who received breast cancer screening were least likely to report their physician discussed screening benefits or risks (12,13), often did not perceive they had a choice (12,14) and demonstrated low decision processing (15).
Decision Aids (DAs) have established a link to increased SDM (16,17) with growing emphasis on mammography (18 –20). Decision Aids are designed to support complex health decisions by supplementing the patient–provider interaction and promoting SDM. Yet, physicians feel ill-equipped in their understanding of breast cancer risk factors and mammography guidelines, with limited time to address this in the visit (21). For true SDM, DAs need to accurately estimate individual risk for breast cancer, clarify patient-centered recommendations when guidelines conflict, and facilitate a time efficient conversation between physicians and patients (21,22).
Our team collaborated with HealthDecision to develop and implement the Breast Cancer Risk Estimator & Decision Aid (BCARE-DA). The BCARE-DA is an interactive, web accessible, or electronic medical record (EMR)-embedded SDM tool uniquely intended for collaborative use by physicians and patients during the clinic encounter. Using the Breast Cancer Surveillance Consortium Risk Calculator, it determines individual baseline risk of breast cancer incidence and mortality in the next 10 years and provides graphical displays to illustrate overdiagnosis, false alarms, and mortality reduction (see details and access BCARE-DA at www.healthdecision.org/tool#/tool/mammo).
This preliminary investigation evaluates mammography SDM quality for women aged 40 to 49 years and their physicians who utilized BCARE-DA during the clinical encounter and their satisfaction.
Materials and Methods
Approval was granted by the University of Wisconsin Health Sciences Institutional Review Board.
Participants
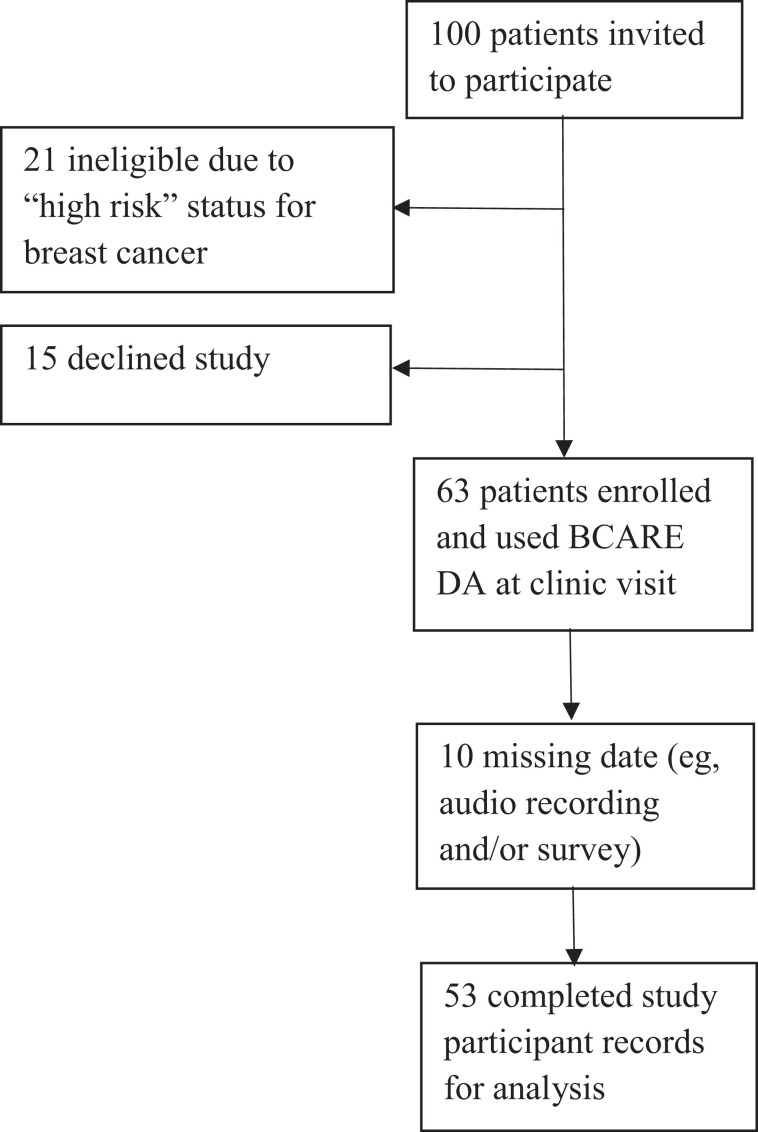
Participants were recruited from May 2017 through May 2018. Eleven primary care physicians within a Midwestern US academic health care system consented to participate in the study. A convenience sample of 100 patients (non-high-risk, English speaking, women aged 40-49 years) scheduled for an appointment with an enrolled physician in which discussion of mammography screening would be appropriate were invited via a letter to participate. Following subsequent phone screening, 21 patients were ineligible because of high risk for breast cancer (new breast symptoms, history of breast cancer, prior chest radiation, known genetic markers); 15 patients declined participation due to lack of interest or concerns with audio recording. Sixty-three patients enrolled in the study and provided informed consent. Due to missing data, 53 complete patient records are utilized (Figure 1). Patients were primarily Caucasian (94.4%), college-educated (72.2%), with annual household income greater than US$100 000 (52.8%). Physicians (10 female, 1 male) had 9 to 36 years in practice. Training on BCARE-DA was not provided as this initial evaluation of BCARE-DA was to inform DA modifications, future training, and clinical implementation.
