Abstract
The cardiac natriuretic peptides (NPs) are well established as regulators of blood pressure and fluid volume, but they also stimulate adipocyte lipolysis and control the gene program of nonshivering thermogenesis in brown adipose tissue. The NP “clearance” receptor C (NPRC) functions to clear NPs from the circulation via peptide internalization and degradation and thus is an important regulator of NP signaling and adipocyte metabolism. It is well known that the Nprc gene is highly expressed in adipose tissue and dynamically regulated upon nutrition and environmental changes. However, the molecular basis for how Nprc gene expression is regulated is still poorly understood. Here, we identified the nuclear receptor transcription factor peroxisome proliferator–activated receptor gamma (PPARγ) as a transcriptional regulator of Nprc expression in mouse adipocytes. During 3T3-L1 adipocyte differentiation, levels of Nprc expression increase in parallel with PPARγ induction. Rosiglitazone, a classic PPARγ agonist, increases, whereas siRNA knockdown of PPARγ reduces, Nprc expression in 3T3-L1 adipocytes. By using chromosome conformation capture and luciferase reporter assays, we demonstrate that PPARγ controls Nprc gene expression in adipocytes through its long-range distal enhancers. Furthermore, the induction of Nprc expression in adipose tissue during high-fat diet feeding is found to be associated with increased PPARγ enhancer activity. Our findings define PPARγ as a mediator of adipocyte Nprc gene expression and establish a new connection between PPARγ and the control of adipocyte NP signaling in obesity.
Keywords: Nprc, PPARγ, adipocyte, natriuretic peptide, gene expression, enhancer, obesity
Abbreviations: 3C, chromosomal conformation capture assay; AP, alkaline phosphatase; BAT, brown adipose tissue; BNP, B-type NP; ChIP-Seq, chromatin immunoprecipitation sequencing; DMEM, Dulbecco's modified Eagle's medium; eRNA, enhancer RNA; Fabp4, fatty acid–binding protein 4; FBS, fetal bovine serum; HFD, high-fat diet; iWAT, inguinal white adipose tissue; NIH, National Institutes of Health; NP, natriuretic peptide; NPRA, NP receptor A; NPRC, NP receptor C; PPARγ, peroxisome proliferator–activated receptor gamma; PPRE, PPARγ response element; qPCR, quantitative PCR
The cardiac natriuretic peptides (NPs), including atrial NP and B-type NP (BNP), were first discovered as factors in atrial extracts that evoked a strong decrease in blood pressure when injected into rodents (1). They are now known to be secreted from atrial cardiomyocytes in response to increases in blood volume and cardiac wall stress. However, in conditions of persistent high blood pressure, some reports suggest that BNP can also be produced in the ventricle (2). Thus, the most well-established physiological function of NPs is to maintain blood pressure and fluid volume (3). NPs have also been shown to stimulate adipocyte lipolysis to liberate fatty acids from adipose tissue with a potency comparable to the catecholamines (4, 5). This places the heart in a position of being both a consumer of fatty acids as well as a regulator of their release from adipose tissue. Furthermore, work from our laboratory has demonstrated that NPs could also promote the thermogenic program of brown adipocytes by increasing the gene expression programs of mitochondrial biogenesis, fatty acid oxidation, and the key thermogenic protein uncoupling protein-1 (6).
The physiological effect of the NPs is mediated by the cGMP–protein kinase G signaling cascade in target cells. There are two receptors for the cardiac NPs: NP receptor A (NPRA) and NP receptor C (NPRC). Binding of NPs to NPRA activates its intracellular guanylyl cyclase activity, increases cGMP production, and triggers the protein kinase G–dependent signaling cascade (7). In contrast, binding of NPs to NPRC, which lack an intracellular guanylyl cyclase domain, results in the internalization and degradation of the peptides (7). NPRC-mediated NP degradation is an important mechanism to modulate the available pool of NPs for target cell activation.
NPRC appears to be an important regulator of adipose tissue NP signaling and energy metabolism. In humans, increases in circulating NPs are associated with weight loss, whereas obese human subjects across ethnic groups with metabolic syndrome often show reduced circulating NPs and biological efficacy (e.g., elevated blood pressure) (8, 9, 10, 11). This biological response to NPs depends on the relative amount of the guanylyl cyclase receptor NPRA to the “clearance” receptor NPRC. In mice, it was shown that genetic deletion of NPRC did not change the plasma levels of atrial NP and BNP, but their circulating half-life was substantially increased (12). In adipocytes, the levels of NPRC are dynamically regulated in response to nutritional and hormonal status. In obese humans and rodent models, the levels of NPRC are elevated in adipose tissue (9, 13, 14, 15, 16, 17, 18). This is of physiological significance because it has been proposed that greater removal of NPs from circulation by NPRC in adipose tissue could explain what has been referred to as a “natriuretic handicap” linking obesity and hypertension (9, 10, 11). We previously reported that deletion of Nprc specifically in adipose tissue increases NP signaling and protects against diet-induced obesity and insulin resistance (16). As a result, the level of NPRC in adipose tissues is crucial for NP action. In spite of this important biology, there is little known about how the Nprc gene is regulated. To tackle this problem, we used in vitro adipocyte culture and diet-induced obesity mouse models to investigate the mechanisms by which Nprc gene expression is regulated in adipocytes and adipose tissues. Here, we describe our results that identified peroxisome proliferator–activated receptor gamma (PPARγ) as a transcriptional regulator of Nprc expression and showed that the PPARγ enhancer activity in the Nprc gene is related to Nprc mRNA induction in response to high-fat diet (HFD) feeding. Although there are probably additional factors to control Nprc gene expression, our data provide the first insights on Nprc transcriptional regulation and establish a new role for PPARγ to control adipocyte NP signaling and metabolism during obesity.
