Abstract
Introduction
Diabetes mellitus causes serious complications such as diabetic nephropathy. Diabetic nephropathy is now the most common reason of chronic kidney disease. Inflammation plays a crucial role in development and progression of diabetic nephropathy. The aim of this study was to evaluate the relationship of Inflammatory markers (neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio) with diabetic nephropathy in Syrian patients.
Materials and methods
A total of 158 patients with type 2 diabetes mellitus were distributed into three groups according to urinary albumin-to-creatinine ratio: Group A, type 2 diabetic patients with normoalbuminuria (urinary albumin-to-creatinine ratio <30 mg/g); Group B, type 2 diabetic patients with microalbuminuria (urinary albumin-to-creatinine ratio = 30–300 mg/g); Group C, type 2 diabetic patients with macroalbuminuria (urinary albumin-to-creatinine ratio ≥300 mg/g). Levels of inflammatory markers (neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio) were recorded and compared among the three groups.
Results
Significant differences were detected between the groups in terms of neutrophil-to-lymphocyte ratio (p = 0.000) and platelet-to-lymphocyte ratio (p = 0.000). Receiver operating characteristic curve analysis of inflammatory markers and microalbuminuria prediction demonstrated an area under curve (AUC) of 0.869 for neutrophil-to-lymphocyte ratio (confidence interval: 0.813–0.926, p = 0.000) and 0.739 for platelet-to-lymphocyte ratio (confidence interval: 0.662–0.815, p = 0.000).
Conclusion
Increased neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio were significantly correlated with diabetic nephropathy, and high neutrophil-to-lymphocyte ratio & platelet-to-lymphocyte ratio may be served as a predictor and a prognostic risk marker of diabetic nephropathy.
Keywords: Diabetic nephropathy, Urinary albumin-to-creatinine ratio, Neutrophil-to-lymphocyte ratio, Platelet-to-lymphocyte ratio
Diabetic nephropathy, Urinary albumin-to-creatinine ratio, Neutrophil-to-lymphocyte ratio, Platelet-to-lymphocyte ratio.
1. Introduction
Diabetes Mellitus (DM) is a serious threat to global health with an increasing prevalence and incidence rates. The number of people who had DM was 463 million in 2019. It is estimated that this number will have reached 700 million by 2045 according to International Diabetes Federation (IDF) [1].
DM is a chronic metabolic disease characterized by high sugar levels (hyperglycemia) due to impairment of insulin secretion, cellular resistance to insulin or both [2]. DM is classified into two major types; type-1 in which the pancreas is unable to produce insulin and type -2 in which insulin secretion is not adequate or the body is unable to respond to it proficiently [3]. DM causes serious complications such as diabetic nephropathy (DN), diabetic retinopathy, and diabetic neuropathy (microvascular complications) in addition to stroke, cardiovascular diseases (CVDs), and peripheral vascular diseases (macrovascular complications) [4, 5].
DN or diabetic kidney disease is a syndrome described by the presence of pathological amounts of proteinuria, diabetic glomerular lesions, and decrease of glomerular filtration rate (GFR) in diabetic patients [6]. DN is now the most common reason of chronic kidney disease (CKD) [7], yet DN pathogenesis is not fully understood. Both types of diabetes can cause chronic kidney disease and eventually end – stage renal disease (ESRD) [9]. But the prevalence of type 2 diabetes is much higher than type 1, often patients with ESRD have type 2 diabetes [10]. An increase in urinary albumin excretion is a clinical manifestation for DN, starting from microalbuminuria to macroalbuminuria and eventually ESRD [8, 9]. Current diagnosis of DN is depended on albuminuria as a biomarker [11]. However, its diagnostic value in early-stage DN is limited because renal injury commonly precedes urinary albumin secretion [6].
Inflammation plays a crucial role in development and progression of DN, as many inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-8 (IL-18), and tumor necrosis factor α (TNF-α) contribute in the pathogenesis of DN [12]. However, the measurement of these inflammatory markers is not used in daily clinical practice because of their costs and technical difficulties in application [13].
Total and differential white blood cell (WBC) count is a sensitive indicator of inflammation which can be done easily in laboratory. It is a cost-effective and routine test. In this respect, neutrophil-to-lymphocyte ratio (NLR) has emerged as a novel alternative marker [11].
Furthermore, Turkmen et al. mentioned that platelets may involve in atherosclerosis through secreting proinflammatory cytokines and its bounding to endothelial cells [14]. Platelets release the thromboxanes and other mediators, this may cause increased inflammation in patients with higher platelets [15].