Figure 1.
CONSORT diagram of study recruitment and retention.
Data Collection
When an enrolled patient arrived for her scheduled physician appointment, a digital audio recorder was placed in the examination room for capturing discussion about screening mammography. Physicians started recording when conversation about screening mammography initiated. Physicians accessed BCARE-DA via EMR. Audio recordings were transcribed by a professional service. Patient surveys were mailed within a week of their visit, with a reminder postcard sent 4 days later; if no response was received within 3 weeks, a duplicate survey was mailed. Physicians completed a single web-based survey after all study clinical encounters had been conducted.
Measures
Transcription evaluation for SDM
Observer OPTION-5
The Observer OPTION-5 (23) assesses physician’s effort to involve the patient in SDM (0 = no effort to 4 = exemplary effort) with 5 items: (1) identifying need for a decision exists, (2) describing options, (3) information exchange, (4) preference elicitation, and (5) preference integration. OPTION-5 has good validity and reliability (24,25). Two coders (LD and VO) independently coded each transcription. Threshold for acceptable inter-rater variance was set as follows: per patient, variances could not exist for more than 3 of the 5 items, and any given item variance could not exceed 1 point. Items exceeding this threshold were deliberated and agreement achieved between coders. Item means between raters were used for analysis.
Breast Cancer Screening Decision Core Components Checklist
The Breast Cancer Screening Decision (BCSD) Core Components Checklist is derived from our previous work (26,27) and identifies 24 core components of breast cancer screening SDM that can be objectively observed from transcripts. A single coder (LD) evaluated transcripts to determine whether (“1”) or not (“0”) each component occurred within the clinical encounter. Scale score is the sum of the 24 items (0-24). The Checklist offers observations of specific elements of the interaction, whereas the OPTION-5 offers observations of 5 general SDM concepts.
Physician survey
Physicians responded to newly developed numerical scales and open-ended questions assessing their satisfaction with BCARE-DA. Survey provided in Supplemental Appendix A.
Patient Survey
Patient surveys included the Decisional Conflict Scale (28) and Likert-type and open-ended items assessing satisfaction.
Decisional Conflict Scale
This validated instrument (28) measures patient’s perceptions of (1) uncertainty in choosing options, (2) modifiable factors contributing to uncertainty, and (3) effective decision-making. Items are scored on a 0 (“yes”) to 4 (“no”) scale. Scale and subscale scores are calculated as item means multiplied by 25 (0-100; greater values indicate greater decisional conflict). This is a 16-item scale, however we inadvertently eliminated 1 item “Do you know the risks of each option,” which contributes to the Informed subscale.
Patient satisfaction items
The survey included the Likert-type item “How valuable was the experience of using the B-CARE DA for you?” scored 1 (“not at all”) to 5 (“extremely”). Open-ended survey items are “What did you like about the BCARE-DA?” and “What didn’t you like about the BCARE-DA?”
Discussion time
Discussion time is the length of the audio recording, rounded to nearest minute. Audio recording started when either the physician or patient initiated talk about mammography screening and ended when the screening discussion concluded.