Results
Regulation of Nprc expression in adipocytes by PPARγ
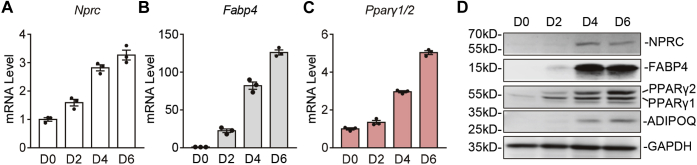
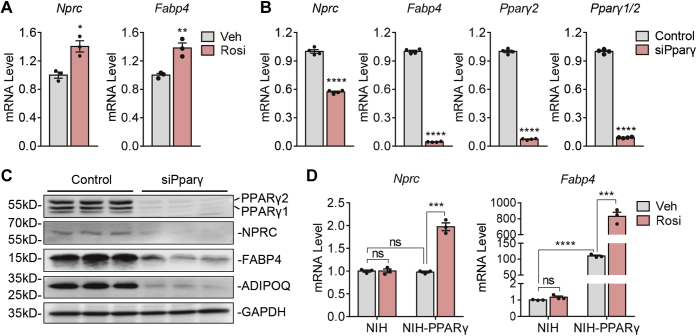
In efforts to understand how Nprc expression is regulated in adipocytes, we first examined the expression of Nprc over the time course of 3T3-L1 adipocyte differentiation. As shown in Figure 1A, Nprc mRNA levels progressively increased throughout the differentiation process. Meanwhile, the expression of Pparγ, the master regulator of adipocyte differentiation (19), and fatty acid–binding protein 4 (Fabp4), a well-characterized PPARγ target gene (20, 21), also increased over the course of differentiation (Fig. 1, B and C). In line with this, although Nprc protein was not detectable in the preadipocytes, it increased along with the differentiation process, in parallel to the protein levels of PPARγ and its targets FABP4 and adiponectin (ADIPOQ) (22) (Fig. 1D). When fully differentiated, adipocytes treated with rosiglitazone, a potent PPARγ agonist, for 6 h showed a further increase in Nprc transcript levels, in a similar fashion to Fabp4 (Fig. 2A). In contrast, siRNA knockdown of Pparγ resulted in a significant reduction of Nprc levels compared with the scramble siRNA control (Fig. 2B). Similarly, NPRC protein was also significantly reduced with PPARγ knockdown as expected, like the other two PPARγ targets FABP4 and ADIPOQ (Fig. 2C). These data suggested that PPARγ controls Nprc expression in adipocytes. We further confirmed this observation in National Institutes of Health (NIH)-3T3 fibroblasts that stably express PPARγ (NIH-PPARγ) (23, 24). In NIH-PPARγ cells, Nprc mRNA increased in response to rosiglitazone treatment as expected, though to a lesser extent than Fabp4. In contrast, in the parental NIH-3T3 cells that lack PPARγ, Nprc as well as Fabp4 transcripts did not increase after rosiglitazone treatment (Fig. 2D). These data further support the conclusion that expression of Nprc is regulated by PPARγ.
Figure 1.
Nprc mRNA expression during 3T3-L1 adipocyte differentiation.A–C, mRNA levels of Nprc (A), Fabp4 (B), and Pparγ1/2 (C) during 3T3-L1 adipocyte differentiation. Data were normalized with 36B4. D, protein levels of NPRC, FABP4, PPARγ1/2, and ADIPOQ during 3T3-L1 adipocyte differentiation (Day 0, 2, 4, and 6). FABP4, fatty acid–binding protein 4; Nprc, NP receptor C; PPARγ1/2, peroxisome proliferator–activated receptor gamma1/2.
Figure 2.
Modulation of Nprc expression by rosiglitazone and PPARγ.A, mRNA levels of Nprc and Fabp4 in 3T3-L1 adipocytes after treatment with 1 μM rosiglitazone (Rosi) or vehicle (Veh) for 6 h. B, mRNA levels of Nprc, Fabp4, and Pparγ1/2 mRNA in 3T3-L1 adipocytes after siRNA knockdown of Pparγ (siPparγ). C, protein levels of PPARγ1/2, NPRC, FABP4, and ADIPOQ in 3T3-L1 adipocytes after siPparγ. D, mRNA levels of Nprc and Fabp4 in NIH-3T3 cells (NIH) and NIH-3T3 cells stably expressing PPARγ (NIH-PPARγ) after treatment with 1 μM rosiglitazone (Rosi) or vehicle (Veh) for 6 h. Quantitative PCR data were normalized with 36B4. Student's t test, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. Fabp4, fatty acid–binding protein 4; NIH, National Institutes of Health; Nprc, NP receptor C; ns, not significant; PPARγ, peroxisome proliferator–activated receptor gamma.
Interaction of distal PPARγ enhancers with Nprc promoter in adipocytes
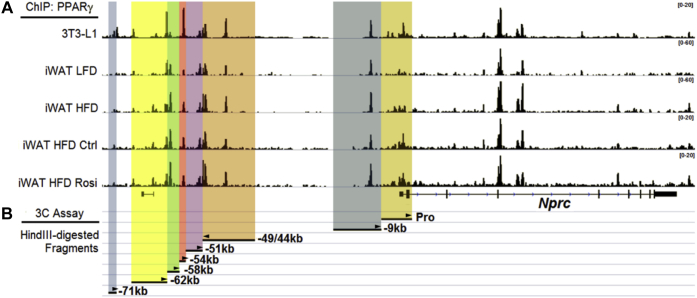
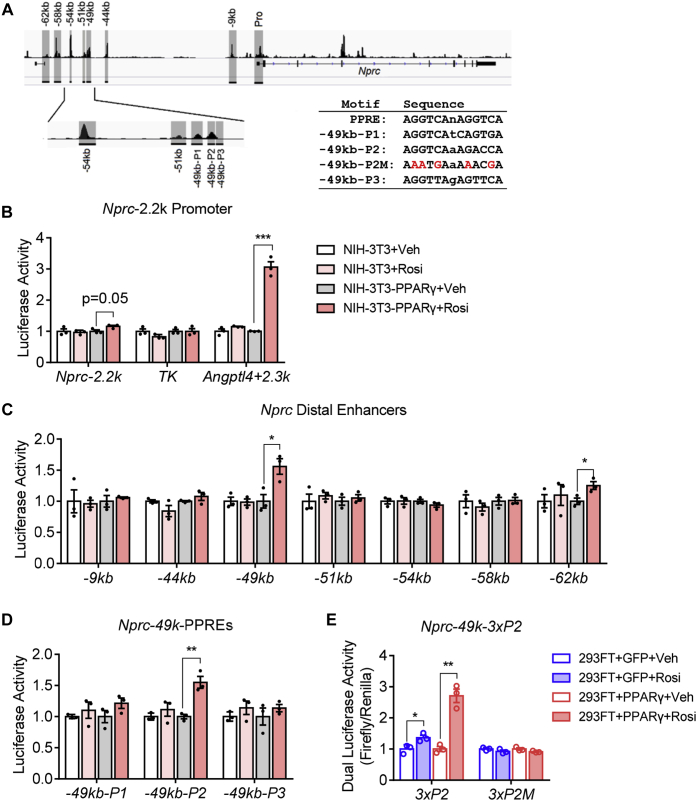
To determine the mechanism by which PPARγ regulates Nprc expression, we next searched for PPARγ-binding sites in the mouse Nprc locus by analyzing a previously published PPARγ chromatin immunoprecipitation sequencing (ChIP-Seq) dataset from 3T3-L1 adipocytes and mouse subcutaneous inguinal white adipose tissue (iWAT) (25). As shown in Figure 3A, there were several PPARγ-binding sites in the regions −9 kb, −44 kb, −49 kb, −54 kb, −58 kb, −62 kb, and −71 kb upstream of the Nprc transcription start site. These PPARγ-binding peaks were well harmonized between 3T3-L1 adipocytes and the iWAT depot. It was also noted that the PPARγ-binding activity was higher in the iWAT of mice fed with HFD and further increased with rosiglitazone administration (Fig. 3A). These data suggest that the control of Nprc expression by PPARγ could be mediated by long-range enhancers.
Figure 3.