NLR and platelet-to-lymphocyte ratio (PLR) have been proposed as surrogate markers for endothelial dysfunction and inflammation, so that the present study aimed to evaluate the association of NLR and PLR with DN in Syrian patients and whether or not NLR and PLR can be used as predictors and prognostic risk markers of DN to lower the development of the complications upon uncontrolled diabetic patients, and to help doctors to give attention to their patients early.
2. Materials and methods
2.1. Design and sample
Many medical records of patients with diabetes from the outpatient clinics at Al-Assad University Hospital, Damascus, Syria, during the years 2017–2019 were reviewed and examined without any bias. The data from 158 patients of these records with type 2 diabetes mellitus (T2DM) met the inclusion criteria of the study. This study included patients who had T2DM for at least 4 years.
Exclusion Criteria: Patients with diabetes type 1, acute and chronic infection or inflammation, cancer, medications affecting on the number of leukocytes (such as steroids, etc.), hypertension, heart diseases, systematic disorders, any other known renal diseases, blood diseases, autoimmune disorders, diseases affecting urinary protein excretion, dehydration, low glomerular filtration rate without microalbuminuria and alcohol.
This study was approved by Human Research Ethics Committee at both Faculty of Pharmacy, Damascus University and Al-Assad University Hospital. Patients were classified into three groups according to urinary albumin-to-creatinine ratio (UACR) as follows: Group A, type 2 diabetic patients with normoalbuminuria (UACR <30 mg/g); Group B, type 2 diabetic patients with microalbuminuria (UACR = 30–300 mg/g); Group C, type 2 diabetic patients with macroalbuminuria (UACR ≥300 mg/g).
Demographic, clinical, and laboratory data were recorded from patient file records regarding age, gender, smoking, weight, height, duration of diabetes, hemoglobin A1c (HbA1c), blood pressure, serum urea, serum creatinine, serum albumin, UACR, glomerular filtration rate (GFR), C- reactive protein (CRP) and complete blood count (CBC) including total white blood cell count (WBC), neutrophil count, lymphocyte count, neutrophil/lymphocyte ratio (NLR), monocyte count, total red blood cell count (RBC), hemoglobin (Hb), platelet count (PLT), and platelet/lymphocyte ratio (PLR).
Body mass index (BMI) (kg/m2) was calculated using the following formula BMI = weight/(height)2. The estimated GFR was calculated according to the Modification of Diet in Renal Disease (MDRD) formula. Assessment of NLR by dividing the absolute neutrophil count on the absolute lymphocyte count, and PLR by dividing the absolute platelet count on the absolute lymphocyte count. For each patient, two last consecutive blood tests were evaluated and checked for consistency of the parameters recorded, to exclude irregularities.
Uncontrolled DM was considered when HbA1c > 7 %. Evaluation of DN (level of proteinuria) was done by examining UACR (mg albumin/g creatinine) in a spot urine sample to classify proteinuria; no proteinuria or normoalbuminuria (UACR <30 mg/g), microalbuminuria or early-stage DN (UACR = 30–300 mg/g), macroalbuminuria or overt diabetic nephropathy (UACR >300 mg/g) [16].
2.2. Statistical analysis
Continuous variables are presented as mean ± standard deviation, and categorical variables are given as frequencies and percentages. The Kolmogorov-Smirnov test was conducted to evaluate variable distribution. One-way analysis of variance (ANOVA) test was used to compare among the three study groups. Correlations were assessed using Pearson's test. Receiver operating characteristic (ROC) curve analysis was used to determine the optimum cutoff levels of inflammation markers NLR and PLR to predict microalbuminuria. P value <0.05 was considered statistically significant. Statistical Package for Social Science (SPSS for Windows, version 20.0; SPSS, Inc., Chicago, IL, USA) was used for statistical calculations.
3. Results
A total of 158 type 2 diabetic patients were enrolled in this study. The patients distributed into three groups according to their level of albumin-to-creatinine ratio, designated as normoalbuminuria (N = 67, 42.4%), microalbuminuria (N = 50, 31.6%) and macroalbuminuria (N = 41, 25.9%). Table 1 summaries one-way ANOVA results regarding the demographic, clinical, and laboratory characteristics of the study groups.
Table 1.