Analysis
Mixed methods analyses are employed. Quantitative analyses include descriptive statistics for scaled measures (OPTION-5, Components Checklist, Decisional Conflict Scale), Likert-type items, and discussion time. Correlations test relationships between Decision Conflict and discussion time with both OPTION-5 and Components Checklist. Analysis of variance tests physician differences for OPTION-5 and discussion time. Qualitative analyses examine open-ended satisfaction survey items. Conventional exploratory content analysis coded for themes to allow for new and important material to be elucidated. Constant comparison technique identified initial categories and subcategories with revisions based on further evaluation of content within these frameworks.
Results
Shared Decision-Making
Table 1 lists OPTION-5 means and standard deviations. On average, physicians demonstrated moderate effort toward including patients in SDM (mean [M] = 10.72). Physicians demonstrated lowest effort for team talk (M = 1.51) and greatest effort offering alternative options (M = 2.79). Each scale and the total scale had significant differences between physicians (Table 1).
Table 1.
Observer OPTION-5 Scale Means, Standard Deviations, and ANOVA Tests of Between Physician Differences.
| OPTION-5 Scales (score ranges) | Between physician difference | |||
|---|---|---|---|---|
| M | SD | F 10, 42 | P | |
| Option Talk: alternate options exist (0-4) | 2.79 | .72 | 2.91 | .007 |
| Team Talk: support deliberation/forming partnership (0-4) | 1.15 | .92 | 2.38 | .025 |
| Option Talk: information about options (0-4) | 2.15 | .43 | 2.42 | .023 |
| Decision Talk: elicit preferences (0-4) | 2.6 | .84 | 4.57 | .000 |
| Decision Talk: integrating preferences (0-4) | 2.09 | .84 | 3.24 | .004 |
| Total score (0-20) | 10.72 | 2.59 | 4.64 | .000 |
Abbreviations: ANOVA, analysis of variance; SD, standard deviation.
Table 2 reports BCSD Core Components Checklist frequencies. Physicians demonstrated most elements of SDM consistently (M = 18.44); 2/3 of core elements were demonstrated in 80% to 100% of encounters. Four components were demonstrated in less than 1/3 of encounters: checking the woman’s understanding (26.9%), explaining risk information has uncertainties (7.7%), recommendation to discuss at regular intervals (19.2%), and discussion of a woman’s values in general (15.4%).
Table 2.
Frequencies of Components Observed for Breast Cancer Screening Decision Core Components Checklist.
| Core component item | N (of 52) and % cases observed | |
|---|---|---|
| 1. MD tells the woman that her risk of breast cancer is based on her personal risk factors | 48 | 92 |
| 2. MD identifies the woman’s personal breast cancer risk factors (at least 1 risk factor mentioned, eg, family history, breast density, past biopsy, weight, smoking) | 50 | 96 |
| 3. MD tells the woman how her personal risk of breast cancer compares to the general population | 48 | 92 |
| 4. MD tells the woman that expert groups such as the US Preventive Services Task Force and the American Cancer Society differ in their recommendations on what age to begin mammography screening and how frequently to be screened | 42 | 80 |
| 5. MD presents both sides of the decision to have mammography or not | 47 | 90 |
| 6. MD tells the woman the benefits of having mammography screening (eg, reduce the risk of negative outcome such as cancer getting worse, death, reduce worry about cancer) | 49 | 94 |
| 7. MD tells the woman the risks of having mammography screening (at least 1, eg, false positive, over treatment, callbacks for second view/biopsy that are benign, anxiety) | 50 | 96 |
| 8. MD tells the woman the risks of overtreatment | 34 | 65 |
| 9. MD identifies the alternatives to mammography screening (eg, annual, biannual, no screening). | 50 | 96 |
| 10. MD tells the woman the benefits of not having mammography screening (at least 1, eg, avoid false positive, avoid stress/anxiety of false positive) | 41 | 78 |
| 11. MD tells the woman the risks of not having mammography screening (at least 1, eg, missed diagnosis of treatable cancer; loss of peace of mind) | 43 | 82 |
| 12. MD presented choices in an unbiased, nonpersuasive manner | 48 | 92 |
| 13. MD explains that information relating to risk has uncertainties and is not a guarantee | 4 | 7.7 |
| 14. MD recommends that they discuss the decision about whether to have mammography screening on a regular basis at future appointments (eg, “we can have this discussion again next year” | 10 | 19 |
| 15. MD checks with the woman to make sure she understands the information (more than “okay?” At minimum need to ask for questions or understanding, or if patient offers summary or teach-back then MD does not have to ask explicitly) | 14 | 26 |
