Abstract
Objective
To compare the effect of the modified Paleolithic elimination (Wahls) and low-saturated fat (Swank) diets in relapsing-remitting MS (RRMS).
Methods
Individuals (n = 87) with RRMS were randomized to the Swank or Wahls diets in a parallel group clinical trial consisting of four timepoints: 1) run-in, 2) baseline, 3) 12-weeks, and 4) 24-weeks.
Results
77 participants completed 12 weeks and 72 completed 24 weeks. The 12-week change from baseline in fatigue was -0.94 ± 0.18 (FSS) and -9.87 ± 1.93 (MFIS; both p < 0.0001) for Swank, and -0.71 ± 0.24 (FSS; p = 0.004) and -14.41 ± 2.22 (MFIS; p ≤ 0.0001) for Wahls. Physical MSQoL scores improved by 6.04 ± 2.18 (p = 0.006) for Swank and by 14.5 ± 2.63 (p < 0.0001) for Wahls. Mental MSQoL scores improved by 11.3 ± at 2.79 (p < 0.0001) for Wahls while the Swank did not change (3.85 ± 2.63; p = 0.14). Neither group showed significant changes in 6-minute walking distance at 12 weeks. All outcomes were maintained or further improved at 24 weeks.
Conclusions
Both diets were associated with clinically meaningful within-group reductions in fatigue and improvements in QoL.
Trial Registration: Clinicaltrials.gov Identifier: NCT02914964
Keywords: Multiple sclerosis, fatigue, quality of life, 6-minute walk test, low-saturated fat diet, paleolithic diet
Introduction
Multiple sclerosis (MS) is a chronic immune-mediated neuroinflammatory condition that affects nearly one million people in the United States. 1 Fatigue is one of the most common and debilitating symptoms, 2 and is associated with increased disability and reduced quality of life (QoL). 3 Pharmacological treatment options for MS-related fatigue have limited efficacy 4 ; thus, many individuals with MS seek non-pharmacologic therapies to reduce their fatigue burden.
Despite a lack of consistent evidence for any specific therapeutic diet for MS, 5 surveys observe that half of individuals with MS report implementing dietary modifications.6,7 Due to the lack of evidence demonstrating diet intervention-related reduced disease activity 5 and the limited role of the neurologist in providing dietary recommendations, 8 people newly diagnosed with MS receive little dietary advice 9 which forces this information to be sought from internet sources that are often not evidence-based. 10 Two dietary approaches with supporting preliminary evidence are the low-saturated fat diet developed by Dr. Swank and the modified Paleolithic diet developed by Dr. Wahls. 5
Based on epidemiological evidence that regions with higher saturated fat intake experience higher incidences of MS, 11 Swank began recommending his patients consume a low saturated fat diet, 12 and followed them for up to 50 years. 13 His studies observed that patients who consumed the least amount of saturated fat were less likely to have exacerbations, more likely to continue to ambulate, and had reduced risk of mortality.14,15 In recent years, randomized controlled trials using plant-based and omega-3 supplemented low-fat diets have demonstrated favorable outcomes on fatigue and QoL among individuals with RRMS.16,17
Wahls believed that a Paleolithic-based diet that eliminated specific dietary antigens (gluten, casein, and lectins) and maximized micronutrient density could optimize health and prevent disease progression. In a single-arm study using this dietary approach as part of a multimodal intervention, improvements in fatigue, QoL, and gait, were observed in half of a cohort with progressive MS.18,19 Due to the multimodal intervention in that study it was not possible to determine the effect attributable to the diet; however, two small follow-up randomized, controlled trials comparing the modified Paleolithic diet to usual diet demonstrated favorable outcomes for fatigue and QoL among individuals with progressive or RRMS.20,21
Both the low-saturated fat and modified Paleolithic diets are popular among individuals with MS 6 ; however, due to a lack of data from high-quality randomized controlled trials, it is not possible for either of these diets to be recommended in clinical practice. The objective of this study was to assess and compare the effect of the Swank and Wahls diets on perceived fatigue and QoL in individuals with RRMS.
Participants and methods
Study design and participants
This 36-week, randomized, parallel-group, single-blinded trial was conducted at the University of Iowa Prevention Intervention Center, with participant recruitment taking place from August 2016 to May 2019 and follow-up from February 2017 to January 2020. The trial protocol 22 was approved by the University of Iowa Institutional Review Board. Written and informed consent was obtained from all participants, and data safety and management of the trial was monitored by a National Multiple Sclerosis Society (NMSS) data safety monitoring board. This trial followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines. 23
Participants were recruited from within a 500-mile radius of Iowa City, Iowa. The research team worked with local NMSS support groups, regional MS centers, the North American Research Committee on Multiple Sclerosis, the University of Iowa Hospitals & Clinics Department of Neurology, the Iowa City VA Health Care System neurology clinic, the Swank Foundation, terrywahls.com, and other organizations to recruit study participants.
Participants aged 18-70 years were eligible for enrollment if they had: 1) neurologist-confirmed RRMS based on the 2010 McDonald criteria, 24 2) moderate to severe fatigue (FSS ≥ 4.0), 3) an ability to walk 25 feet with unilateral or no support, 4) were not pregnant or planning on becoming pregnant, and 5) were willing to comply with all aspects of the study intervention and assessments. Major exclusion criteria included: 1) MS-relapse or change in disease modifying drug use within the previous 12 weeks, 2) change in medication to manage MS symptoms, 3) low body weight (BMI < 19 kg/m2), 4) severe mental impairment, 5) self-reported adverse reactions to gluten-containing foods, 6) diagnosed conditions including eating disorders, severe psychiatric disorders, celiac disease, kidney stones, heart failure, angina, or liver cirrhosis, and 7) insulin, warfarin, radiation, or chemotherapy use. An exhaustive list of inclusion and exclusion criteria can be found in Supplemental Table 1 or the published protocol. 22 The study consisted of four study visits for data collection: 1) run-in, 2) baseline, 3) 12-weeks, and 4) 24-weeks. Eligible participants were enrolled in a 12-week run-in phase for observation prior to treatment randomization at baseline. Participants who did not complete all study procedures during the run-in phase were excluded from the study.