One-way ANOVA results among the study groups.
| Parameter | Normoalbuminuria (N = 67) Mean ± Std.Dev |
Microalbuminuria (N = 50) Mean ± Std.Dev |
Macroalbuminuria (N = 41) Mean ± Std.Dev |
P-value |
|---|---|---|---|---|
| Age (year) | 54 ± 10 | 58 ± 7 | 61 ± 6 | 0.000 |
| Male/Female (N) | 39/28 | 29/21 | 24/17 | NS |
| Smoking (Yes/No) | 27/40 | 21/29 | 16/25 | NS |
| BMI (kg/m2) | 27.66 ± 2.4 | 27.27 ± 2.37 | 27.5 ± 1.39 | NS |
| Duration of Diabetes (year) | 6.7 ± 1.48 | 10.32 ± 1.85 | 12 ± 1.88 | 0.000 |
| HbA1c (%) | 7.95 ± 1.49 | 8.55 ± 1.29 | 8.92 ± 1.35 | 0.002 |
| Systolic Blood Pressure (mmHg) | 12.16 ± 0.88 | 13 ± 0.76 | 14.37 ± 1.11 | 0.000 |
| Diastolic Blood Pressure (mmHg) | 8 ± 0 | 8.44 ± 0.76 | 8.93 ± 0.82 | 0.000 |
| Serum urea (mg/dl) | 23.91 ± 8.14 | 40.14 ± 12.54 | 41.95 ± 7.83 | 0.000 |
| Serum creatinine (mg/dl) | 0.73 ± 0.08 | 0.87 ± 0.14 | 1.04 ± 0.18 | 0.000 |
| Serum albumin (g/dl) | 4.25 ± 0.33 | 4.23 ± 0.37 | 4.16 ± 0.39 | NS |
| GFR (ml/min/1.73m2) | 107.7 ± 14.75 | 87.04 ± 12.2 | 70.32 ± 7.23 | 0.000 |
| WBC (×103/μl) | 7.39 ± 1.68 | 8.27 ± 1.82 | 8.92 ± 1.47 | 0.000 |
| Absolute Neutrophil count (/μl) | 4145.23 ± 1318.34 | 5183.63 ± 1457.24 | 5961.7 ± 1198.02 | 0.000 |
| Absolute Lymphocyte count (/μl) | 2448.04 ± 638.35 | 2290.26 ± 527.6 | 1992.93 ± 446.27 | 0.000 |
| NLR | 1.73 ± 0.47 | 2.3 ± 0.58 | 3.03 ± 0.46 | 0.000 |
| Absolute Monocyte count (/μl) | 430.8 ± 165.72 | 459.92 ± 149.76 | 454 ± 130.35 | NS |
| RBC (× 106/μl) | 4.82 ± 0.62 | 4.83 ± 0.39 | 4.62 ± 0.54 | NS |
| Hb (g/dl) | 13.4 ± 1.45 | 13 ± 2.6 | 11.9 ± 2.02 | 0.000 |
| PLT (×103/μl) | 232.67 ± 63.6 | 256.08 ± 67.72 | 276.93 ± 60.97 | 0.003 |
| PLR | 98.35 ± 27.15 | 115.45 ± 32.12 | 143.6 ± 35.95 | 0.000 |
| CRP (mg/dl) | 0.22 ± 0.14 | 0.55 ± 0.22 | 1.32 ± 0.33 | 0.000 |
| Albumin/creatinine (mg/g) | 9.90 ± 3.93 | 103.71 ± 65.15 | 530.40 ± 168.49 | 0.000 |
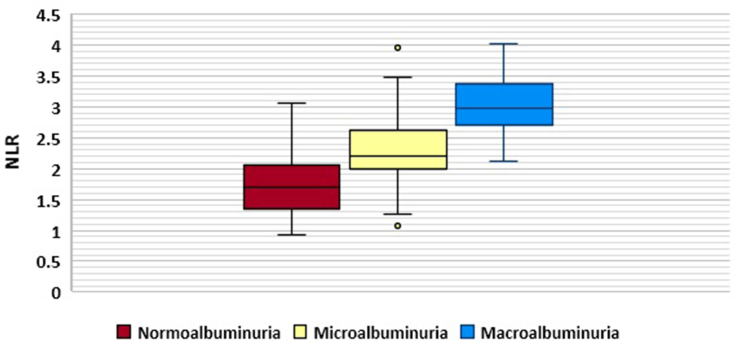
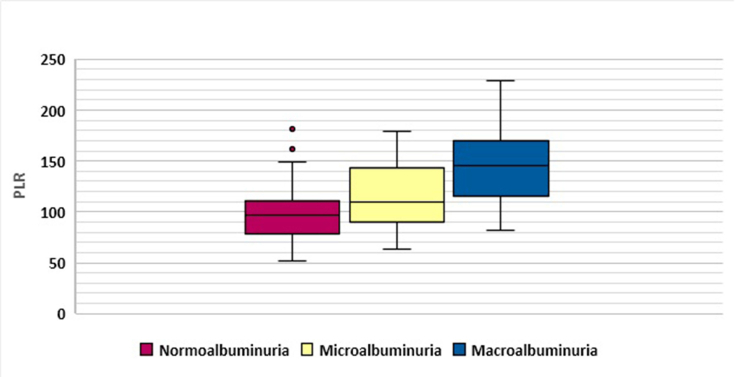
No significant difference was observed between the groups with regard to BMI, serum albumin, monocyte count, and RBC. However, there were significant differences among the three groups regarding age (p = 0.000), duration of diabetes (p = 0.000), HbA1c (p = 0.000), blood pressure (p = 0.000), serum urea (p = 0.000), serum creatinine (p = 0.000), GFR (p = 0.000), total WBC (p = 0.000), absolute neutrophil count (p = 0.000), absolute lymphocyte count (p = 0.000), Hb (p = 0.000), PLT (p = 0.003), and inflammatory markers NLR (p = 0.000), PLR (p = 0.000) and CRP (p = 0.000). Graphical representations of NLR and PLR distribution is shown by box plot in Figure 1 and Figure 2, respectively.
