Abstract
The incidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exposure in shared patient rooms was low at our institution: 1.8 per 1,000 shared-room patient days. However, the secondary attack rate (21.6%) was comparable to that reported in household exposures. Lengthier exposures were associated with SARS-CoV-2 conversion. Hospitals should implement measures to decrease shared-room exposures.
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Because patients with COVID-19 may be asymptomatic or presymptomatic (in their incubation period) at the time of hospital admission, early identification and isolation are ideal but challenging.1,2
Patients hospitalized for reasons other than COVID-19 may be placed in shared rooms. Shared rooms maximize the number of patients able to receive care but have been shown to increase the risk of hospital-associated infections.3 Few studies have quantified SARS-CoV-2 exposures and transmission in shared-patient rooms. We investigated the incidence of patient-to-patient SARS-CoV-2 exposures and subsequent conversion associated with shared rooms.
Methods
The University of Iowa Hospitals & Clinics (UIHC) is an 811-bed tertiary-care center, and 16% of patient days are spent in shared rooms. During July 2020–May 2021, healthcare personnel wore personal protective equipment (PPE) for all patient contact (minimum of a medical-grade mask and eye protection). Most patients did not wear a mask at all times while in their room. During this time, we tried to minimize aerosol-generating procedures (AGPs), especially in shared rooms.
We performed active SARS-CoV-2 surveillance for inpatients: nasopharyngeal SARS-CoV-2 polymerase chain reaction (PCR) testing at admission and every 5 days thereafter. Samples were tested using the ThermoFisher TaqPath PCR assay (Thermo Fisher, Waltham, MA) targeting ORF1ab, N gene, and S gene. All positive SARS-CoV-2 PCR results for inpatients were reviewed by the program of hospital epidemiology: We reviewed the electronic health record (Epic, Verona WI) for symptoms, imaging results, and history of any previous positive SARS-CoV-2 results. We then used the Epic patient-trace function to determine whether a patient had been in a shared room during their infectious period (2 days before symptom onset or first positive SARS-CoV-2 PCR if asymptomatic). Symptomatic patients were deemed infectious regardless of cycle threshold (Ct) values. Because SARS-CoV-2 PCR can remain positive for months after an acute infection, we assessed infectiousness of asymptomatic patients using Ct values of 2 PCR tests (collected at least 24 hours apart), SARS-CoV-2 serology results, and clinical history.1,2 Asymptomatic patients were categorized as infectious (Ct values <30 in at least 1 sample) or noninfectious if 2 samples had Ct values ≥30.1,4 Other evidence of being noninfectious was a positive SARS-CoV2 IgG or history of a positive result in the previous 90 days.
Roommates were considered to have been exposed if they shared a room with an infectious patient during that patient’s infectious period. Patients with a positive SARS-CoV-2 test within 90 days of the exposure were considered not exposed. Exposed patients were notified, quarantined (private room for inpatients, home quarantine if discharged), and follow-up testing was arranged following CDC and public health guidelines. Conversion was defined as having a negative test ≤2 days before exposure or after, followed by a subsequent positive within 14 days after exposure.
We calculated the incidence of SARS-CoV-2 exposures: number of exposed patients in shared rooms divided by the number of shared-room patient days (ie, hospitalization days in shared patient rooms). The secondary attack rate was calculated as number of exposed patients in shared rooms who converted divided by the number of exposed roommates in a shared room with follow-up data. Shared-room–associated COVID-19 incidence was calculated as the incidence of SARS-CoV-2 exposures multiplied by the secondary attack rate.
The distance between beds (head center to head center) were retrospectively measured for all rooms included in the study. Air exchanges for each room were retrospectively measured by taking 2 separate measurements 1 week apart and averaging the measurements. Airflow was measured with a certified balometer (Shortridge Instruments, Scottsdale, AZ), and measurements in cubic feet per minute (CFM) were converted to air changes per hour (ACH) = (CFM×60) divided by room volume.
We performed univariable logistic regression with clinically significant variables using Stata statistical software (StataCorp, College Station, TX). Characteristics of exposed patients who converted were compared to those who did not. This study was approved by the Institutional Review Board of the University of Iowa.
Results
There were 38,142 shared-room patient days during July 2020–May 2021. We identified 53 COVID-19 inpatients who had a roommate during their infectious period, for a total of 70 exposed roommates. The incidence of SARS-CoV-2 exposure in a shared room was 1.8 per 1,000 shared-room patient days. In total, 37 (52.9%) exposed roommates completed follow-up testing. Of these, 8 (21.6%) converted. The median time to conversion was 5 days (range, 2–11). The incidence of conversion after exposure was 0.4 per 1,000 shared-room patient days. None of the conversions were related to an AGP. There were no statistically significant differences in age, sex, or presence of symptoms in infectious patients (source) between those who converted and those who did not (Table 1). Median exposure time was 1.9 days (range, 0.1–3 days) for converters and 0.6 days (range, 0.04–3.1) for nonconverters (odds ratio, 1.05; 95% confidence interval, 1.05–5.38).