Randomization and masking
Eligible participants were randomized 1:1 to follow either the modified Paleolithic elimination (Wahls) or low-saturated fat (Swank) diet at baseline. Two randomization tables were used to achieve similar mean FSS values at baseline—one for moderate perceived fatigue (FSS ≥ 4 and < 5.5) and the other for high perceived fatigue (FSS ≥ 5.5). Password-protected randomization tables were accessible only by the intervention registered dietitians (RDs). Study participants, intervention RDs, and the statistician overseeing randomization and data management were not masked to treatment randomization. The study principal investigator, co-investigators, study coordinator, data analysis statistician, and outcome assessors were masked to treatment randomization. All analyses were performed by the masked statistician (PTE) with groups coded “X” and “Y” and then decoded by the unmasked statistician (LMR).
Procedures
Following a 12-week run-in period for observation of usual diet and stability of pre-intervention outcomes, participants were randomized to either the Wahls or the Swank diets for 24 weeks. During the first 12 weeks post-randomization, participants received two in-person and five telephone-based nutrition counseling sessions from an intervention RD. Participants also received personalized emails with feedback on their diet checklists every 4 weeks. At week 12 of the intervention period, in-person and telephone counseling sessions were discontinued, but participants were allowed to contact the intervention RD at any time for additional support.
Intervention diets
The Swank diet restricts saturated fat to ≤ 15 g per day and provides 20-50 g (4-10 teaspoons) unsaturated fat per day and four servings each of grains, whole preferred, and fruits and vegetables (FV; Supplemental Table 2). The Wahls diet recommends 6-9 servings of FV and provides 6-12 ounces meat per day according to gender. It excludes all grain, legumes, eggs, and dairy (except for clarified butter or ghee). Nightshade vegetables were also excluded in the Wahls group during the first 12-week period from baseline and then the intervention RDs provided guidance to reintroduce nightshades during the second 12-week period on the diet. A review of the published research and potential mechanisms of both diets can be found elsewhere. 25 Participants in both groups were instructed to follow their assigned diet ad libitum and were given the following daily supplement regimen: 1 teaspoon cod liver oil, 1,000 µg methyl-B12, 1,000 µg methylfolate, a multivitamin without iron, and 5,000 IU vitamin D3, the latter of which was adjusted based on serum levels with a target range of 40 to 80 ng/mL. 22
Outcomes
The primary endpoint of the trial was the change in perceived fatigue from baseline to 12 weeks as assessed by the Fatigue Severity Scale (FSS). Secondary endpoints included perceived fatigue assessed by the Modified Fatigue Impact Scale (MFIS), mental and physical QoL assessed by the Multiple Sclerosis Quality of Life-54 (MSQoL-54), and the 6-minute walk test (6MWT) and an additional 12-week follow-up period. Clinically meaningful changes were defined as 0.45 for FSS, 26 4.0 for MFIS, 26 5.0 points for both mental and physical MSQoL-54, 27 and 6% change for 6MWT. 28 Adherence to diet specific food components (i.e., grams of gluten for the Wahls group and grams of saturated fat for the Swank group) was monitored using three-day weighed food records collected on three consecutive days including one weekend day in the week prior to each study visit and were analyzed at the University of Minnesota Nutrition Coordinating Center using Nutrition Data System for Research software.
Statistical analysis
The trial was powered to detect a group mean difference of 0.7 ± 0.9 for FSS within and between groups. With two-sided significance set at α = 0.05 and power of 85%, we determined the required sample size to be 34 participants per group. Assuming 25% attrition based on our previous experience, we aimed to recruit 43 participants per group.
Descriptive statistics were calculated for every variable at enrollment using frequencies, means ± standard error (SE), and medians (interquartile range). Outliers were checked for accuracy and possible data entry errors. Distributions of continuous variables were evaluated for normality by graphical observation. Generalized linear mixed models 29 were used to test the interacting effects of diet and time on outcome measures while accounting for repeated measures for each participant. Other potentially important variables (age, sex, BMI, smoking status, alcohol consumption, walking assistance, years since MS diagnosis, disease modifying drug use, baseline vitamin D, baseline six-minute walk distance) were considered for inclusion in each model to assess their relationship with the outcome and whether they modified the estimates for the diet and time interaction. For each outcome, the model with the smallest Akaike information criterion (AIC) 30 was deemed to have the optimal predictor set. Point estimates, 95% confidence intervals, and p-values of the group mean changes in outcome measures over visits were generated for each optimal model. For primary data analysis, data from all participants completing 12- and 24-week assessments were included in an intention-to-treat analysis. A secondary per-protocol analysis was used to examine the participants who successfully met study compliance requirements for their assigned diet intervention. Participants were included in the per-protocol analysis if weighed food records indicated that they consumed within 20% of recommendations for key diet components (≤ 0.2 grams of gluten or ≤ 18 grams of saturated fat for the Wahls or Swank diets, respectively) on a minimum of two of three food records at 12 weeks. All analyses were conducted with two-sided tests (α = 0.05) using SAS software (version 9.4, SAS Institute, Inc.).