ChIP-Seq identified PPARγ enhancers in the upstream distal region of Nprc.A, PPARγ-binding sites in the upstream distal region of the Nprc promoter were identified by ChIP-Seq in 3T3-L1 adipocytes and inguinal white adipose tissue (iWAT) from mice fed with low-fat diet (LFD), high-fat diet (HFD), HFD plus vehicle (Ctrl), and HFD plus rosiglitazone (Rosi) (Soccio et al., 2017 (25)). B, HindIII-digested restriction fragments containing Nprc promoter (Pro) and the PPARγ-binding sites (−9 kb, −44/49 kb, −51 kb, −54 kb, −58 kb, 62 kb, and −71 kb) are illustrated for the chromosomal conformation capture (3C) analysis as described for Figure 4. The arrowheads indicate the primers used for 3C PCR in each fragment. ChIP-Seq, chromatin immunoprecipitation sequencing; Nprc, NP receptor C; PPARγ, peroxisome proliferator–activated receptor gamma.
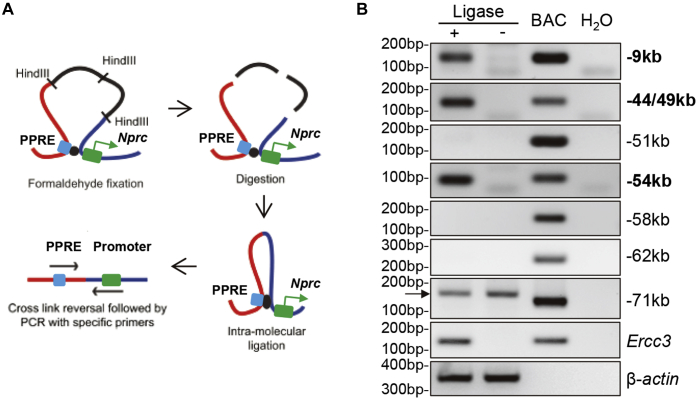
To determine whether these PPARγ-binding sites are directly involved in the regulation of Nprc expression, we utilized chromosomal conformation capture (3C) assay assays to examine the interaction of these enhancers with the proximal Nprc promoter (Figs. 3B and 4A). Among those HindIII-digested restriction fragments that contained PPARγ enhancers, only the −9 kb, −44/49 kb, and −54 kb fragments could form chimeric ligation products with the Nprc promoter fragment (Fig. 4B), suggesting that the distal enhancers in these three fragments could interact with the Nprc promoter by forming a looping structure, which is essential for the function of distal enhancer elements (26).
Figure 4.
Interaction of PPARγ-binding fragments with Nprc promoter.A, procedure of chromosomal conformation capture (3C) analysis (modified from Cope and Fraser, 2009 (48)). B, 3C analysis with C3H10T1/2 adipocytes to determine the interaction between Nprc promoter and PPARγ-binding sites within each HindIII-restriction fragment (−9 kb, −44/49 kb, −54 kb, −58 kb, −62 kb, and −71 kb; see also Fig. 3B for details). Ercc3 was positive control for constitutive enhancer interaction. β-actin was DNA input control. Ligase+, HindIII-digested chromatin with intramolecular religation. Ligase−, HindIII-digested chromatin without intramolecular religation. BAC, bacterial artificial chromosome DNA covering Nprc (RP23-305L10) and Ercc3 (RP23-148C24) genes was digested with HindIII and randomly religated as positive control. Arrowhead indicates nonspecific amplification products. Nprc, NP receptor C; PPARγ, peroxisome proliferator–activated receptor gamma; PPRE, PPARγ response element.
Functionality of Nprc promoter and the distal PPARγ enhancers
To determine the functionality of the Nprc promoter and these distal enhancers, we next cloned a 2.2 kb Nprc promoter fragment and the PPARγ enhancer fragments (as shown in Fig. 5A) into a luciferase reporter system to test their reporter activity in response to rosiglitazone treatment. The Nprc-2.2k promoter did not show any luciferase activity in NIH-PPARγ cells in response to rosiglitazone (Fig. 5B). The TK promoter and the Angptl4 enhancer were used as negative and positive controls, respectively, as described in a previous report (27). Among all the distal enhancer fragments tested, the −49 kb and −62 kb PPARγ enhancer fragments showed significant increases in luciferase activities in response to rosiglitazone (Fig. 5C). Since the −62 kb fragment did not interact with Nprc promoter (Fig. 4B), it may be that it participates in the regulation of a neighboring gene other than Nprc. Therefore, our further analysis focused on the −49 kb fragments. More detailed sequence analysis showed that there were three putative PPARγ response element (PPRE) motifs within the −49 kb fragment (Fig. 5A). These three PPREs were further cloned into the luciferase reporter vector. The −49 kb-P2 showed increased luciferase activity with rosiglitazone treatment, illustrating that the −49 kb-P2 is a functional PPARγ-binding site, although the other candidate sites in the −49 kb region may function only in the context of the intact chromatin. To further validate the functionality of the P2 element, the intact and mutated versions of P2 element were subcloned into the TK-Luc vector as triplet repeats (3xP2 and 3xP2M). Their activity was assayed with a previously established dual-luciferase assay protocol in human embryonic kidney 293FT (HEK293FT) cells (28). As shown in Figure 5E, the triplet P2 element, but not its mutant P2M, displayed robust luciferase activity with rosiglitazone treatment. While the fold induction of the individual P2 element is modest, the agonist effect is very specific and reproducible, and it might work together with the P1 and P3 elements within the context of intact chromatin in a cooperative or synergistic manner. Note that in the parental NIH-3T3 cells, which do no express PPARγ, none of the enhancer and PPRE fragments, including the positive control Angptl4 enhancer, showed any luciferase activity after rosiglitazone. This is consistent with a specific role for PPARγ in NIH-PPARγ cells. Taken together, these data suggest that PPARγ controls Nprc gene expression through long-range distal enhancers.
Figure 5.
Rosiglitazone increases Nprc distal enhancer activity but not proximal promoter activity alone.A, Nprc promoter (Pro), distal PPARγ enhancers (−9 kb, −44 kb, −49 kb, −51 kb, −54 kb, −58 kb, and 62 kb), and the three PPARγ response elements (−49 kb—P1, P2, and P3) are shown in gray and were cloned for luciferase reporter analysis. The sequences of the consensus PPRE motif and the Nprc PPREs are listed in inset. B, luciferase activity of Nprc promoter (−2233 bp to +1 bp, mNprc-2.2k). TK promoter alone (TK) and TK promoter with a PPARγ enhancer from the Angptl4 gene (Angptl4+2.3k) were used as the negative and positive controls, respectively. C, luciferase activity of the Nprc distal enhancer fragments (−9 kb, −44 kb, −49 kb, −51 kb, −54 kb, −58 kb, and −62 kb). D, enhancer activity of the Nprc distal PPREs in the −49 kb fragment (P1–P3). For reporter assays, NIH-3T3 and NIH-3T3 stably expressing PPARγ (NIH-3T3-PPARγ) cells were transfected with reporter plasmids and treated with vehicle (Veh) or 1 μM rosiglitazone (Rosi) for 48 h. Luciferase activity was normalized to protein concentrations. E, dual-luciferase assay of the −49kb-3xP2. 293FT cells were transfected with indicated reporter plasmids in combination with GFP or PPARγ and treated with vehicle (Veh) or 1 μM rosiglitazone (Rosi) for 24 h. Data were representative of at least three independent experiments. Student's t test, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. NIH, National Institutes of Health; Nprc, NP receptor C; PPARγ, peroxisome proliferator–activated receptor gamma; PPRE, PPARγ response element.