Figure 1.
Neutrophil –to-lymphocyte ratio (NLR) distribution in the study groups.
Figure 2.
Platelet –to-lymphocyte ratio (PLR) distribution in the study groups.
Univariate analysis (Pearson) found significant correlation between NLR and duration of diabetes, HbA1c, blood pressure, urea, creatinine, GFR, albumin/creatinine ratio, WBC, PLR, and CRP. Furthermore, Pearson test demonstrated significant correlation between PLR and duration of diabetes, HbA1c, blood pressure, creatinine, GFR, albumin/creatinine ratio, NLR, PLT, and CRP (Table 2).
Table 2.
Pearson's correlation analysis of NLR and PLR.
| Variable | NLR |
PLR |
||
|---|---|---|---|---|
| r | P value | r | P value | |
| BMI | -0.036 | 0.654 | 0.003 | 0.971 |
| Duration of Diabetes | 0.537 | 0.000 | 0.398 | 0.000 |
| HbA1c | 0.343 | 0.000 | 0.179 | 0.025 |
| Systolic Blood Pressure | 0.431 | 0.000 | 0.374 | 0.000 |
| Diastolic Blood Pressure | 0.410 | 0.000 | 0.305 | 0.000 |
| Serum urea | 0.407 | 0.000 | 0.133 | 0.096 |
| Serum creatinine | 0.537 | 0.000 | 0.184 | 0.021 |
| Serum albumin | -0.036 | 0.650 | -0.044 | 0.579 |
| Albumin/Creatinine | 0.659 | 0.000 | 0.374 | 0.000 |
| GFR | -0.626 | 0.000 | -0.408 | 0.000 |
| WBC | 0.431 | 0.000 | -0.100 | 0.213 |
| Neutrophil count | 0.660 | 0.000 | 0.023 | 0.773 |
| Lymphocyte count | -0.418 | 0.000 | -0.564 | 0.000 |
| NLR | 0.483 | 0.000 | ||
| RBC | -0.059 | 0.460 | -0.039 | 0.626 |
| Hb | -0.290 | 0.000 | -0.292 | 0.000 |
| PLT | 0.153 | 0.055 | 0.594 | 0.000 |
| PLR | 0.483 | 0.000 | ||
| CRP | 0.653 | 0.000 | 0.392 | 0.000 |
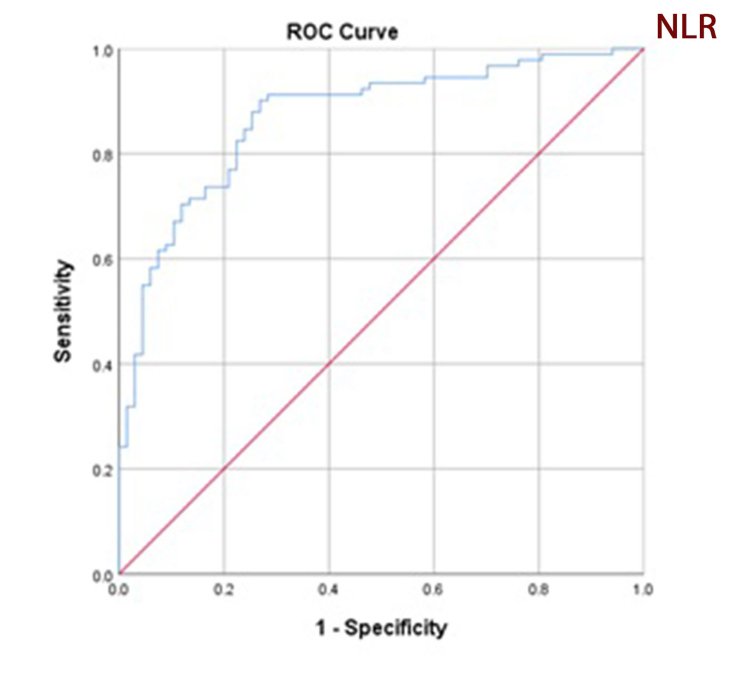
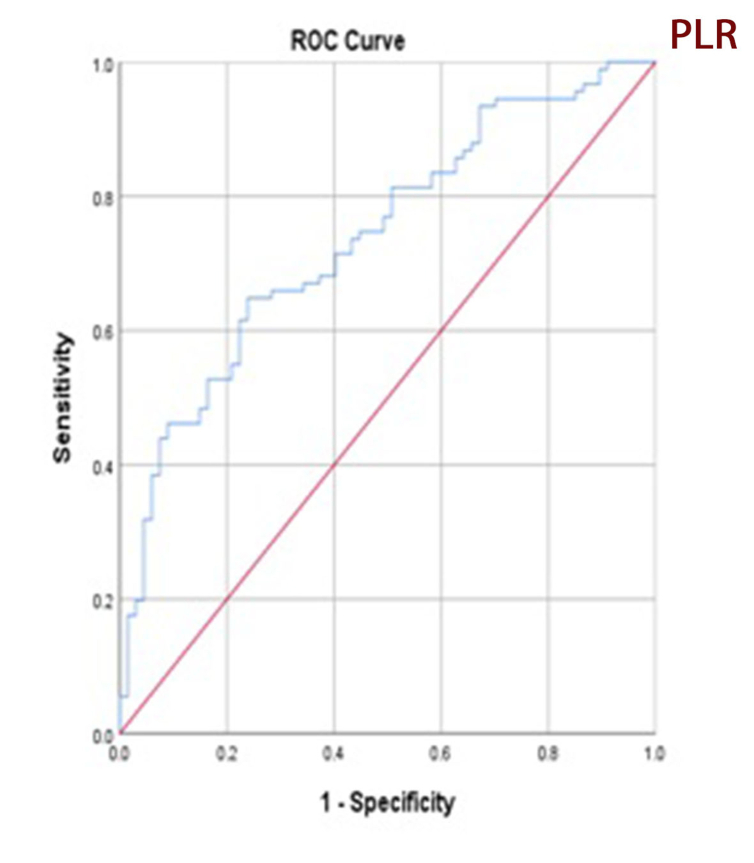
