Highlights
-
•
SBCs can mostly be treated conservatively.
-
•
In ABCs a biopsy is compulsory.
-
•
ABCs can be treated by polidocanol instillations adequately.
Abbreviations: ABC, aneurysmal bone cyst; SBC, solitary bone cyst
Keywords: Aneurysmal bone cyst, Solitary bone cyst, Simple bone cyst, Juvenile bone cyst
Abstract
This review of the literature aims to compare the etiology, the pathogenesis, the clinical diagnostics and the relevant treatment options of two different types of cystic bone lesions: the solitary bone cyst (SBC) and the aneurysmal bone cyst (ABC). Whereas the clinical symptoms and the radiographic appearance can be similar, the diagnostic pathway and the treatment options are clearly different.
The solitary bone cyst (SBC) represents a tumor-like bone lesion, occurring most frequently in the humerus and femur in children and adolescents. Pain caused by intercurrent pathological fractures is often the first symptom, and up to 87% of the cysts are associated with pathological fractures. In the majority of cases SBCs can be treated conservatively, especially in the upper extremity. However, if a fracture is completely dislocated, joint affecting, unstable or open, surgical treatment is necessary. Pain under weight bearing or regaining the ability to mobilize after fracture timely can necessitate surgical treatment in SBCs affecting the lower extremity. Spontaneous resolution can be seen in rare cases.
The aneurysmal bone cyst (ABC) is a benign, locally aggressive tumor that occurs in childhood and early adulthood. It usually affects the metaphysis of long bones but can also occur in the spine or the pelvis. ABC can be primary but also secondary to other bone pathologies. The diagnosis has to be confirmed by biopsy and histopathological examinations. With cytogenetic studies and the detection of specific translocations of the ubiquitin-specific protease (USP) 6 gene primary ABCs can be differentiated from secondary ABCs and other bone lesions. Among various modalities of treatment i.e. en bloc resection, intralesional curettage with adjuvants, embolization or the systemic application of denosumab, intralesional sclerotherapy using polidocanol is an effective and minimally invasive treatment of primary ABCs.
Solitary (SBC) and aneurysmal bone cysts (ABC) are benign, fluid filled bone lesions. They appear in childhood or early adulthood. The clinical symptoms and the radiographic appearance can be similar, but the diagnostic pathway and the treatment options are clearly different.
1. Solitary bone cysts
Solitary bone cysts (SBCs) were first described by the german pathologist Rudolph Virchow in 1876 [1]. They are benign, single chambered and fluid filled.
1.1. Epidemiology
The average age of onset is 8–14 years [2]. The male-to-female ratio is 2:1 to 4:1 [3].
1.2. Localization
SBCs represent tumor-like bone lesions, occurring most frequently in the proximal aspect of the humerus and femur in children and adolescents [4].
1.3. Clinical symptomatology and radiographic findings
The majority of patients with SBCs is asymptomatic and the diagnosis is mainly incidental. If symptomatic, pain caused by intercurrent pathological fractures is often the first symptom, and 63–87% of the cysts are combined with pathological fractures [5]. SBCs show specific radiographic features on conventional radiographs and MRI scans (Fig. 1). Usually, they appear as a centrally located, radiolucent, well-marginated and slightly expansile lesion in the proximal metaphysis [4]. In MRI scans non or only a few septae and fluid-fluid levels compared to aneurysmal bone cysts can be seen. Pathological fractures may occur due to thinned and weakened cortex. The fallen leave sign is a typical radiographic finding [6]. In case of pathological fracture patients are often unaware of the presence of a preexisting cystic bone lesion.
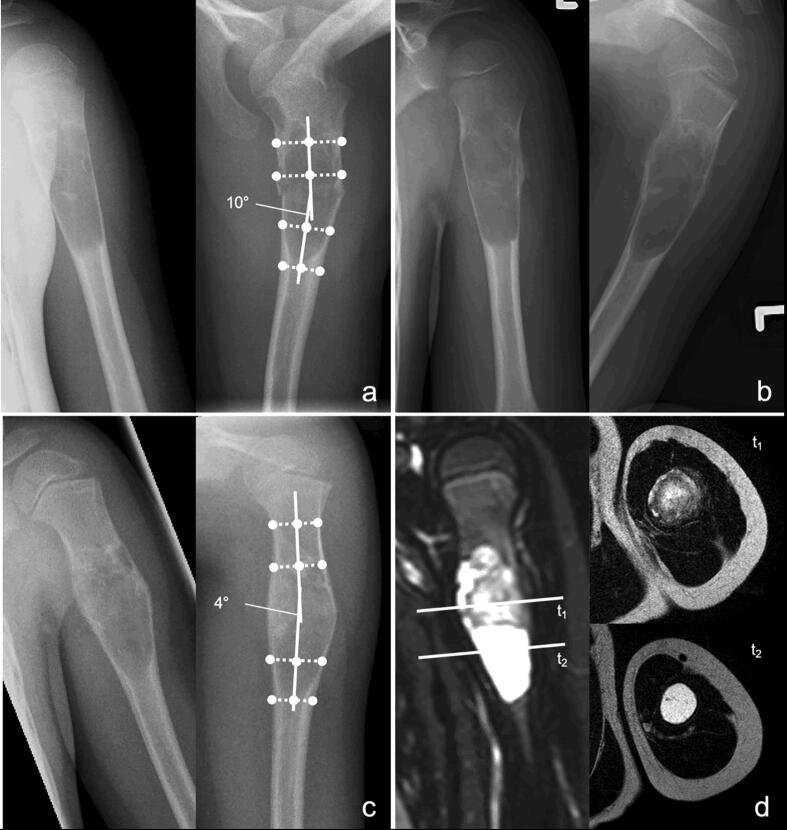
Fig. 1.