HFD-induced Nprc gene expression in adipose tissue is associated with increased PPARγ enhancer activity
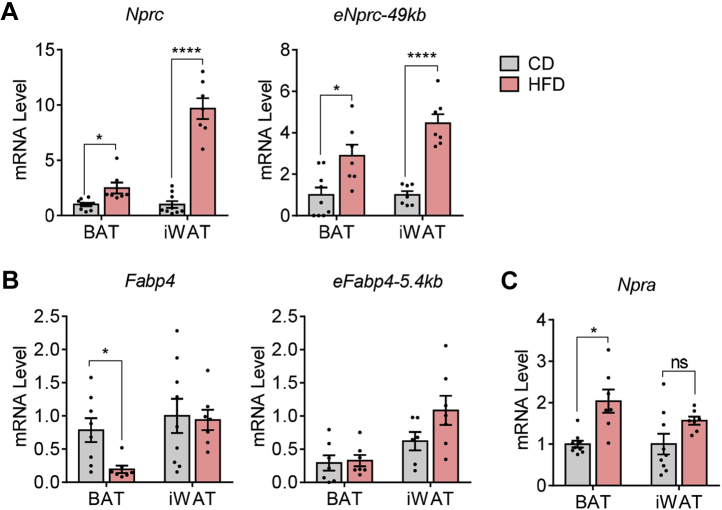
We and others have shown that Nprc expression is increased in rodent adipose tissue in response to HFD feeding (15, 16, 17, 18). Coupled with the observation that there is more PPARγ binding to these distal enhancers in the ChIP-Seq data (Fig. 3A; (25)), we next questioned whether the increase of Nprc expression in HFD-fed mice is mediated by PPARγ. To test this hypothesis, we examined the −49 kb PPARγ enhancer activities in adipose tissues by measuring their enhancer RNA (eRNA) synthesis via quantitative PCR (qPCR) (29). Interestingly, the eRNA level of −49 k PPARγ enhancer was significantly increased in the brown adipose tissue (BAT) and iWAT of HFD-fed mice, in parallel to the changes in Nprc gene expression itself (Fig. 6A). The level of Fabp4 mRNA was slightly decreased in BAT, but its −5.4 kb PPARγ eRNA was not altered with HFD feeding (Fig. 6B), and suggested a tissue and locus-specific effect of PPARγ action. The level of Npra mRNA was modestly increased in BAT and unchanged in iWAT with HFD (Fig. 6C). In sum, our results lead us to conclude that obesity increases Nprc expression in the adipose tissue through a PPARγ-dependent mechanism, the effect of which is the suppression of NP signaling, decreased energy expenditure, and impaired glucose homeostasis (Fig. 7).
Figure 6.
HFD induces Nprc expression and PPARγ enhancer activity in adipose tissue.A, levels of Nprc mRNA and the −49 kb PPARγ enhancer RNAs (eNprc-49k) in brown adipose tissue (BAT) and inguinal white adipose tissue (iWAT) of wildtype mice fed with control diet (CD) or HFD. B, levels of Fabp4 and the −5.4 kb PPARγ enhancer RNA (eFabp4-5.4kb) in BAT and iWAT of mice fed with CD and HFD. C, levels of Npra mRNA in the BAT and iWAT of mice fed with CD and HFD. Student's t test, ∗p < 0.05 and ∗∗∗∗p < 0.0001. Fabp4, fatty acid–binding protein 4; HFD, high-fat diet; Npra, NP receptor A; Nprc, NP receptor C; ns, not significant; PPARγ, peroxisome proliferator–activated receptor gamma.
Figure 7.
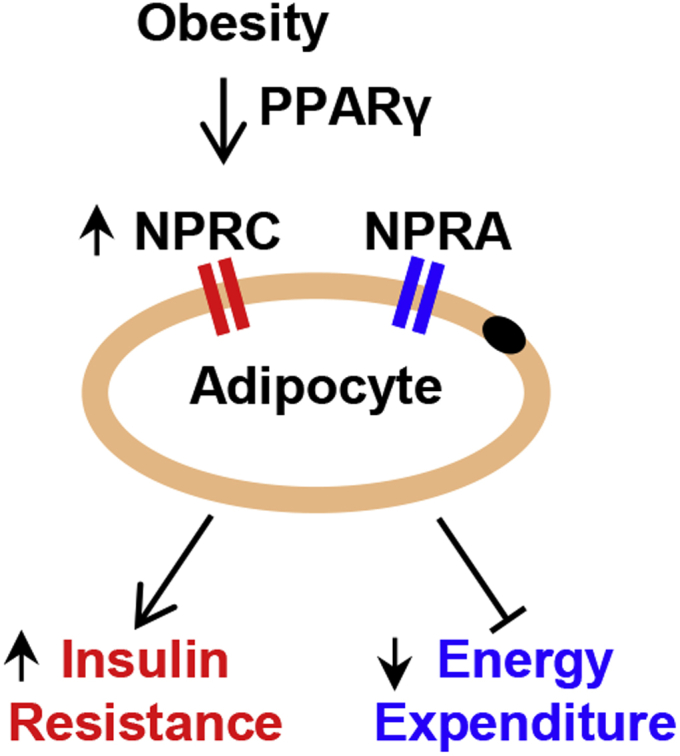
Obesity induces NPRC expression in adipocytes. Obesity induces NPRC expression in adipocytes potentially though a PPARγ-dependent mechanism. The increase in NPRC lowers the NPRA/NPRC ratio and reduces adipocyte natriuretic peptide signaling and results in decreased energy expenditure and impaired insulin sensitivity. NPRA, NP receptor A; NPRC, NP receptor C; PPARγ, peroxisome proliferator–activated receptor gamma.
Discussion
Transcriptional regulation of Nprc in adipocytes by PPARγ via long-range distal enhancers
Although the regulation of Nprc expression has been linked to glucose levels, insulin action, estrogen, and adrenergic receptor signaling in previous reports (30, 31, 32, 33), our current study is the first to identify PPARγ, the master regulator of adipogenesis (19), as a transcription factor that directly controls Nprc expression in adipocytes. We showed that PPARγ stimulates Nprc expression though the action of its long-range distal enhancers. We further demonstrated that induction of Nprc expression in adipose tissues by HFD is also potentially mediated by PPARγ. The PPARγ eRNA synthesis, an indicator of enhancer activity that could be evaluated by qPCR (29), was significantly increased in parallel to Nprc mRNA expression.