Receiver operating characteristic curve analysis of NLR and PLR for microalbuminuria prediction found an area under curve of 0.869 for NLR (confidence interval: 0.813–0.926, p = 0.000) and 0.739 for PLR (confidence interval: 0.662–0.815, p = 0.000) (Figures 3 and 4). A NLR cutoff point of 2.2 has 72.0 % sensitivity and 78.0 % specificity; a PLR cutoff point of 115.6 has 78.1 % sensitivity and 88 % specificity which suggest sufficient accuracy. ROC curve analysis and selected cutoff points for NLR and PLR are presented in Table 3.
Figure 3.
Receiver operating characteristic (ROC) curve analysis of neutrophil-to-lymphocyte ratio for microalbuminuria prediction. Area under curve is 0.869.
Figure 4.
Receiver operating characteristic (ROC) curve analysis of platelet-to-lymphocyte ratio for microalbuminuria prediction. Area under curve is 0.73.
Table 3.
Receiver operating characteristic (ROC) curve analysis for prediction of microalbuminuria using NLR and PLR cutoff values.
| Area under curve | cut off | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|
| NLR | 0.869 | 2.2 | 72.0 | 78.0 |
| PLR | 0.739 | 115.6 | 78.1 | 88.0 |
4. Discussion
The main purpose of this study was to investigate and evaluate the predictive value of NLR and PLR for DN in type 2 diabetic patients. The sample consisted of patients with type 2 diabetes who were divided into three groups according to their albumin-to-creatinine ratio. Levels of inflammatory markers (NLR, PLR) and other parameters were compared among the three groups. Results indicated that the NLR and PLR values were significantly higher in the diabetic patients with macroalbuminuria than in those with microalbuminuria and those without albuminuria.