Table 1.
Comparison of Patients Who Converted After Exposure and Those Who Did Not in A Tertiary-Care Center in Iowa, July 2020–May 2021
| Characteristic | Converted (N=8) | Did Not Convert (N=29) | Odds Ratio | 95% CI | ||
|---|---|---|---|---|---|---|
| Exposed patient characteristics | ||||||
| Median age, y (range) | 66.5 | (49–87) | 68 | (36–97) | 1.01 | 0.95–1.06 |
| Sex, male | 7 | (88%) | 20 | (69%) | 3.15 | 0.34–29.53 |
| Median exposure time, d (range) | 1.9 | (0.1–3) | 0.6 | (0.04–3.1) | 2.38 | 1.05–5.38 |
| Source patient characteristics | ||||||
| Median Ct value (range) | 19 | (9–29) | 23.5 | (9–37) | 0.94 | 0.83–1.06 |
| Detected with active surveillance | 5 | (63%) | 21 | (72%) | 0.63 | 0.12–3.30 |
| Symptomatic | 3 | (38%) | 14 | (48%) | 0.64 | 0.13–3.20 |
| Shared room characteristics | ||||||
| Median bed distance, meters (range) | 4.2 | (1.9–5.0) | 4.2 | (2.0–7.0) | 0.99 | 0.80–1.22 |
| Median air exchanges, ACH (range) | 3.1 | (0.1–4) | 3.3 | (0.1–12.5) | 0.76 | 0.46–1.27 |
Note. Ct, cycle threshold; ACH, air changes per hour.
Of the patients who converted, 3 were discharged before testing positive and 1 of these had a documented household exposure before converting. Also, 2 patients were located in a psychiatric unit during an outbreak and were possibly exposed to additional infectious patients. Of those who converted, 2 were asymptomatic, 5 had mild-to-moderate symptoms, and 1 had severe symptoms.
Discussion
The incidence of SARS-CoV-2 exposure in shared rooms was 1.8 per 1,000 shared-room patient days. Of exposed patients, 21.6% developed COVID-19. Lengthier exposures were associated with conversions. Shared rooms play a role in SARS-CoV-2 hospital transmission. Hospital design guidelines and infection prevention protocols should address and mitigate risks associated with these rooms.
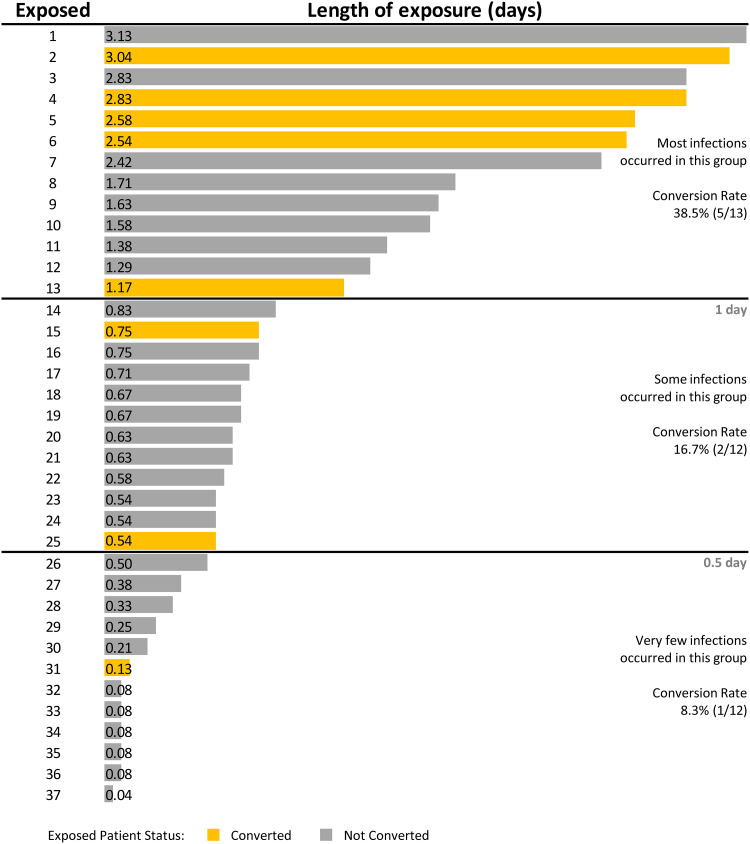
Although the incidence of SARS-CoV-2 exposures was low, we demonstrated that 21.6% of exposed patients in shared rooms subsequently developed COVID-19. This secondary attack rate is comparable to estimated SARS-CoV-2 household transmission rates of 16.6%, 29%, and 32.4% reported in recent studies.5–7 Madewell et al5 revealed that household secondary attack rates were higher for symptomatic index cases (18%) than for asymptomatic index cases (0.7%). However, we did not detect a difference in symptoms among source patients. Instead, we demonstrated that lengthier exposures were associated with conversion. Most conversions occurred after exposure times ≥1 day. Only 1 patient converted after an exposure ≤0.5 day (Fig. 1). Munier-Marion et al8 reported that the incidence of hospital-associated influenza in a shared room was 2 per 100 patient days compared to 0.7 per 100 patient days in a private room.8 Our study reported a much lower incidence of acquiring SARS-CoV-2 in a shared room: 0.4 per 1,000 shared-room patient days. This lower finding can likely be attributed to the implementation of extensive COVID-19 surveillance, including admission screening and serial testing.