Results
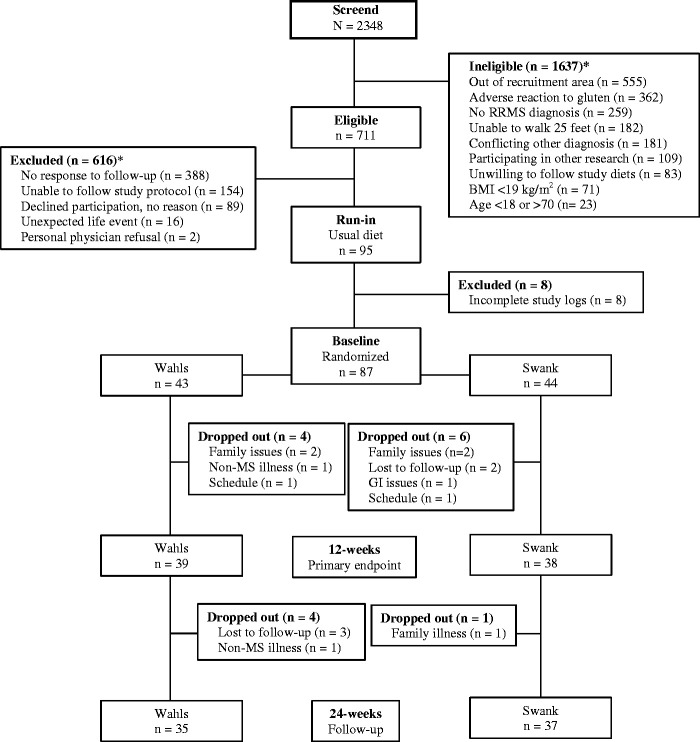
A total of 95 participants enrolled in the 12-week run-in period, of whom 8 were excluded for not providing complete study logs during the run-in period (Figure 1). Of the remaining 87 randomized participants, 43 were randomized to the Wahls diet and 44 to the Swank diet. There were no significant group differences at baseline (Table 1) and baseline outcome values were largely stable compared to run-in values (Table 2). A total of 77 participants (39 Wahls and 38 Swank) completed the primary endpoint at 12 weeks and 72 (35 Wahls and 37 Swank) completed follow-up at 24 weeks. No serious adverse events were reported. Diet adherence was high for both groups. At 12 weeks, 86.8% (33 of 38) of the Swank group and 79.5% (31 of 39) of the Wahls group participants were considered adherent to the respective study diets and were included in the secondary per-protocol analysis. At 24 weeks, diet adherence was 81.1% (30 of 37) for the Swank group and 74.3% (26 of 35) for the Wahls group.
Figure 1.
CONSORT diagram of study recruitment and participant flow. Reasons for ineligibility or exclusion may not add up to value of ineligible or excluded because some participants were found ineligible or were excluded for multiple reasons.
Table 1.
Baseline characteristics of participants who completed a 12-week intervention of the Swank or Wahls diets.a
| Characteristics | Swank | Wahls | p-valueb |
|---|---|---|---|
| N | 38 | 39 | – |
| Age (years) | 46.9 ± 1.7 | 46.4 ± 1.5 | 0.84 |
| Gender (female) | 35 (92.1) | 32 (82.1) | 0.31 |
| MS duration (years) | 12.1 ± 1.6 | 9.3 ± 1.0 | 0.14 |
| Disease modifying drug use | 0.83 | ||
| None | 13 | 10 | |
| Oral | 11 | 11 | |
| Injectable | 10 | 12 | |
| Infused | 4 | 6 | |
| Race (Caucasian) | 36 (94.7) | 38 (97.4) | 0.99 |
| Education | 0.32 | ||
| High school | 0 (0.0) | 3 (7.7) | – |
| Some college | 12 (31.6) | 10 (25.6) | – |
| 4-year degree | 11 (28.9) | 8 (20.5) | – |
| Advanced degree | 15 (39.5) | 18 (46.2) | – |
| Smoking status | 0.13 | ||
| Never | 29 (76.3) | 23 (59.0) | – |
| Former | 3 (7.9) | 2 (5.1) | – |
| Current | 6 (15.8) | 14 (35.9) | – |
| Alcohol drinks per monthc | 0.99 | ||
| None | 6 (15.8) | 7 (17.9) | – |
| Within recommendations | 29 (76.3) | 29 (74.4) | – |
| Above recommendations | 3 (7.9) | 3 (7.7) | – |
| BMI (kg/m2) | 27.6 ± 0.94 | 30.2 ± 1.3 | 0.11 |
| 6-minute walk distance (meters) | 481 ± 16.3 | 459 ± 10.3 | 0.40 |
| Walking assistive device used (y) | 5 (13.2) | 4 (10.3) | 0.73 |
| Serum vitamin D (nmol/L) | 47.9 ± 3.9 | 50.9 ± 3.2 | 0.55 |
| Fatigue Severity Scored | 5.3 ± 0.2 | 5.2 ± 0.2 | 0.62 |
aData are shown as mean ± SEM or N (%). There were no significant differences in baseline values between groups.
bSignificance determined by Fisher’s exact test or generalized linear models.
cAlcohol recommendations defined as ≤ 1 standard drink per day for females and ≤ 2 standard drinks per day for males.
dParticipants were randomized at baseline based on run-in FSS values.
Table 2.