In our study, we compared the expression of Nprc with another well-characterized PPARγ target Fabp4 (20, 21). The effect of PPARγ and its agonist rosiglitazone on Nprc and Fabp4 expression was not uniform in some instances. For example, under basal conditions without PPARγ agonist stimulation, the expression of Fabp4 was significantly higher in NIH-PPARγ cells than in the parental NIH-3T3 cells, but basal Nprc mRNA levels were comparable in these two cell types (Fig. 2D). This suggested that Nprc expression is more dependent on the action of a PPARγ ligand for maximal PPARγ activation (34, 35). In addition, PPARγ can bind to both the distal enhancer and the proximal promoter of Fabp4 (21, 36), which might be more potent than binding the distal enhancer alone; thus, it is possible that Fabp4 expression could still be stimulated by PPARγ without its full activation by the ligand. In line with this, the induction of Fabp4 mRNA in NIH-PPARγ cells was also more robust than that of Nprc after rosiglitazone treatment (Fig. 2D). Second, while Nprc expression was increased in the brown fat of HFD-fed mice, Fabp4 mRNA was actually lower even though its −5.4 kb PPARγ enhancer activity was unaffected by the HFD compared with chow. This again may be due to the fact that PPARγ could bind to both Fabp4 distal enhancer and proximal promoter (21, 36), so that the activity of the enhancer alone did not fully represent its overall mRNA expression levels. Moreover, it has been reported that FABP4 can attenuate PPARγ by triggering PPARγ ubiquitination and subsequent proteasomal degradation (37). Finally, while it appears that PPARγ is the major regulator of Fabp4 gene expression (20, 21), Nprc gene expression is likely regulated by other factors in addition to PPARγ (30, 31, 32, 33).
Our analysis of the long-range distal PPARγ enhancers only covered a 71 kb region upstream of Nprc promoter. However, enhancer elements may also reside in the downstream or intronic regions. As discussed by others (38), these enhancer elements do not necessarily act on the respective closest promoter but can bypass neighboring genes to regulate genes located more distantly along a chromosome. As such, we cannot exclude the possibility that additional long-range distal elements may exist and function synergistically with the −49 kb enhancer that we identified in this study to control Nprc gene expression. Unbiased approaches, such as the circular chromosome conformation capture assay (39) or imaging-based 3D genome technologies (40), will be required to identify and validate these additional elements in future study.
The physiological significance of PPARγ-dependent regulation of Nprc expression
Our study presents the first evidence to directly link Nprc gene expression to PPARγ, the master regulator of adipocyte formation. This connection may, at least partially, explain the well-appreciated fact that Nprc is abundantly expressed in adipocytes and the adipose tissue (6, 15). It will be interesting to determine whether Nprc expression could also be regulated by PPARγ in the other related tissues, such as the macrophage and cardiovascular system (41, 42, 43). In addition, we observed that Nprc expression was significantly higher in the male epidydimal white adipose tissue than in the female parametrial white adipose tissue, and this difference was mirrored by the PPARγ enhancer activity (data not shown). This apparent sexually dimorphic and depot-specific pattern of PPARγ activity needs further investigation.
It is well known that activation of PPARγ improves insulin sensitivity through a combination of metabolic actions, including partitioning of lipid stores and the regulation of metabolic and inflammatory mediators such as adiponectin (44). Our study showed that HFD-dependent Nprc expression could be potentially mediated by the action of PPARγ. An increase in NPRC expression in the adipose tissue results in reduced NPRA to NPRC ratio and thus suppresses adipose tissue NP signaling in obesity. As we previously showed, deletion of Nprc in the adipose tissue enhances NP signaling, stimulates energy expenditure, improves glucose homeostasis, and protects against diet-induced obesity (6, 16). Given the substantial role of NPRC for the control of NP signaling, our study provides a new connection to PPARγ for modulating NP signaling and energy metabolism during obesity.
Our preliminary analysis of a recent promoter Capture Hi-C dataset from human adipose tissue suggested the existence of several promoter-interacting elements for the human NPRC gene (45). Motif and integration analysis with human adipocyte PPARγ ChIP-Seq data suggested the existence of PPARγ-binding sites within these NPRC promoter-interacting sequences. This indicates that regulation of NPRC expression via long-range distal PPARγ enhancers is a conserved mechanism across species. Furthermore, several studies including ours have demonstrated that adipose NPRC levels are increased with obesity in both mouse and human (9, 13, 14, 15, 16, 17, 18), thus the obesity-associated increase in NPRC expression is conserved between mouse and human. However, PPARγ-mediated NPRC gene expression in human adipocytes and adipose tissue will need to be further investigated.
In conclusion, our current study demonstrates that PPARγ regulates Nprc expression in adipocytes through its long-range distal enhancers, and the diet-dependent increase in Nprc expression in obesity is potentially mediated by the actions of PPARγ. Although we expect that PPARγ is not the sole regulator of Nprc expression in adipocytes, our study provides the first insight into the transcriptional regulation of the Nprc gene by establishing a new connection for how PPARγ may control NP signaling in the adipocyte.
Experimental procedures
Cell lines
Mouse 3T3-L1 preadipocytes were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. C3H10T1/2 preadipocytes were maintained in DMEM supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. When reaching confluence, adipocyte differentiation was induced by a cocktail of 5 μg/ml insulin, 1 μM dexamethasone, 0.5 mM isobutyl methylxanthine, 1 μM rosiglitazone in DMEM with 10% FBS for 3 days, and then maintained in DMEM with 10% FBS until day 6 to 7 when ready for experiment. NIH-3T3 fibroblasts and a clonal line that stably expresses PPARγ (NIH-PPARγ) were cultured as described (23, 24).
siRNA
Knockdown of Pparγ in differentiating 3T3-L1 adipocytes was performed with Pparγ esiRNA (Sigma; EMU041151) as previously described (46). Briefly, at day 4 of differentiation per the aforementioned protocol, 3T3-L1 adipocytes were trypsinized and resuspended in growth medium at a density of 2.4 × 106 cells/ml. A mixture of 40 pmol siRNA and 24 μl RNAiMAX (Invitrogen) were prepared with OptiMEM medium in a final volume of 160 μl, incubated at RT for 5 min, and then added to a collagen (Gibco; A10644-01)-coated well of a 12-well plate. 3T3-L1 adipocytes were plated onto the 12-well plates at a density of 4.64 × 105 cells with a final media volume of 0.8 ml per well for 6 h. After this step, cells were replaced with fresh media and cultured for another 2 days before harvesting for RNA and Western blot analysis.
3C
3C was performed according to previous reports (47, 48, 49). Briefly, 1 × 107 C3H10T1/2 adipocytes at day 6 of differentiation were trypsinized, crosslinked by 2% formaldehyde at RT for 10 min and quenched with 0.125 M glycine at RT for 5 min. Adipocytes were washed and lysed in a buffer (10 mM Tris–HCl, pH 8.0, 10 mM NaCl, 0.2% NP-40, and 1× cOmplete Protease Inhibitor Cocktail from Roche) on ice for 30 min with shaking. The nuclei were harvested and resuspended in 0.5 ml cold 1.2× NEBuffer 2 (New England Biolabs), incubated with 0.3% SDS for 1 h at 37 °C, and with 1.8% Triton X-100 for 1 h at 37 °C. The isolated chromatin was digested with 1500 U of HindIII (NEB) overnight with shaking at 37 °C and then incubated with 1.6% SDS at 65 °C for 20 min. The samples were transferred to 7 ml of 1.15× T4 ligation buffer (NEB), incubated with 1% Triton X-100 for 1 h at 37 °C, then ligated with 800 U T4 ligase (NEB) for 4 h at 16 °C followed by 30 min at RT. The chromatin was then digested with 300 μg proteinase K overnight at 65 °C and by 30 μg RNaseA for 1 h at 37 °C. The DNA was purified with phenol/chloroform extraction, precipitated with ethanol, dissolved in 150 μl of Tris buffer (10 mM; pH 7.5), and quantified with NanoDrop (Thermo Scientific). Two hundred nanograms of DNA sample was used for PCR with an anchor primer in the Nprc promoter fragment and a bait primer in each of the distal enhancer fragments to determine the Nprc promoter and enhancer interaction (Fig. 3B). The 3C PCR primers are listed in the Supporting Table. For positive controls, BAC DNA that cover Nprc (RP23-305L10) and Ercc3 (RP23-148C24) genes were digested with HindIII and randomly religated to generate all possible ligation products as described previously (49).