| 16. MD shows the woman they are knowledgeable (eg, provides necessary information, answers questions about issues related to breast cancer screening) | 52 | 100 |
| 17. MD helps the woman decide how often to get screened | 45 | 86 |
| 18. MD tells the woman there is a decision to make about breast cancer screening | 49 | 94 |
| 19. MD explains the nature of the decision to be made (whether and how often to use mammography for breast cancer screening) | 49 | 94 |
| 20. MD identifies that the woman has a role in decision-making | 41 | 78 |
| 21. MD and woman discuss the woman’s values regarding a screening mammogram (eg, factors she considers in her decision such as risk, false alarm, anxiety, logistical concerns like cost) | 37 | 71 |
| 22. MD and woman identify woman’s preferences regarding a screening mammogram (MD elicits and/or woman states her preference for timing of mammogram or preference to defer decision) | 49 | 98 |
| 23. MD supports decision regardless whether it reduces risk | 51 | 98 |
| 24. MD and woman discuss the woman’s personal values and preferences in general | 8 | 15 |
Physician Evaluations
All physicians reported BCARE-DA changed the way they communicated with their patients about breast cancer screening (Item 3, Supplemental Appendix A) “somewhat” (n = 5), “very much” (n = 4), or “extremely (n = 2). Content analysis generated 2 themes for ways BCARE-DA changed physician communication with their patients: communication style and communication of risk. Physicians perceived communication style as more open, personalized, evidence-base and structured. They perceived increased ability to communicate risk, including identifying patient’s individual breast cancer risk, identifying patient’s personal risk/benefits for screening options (eg, overtreatment with more frequent screenings), and aiding knowledge transfer through graphic presentations of risk. One physician summarized “The figure showing the impact on survival with yearly vs. biannual mammography surprised a lot of women and provided a basis for a frank, evidence-based discussion on the utility of mammography.”
All physicians reported BCARE-DA facilitated SDM about mammography with their patients (Item 5, Supplemental Appendix A). Content analysis of ways BCARE-DA facilitated SDM yielded 6 themes: (1) informed patient’s choice, (2) increased awareness and understanding of false positives and over-treatment, (3) elicited patient’s priorities and goals, (4) visual aids facilitated understanding of risk, and (5) aided collaboration. One physician explained, BCARE-DA “helped me elucidate patient priorities and goals, also helped me feel confident that patients were making an informed decision, rather than deciding to do a mammogram because they are ‘supposed to.’” Another physician illustrates these themes: “[BCARE-DA] provided specific information about false positives and overdiagnosis. During the description of these issues, I am able to start to get a sense of her values.” This compliments quantitative findings that 10 providers reported BCARE-DA aided them in eliciting patient values (Item 7, Supplemental Appendix A).
Response to the open-ended question eliciting challenges in using BCARE-DA (Item 8, Supplemental Appendix A) elucidated the following physician barriers to utilizing the DA: forgetting to use BCARE-DA, not enough time for discussion, patient difficulty in viewing the computer screen, and lack of patient interest. One physician explained, “Some women were absolute in wanting/not wanting a specific plan no matter what the tool would show.” One physician expressed concern that women may choose not to get a mammogram.
Regarding factors that facilitated BCARE-DA use (Item 9, Supplemental Appendix A), content analysis of physicians’ responses yielded (1) easy access in the EMR, (2) ease of use, (3) helpful infographics, and (4) facilitating interesting discussions with patients.
Patient Evaluations
Patient decision conflict
Table 3 reports Decision Conflict Scale means and standard deviations. On average, patients reported low levels of conflict across all factors of breast cancer screening decision-making. Decision conflict did not correlate with OPTION-5 (r = .072, P = .608) nor Core Components Checklist scores (r = .186, P = .187).
Table 3.
Descriptive Statistics for Patient Decision Conflict Scale and Subscales.
| Decision Conflict Scale and Subscales | Rangea | M | SD |
|---|---|---|---|
| Decision Conflict Full Scale | 0-53.33 | 8.57 | 11.74 |
| Informed subscale | 0-50.00 | 9.43 | 14.16 |
| Values Clarity subscale | 0-62.50 | 11.09 | 17.79 |
| Support subscale | 0-50.00 | 4.40 | 10.02 |
| Uncertainty subscale | 0-75.00 | 12.42 | 18.39 |
| Effective Decision subscale | 0-43.75 | 6.84 | 11.32 |
Abbreviation: SD, standard deviation.
a Possible values of 0 to 100, greater scores indicate greater decision conflict.