Imaging of a 6-year-old male patient with SBC and pathological fracture of the proximal humerus. Anteroposterior and lateral radiographs showing the fracture with 10° axis deviation in the lateral plane (a). Anteroposterior and lateral radiographs four weeks after conservative treatment showing progressive consolidation of the fracture (b). Anteroposterior and lateral radiographs (c) and MRI scans (d) twelve months after fracture showing partial sclerosis of the SBC and complete consolidation of the fracture.
1.4. Histological findings
Histologically, the wall of the cyst is lined with a thin fibrous membrane. The membrane contains immature, calcified, flakey cement-like bone matter. In case of fracture, the fibrous membrane thickens, becoming cellular, with fibroblastic reaction, osteoclastic giant cells, inflammatory cellular elements and hemosiderin and cholesterol deposits [7].
1.5. Etiology and pathogenesis
For explaining the pathogenesis of SBCs various hypotheses were formulated. Among them bone resorptive properties of the cyst lining, vascular obstruction, increase in intra-cavitary osseous pressure, inflammation or traumatic causes are proposed as cause for the genesis [8], [9], [10], [11].
1.6. Treatment
For symptomatic SBCs different treatment options of SBCs are controversially discussed in literature, mainly regarding the particularities of the affected limb, possible growth plate involvement and the presence and type of a pathological fracture. Among the therapeutical options are simple observation [12], decompression of the cyst with screws or nails [13], intracavitary injection of steroids and autologous red bone marrow [14], intralesional curettage and defect reconstruction with bone substitute [15] or elastic intramedullary nailing [2] with or without use of bone marrow and/or bone substitute. In case of pathological fracture, conservative and different surgical treatment modalities are discussed [12]. As the cysts tend to resolve spontaneously after puberty [16] every surgical procedure has to be critically discussed with the patient and his family. Hardes et al. [13] regard a stable situation without clinical symptoms, lack of radiographic progression of the cyst or an incomplete healing as an acceptable intermediate result. In a recent study of our institution, we analyzed 68 cases of SBCs who underwent a conservative or surgical treatment between 2009 and 2020. The patient collective included 50 male (73.5%) and 18 female (26.5%) patients with a mean age of 9.1 years. The most common locations were the proximal humerus (69.2%) and femur (16.2%). In 43 cases (63.2%) a pathological fracture occurred. 50 patients (73.5%) underwent a conservative treatment. In 11 cases (16.2.1%; upper limb n = 4, lower limb n = 7) an intralesional curettage and defect reconstruction with bone substitute without stabilization were performed. Five patients (7.3%) received an osteosynthesis, in two cases (2,9%) combined with an intralesional curettage and defect reconstruction with bone substitute. All 32 pathological fractures treated conservatively, healed within six weeks; 17/43 patients (39.5%) suffered at least one second fracture. After intralesional curettage and defect reconstruction with bone substitute local recurrence was observed in 5/13 cases (38.5%). Spontaneous consolidation, at least partially, was observed in three cases (4.4%) following conservative treatment after fracture. No relevant secondary angular or torsional deformity was observed after treatment (Fig. 1). Nevertheless, the absolute volume of SBCs increased due to the natural bone growth of children and adolescents. Ambacher et al. [12] did not see the need for surgical intervention in every case of SBC. In uncomplicated cysts with or without pathological fracture the authors rather underlined the possibility of spontaneous healing. However, the authors considered surgical treatment in completely dislocated, joint affecting, instable or open fractures [12]. In such cases, elastic intramedullary nailing was described as surgical treatment option with or without the use of bone substitute [2]. Irrespective of the affected limb Rapp al. [17] recommended elastic intramedullary nailing in combination with artificial bone substitute and autologous platelet rich plasma to treat pathological fractures due to SBCs. Zhang et al. [2] saw significant advantages in bone healing and recurrence rates in a comparative study between the treatment of SBCs by intralesional curettage with and without additional nailing in favour of the combined technique.