Luciferase reporter assays
A −2.2 kb fragment of the mouse Nprc gene promoter (−2233 bp to +1 bp, mNprc-2.2k) was amplified by PCR with overhangs in the forward (5′-ATGACTCGAG-3′) and reverse (5′-ATTAAAGCTT-3′) primers, digested with XhoI and HindIII, and cloned into pGL-4.14 vector. The positive control plasmid with a PPARγ enhancer from Angptl4 gene in pUC18 HSV TK-Luc vector (Angptl4+2.3k) was constructed as described (27, 50). The individual enhancer fragments were amplified by PCR with overhangs in the forward (5′-ATTGTTGGATCC-3′) and reverse (5′-ATTGTTGTCGAC-3′) primers, digested with SalI and BamHI, and cloned into pUC18 HSV TK-Luc vector. The PPRE-49kb-P2 element and the mutant P2M were synthesized with SalI overhangs in the forward and reverse oligos and subcloned into pUC18 HSV TK-Luc vector. The triplet repeat clones were confirmed with sequencing. The reporter plasmid cloning primers and oligos are listed in the Supporting Table. For luciferase assay, NIH-3T3 and NIH-PPARγ fibroblasts were plated in 12-well culture plates at a density of 1.2 × 105 cells/well and reached 80% confluence the following day when ready for transfection. Cells were transfected with 1.25 μg/well of the reporter plasmids by polyethyleneimine reagent (Polysciences; #23966) and differentiated in DMEM/10% FBS with 1 μM rosiglitazone for 2 days. Luciferase activity was assayed according to the manufacturer's instruction (Promega; E1501) and normalized to total protein concentration (bicinchoninic acid; Pierce). The HEK293FT dual-luciferase assay was performed according to a previous report (28). Briefly, HEK293FT cells were plated in 24-well plate at a density of 3 × 104 cells/well. On the following day (day 1), cells were transfected with 12.5 ng/well reporter plasmid, 0.125 ng/well pRL-TK control plasmid, and 350 ng/well PPARγ or GFP plasmids by polyethyleneimine reagent. On day 2, cells were treated with 1 μM rosiglitazone or vehicle for 24 h before harvesting the cell lysates. The dual-luciferase assay was performed according to the manufacturer's instruction (Promega; E1910).
HFD experiment
Eight-week-old wildtype male mice were fed with an HFD (60% of calories from fat; Research Diet; D12492) for 12 weeks. All animal studies were approved by the Institutional Animal Care and Use Committee of Vanderbilt University Medical Center and in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
qPCR
RNA was extracted with TRIzol (Invitrogen), purified with RNeasy Mini columns (Qiagen) or Quick-RNA columns (Zymo Research), and reversed transcribed with High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems). For eRNA evaluation, complementary DNA was prepared with iScript gDNA Clear cDNA Synthesis Kit (Bio-Rad) to avoid genomic DNA contamination. qPCR was performed with PowerUp SYBR Green Master Mix (Life Technologies) according to manufacturer's instruction. qPCR results were analyzed with the ΔΔCt method, normalized to the internal control gene 36B4, and presented as fold change relative to the control group. qPCR primers are listed in the Supporting Table.
Western blot
Protein was extracted from cells as previously described (51). For Western blotting analysis, 30 to 40 μg of protein was resolved by 10% SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes (Bio-Rad), incubated overnight at 4 °C with specific primary antibodies in blocking buffer (Tris-buffered saline, 0.1% Tween-20, and 5% milk or bovine serum albumin), and detected with alkaline phosphatase (AP)–conjugated secondary antibody. The antibodies used were anti-NPRC (1:2000; Novus Biologicals; #NBP1-31365), anti-PPARγ (1:2000; Cell Signaling Technology; #2435), anti-FABP4 (1:1000; Cell Signaling Technology; #2120), anti-ADIPOQ (1:1000; Cell Signaling Technology; #2789), anti-GAPDH (1:2000; Protein Tech; #10494-1-AP), and anti-rabbit IgG-AP (1:20,000; Sigma; #A3687).
ChIP-Seq data analysis
A previously published PPARγ ChIP-Seq dataset in 3T3-L1 adipocyte and adipose tissues (Gene Expression Omnibus; GSE92606) (25) was downloaded and analyzed with Integrative Genomics Viewer (Broad Institute) (52).
Statistical analysis
GraphPad Prism 7 (GraphPad Software, Inc) was used for statistical analysis. All data were presented as means ± SEM. Unpaired two-tailed Student's t tests were used to determine the differences between groups. Statistical significance was defined as p < 0.05.
Data availability
All data are contained within this article. Reagents and plasmids described in this article are available upon request.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
The authors thank Ms Wei Zhang for animal husbandry assistance, Dr Ryan P. Ceddia for advice and comments on the article, and Dr Stephen Farmer for the NIH-3T3 and NIH-PPARγ cells.
Author contributions
F. S., Z. S., L. N., and S. C. conceptualization; F. S., Z. S., L. N., and S. C. funding acquisition; F. S., Z. S., L. N., and S. C. investigation; F. S., Z. S., L. N., and S. C. writing–original draft; F. S., Z. S., L. N., and S. C. project administration; F. S., Z. S., L. N., and S. C. writing–review and editing; Z. S., L. N., and S. C. data curation; Z. S., L. N., and S. C. formal analysis.
Funding and additional information
This work was supported by NIH R01 DK103056 (to S. C.), R56 DK126890 (to S. C.), and R01 DK115924 (to L. N.). F. S. is supported by the American Diabetes Association Postdoctoral Fellowship Award (1-18-PDF-110). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Edited by Qi-Qun Tang
Footnotes
Present address for Zoltan Simandi: Department of Bioengineering, University of Pennsylvania, Philadelphia, Pennsylvania 19104, USA.