DN is a common severe complication in patients with diabetes, but its exact pathogenesis remains unclear [17]. Although microalbuminuria is a strong marker for DN diagnosis and progression, glomerular damage is considered as early sign of DN and precedes the appearance of microalbuminuria [18]. It is known that a cascade of pathological events (glomerular damage gives rise to proteinuria, followed by progressive renal damage, fibrosis, inflammation, and finally loss of functional nephrons) is involved in the development and progression of DN. Accumulated evidences have demonstrated that chronic inflammation plays a key role in the development of DM-associated complications [13]. Several studies have associated DN with chronic inflammation, as various inflammatory molecules such as adipokines, chemokines, adhesion molecules, and cytokines could contribute in the development of DN [19]. Spranger et al. suggested that the circulating inflammatory cytokines modify the risk for type 2 diabetes particularly a joint elevation of IL-1 and IL-6 which increased the risk of type 2 DM [20].
White blood cell count and their subtypes have been extensively considered as inflammation markers, where neutrophilia and relative lymphocytopenia are independent markers of many diseases, including DM-complications such as DN [21]. However, DN diagnosis based on WBC, neutrophil, or lymphocyte counts has biases. NLR, a readily available and cheap index calculated by blood routine examination has been considered a novel inflammatory biomarker reflecting both adaptive immune response (mediated by lymphocytes) and innate immune response (mediated by neutrophil) and its stability is better and less influenced by physiological and pathological status [13]. Thus, evaluating the associations between the NLR level and different diabetic complications is important.
NLR was recognized as a predictive marker in cardiovascular diseases (such as coronary artery disease, acute coronary syndromes, and heart failure) and in several types of cancer. Additionally, NLR has a positive correlation with not only the presence but also the severity of metabolic syndrome [22]. Recently, multiple studies have indicated that NLR could play as predictor for assessing of microvascular complications of DM.
Wan et al. reported that a higher NLR level was associated with an increased prevalence of cardiovascular and cerebrovascular diseases, and diabetic kidney disease in diabetic adults [13]. A study by Öztürk et al. showed that NLR is an independent predictor for microvascular complications in geriatric diabetic subjects [23]. Moursy et al. indicated that NLR is not only an efficient and stable index of inflammation, but also a crucial predictor for the presence of microvascular diabetic complications in Egyptian patients with type 2 diabetes [24]. Ulu et al. showed that NLR is a rapid and reliable prognostic index for retinopathy and its severity [25]. Another study by Ulu et al. found NLR could play as predictive and prognostic marker for sensorineural hearing loss in diabetic patients [26]. In a 3-year follow-up study, NLR seems to have a critical role in prediction of worsening renal function in diabetics [27].
Moreover, our study revealed a positive correlation between albuminuria levels and NLR, whereas there was a negative correlation between GFR and NLR levels. In concordance with our results, Kahramn et al. demonstrated that there were strong correlations among albuminuria, GFR and NLR levels in type 2 diabetic patients [28], thus NLR may represent an inflammatory marker of DN. Khandare et al. showed significant relation between NLR and DN, implying that inflammation and endothelial dysfunction could be involved in DN. Therefore, that study considered NLR as a predictor and a prognostic risk marker of DN [29]. Another study by Huang et al. concluded that increased NLR values were independently correlated to DN and NLR is a reliable predictive marker for early-stage diabetic nephropathy [17].
PLR is considered as a potential inflammatory marker in cardiac, oncologic disorders [30], and chronic kidney disease [31]. Furthermore, PLR like NLR, which can be calculated by complete blood count, may be utilized as cheap predictors of diabetic microvascular complications [32]. Mineoka et al. reported that PLR can be serve as a useful marker for assessment of high risk diabetic foot and foot ulcers in patients with type 2 diabetes [33]. In our study, PLR has positive correlation with albuminuria levels and negative correlation with GFR. In agreement with these results, Abdelaziz et al. found that NLR and PLR were significantly related to DN, NLR & PLR may be served as predictor and prognostic risk markers for DN [34].
The current study has revealed significant increase in serum creatinine levels in parallel to albuminuria, this may indicate alteration in kidney function.
Hypertension also contributes in DN initiation and development [7]; this agrees with our results that revealed elevation in blood pressure. This study also showed a negative correlation between NLR, PLR and hemoglobin, and a positive correlation between NLR, PLR and CRP. These results suggest that NLR and PLR can be used as markers of systemic inflammation.
In reference to glycemic parameters, there were significant differences among the groups, HbA1c values were higher than 7% in all study groups, this may have indicated poor glycemic control in type 2 diabetic patients. It can also be considered as a disease monitoring tool during the follow-up of patients with diabetes.