Fatigue and QoL values among participants with RRMS assigned to the Swank or Wahls dietary interventions.a
| Study visit | ||||
|---|---|---|---|---|
| Outcome (range) | Run-in | Baseline | 12 Weeks | 24 Weeks |
| Swank | ||||
| FSS (1–9) | 5.45 ± 0.15 | 5.32 ± 0.18 | 4.39 ± 0.22*** | 4.32 ± 0.25*** |
| MFIS total (0–84) | 43.6 ± 2.50 | 40.7 ± 2.40 | 30.8 ± 2.35*** | 30.2 ± 2.63*** |
| MFIS physical (0–36) | 19.3 ± 1.26 | 18.9 ± 1.36 | 13.8 ± 1.29*** | 14.7 ± 1.40*** |
| MFIS cognitive (0–40) | 20.2 ± 1.40** | 17.6 ± 1.37 | 14.0 ± 1.28** | 13.5 ± 1.37** |
| MFIS psychosocial (0–8) | 4.08 ± 0.37 | 4.18 ± 0.40 | 3.05 ± 0.31*** | 3.03 ± 0.34*** |
| MSQoL-54 mental (0–100) | 63.2 ± 3.11* | 67.7 ± 2.90 | 71.6 ± 2.93 | 73.6 ± 2.81* |
| MSQoL-54 physical (0–100) | 53.2 ± 2.85 | 55.6 ± 3.01 | 61.7 ± 3.27** | 64.9 ± 3.15*** |
| 6MWT (meters) | 460 ± 19.1 | 481 ± 16.3 | 485 ± 15.7 | 491 ± 16.6 |
| Wahls | ||||
| FSS (1–9) | 5.36 ± 0.15 | 5.19 ± 0.20 | 4.47 ± 0.22** | 3.87 ± 0.27*** |
| MFIS total (0–84) | 45.4 ± 2.33 | 45.6 ± 1.99 | 31.2 ± 2.72*** | 26.5 ± 3.00*** |
| MFIS physical (0–36) | 20.5 ± 1.05 | 20.6 ± 0.98 | 13.7 ± 1.23*** | 11.3 ± 1.17*** |
| MFIS cognitive (0–40) | 20.4 ± 1.47 | 20.4 ± 1.24 | 14.5 ± 1.58*** | 12.8 ± 1.73*** |
| MFIS psychosocial (0–8) | 4.56 ± 0.31 | 4.59 ± 0.31 | 2.92 ± 0.37*** | 2.37 ± 0.35*** |
| MSQoL-54 mental (0–100) | 60.0 ± 3.33 | 62.3 ± 3.49 | 73.7 ± 3.43*** | 76.3 ± 3.59*** |
| MSQoL-54 physical (0–100) | 54.3 ± 2.89 | 53.8 ± 3.05 | 68.2 ± 3.02*** | 71.0 ± 3.20*** |
| 6MWT (meters) | 468 ± 19.3 | 459 ± 10.3 | 468 ± 20.3 | 495 ± 18.7** |
FSS: Fatigue Severity Scale; MFIS: Modified Fatigue Impact Scale; MSQoL-54: Multiple Sclerosis Quality of Life-54 Scale; 6MWT: 6-minute walk test.
aAll values mean ± SEM.
*indicates statistical significance compared to baseline values (P ≤ 0.05), **indicates statistical significance compared to baseline values (P ≤ 0.01), ***indicates statistical significance compared to baseline values (P ≤ 0.001).
Significant mean reductions from baseline were observed in the primary outcome of FSS at both 12 and 24 weeks for the Swank (-0.94 ± 0.18 and -1.01 ± 0.24, respectively; p ≤ 0.0001 for both) and Wahls (-0.71 ± 0.24 and -1.31 ± 0.29, respectively; p ≤ 0.004 for both) groups, which exceeded the clinically meaningful threshold, defined as 0.45 FSS points, 26 at all time points (Figure 2(a)). There were no differences in the magnitude of 12- and 24-week FSS changes between groups. In addition, both groups had significant reductions in MFIS, the 12- and 24-week mean differences for the Swank group were -9.87 ± 1.93 and -10.5 ± 2.46, respectively (p ≤ 0.0001 for both) and for the Wahls group were -14.4 ± 2.22 and -19.1 ± 2.66, respectively (p ≤ 0.0001 for both), which exceeded the clinically meaningful threshold, defined as 4.0 MFIS points, 26 at all time points (Figure 2(b)). The Wahls group experienced a 24-week MFIS mean reduction that was significantly greater than the Swank group (p = 0.02).
Figure 2.
Change from baseline for (A) Fatigue Severity Scale (FSS), (B) total Modified Fatigue Impact Scale (MFIS), (C) Multiple Sclerosis Quality of Life-54 (MSQoL-54) mental, (D) MSQoL-54 physical, and (E) 6-minute walk test among study participants with RRMS randomized to either the Swank diet (grey bars) or the Wahls elimination diet (black bars) for 24 weeks. The dashed lines represent thresholds of clinically meaningful changes. Statistical significance determined by generalized linear mixed models, statistical significance is represented by * = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001.
Significant improvements occurred in both the mental and physical MSQoL-54 subscales for each group. Among the Swank group, the 12-week mental MSQoL-54 mean difference (3.85 ± 2.63; p = 0.14) did not achieve clinical significance defined as 5.0 MSQoL points, 27 though the 24-week difference (5.87 ± 2.65; p = 0.03) did achieve clinical significance (Figure 2(c)). The Wahls group had a clinically meaningful mean difference in mental MSQoL-54 scores at both 12 and 24 weeks (11.3 ± 2.79 and 14.0 ± 3.15, respectively; p ≤ 0.0001 for both), which were significantly greater than the 12- and 24-week differences experienced by the Swank group (both time periods p = 0.05). The 12- and 24-week mean differences in physical MSQoL-54 scores were clinically meaningful for both the Swank (6.04 ± 2.18 and 9.25 ± 2.12, respectively; p ≤ 0.006 for both) and the Wahls (14.5 ± 2.63 and 17.24 ± 2.84, respectively; p ≤ 0.0001 for both) groups, which exceeded clinically meaningful threshold at all time points (Figure 2(d)). The Wahls group had 12- and 24-week physical MSQoL-54 mean improvements that were significantly greater than those experienced by the Swank group (both time periods p ≤ 0.05).