Patient satisfaction items
In response to “How valuable was the BCARE experience to you?” most women found BCARE-DA somewhat (32%), very (35%), or extremely (21%) valuable. Content analysis of patient responses regarding likes about BCARE-DA yielded 7 themes: (1) ease of use, (2) content clarity and understandability, (3) graphic presentations of personal risk statistics, (4) risk for false positives, (5) individualized risk information, (6) evidence-base information, and (7) facilitated communication with their physician. One patient described, “Visually clear/simple to understand. I liked seeing the tool as I was discussing the risks/benefits of mammography with my physician. Nice reference for the discussion.” Another patient commented, “The confidence it gave me to know when to have my next mammogram. And the clarity. The choice became obvious.”
Patient responses to what they did not like about BCARE-DA yielded 4 theme areas of dissatisfaction or needing improvement: (1) access, (2) content, (3) technology, and (4) nature of SDM. Some patients would like BCARE-DA access beyond the clinical encounter, either prior to or after the visit. Areas for content improvements included desire for risk statistics for varying screening intervals beyond current paradigms, definitions for terms (eg, “overtreatment”), and ability to select multiple races/ethnicities. Technology concerns included a general bias against using computer systems; “I trust my doctor and respect and trust his advice. So, I feel like he knows me and my situation better than a computer.” Finally, some patients’ dislikes reflect the very nature of SDM and the breast cancer screening dilemma: a definite directive is not provided, with low risk a woman may perceive little difference in outcomes between options, and “It’s not exact, it’s still uncertain.” One patient expressed her uneasiness with the patient-centered approach, “Because it made me really think about my options, it challenged previously held beliefs about annual mammograms. I know I made the best choice for me but there’s a tinge of worry in letting go of those old beliefs about what is good practice.”
Discussion Time
Discussions ranged from 2 to 14 minutes (M = 7.67, standard deviation = 3.10); 26% of discussions took 10 minutes or longer; 24% were 5 minutes or less. Physicians differed significantly in discussion time, F 10, 53 = 2.06, P = .049. Physicians saw 1 to 7 enrolled patients, with individual mean discussion times ranging from 5.33 to 14.0 minutes. Discussion time had a weak but significant correlation with OPTION-5 total score, r = .347, P = .011, but no correlation with Core Component Checklist, r = .207, P = .140, nor Decision Conflict, r = −.007, P = .960.
Discussion
Physicians and women were receptive to using BCARE-DA and generally offered positive evaluations. Physicians reported BCARE-DA improved their communication with patients about screening mammography and facilitated their discussion about personal risks for cancer and potential risks involved with each screening option. Patients noted BCARE-DA facilitated their understanding of personal risks, facilitated discussion with their physician, and clarified their decisions. In turn, patients also offered feedback for improving their effective use of BCARE-DA (eg, access outside of the encounter, additional screening intervals, ability for more racial/ethnic specificity).
Preliminary evaluations indicate BCARE-DA can facilitate breast cancer screening SDM in the clinical encounter for non-high-risk US women aged 40 to 49 years, where screening guidelines suggest personalized decision-making (2,3). The BCARE-DA appears to address several gaps identified in our earlier work (21). Although physicians indicated less confidence in their knowledge of personal risk factors for breast cancer (21), here, in over 90% of encounters physicians explained the woman’s risk for breast cancer is based on personal risk factors, identified the woman’s personal risk factors, and compared her personal risk for breast cancer with that of the general population. However, physicians rarely explained risk information has uncertainties with no guarantee, a problematic phenomenon because current risk prediction models are far from perfect (29). Further, whereas women felt ill-prepared for callbacks for further imaging (21), here 96% of encounters included physicians providing information about risks of false positives and callbacks for noncancerous findings.
In comparison to national US survey results of patients aged 40+ years (12 –14), this preliminary evaluation does suggest some advantage in clinical encounters utilizing BCARE-DA. National surveys demonstrated low rates of physicians discussing benefits or risks of breast cancer screening (12,13), whereas they were discussed in nearly all encounters utilizing BCARE-DA. Also, women infrequently perceived they had a choice in mammography screening (12,14), whereas here physicians clarified there is a decision to be made in 94.2% of encounters and explicitly addressed the woman’s role in the decision-making process in 78.8% of encounters.