Moreover, NLR and PLR are influenced by genetic and non-genetic factors (sex, age, seasonal conditions, lifestyle and diseases) [35]. So that, this study has some limitations namely, small number of patients (n = 158), retrospective design, the lack of accessibility to some data such as, cholesterol, 24-hour urinary albumin excretion, etc.
5. Conclusion
Increased NLR & PLR were significantly correlated with DN, and high NLR & PLR may be served as a predictor and a prognostic risk marker of DN. These parameters are easy to calculate in the laboratory. NLR and PLR tests are simple, cost-effective, and done routinely. They can be beneficial as alternative markers for inflammation. More research investigations are still required with follow-up and larger samples to confirm their effectiveness as probable risk factors for DN.
Declarations
Author contribution statement
Marwa Jaaban: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Almoutassem Billah Zetoune: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Sondos Hesenow: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Razan Hessenow: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The authors do not have permission to share data.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References:
- 1.Williams R. 9th. International Diabetes Federation; 2019. IDF Diabetes Atlas Ninth Edition 2019. (IDF Diabetes Atlas). [Google Scholar]
- 2.Punthakee Z., Goldenberg R., Katz P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can. J. Diabetes. 2018;42:S10–S15. doi: 10.1016/j.jcjd.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Latha S., V. R. The facts about diabetes mellitus- A review. GIJHSR. 2019;4(2):64–75. [Google Scholar]
- 4.Adapa D., Sarangi T. A review on diabetes mellitus: complications, management and treatment modalities. J. Med. Health. Sci. 2015;4(3) [Google Scholar]
- 5.Okur M.E., Karantas I.D., Siafaka P.I. Diabetes Mellitus: a review on pathophysiology, current status of oral pathophysiology, current status of oral medications and future perspectives. ACTA Pharm. Sci. 2017;55(1) [Google Scholar]
- 6.Lim A.K. Diabetic nephropathy–complications and treatment. Int. J. Nephrol. Renovascular Dis. 2014;7:361. doi: 10.2147/IJNRD.S40172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alicic R.Z., Rooney M.T., Tuttle K.R. Diabetic kidney disease: challenges, progress, and possibilities. Clin. J. Am. Soc. Nephrol. 2017;12(12):2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pugliese G. Diabetic kidney disease: new clinical and therapeutic issues. Joint position statement of the Italian Diabetes Society and the Italian Society of Nephrology on “The natural history of diabetic kidney disease and treatment of hyperglycemia in patients with type 2 diabetes and impaired renal function”. Nutr. Metabol. Cardiovasc. Dis. 2019;29(11):1127–1150. doi: 10.1016/j.numecd.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Gluhovschi C. Urinary biomarkers in the assessment of early diabetic nephropathy. J. Diabet. Res. 2016;2016 doi: 10.1155/2016/4626125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahbazian H., Rezaii I. Diabetic kidney disease; review of the current knowledge. J. Ren. Inj. Prev. 2013;2(2):73. doi: 10.12861/jrip.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winter L. Use of readily accessible inflammatory markers to predict diabetic kidney disease. Front. Endocrinol. 2018;9:225. doi: 10.3389/fendo.2018.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uwaezuoke S.N. The role of novel biomarkers in predicting diabetic nephropathy: a review. Int. J. Nephrol. Renovascular Dis. 2017;10:221. doi: 10.2147/IJNRD.S143186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan H. Associations between the neutrophil-to-lymphocyte ratio and diabetic complications in adults with diabetes: a cross-sectional study. J. Diabet. Res. 2020:2020. doi: 10.1155/2020/6219545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turkmen K. Platelet-to-lymphocyte ratio better predicts inflammation than neutrophil-to-lymphocyte ratio in end-stage renal disease patients. Hemodial. Int. 2013;17(3):391–396. doi: 10.1111/hdi.12040. [DOI] [PubMed] [Google Scholar]
- 15.Balta S., Demırkol S., Kucuk U. The platelet lymphocyte ratio may be useful inflammatory indicator in clinical practice. Hemodial. Int. 2013;4(17):668–669. doi: 10.1111/hdi.12058. [DOI] [PubMed] [Google Scholar]