Neither group experienced significant improvements in distance walked during the 6MWT at 12 weeks (Table 2). The 12-week mean differences were 3.7 ± 5.5 meters for the Swank group (p = 0.50) and 9.3 ± 7.8 meters for the Wahls group (p = 0.23). By 24 weeks, the mean difference from baseline for the Swank group did not change (10.0 ± 6.7 meters; p = 0.13), whereas the Wahls group walked 36.6 ± 13.5 meters further compared to baseline distance (p = 0.007) and achieved clinical significance defined as 6% change 28 (Figure 2(e)). After removal of non-adherent participants in the secondary per-protocol analysis, the 24-week mean difference within each group was significant at 18.6 ± 5.7 meters for the Swank group (p = 0.001) and 39.9 ± 16.3 meters for the Wahls group (p = 0.02; Supplemental Figure 1).
Significant within-group 12- and 24-week mean improvements were observed in all outcomes assessed for the Wahls group and nearly all outcomes for the Swank group (Table 2). In the primary analysis, 47.4% (mental) and 50.0% (physical) of participants in the Swank group experienced clinically meaningful improvements in QoL while 68.4% (mental) and 60.5% (physical) of participants in the Wahls group experienced clinically meaningful improvements in QoL (p > 0.05 for both; Table 3). However, after removal of non-adherent participants in the secondary per-protocol analysis, the percentage of participants at 12 weeks who achieved clinically meaningful improvements in the mental MSQoL-54 subscale was higher in the Wahls group (74.2%) compared to the Swank group (45.5%; p = 0.02). In addition, in the primary analysis the proportion of participants at 12 weeks in the Swank group that experienced clinically meaningful reductions in fatigue was 59.5% (FSS) and 73.7% (MFIS) compared to 56.8% (FSS) and 76.3% (MFIS) of participants in the Wahls group (p > 0.05 for both). No individuals in the Swank group and 5.4% of individuals in the Wahls group achieved clinically meaningful improvements in the 6MWT at 12 weeks (p = 0.49). For all outcomes, the proportion of participants that experienced clinically meaningful improvements was lower at 24 weeks compared to respective values at 12 weeks. Additional results of the secondary per-protocol analysis can be found in Supplemental Table 3.
Table 3.
Percent (95% CI) of participants with clinically meaningful improvements in fatigue and MSQoL scores at 12 and 24 weeks compared to baseline.
| Outcome | Thresholda |
Primary intention-to-treat analysis |
Secondary per-protocol analysis |
||||
|---|---|---|---|---|---|---|---|
| Swank | Wahls | p-valueb | Swank | Wahls | p-valueb | ||
| FSS | 0.45 26 | ||||||
| 12 weeks | 59.5 (43.6, 75.3) | 56.8 (40.8, 72.7) | 0.81 | 62.5 (43.7, 78.9) | 56.7 (37.4, 74.5) | 0.80 | |
| 24 weeks | 45.9 (29.9, 62.0) | 48.6 (32.5, 64.8) | 0.82 | 50.0 (32.7, 67.3) | 46.7 (28.8, 64.5) | 0.79 | |
| MFIS | 4.0 26 | ||||||
| 12 weeks | 73.7 (59.7, 87.7) | 76.3 (62.8, 89.8) | 0.91 | 72.7 (54.5, 86.7) | 80.0 (61.4, 92.3) | 0.56 | |
| 24 weeks | 55.3 (39.5, 71.1) | 68.4 (53.6, 83.2) | 0.24 | 54.5 (37.6, 71.5) | 70.0 (53.6, 86.4) | 0.21 | |
| MSQoL-54 mental | 5.0 27 | ||||||
| 12 weeks | 47.4 (31.5, 63.2) | 68.4 (53.6, 83.2) | 0.06 | 45.5 (28.1, 63.7) | 74.2 (55.4, 88.1) | 0.02 | |
| 24 weeks | 36.8 (21.5, 52.2) | 52.6 (36.8, 68.5) | 0.17 | 36.4 (20.0, 52.8) | 58.1 (40.7, 75.4) | 0.08 | |
| MSQoL-54 physical | 5.0 27 | ||||||
| 12 weeks | 50.0 (34.1, 65.9) | 60.5 (45.0, 76.1) | 0.36 | 48.5 (30.8, 66.5) | 61.3 (42.2, 78.2) | 0.33 | |
| 24 weeks | 42.1 (26.4, 57.8) | 50.0 (34.1, 65.9) | 0.49 | 39.4 (22.7, 56.1) | 54.8 (37.3, 72.4) | 0.22 | |
| 6MWT | 6% 28 | ||||||
| 12 weeks | 0.0 (0.0, 0.0) | 5.4 (0.7, 18.2) | 0.49 | 0.0 (0.0, 0.0) | 6.9 (0.9, 22.8) | 0.22 | |
| 24 weeks | 0.0 (0.0, 0.0) | 3.1 (0.1, 16.2) | 0.48 | 0.0 (0.0, 0.0) | 4.0 (0.1, 20.3) | 0.45 | |
FSS: Fatigue Severity Scale; MFIS: Modified Fatigue Impact Scale; MSQoL-54: Multiple Sclerosis Quality of Life-54 Scale; NA: not applicable; 6MWT: 6-minute walk test.
aThershold value corresponds to clinically meaningful change in outcome.
bBetween-group significance determined by Pearson’s chi-square test.