However, physicians expressed concerns for the added time SDM requires. Given the individual differences between physicians and weak to no correlation between time and SDM outcomes, lengthy interactions are likely due to factors beyond SDM. Still, increasing familiarity with BCARE-DA’s content and related discussions may facilitate shorter durations to accomplish effective SDM.
Utilizing complementing measures of the OPTION-5 general SDM concepts and the Core Components Checklist’s specific elements of the interaction allowed further illumination of areas for improvement. For example, physicians engaged in a lower-end moderate level of effort for the OPTION-5 item “gives information or checks understanding of options.” The Checklist affords further detailed understanding of strengths and weaknesses in conveying information. Physicians often explain a woman’s risk is based on personal risk factors, identify those factors and compare her risk to the general population, identify risks and benefits of screening, mammogram alternatives, and risk of not having mammogram. However, physicians less frequently identify benefits of not having mammograms. Physicians rarely discussed the risk of overtreatment specifically, explained risk estimates are uncertainties and not guaranteed, and checked women’s understanding of information. The Checklist may further efforts to identify explicit elements of breast cancer screening SDM that impact outcomes and may have valuable utility when training physicians.
Limitations
As a preliminary evaluation, a notable limitation is the lack of a comparison group to clearly test BCARE-DA’s effectiveness on SDM outcomes. Further, small physician and patient sample sizes and limited variance in SDM outcomes may cloud findings. This is a geographically and demographically limited sample of predominantly Caucasian, insured women receiving care in an urban/suburban academic medical center with an established primary care physician. Best SDM practices need to consider cultural and health literacy perspectives. For example, some African American and Latina women have lower confidence and/or desire to take an active role in decision-making (30). It is unclear how BCARE-DA may impact encounters for women from diverse backgrounds.
Two measurement considerations are noteworthy. Due to limited resources, the newly developed BCSD Core Component Checklist had a single rater, eliminating interrater reliability; further evaluation of this measure is needed. Also, the omission of one Decision Conflict item has an unknown impact on that outcome. The 3-item Informed subscale has demonstrated strong internally consistency (0.92) (31), suggesting the item omission may have limited impacted on scale scores. This is further supported in that women generally reported low decision conflict across full scale and subscales.
As physicians often influence outcomes, individual provider differences need to be examined within a larger pool of providers who repeatedly use BCARE-DA. If truly individualized patient screening is to be realized, patient individual differences must overcome provider differences. Efforts are needed to understand and minimize unwarranted variation in practice (32). This study did not offer training to physicians. Future work, utilizing standardized training protocols, will illuminate roles of training in increasing SDM quality and potential to reduce physician variance.
Conclusion
This preliminary study demonstrated BCARE-DA’s potential to facilitate SDM in primary care visits for non-high-risk US women for which individualized screening plans are recommended (2,3). Although further controlled studies are necessary, we are encouraged that a shared DA utilized within the patient–clinician encounter can meet existing gaps between patients’ expectations and providers’ confidence (21) in SDM for breast cancer screening. Specifically, such DAs demonstrate promise to facilitate physicians’ and women’s consideration of individual risk for each screening option. However, some SDM elements likely require additional facilitating.
Supplemental Material
Supplemental Material, sj-pdf-1-jpx-10.1177_23743735211034039 for Preliminary Evaluation of a Breast Cancer Screening Shared Decision-Making Aid Utilized Within the Primary Care Clinical Encounter by Lori DuBenske, Viktoriya Ovsepyan, Terry Little, Sarina Schrager and Elizabeth Burnside in Journal of Patient Experience
Footnotes
Authors’ Note: Ethical approval to report this research article was obtained from The University of Wisconsin Health Sciences Institutional Review Board (2015-0022-CR006)*. All procedures in this study were conducted in accordance with The University of Wisconsin Health Sciences Institutional Review Board’s (2015-0022-CR006)* approved protocols. Written informed consent was obtained from the patients and physicians for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Community-Academic Partnerships core of the University of Wisconsin Institute for Clinical and Translational Research (UW ICTR), grant 9 U54 TR000021 from the National Center for Advancing Translational Sciences (previously grant 1 UL1 RR025011 from the National Center for Research Resources), the National Institutes of Health, National Cancer Institute, grant K24 CA194251, and the University of Wisconsin Carbone Cancer Center, support grant P30 CA014520. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funders.