- 16.Persson F., Rossing P. Diagnosis of diabetic kidney disease: state of the art and future perspective. Kidney Int. Suppl. 2018;8(1):2–7. doi: 10.1016/j.kisu.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang W. Neutrophil–lymphocyte ratio is a reliable predictive marker for early-stage diabetic nephropathy. Clin. Endocrinol. 2015;82(2):229–233. doi: 10.1111/cen.12576. [DOI] [PubMed] [Google Scholar]
- 18.Fiseha T. Urinary biomarkers for early diabetic nephropathy in type 2 diabetic patients. Biomark Res. 2015;3(1):1–7. doi: 10.1186/s40364-015-0042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J. The association of neutrophil to lymphocyte ratio, mean platelet volume, and platelet distribution width with diabetic retinopathy and nephropathy: a meta-analysis. Biosci. Rep. 2018;38(3) doi: 10.1042/BSR20180172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spranger J. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52(3):812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg R.B. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J. Clin. Endocrinol. Metab. 2009;94(9):3171–3182. doi: 10.1210/jc.2008-2534. [DOI] [PubMed] [Google Scholar]
- 22.Buyukkaya E. Correlation of neutrophil to lymphocyte ratio with the presence and severity of metabolic syndrome. Clin. Appl. Thromb. Hemost. 2014;20(2):159–163. doi: 10.1177/1076029612459675. [DOI] [PubMed] [Google Scholar]
- 23.Öztürk Z. Is there a link between neutrophil-lymphocyte ratio and microvascular complications in geriatric diabetic patients? J. Endocrinol. Invest. 2013;36(8):593–599. doi: 10.3275/8894. [DOI] [PubMed] [Google Scholar]
- 24.Moursy E.Y. Relationship between neutrophil lymphocyte ratio and microvascular complications in Egyptian patients with type 2 diabetes. Am. J. Intern. Med. 2015;3(6):250–255. [Google Scholar]
- 25.Ulu S.M. Neutrophil-to-lymphocyte ratio as a quick and reliable predictive marker to diagnose the severity of diabetic retinopathy. Diabetes Technol. Therapeut. 2013;15(11):942–947. doi: 10.1089/dia.2013.0097. [DOI] [PubMed] [Google Scholar]
- 26.Ulu S. Neutrophil–lymphocyte ratio as a new predictive and prognostic factor at the hearing loss of diabetic patients. Eur. Arch. Oto-Rhino-Laryngol. 2014;271(10):2681–2686. doi: 10.1007/s00405-013-2734-3. [DOI] [PubMed] [Google Scholar]
- 27.Azab B. Neutrophil-to-lymphocyte ratio as a predictor of worsening renal function in diabetic patients (3-year follow-up study) Ren. Fail. 2012;34(5):571–576. doi: 10.3109/0886022X.2012.668741. [DOI] [PubMed] [Google Scholar]
- 28.Kahraman C. The relationship between neutrophil-to-lymphocyte ratio and albuminuria in type 2 diabetic patients: a pilot study. Arch. Med. Sci. 2016;12(3):571. doi: 10.5114/aoms.2016.59931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khandare S.A. Study of neutrophil-lymphocyte ratio as novel marker for diabetic nephropathy in type 2 diabetes. Indian J. Endocrinol. Metab. 2017;21(3):387. doi: 10.4103/ijem.IJEM_476_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akkaya E., Gul M., Ugur M. Platelet to lymphocyte ratio: a simple and valuable prognostic marker for acute coronary syndrome. Int. J. Cardiol. 2014;177(2):597–598. doi: 10.1016/j.ijcard.2014.08.143. [DOI] [PubMed] [Google Scholar]
- 31.Valga F. Nefrología; 2019. Neutrophil-to-lymphocyte and Platelet-To-Lymphocyte Ratios as Biological Markers of Interest in Kidney Disease. (English Edition) [DOI] [PubMed] [Google Scholar]
- 32.Onalan E., Gozel N., Donder E. Can hematological parameters in type 2 diabetes predict microvascular complication development? Pak. J. Med. Sci. 2019;35(6):1511. doi: 10.12669/pjms.35.6.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mineoka Y. Platelet to lymphocyte ratio correlates with diabetic foot risk and foot ulcer in patients with type 2 diabetes. Endocr. J. 2019;66(10):905–913. doi: 10.1507/endocrj.EJ18-0477. [DOI] [PubMed] [Google Scholar]
- 34.Abdallah A.I. Study of neutrophil lymphocyte ratio (NLR) and platelet lymphocyte ratio (PLR) as a predictor inflammatory marker for diabetic nephropathy in type 2 diabetic patients. Egypt. J. Hosp. Med. 2018;72(7):4800–4807. [Google Scholar]
- 35.Bedel C., Korkut M., Armağan H.H. NLR, d-NLR and PLR can be affected by many factors. Int. Immunopharm. 2020 Nov 21:107154. doi: 10.1016/j.intimp.2020.107154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.