Discussion
The findings from this trial confirm those of preliminary trials that the Wahls and Swank diets are associated with significant reductions in fatigue and improvements in QoL among RRMS participants. In both diet groups, between 1/2 to 3/4 of participants reported clinically meaningful reductions in fatigue, depending on the scale used, at 12 weeks and were maintained by most individuals at 24 weeks despite receiving no active RD support in the final 12 weeks. In the Wahls group, the 12-week change in FSS scores (-0.71 ± 0.24) is approximately half the magnitude compared to findings from the 12-week preliminary trial 20 ; however, the 24-week change in FSS scores in this study (-1.31 ± 0.29) is similar to the preliminary findings. Similarly, the 12- and 24-week FSS differences among the Swank group (-0.94 ± 0.18 and -1.01 ± 0.24, respectively) confirm the three- and six-month changes observed in the plant-based low-fat diet preliminary trial. 17 Furthermore, FSS changes in both groups are similar to those observed in pharmacological interventions for MS-related fatigue, 4 suggesting significant benefit of these two dietary interventions for MS-related fatigue.
Given fatigue is inversely associated with QoL among individuals with MS, 3 it is not surprising that significant improvements in QoL were also observed in this study. The 12-week change in mean values from baseline in both mental and physical MSQoL-54 scores among the Wahls group (11.3 ± 2.79 and 14.5 ± 2.63, respectively) confirm preliminary findings. 20 The Swank group experienced greater improvement in the physical compared to the mental MSQoL-54 subscale, which corresponds well to preliminary findings.16,17 These findings demonstrate that adoption of the Swank or Wahls diets is associated with reduced fatigue and improved QoL for up to 24 weeks.
High diet adherence was observed in both groups at 12 and 24 weeks, demonstrating that these dietary approaches can be adopted with RD support and then maintained without support. The mechanism by which diet affects MS-related fatigue and QoL is not known; however, results from preliminary trials suggest that modulation of inflammation or oxidative stress is likely.17,20 In addition, two trials show that dietary modification improves the mass and diversity of the gut microbiota in people with MS.31,32 Evidence suggests that people with MS have reduced mass and diversity of their gut microbiota compared to healthy controls, 31 which likely promotes inflammation. 33 There are currently no studies investigating changes in inflammatory profiles or gut microbiota following adoption of the Swank or Wahls diets; however, both diets are rich in fiber and plant-derived phytochemicals that are known to be beneficial to the gut microbiota 34 and modulate neuroinflammation. 35
Due to the significant and consistent improvements observed in both groups in this study, it is important to consider how these two diets are similar rather than how they differ. Both diets include recommendations for high intake of fruits, vegetables, and unsaturated fats and for limited intake of highly processed foods. These recommendations are consistent with diet quality indexes that are associated with reduced symptom burden in observational studies. 36
The consistency of run-in and baseline values, similar conclusions from the primary and secondary analyses, and the use of a blinded statistician are strengths of this study. This study is limited by the short duration of the intervention, the use of participant-reported data for primary and secondary outcomes, lack of racial/ethnic diversity in the study participants, the lack of a usual diet comparison group, and that dietary adherence was not evaluated with a biomarker. Due to these limitations and the wide range of exclusion criteria for this study, the generalizability of the observed findings is limited to fatigued individuals with RRMS.
The observation that both dietary approaches in this study are associated with significant reductions in fatigue and improvements in QoL greatly benefits the MS community in that it allows for patient preference for either diet and suggests that the benefits of dietary approaches are due to underlying mechanisms rather than unique characteristics of specific diets. This study was unable to show significant improvements in the 6-minute walk test at 12-weeks; however, the Wahls group in the primary analysis and both groups in the secondary analysis showed significant increases in meters walked during the 6MWT at 24-weeks. It is possible that walking endurance takes longer to develop than participant-reported outcomes such as fatigue and QoL with dietary interventions. The results from this study provide justification for future randomized controlled trials with larger sample sizes and longer duration to evaluate changes in brain MRI-evaluated disease activity and exploration of underlying mechanisms by which diet may affect the MS disease course.
Supplemental Material
Supplemental material, sj-pdf-1-mso-10.1177_20552173211035399 for Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: The WAVES randomized parallel-arm clinical trial by Terry L Wahls Tyler J Titcomb Linda MRubenstein Warren G Darling Linda G Snetselaar in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-pdf-2-mso-10.1177_20552173211035399 for Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: The WAVES randomized parallel-arm clinical trial by Terry L Wahls Tyler J Titcomb Linda MRubenstein Warren G Darling Linda G Snetselaar in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-pdf-3-mso-10.1177_20552173211035399 for Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: The WAVES randomized parallel-arm clinical trial by Terry L Wahls Tyler J Titcomb Linda MRubenstein Warren G Darling Linda G Snetselaar in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-pdf-4-mso-10.1177_20552173211035399 for Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: The WAVES randomized parallel-arm clinical trial by Terry L Wahls Tyler J Titcomb Linda MRubenstein Warren G Darling Linda G Snetselaar in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-pdf-5-mso-10.1177_20552173211035399 for Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: The WAVES randomized parallel-arm clinical trial by Terry L Wahls Tyler J Titcomb Linda MRubenstein Warren G Darling Linda G Snetselaar in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Acknowledgments
The authors would like to acknowledge the following contributors: Mary Ehlinger for her role in study recruitment and as the primary study coordinator; Karen Smith for her role in nutrition data acquisition and as an intervention dietitian; Lisa Brooks for her role as an intervention dietitian; Michaella Edwards for her role in study coordination and data acquisition; Zaidoon Al-Share for his role in data acquisition; and the students who supported recruitment and data acquisition. The authors also thank Kristina Greiner for her editing assistance.
Authors’ contribution: The authors’ responsibilities were as follows: TJT cleaned data and wrote the first draft of the manuscript; PTE performed all blinded statistical analyses; LR managed unblinded data and confirmed statistical analyses; BB, LC, WD, KH, and JK trained staff, assisted in data acquisition and interpretation, and oversaw study procedures; JK was the study team neurologist who confirmed diagnoses; TW and LS designed the study and oversaw all study procedures. All authors have read and approve the final version of the manuscript.