Generally, it has to be called into question if intramedullary nailing without curettage of the cyst is advisable in case of an uncomplicated fracture of the upper extremity. As every fracture in in the above-mentioned study of our institution healed without secondary axis deviation under conservative treatment timely, the necessity of an operative treatment only for stabilization of the fracture without addressing the underlying cyst is questionable.
Intralesional curettage with filling up the resulting cavity with bone substitute is a common surgical procedure in case of a symptomatic SBC and an alternative treatment to simple observation. Curettage and defect reconstruction with bone substitute in cases without pathological fracture are performed due to pain under weight bearing in the lower extremity and due to the prevention of frequent fractures of the humerus in the upper extremity. After intralesional curettage and defect reconstruction with bone substitute complete healing of the cyst can be observed in one-third of the cases, while a local recurrence can be expected in 30–40% of the patients [2]. Traub et al. reported no recurrence of SBCs after curettage and local steroid application in a small cohort of eight patients. In contrast the authors described failure rates after initial treatment of 36.6% with steroids, 50.0% with intramedullary nailing alone and 21.4% with intramedullary nailing and additional steroid application [4]. Sakamoto et al. achieved promising results in a study with 13 patients with SBCs treated surgically by curettage and local steroid injection [18]. Only one of the 13 patients in this study developed a pathological fracture and underwent bone grafting. The remaining 12 patients achieved bone consolidation, after an average of 1.6 injections. Nevertheless, due to the low number of patients in the above-mentioned studies, larger, especially prospective studies are necessary to verify the described treatment success.
Concluding, symptomatic but unfractured SBCs of the upper extremity do not need any treatment. Regarding the uncomplicated healing potential and the lack of relevant secondary axis deviations the majority of pathological fractures caused by SBCs of the upper extremity can successfully be treated conservatively. However, if the fracture is completely dislocated, joint affecting, unstable or open, surgical treatment is advisable. In symptomatic SBCs affecting the lower extremity surgical treatment is recommended in most cases with pathological fracture to regain the ability to mobilize timely and to prevent secondary axis deviations. Contrary to the upper limb surgical stabilization can also be considered in symptomatic unfractured SBCs of the lower extremity due to load-dependent pain and increased risk of fracture. In these conditions intralesional curettage and defect reconstruction with bone substitute might be preferable. However, the considerable risk of local recurrence has to be considered.
2. Aneurysmal bone cyst
The aneurysmal bone cyst (ABC) was first described by Jaffé and Lichtenstein in 1942 [19]. It is a relatively rare, benign, mostly local aggressive bone tumor that occurs in childhood and early adulthood.
2.1. Epidemiology
For ABCs the literature describes a percentage of 1–6% of all primary, benign bone tumors [20], [21], [22], [23], [24] and an incidence of 0.14/ 100.000 per year [20], [24], [25], [26]. ABCs occur in 75–90% in patients younger than twenty years [7], [21], [24] but can also occur in any older age [20], [27], [28]. Regarding the gender distribution, some authors describe an equal incidence for both genders, other authors as for example Leithner et al. [26] describe a higher incidence for females [19], [21], [24], [25], [26], [27].
2.2. Localization
Most common localization are the metaphysis of long bones and the posterior part of spine, but ABCs can also affect any other localization [20], [21], [24], [27], [29], [30], [31].
2.3. Clinical symptomatology and radiographic findings
Patients usually complain of pain and swelling in the affected region and sometimes pathological fractures can be observed. In plain radiographs a relatively well-defined osteolytic, “expansile” lesion with possible blow out of the periosteum and a soap-bubble appearance can be found [7], [21], [24], [29], [31]. The magnetic resonance imaging shows cystic formations with typical fluid-fluid levels due to blood sedimentation [22]. A biopsy is compulsory, and the results of the histopathological examinations have to be seen in synopsis with the clinic history, the radiographs and MR scans.
2.4. Histological findings
Histologically, an ABC appears as solitary, multicystic lesion, which grows rapidly and locally destructive [23]. Septae of variable thickness divide the ABC in numerous blood-filled cavities of different sizes [23], [24], [27], [32]. The fibrous septae consist of osteoclast-like giant cells, stromal mononuclear cells, spindle cells and even small strands of osteoid and reactive woven bone (Fig. 2).