Supporting information
References
- 1.de Bold A.J., Borenstein H.B., Veress A.T., Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa T., Linz W., Stevenson M., Bruneau B.G., Kuroski de Bold M.L., Chen J.H., Eid H., Scholkens B.A., de Bold A.J. Evidence for load-dependent and load-independent determinants of cardiac natriuretic peptide production. Circulation. 1996;93:2059–2067. doi: 10.1161/01.cir.93.11.2059. [DOI] [PubMed] [Google Scholar]
- 3.Schlueter N., de Sterke A., Willmes D.M., Spranger J., Jordan J., Birkenfeld A.L. Metabolic actions of natriuretic peptides and therapeutic potential in the metabolic syndrome. Pharmacol. Ther. 2014;144:12–27. doi: 10.1016/j.pharmthera.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Sengenes C., Berlan M., De Glisezinski I., Lafontan M., Galitzky J. Natriuretic peptides: A new lipolytic pathway in human adipocytes. FASEB J. 2000;14:1345–1351. [PubMed] [Google Scholar]
- 5.Sengenes C., Zakaroff-Girard A., Moulin A., Berlan M., Bouloumie A., Lafontan M., Galitzky J. Natriuretic peptide-dependent lipolysis in fat cells is a primate specificity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R257–R265. doi: 10.1152/ajpregu.00453.2001. [DOI] [PubMed] [Google Scholar]
- 6.Bordicchia M., Liu D., Amri E.Z., Ailhaud G., Dessi-Fulgheri P., Zhang C., Takahashi N., Sarzani R., Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potter L.R. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011;278:1808–1817. doi: 10.1111/j.1742-4658.2011.08082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dessi-Fulgheri P., Sarzani R., Rappelli A. The natriuretic peptide system in obesity-related hypertension: New pathophysiological aspects. J. Nephrol. 1998;11:296–299. [PubMed] [Google Scholar]
- 9.Wang T.J., Larson M.G., Levy D., Benjamin E.J., Leip E.P., Wilson P.W., Vasan R.S. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 10.Fox E.R., Musani S.K., Bidulescu A., Nagarajarao H.S., Samdarshi T.E., Gebreab S.Y., Sung J.H., Steffes M.W., Wang T.J., Taylor H.A., Vasan R.S. Relation of obesity to circulating B-type natriuretic peptide concentrations in blacks: The Jackson Heart Study. Circulation. 2011;124:1021–1027. doi: 10.1161/CIRCULATIONAHA.110.991943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clerico A., Giannoni A., Vittorini S., Emdin M. The paradox of low BNP levels in obesity. Heart Fail. Rev. 2012;17:81–96. doi: 10.1007/s10741-011-9249-z. [DOI] [PubMed] [Google Scholar]
- 12.Matsukawa N., Grzesik W.J., Takahashi N., Pandey K.N., Pang S., Yamauchi M., Smithies O. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7403–7408. doi: 10.1073/pnas.96.13.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dessi-Fulgheri P., Sarzani R., Tamburrini P., Moraca A., Espinosa E., Cola G., Giantomassi L., Rappelli A. Plasma atrial natriuretic peptide and natriuretic peptide receptor gene expression in adipose tissue of normotensive and hypertensive obese patients. J. Hypertens. 1997;15:1695–1699. doi: 10.1097/00004872-199715120-00074. [DOI] [PubMed] [Google Scholar]
- 14.Sarzani R., Strazzullo P., Salvi F., Iacone R., Pietrucci F., Siani A., Barba G., Gerardi M.C., Dessi-Fulgheri P., Rappelli A. Natriuretic peptide clearance receptor alleles and susceptibility to abdominal adiposity. Obes. Res. 2004;12:351–356. doi: 10.1038/oby.2004.44. [DOI] [PubMed] [Google Scholar]
- 15.Kovacova Z., Tharp W.G., Liu D., Wei W., Xie H., Collins S., Pratley R.E. Adipose tissue natriuretic peptide receptor expression is related to insulin sensitivity in obesity and diabetes. Obesity (Silver Spring) 2016;24:820–828. doi: 10.1002/oby.21418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu W., Shi F., Liu D., Ceddia R.P., Gaffin R., Wei W., Fang H., Lewandowski E.D., Collins S. Enhancing natriuretic peptide signaling in adipose tissue, but not in muscle, protects against diet-induced obesity and insulin resistance. Sci. Signal. 2017;10 doi: 10.1126/scisignal.aam6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coue M., Badin P.M., Vila I.K., Laurens C., Louche K., Marques M.A., Bourlier V., Mouisel E., Tavernier G., Rustan A.C., Galgani J.E., Joanisse D.R., Smith S.R., Langin D., Moro C. Defective natriuretic peptide receptor signaling in skeletal muscle links obesity to type 2 diabetes. Diabetes. 2015;64:4033–4045. doi: 10.2337/db15-0305. [DOI] [PubMed] [Google Scholar]
- 18.de Andrade E.N., Goncalves G.K., de Oliveira T.H., Santos C.S., Souza C.L., Firmes L.B., de Magalhaes A.C., Soares Tde J., Reis A.M., Belo Nde O. Natriuretic peptide system: A link between fat mass and cardiac hypertrophy and hypertension in fat-fed female rats. Regul. Pept. 2011;167:149–155. doi: 10.1016/j.regpep.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Tontonoz P., Hu E., Spiegelman B.M. Stimulation of adipogenesis in fibroblasts by PPARg2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 20.Tontonoz P., Hu E., Graves R.A., Budavari A.I., Spiegelman B.M. mPPAR gamma 2: Tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 21.Tontonoz P., Graves R.A., Budavari A.I., Erdjument-Bromage H., Lui M., Hu E., Tempst P., Spiegelman B.M. Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPAR gamma and RXR alpha. Nucleic Acids Res. 1994;22:5628–5634. doi: 10.1093/nar/22.25.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda N., Takahashi M., Funahashi T., Kihara S., Nishizawa H., Kishida K., Nagaretani H., Matsuda M., Komuro R., Ouchi N., Kuriyama H., Hotta K., Nakamura T., Shimomura I., Matsuzawa Y. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 23.Wu Z., Xie Y., Morrison R.F., Bucher N.L., Farmer S.R. PPARgamma induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPalpha during the conversion of 3T3 fibroblasts into adipocytes. J. Clin. Invest. 1998;101:22–32. doi: 10.1172/JCI1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon T.M., Daniel K.W., Farmer S.R., Collins S. CAATT/Enhancer binding protein-a is required for transcription of the b3AR gene during adipogenesis. J. Biol. Chem. 2001;276:722–728. doi: 10.1074/jbc.M008440200. [DOI] [PubMed] [Google Scholar]
- 25.Soccio R.E., Li Z., Chen E.R., Foong Y.H., Benson K.K., Dispirito J.R., Mullican S.E., Emmett M.J., Briggs E.R., Peed L.C., Dzeng R.K., Medina C.J., Jolivert J.F., Kissig M., Rajapurkar S.R. Targeting PPARγ in the epigenome rescues genetic metabolic defects in mice. J. Clin. Invest. 2017;127:1451–1462. doi: 10.1172/JCI91211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoenfelder S., Fraser P. Long-range enhancer-promoter contacts in gene expression control. Nat. Rev. Genet. 2019;20:437–455. doi: 10.1038/s41576-019-0128-0. [DOI] [PubMed] [Google Scholar]