Conflict of Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Terry Wahls personally follows and promotes the Wahls™ diet. She has equity interest in the following companies: Terry Wahls LLC; TZ Press LLC; The Wahls Institute, PLC; FBB Biomed Inc; and the website http://www.terrywahls.com. She also owns the copyright to the books Minding My Mitochondria (2nd edition) and The Wahls Protocol, The Wahls Protocol Cooking for Life, and the trademarks The Wahls Protocol® and Wahls™ diet, Wahls Paleo™ diet, and Wahls Paleo Plus™ diets (the Wahls elimination diet is not trademarked). She has completed grant funding from the National Multiple Sclerosis Society for the Dietary Approaches to Treating Multiple Sclerosis Related Fatigue Study. She has financial relationships with BioCeuticals, MCG Health LLC, Genova Diagnostics, and the Institute for Functional Medicine. She receives royalty payments from Penguin Random House. Dr. Wahls has conflict of interest management plans in place with the University of Iowa and the Iowa City VA Health Care System. All other authors report no personal or financial conflicts of interest in this work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by the National Multiple Sclerosis Society grant RG-1506-04312 (TW), the Institute for Clinical and Translational Science (ICTS) at the University of Iowa, and University of Iowa institutional funds. The ICTS is supported by the National Institutes of Health Clinical and Translational Science Award program (grant UL1TR002537). TJT is a research trainee of the University of Iowa Fraternal Order of Eagles Diabetes Research Center (T32DK112751-01) and is supported by the Carter Chapman Shreve Family Foundation and the Carter Chapman Shreve Fellowship Fund for diet and lifestyle research conducted by the Wahls Research team at the University of Iowa. In-kind support was provided by the University of Iowa College of Public Health Preventive Intervention Center.
ORCID iD: Tyler J Titcomb https://orcid.org/0000-0002-8162-4768
Supplemental material: Supplementary material for this article is available online.
Contributor Information
Terry L Wahls, Department of Internal Medicine, University of Iowa, Iowa City, IA, USA.
Tyler J Titcomb, Department of Internal Medicine, University of Iowa, Iowa City, IA, USA; Department of Epidemiology, University of Iowa, Iowa City, IA, USA.
Babita Bisht, Department of Internal Medicine, University of Iowa, Iowa City, IA, USA.
Patrick Ten Eyck, Institute for Clinical and Translational Science, University of Iowa, Iowa City, IA, USA.
Linda M Rubenstein, Department of Epidemiology, University of Iowa, Iowa City, IA, USA.
Warren G Darling, Department of Health and Human Physiology, University of Iowa, Iowa City, IA, USA.
Karin F Hoth, Department of Psychiatry, University of Iowa, Iowa City, IA, USA.
John Kamholz, Department of Neurology, University of Iowa, Iowa City, IA, USA.
References
- 1.Wallin MT, Culpepper WJ, Campbell JD, et al.; US Multiple Sclerosis Prevalence Workgroup. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology 2019; 92: e1029–e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krupp LB, Serafin DJ, Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother 2010; 10: 1437–1447. [DOI] [PubMed] [Google Scholar]
- 3.Hadjimichael O, Vollmer T, Oleen-Burkey M; North American Research Committee on Multiple Sclerosis. North American research committee on multiple S. Fatigue characteristics in multiple sclerosis: the North American research committee on multiple sclerosis (NARCOMS) survey. Health Qual Life Outcomes 2008; 6: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang TT, Wang L, Deng XY, et al. Pharmacological treatments for fatigue in patients with multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci 2017; 380: 256–261. [DOI] [PubMed] [Google Scholar]
- 5.Evans E, Levasseur V, Cross AH, et al. An overview of the current state of evidence for the role of specific diets in multiple sclerosis. Mult Scler Relat Disord 2019; 36: 101393. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald KC, Tyry T, Salter A, et al. A survey of dietary characteristics in a large population of people with multiple sclerosis. Mult Scler Relat Disord 2018; 22: 12–18. [DOI] [PubMed] [Google Scholar]
- 7.Russell RD, Lucas RM, Brennan V, et al. Reported changes in dietary behavior following a first clinical diagnosis of central nervous system demyelination. Front Neurol 2018; 9: 161 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell RD, Black LJ, Begley A. The unresolved role of the neurologist in providing dietary advice to people with multiple sclerosis. Mult Scler Relat Disord 2020; 44: 102304. [DOI] [PubMed] [Google Scholar]
- 9.Russell RD, Black LJ, Sherriff JL, et al. Dietary responses to a multiple sclerosis diagnosis: a qualitative study. Eur J Clin Nutr 2019; 73: 601–608. [DOI] [PubMed] [Google Scholar]
- 10.Beckett JM, Bird ML, Pittaway JK, et al. Diet and multiple sclerosis: scoping review of web-based recommendations. Interact J Med Res 2019; 8: e10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swank RL. Multiple sclerosis; a correlation of its incidence with dietary fat. Am J Med Sci 1950; 220: 421–430. [PubMed] [Google Scholar]
- 12.Swank RL. Treatment of multiple sclerosis with low-fat diet. AMA Arch Neurol Psychiatry 1953; 69: 91–103. [DOI] [PubMed] [Google Scholar]
- 13.Swank RL, Goodwin J. Review of MS patient survival on a swank low saturated fat diet. Nutrition 2003; 19: 161–162. [DOI] [PubMed] [Google Scholar]
- 14.Swank RL. Multiple sclerosis: twenty years on low fat diet. Arch Neurol 1970; 23: 460–474. [DOI] [PubMed] [Google Scholar]
- 15.Swank RL. Treatment of multiple sclerosis with low-fat diet; results of five and one-half years' experience. AMA Arch Neurol Psychiatry 1955; 73: 631–644. [DOI] [PubMed] [Google Scholar]
- 16.Weinstock-Guttman B, Baier M, Park Y, et al. Low fat dietary intervention with omega-3 fatty acid supplementation in multiple sclerosis patients. Prostaglandins Leukot Essent Fatty Acids 2005; 73: 397–404. [DOI] [PubMed] [Google Scholar]