Fig. 1.
Duration of shared room exposures and subsequent conversions in a tertiary-care center in Iowa, July 2020–May 2021.
We were unable to ascertain with certainty how patients who converted acquired COVID-19. Fomite transmission of SARS-CoV-2 has been investigated in recent studies. Kraay et al9 reported that transmission varies in different settings and by surface. Environmental surfaces in all patient rooms and bathrooms are cleaned daily, and recent data show that fomites do not play a large role in SARS-CoV-2 transmission.10 None of the source patients of those who converted had an AGP, and we were unable to investigate the role AGPs played in SARS-CoV-2 transmission in shared rooms. Airborne transmission is likely and depends, we believe, on exposure duration, room area, and air exchanges. Hospitals with shared rooms may want to evaluate these characteristics to reduce transmission. Vaccination status could also be used to determine who is placed in a shared room.
This study has several limitations. Quantitative data on patient masking compliance in rooms were not available, although compliance was known to be low. Visitor information and detailed exposure data, such as patient behaviors which may have increased infection risk, were not collected. Despite being provided quarantine information, discharged patients may have had additional exposures outside the hospital. We were unable to perform whole-genome sequencing on samples to confirm that transmission occurred in shared rooms. Ct values have limitations including sample collection, transport, instrument, and technologist variability. However, these processes were standardized at UIHC during the study period. Symptomatic patients were deemed infectious regardless of Ct values. A sensitivity analysis was performed after removal of symptomatic patients with Ct values ≥30 without a significant effect in our findings. A larger sample size might have shown an association between conversion and air exchanges, Ct values, and bed distance (Supplemental Fig. 2).
In conclusion, COVID-19 exposures in shared patient rooms were infrequent. However, the secondary attack rate was similar to that reported in households. Exposure duration was associated with conversion. Hospitals with shared rooms should consider active COVID-19 surveillance, decrease exposure times, and use vaccination status for bed placement.
Acknowledgments
We thank the University of Iowa Hospital Incident Command System for their support during the COVID-19.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/ice.2021.313.
click here to view supplementary material
References
- 1. Kobayashi T, Trannel A, Holley SA, et al. COVID-19 serial testing among hospitalized patients in a midwest tertiary medical center, July–September 2020. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020;26:672–675. [DOI] [PubMed] [Google Scholar]
- 3. Stiller A, Salm F, Bischoff P, Gastmeier P. Relationship between hospital ward design and healthcare-associated infection rates: a systematic review and meta-analysis. Antimicrob Resist Infect Control 2016;5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rhee C, Baker MA, Kanjilal S, et al. Prospective clinical assessments of hospitalized patients with positive SARS-CoV-2 PCR tests for necessity of isolation. Open Forum Infect Dis 2021. doi: 10.1093/ofid/ofab194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open 2020;3(12):e2031756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lewis NM, Chu VT, Ye D, et al. Household transmission of SARS-CoV-2 in the United States. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa1166. [DOI] [Google Scholar]
- 7. Wu J, Huang Y, Tu C, et al. Household transmission of SARS-CoV-2, Zhuhai, China, 2020. Clin Infect Dis 2020;71:2099–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Munier-Marion E, Benet T, Regis C, Lina B, Morfin F, Vanhems P. Hospitalization in double-occupancy rooms and the risk of hospital-acquired influenza: a prospective cohort study. Clin Microbiol Infect 2016;22:461e7–461e 9. [DOI] [PubMed] [Google Scholar]
- 9. Kraay ANM, Hayashi MAL, Berendes DM, Sobolik JS, Leon JS, Lopman BA. Risk for fomite-mediated transmission of SARS-CoV-2 in child daycares, schools, nursing homes, and offices. Emerg Infect Dis 2021;27:1229–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Science Brief: SARS-CoV-2 and surface (fomite) transmission for indoor community environments. Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/surface-transmission.html. Updated April 5, 2021. Accessed July 13, 2021. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/ice.2021.313.
click here to view supplementary material