- 27.Daniel B., Nagy G., Hah N., Horvath A., Czimmerer Z., Poliska S., Gyuris T., Keirsse J., Gysemans C., Van Ginderachter J.A., Balint B.L., Evans R.M., Barta E., Nagy L. The active enhancer network operated by liganded RXR supports angiogenic activity in macrophages. Genes Dev. 2014;28:1562–1577. doi: 10.1101/gad.242685.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y., Dallner O.S., Nakadai T., Fayzikhodjaeva G., Lu Y.H., Lazar M.A., Roeder R.G., Friedman J.M. A noncanonical PPARγ/RXRα-binding sequence regulates leptin expression in response to changes in adipose tissue mass. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E6039–E6047. doi: 10.1073/pnas.1806366115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W., Notani D., Rosenfeld M.G. Enhancers as non-coding RNA transcription units: Recent insights and future perspectives. Nat. Rev. Genet. 2016;17:207–223. doi: 10.1038/nrg.2016.4. [DOI] [PubMed] [Google Scholar]
- 30.Bordicchia M., Ceresiani M., Pavani M., Minardi D., Polito M., Wabitsch M., Cannone V., Burnett J.C., Jr., Dessi-Fulgheri P., Sarzani R. Insulin/glucose induces natriuretic peptide clearance receptor in human adipocytes: A metabolic link with the cardiac natriuretic pathway. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016;311:R104–R114. doi: 10.1152/ajpregu.00499.2015. [DOI] [PubMed] [Google Scholar]
- 31.Harvell D.M., Richer J.K., Allred D.C., Sartorius C.A., Horwitz K.B. Estradiol regulates different genes in human breast tumor xenografts compared with the identical cells in culture. Endocrinology. 2006;147:700–713. doi: 10.1210/en.2005-0617. [DOI] [PubMed] [Google Scholar]
- 32.Kishimoto I., Yoshimasa T., Suga S., Ogawa Y., Komatsu Y., Nakagawa O., Itoh H., Nakao K. Natriuretic peptide clearance receptor is transcriptionally down-regulated by beta 2-adrenergic stimulation in vascular smooth muscle cells. J. Biol. Chem. 1994;269:28300–28308. [PubMed] [Google Scholar]
- 33.Pivovarova O., Gogebakan O., Kloting N., Sparwasser A., Weickert M.O., Haddad I., Nikiforova V.J., Bergmann A., Kruse M., Seltmann A.C., Bluher M., Pfeiffer A.F., Rudovich N. Insulin up-regulates natriuretic peptide clearance receptor expression in the subcutaneous fat depot in obese subjects: A missing link between CVD risk and obesity? J. Clin. Endocrinol. Metab. 2012;97:E731–E739. doi: 10.1210/jc.2011-2839. [DOI] [PubMed] [Google Scholar]
- 34.Lehrke M., Lazar M.A. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 35.Xu H.E., Li Y. Ligand-dependent and -independent regulation of PPAR gamma and orphan nuclear receptors. Sci. Signal. 2008;1 doi: 10.1126/scisignal.148pe52. [DOI] [PubMed] [Google Scholar]
- 36.Shin J., Li B., Davis M.E., Suh Y., Lee K. Comparative analysis of fatty acid-binding protein 4 promoters: Conservation of peroxisome proliferator-activated receptor binding sites. J. Anim. Sci. 2009;87:3923–3934. doi: 10.2527/jas.2009-2124. [DOI] [PubMed] [Google Scholar]
- 37.Garin-Shkolnik T., Rudich A., Hotamisligil G.S., Rubinstein M. FABP4 attenuates PPARγ and adipogenesis and is inversely correlated with PPARγ in adipose tissues. Diabetes. 2014;63:900–911. doi: 10.2337/db13-0436. [DOI] [PubMed] [Google Scholar]
- 38.Pennacchio L.A., Bickmore W., Dean A., Nobrega M.A., Bejerano G. Enhancers: Five essential questions. Nat. Rev. Genet. 2013;14:288–295. doi: 10.1038/nrg3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Göndör A., Rougier C., Ohlsson R. High-resolution circular chromosome conformation capture assay. Nat. Protoc. 2008;3:303–313. doi: 10.1038/nprot.2007.540. [DOI] [PubMed] [Google Scholar]
- 40.Hu M., Wang S. Chromatin tracing: Imaging 3D genome and nucleome. Trends Cell Biol. 2021;31:5–8. doi: 10.1016/j.tcb.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duan S.Z., Ivashchenko C.Y., Russell M.W., Milstone D.S., Mortensen R.M. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator–activated receptor-γ both induce cardiac hypertrophy in mice. Circ. Res. 2005;97:372–379. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- 42.Babaev V.R., Yancey P.G., Ryzhov S.V., Kon V., Breyer M.D., Magnuson M.A., Fazio S., Linton M.F. Conditional knockout of macrophage PPARγ increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor–deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005;25:1647–1653. doi: 10.1161/01.ATV.0000173413.31789.1a. [DOI] [PubMed] [Google Scholar]
- 43.Hsueh W.A., Bruemmer D. Peroxisome proliferator-activated receptor γ: Implications for cardiovascular disease. Hypertension. 2004;43:297–305. doi: 10.1161/01.HYP.0000113626.76571.5b. [DOI] [PubMed] [Google Scholar]
- 44.Tontonoz P., Spiegelman B.M. Fat and beyond: The diverse biology of PPARgamma. Annu. Rev. Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 45.Pan D.Z., Garske K.M., Alvarez M., Bhagat Y.V., Boocock J., Nikkola E., Miao Z., Raulerson C.K., Cantor R.M., Civelek M., Glastonbury C.A., Small K.S., Boehnke M., Lusis A.J., Sinsheimer J.S. Integration of human adipocyte chromosomal interactions with adipose gene expression prioritizes obesity-related genes from GWAS. Nat. Commun. 2018;9:1512. doi: 10.1038/s41467-018-03554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kilroy G., Burk D.H., Floyd Z.E. High efficiency lipid-based siRNA transfection of adipocytes in suspension. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagege H., Klous P., Braem C., Splinter E., Dekker J., Cathala G., de Laat W., Forne T. Quantitative analysis of chromosome conformation capture assays (3C-qPCR) Nat. Protoc. 2007;2:1722–1733. doi: 10.1038/nprot.2007.243. [DOI] [PubMed] [Google Scholar]
- 48.Cope N.F., Fraser P. Chromosome conformation capture. Cold Spring Harb. Protoc. 2009 doi: 10.1101/pdb.prot5137. [DOI] [PubMed] [Google Scholar]
- 49.Miele A., Gheldof N., Tabuchi T.M., Dostie J., Dekker J. Mapping chromatin interactions by chromosome conformation capture. Curr. Protoc. Mol. Biol. 2006 doi: 10.1002/0471142727.mb2111s74. Chapter 21:Unit 21.11. [DOI] [PubMed] [Google Scholar]
- 50.Szanto A., Balint B.L., Nagy Z.S., Barta E., Dezso B., Pap A., Szeles L., Poliska S., Oros M., Evans R.M., Barak Y., Schwabe J., Nagy L. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33:699–712. doi: 10.1016/j.immuni.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu D., Bordicchia M., Zhang C., Fang H., Wei W., Li J.L., Guilherme A., Guntur K., Czech M.P., Collins S. Activation of mTORC1 is essential for b-adrenergic stimulation of adipose browning. J. Clin. Invest. 2016;126:1704–1716. doi: 10.1172/JCI83532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson J.T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E.S., Getz G., Mesirov J.P. Integrative genomics viewer. Nat. Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within this article. Reagents and plasmids described in this article are available upon request.