- 17.Yadav V, Marracci G, Kim E, et al. Low-fat, plant-based diet in multiple sclerosis: a randomized controlled trial. Mult Scler Relat Disord 2016; 9: 80–90. [DOI] [PubMed] [Google Scholar]
- 18.Bisht B, Darling WG, Shivapour ET, et al. Multimodal intervention improves fatigue and quality of life in subjects with progressive multiple sclerosis: a pilot study. Degener Neurol Neuromuscul Dis 2015; 5: 19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bisht B, Darling WG, White EC, et al. Effects of a multimodal intervention on gait and balance of subjects with progressive multiple sclerosis: a prospective longitudinal pilot study. DNND 2017; 7: 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irish AK, Erickson CM, Wahls TL, et al. Randomized control trial evaluation of a modified paleolithic dietary intervention in the treatment of relapsing-remitting multiple sclerosis: a pilot study. Degener Neurol Neuromuscul Dis 2017; 7: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JE, Titcomb TJ, Bisht B, et al. A modified MCT-based ketogenic diet increases plasma beta-hydroxybutyrate but has less effect on fatigue and quality of life in people with multiple sclerosis compared to a modified paleolithic diet: a waitlist-controlled, randomized pilot study. J Am Coll Nutr 2021; 40: 13– 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wahls T, Scott MO, Alshare Z, et al. Dietary approaches to treat MS-related fatigue: comparing the modified paleolithic (Wahls elimination) and low saturated fat (swank) diets on perceived fatigue in persons with relapsing-remitting multiple sclerosis: study protocol for a randomized controlled trial. Trials 2018; 19: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Hopewell S, Schulz KF, et al.; Consolidated Standards of Reporting Trials Group. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010; 63: e1-37–37. [DOI] [PubMed] [Google Scholar]
- 24.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahls TL, Chenard CA, Snetselaar LG. Review of two popular eating plans within the multiple sclerosis community: low saturated fat and modified paleolithic. Nutrients 2019; 11: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rooney S, McFadyen DA, Wood DL, et al. Minimally important difference of the fatigue severity scale and modified fatigue impact scale in people with multiple sclerosis. Mult Scler Relat Disord 2019; 35: 158–163. [DOI] [PubMed] [Google Scholar]
- 27.Jongen PJ. Health-related quality of life in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs 2017; 31: 585–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baert I, Freeman J, Smedal T, et al. Responsiveness and clinically meaningful improvement, according to disability level, of five walking measures after rehabilitation in multiple sclerosis: a European multicenter study. Neurorehabil Neural Repair 2014; 28: 621–631. [DOI] [PubMed] [Google Scholar]
- 29.Mcculloch CE and Neuhau JM. Generalized linear mixed models. In: Balakrishnan N, Colton T, Everitt B, et al. (eds) Wiley StatsRef: Statistics Reference Online, 2014. 10.1002/9781118445112.stat07540 [DOI] [Google Scholar]
- 30.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr 1974; 19: 716–723. [Google Scholar]
- 31.Swidsinski A, Dorffel Y, Loening-Baucke V, et al. Reduced mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with ketogenic diet. Front Microbiol 2017; 8: 1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saresella M, Mendozzi L, Rossi V, et al. Immunological and clinical effect of diet modulation of the gut microbiome in multiple sclerosis patients: a pilot study. Front Immunol 2017; 8: 1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickard JM, Zeng MY, Caruso R, et al. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev 2017; 279: 70–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riccio P, Rossano R. Diet, gut microbiota, and vitamins D + a in multiple sclerosis. Neurotherapeutics 2018; 15: 75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Song Y, Chen Z, et al. Connection between systemic inflammation and neuroinflammation underlies neuroprotective mechanism of several phytochemicals in neurodegenerative diseases. Oxid Med Cell Longev 2018; 2018: 1972714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fitzgerald K, Mische L, Beier M, et al. Diet quality is associated with mobility and cognitive function in people with multiple sclerosis. Mult Scler J 2018; 24: 144–145. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-mso-10.1177_20552173211035399 for Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: The WAVES randomized parallel-arm clinical trial by Terry L Wahls Tyler J Titcomb Linda MRubenstein Warren G Darling Linda G Snetselaar in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-pdf-2-mso-10.1177_20552173211035399 for Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: The WAVES randomized parallel-arm clinical trial by Terry L Wahls Tyler J Titcomb Linda MRubenstein Warren G Darling Linda G Snetselaar in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-pdf-3-mso-10.1177_20552173211035399 for Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: The WAVES randomized parallel-arm clinical trial by Terry L Wahls Tyler J Titcomb Linda MRubenstein Warren G Darling Linda G Snetselaar in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-pdf-4-mso-10.1177_20552173211035399 for Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: The WAVES randomized parallel-arm clinical trial by Terry L Wahls Tyler J Titcomb Linda MRubenstein Warren G Darling Linda G Snetselaar in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-pdf-5-mso-10.1177_20552173211035399 for Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: The WAVES randomized parallel-arm clinical trial by Terry L Wahls Tyler J Titcomb Linda MRubenstein Warren G Darling Linda G Snetselaar in Multiple Sclerosis Journal – Experimental, Translational and Clinical