ORCID iD: Lori DuBenske  https://orcid.org/0000-0002-4915-951X
https://orcid.org/0000-0002-4915-951X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. American Cancer Society. Cancer facts and figures 2020. 2020. Accessed July 27, 2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/
- 2. American Cancer Society. History of ACS Recommendations for the early detection of cancer in people without symptoms. 2020. Accessed July 27, 2021. https://www.cancer.org/healthy/find-cancer-early/cancer-screening-guidelines/chronological-history-of-acs-recommendations.html
- 3. Siu AL, Bibbins-Domingo K, Grossman DC, LeFevre ML. U. S. preventive services task force. Convergence and divergence around breast cancer screening. Ann Intern Med. 2016;164:301–2. [DOI] [PubMed] [Google Scholar]
- 4. U.S. Preventative Task Force. Screening for breast cancer: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2009;151:716–26, w-236. [DOI] [PubMed] [Google Scholar]
- 5. Qaseem A, Lin JS, Mustafa RA, Horwitch CA, Wilt TJ. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170:547–60. doi:10.7326/M18-2147 [DOI] [PubMed] [Google Scholar]
- 6. Sasieni PD, Smith RA, Duffy SW. Informed decision making and breast cancer screening. J Med Screen. 2015;22:165-7. doi:10.1177/0969141315587344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davey HM, Barratt AL, Butow PN, Houssami N. The impact of different criteria for selecting information to be provided to women undergoing diagnostic breast tests. Patient Educ Couns. 2008;71:86–94. doi:10.1016/j.pec.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 8. Sheridan SL, Harris RP, Woolf SH. Shared decision making about screening and chemoprevention: a suggested approach from the US preventive services task force. Am J Prev Med. 2004;26:56–66. doi:10.1016/j.amepre.2003.09.011 [DOI] [PubMed] [Google Scholar]
- 9. Berlin L. Point: mammography, breast cancer, and overdiagnosis: the truth versus the whole truth versus nothing but the truth. J Am Coll Radio. 2014;11:642–7. doi:10.1016/j.jacr.2014.01.015 [DOI] [PubMed] [Google Scholar]
- 10. Forbes LJ, Ramirez AJ. Expert group on Information about Breast Screening. Offering informed choice about breast screening. J Med Screen. 2014;21:194–200. 10.1177/0969141314555350 [DOI] [PubMed] [Google Scholar]
- 11. Nekhlyudov L, Braddock CH., III An approach to enhance communication about screening mammography in primary care. J Women’s Health. 2009;18:1403–12. 10.1089/jwh.2008.1184. [DOI] [PubMed] [Google Scholar]
- 12. Fowler FJ, Gerstein BS, Barry MJ. How patient centered are medical decisions?: results of a national survey. J Amer Med Assoc Intern Med. 2013;173:1215–21. doi:10.1001/jamainternmed.2013.6172 [DOI] [PubMed] [Google Scholar]
- 13. Zikmund-Fisher BJ, Couper MP, Singer E, Ubel PA, Ziniel S, Fowler FJ, Jr, et al. Deficits and variations in patients’ experience with making 9 common medical decisions: the DECISIONS survey. Med Decis Making. 2010;30:85–95. doi:10.1177/0272989X10380466 [DOI] [PubMed] [Google Scholar]
- 14. Spring LM, Marshall MR, Warner ET. Mammography decision making: trends and predictors of provider communication in the health information national trends survey, 2011 to 2014. Cancer. 2017;123:401–9. doi:10.1002/cncr.30378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoffman RM, Elmore JG, Fairfield KM, Gerstein BS, Levin CA, Pignone MP. Lack of shared decision making in cancer screening discussions. Amer J Prev Med. 2014;47:251–9. doi:10.1016/j.amepre.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 16. O’Connor AM, Stacey D, Entwistle V, Llewellyn-Thomas H, Rovner D, Holmes-Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Syst Rev. 2003. doi:10.1002/14651858.CD001431 [DOI] [PubMed] [Google Scholar]
- 17. O’Connor AM, Llewellyn-Thomas HA, Flood AB. Modifying unwarranted variations in health care: shared decision making using patient decision aids. Health Aff. 2004;23:Var–63. doi:10.1377/hlthaff.var.63 [DOI] [PubMed] [Google Scholar]
- 18. Elkin EB, Pocus VH, Mushlin AI, Cigler T, Atoria CL, Polaneczky MM. Facilitating informed decisions about breast cancer screening: development and evaluation of a web-based decision aid for women in their 40s. BMC Med Inform Decis Mak. 2017;17:29. doi:10.1186/s12911-017-0423-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eden KB, Scariati P, Klein K, Watson L, Remiker M, Hribar M, et al. Mammography decision aid reduces decisional conflict for women in their forties considering screening. J Womens Health. 2015;24:1013–20. doi:10.1089/jwh.2015.5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scariati P, Nelson L, Watson L, Bedrick S, Eden KB. Impact of a decision aid on reducing uncertainty: pilot study of women in their 40 s and screening mammography. BMC Med Inform Decis Mak. 2015;15:89. doi:10.1186/s12911-015-0210-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DuBenske LL, Schrager S, McDowell H, Wilke LG, Trentham-Dietz A, Burnside ES. Mammography screening: gaps in patient’s and physician’s needs for shared decision-making. Breast J. 2017;23:210–4. doi:10.1111/tbj.12779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schrager S, Burnside E. Breast cancer screening in primary care: a call for development and validation of patient-oriented shared decision-making tools. J Womens Health. 2019;28:114–6. doi:10.1089/jwh.2017.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elwyn G, Grande SW, Barr P. Observer OPTION5 Manual. The Dartmouth Institute for Health Policy and Clinical Practice. 2016. [Google Scholar]
- 24. Barr PJ, O’Malley AJ, Tsulukidze M, Gionfriddo MR, Montori V, Elwyn G. The psychometric properties of Observer OPTION(5), an observer measure of shared decision making. Patient Educ Couns. 2015;98:970–6. doi:10.1016/j.pec.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 25. Dillon EC, Stults CD, Wilson C, Chuang J, Meehan A, Li M, et al. An evaluation of two interventions to enhance patient-physician communication using the observer OPTION(5) measure of shared decision making. Patient Educ Couns. 2017;100:1910–17. doi:10.1016/j.pec.2017.04.020 [DOI] [PubMed] [Google Scholar]
- 26. Croes KD, Jones NR, DuBenske LL, Schrager SB, Mahoney JE, Little TA, et al. Core elements of shared decision-making for women considering breast cancer screening: results of a modified Delphi survey. J Gen Intern Med. 2020;35, 1668–77. doi:10.1007/s11606-019-05298-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DuBenske LL, Schrager SB, Hitchcock ME, Kane AK, Little TA, McDowell HE, et al. Key elements of mammography shared decision-making: a scoping review of the literature. J Gen Intern Med. 2018;33:1805–14. doi:10.1007/s11606-018-4576-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O’Connor AM. User Manual – Decisional Conflict Scale (16 item question format). Ottawa Hospital Research Institute; 1993. Updated 2010. Accessed March 19,2020. Accessed January 30, 2020. https://decisionaid.ohri.ca/docs/develop/user_manuals/UM_decisional_conflict.pdf [Google Scholar]
- 29. Burnside ES, Trentham-Dietz A, Shafer CM, Hampton JM, Alagoz O, Cox JR, et al. Age-based versus risk-based mammography screening in women 40-49 years old: a cross-sectional study. Radio. 2019;292:321–328. doi:10.1148/radiol.2019181651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allen JD, Bluethmann SM, Sheets M, Opdyke KM, Gates-Ferris K, Hurlbert M, et al. Women’s responses to changes in US Preventive Task Force’s mammography screening guidelines: results of focus groups with ethnically diverse women. BMC Public Health. 2013;13:1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. [DOI] [PubMed] [Google Scholar]
- 32. King J, Moulton B. Group Health’s participation in a shared decision-making demonstration yielded lessons, such as role of culture change. Health Aff. 2013;32:294–302. doi:10.1377/hlthaff.2012.1067 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-jpx-10.1177_23743735211034039 for Preliminary Evaluation of a Breast Cancer Screening Shared Decision-Making Aid Utilized Within the Primary Care Clinical Encounter by Lori DuBenske, Viktoriya Ovsepyan, Terry Little, Sarina Schrager and Elizabeth Burnside in Journal of Patient Experience