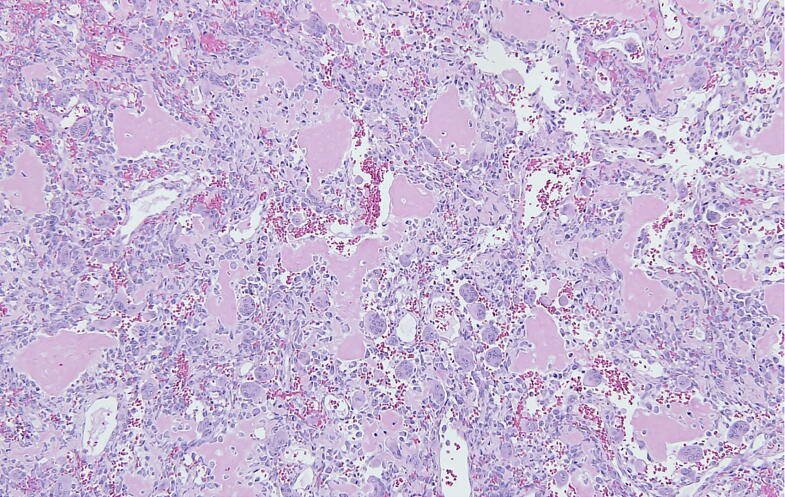
Fig. 2.
Solid parts of an ABC showing scattered mononuclear lesional cells intermingled with multinuclear giant cells and well discernible osteoid formation.
2.5. Etiology and pathogenesis
The precise etiology and pathogenesis of ABCs are not fully clarified. Former theories explained the “characteristic spaces by reference to unspecified hemodynamic disturbances or arteriovenous fistulae” [33], [34], [35]. These theories are no longer accepted for primary ABCs [7]. Since Panoutsakopoulos et al. [36], [37] and Oliveira et al. [37], [38], [39] demonstrated that a recurrent chromosome aberration t(16;17) (q22;p13) leads to a fusion gene of the entire ubiquitin-specific protease 6 (USP6 alias Tre2) coding sequence at 17p13 and the promoter region of the osteoblast cadherin 11 gene (CDH11) at 16q22, ABCs are seen as a primary neoplasm. This translocation of USP6 and the promoter region of cadherin 11 leads to an upregulated expression of the otherwise structurally and functionally intact USP6 [40]. USP6 is part of the deubiquitinase enzyme family that removes ubiquitin from protein substrates [27]. This plays an important role for several regulation processes like e.g. stability of proteins, degradation, cell signaling, angiogenesis and inflammatory response. Oliveira et al. [41] demonstrated that the effect of USP6 in the fibrous stromal component of ABCs leads to the expression of matrix metalloproteinase through activation of nuclear factor κB (NFκB). The biological effects of these processes, e.g. the interaction with the Jakl-STAT3 pathway lead to the typical histological features of the lesion mimicking a hemorrhagic pathology [2]. Oliveira et al. could identify USP6 rearrangements in 69% of primary ABCs and in none of the secondary ABCs using Fluorescence in situ Hybridization (FISH) [38]. By next generation sequencing Guseva et al. [42] and Sekoranja et al. [40] increased the number of detected USP6 involving gene rearrangements to 100%. Both identified new USP6 fusion partners with the help of next generation sequencing and assume still further undiscovered fusion partners [40], [42]. Thus, primary ABCs can be differentiated from secondary and other bone lesions by biopsy and histopathological examinations [43]. Secondary ABCs can occur in cases of a giant cell tumor, a chondroblastoma or a telangiectatic osteosarcoma. Secondary ABCs represent approximately 30% of the whole entity of ABCs and are associated with a preexisting osseus lesion [21], [22], [24], [30]. A malignant transformation is described in rare cases [24]. For secondary ABCs which do not show any chromosomal aberrations involving the USP6 gene, the theory of hemodynamic disturbances remains plausible [38], [44].
2.6. Treatment
Different modalities of treatment are described in literature i.e. intralesional curettage with or without adjuvants, wide resection, embolization, percutaneous intralesional injections using polidocanol or other agents like doxycycline, injection of bone marrow, cryoablation, radiation therapy, radionuclide ablation, or the systemic application of denosumab or bisphosphonates [22], [24], [45]. A USP6 targeted therapy option for ABCs is not described in literature, yet. The optimal treatment is still under discussion.
An en bloc surgical resection results in excellent local control rate but can provoke other complications depending on the dimension of the operation like e.g. bleeding, pain and growth disturbances [22], [46]. The en bloc resection may lead to a defect of the bone, that has to be reconstructed. Depending on the ABC’s localization, this can be challenging. Flont et al. [22], [47] described a non-significant higher rate of complications in en bloc resections than in curettage. They also proposed the wide resection to be considered in cases of local recurrence [22], [47]. Because of these aspects, the predominant therapy for ABCs is a less radical, intralesional curettage (Fig. 3, Fig. 4). Nevertheless, the curettage is performed as open surgery and comes along with possible complications as well. A local recurrence after curettage of over 50% is described in literature [31]. Due to this, the use of various adjuvants has been described in literature [22], [24]. Adjuvants can be hydrogen peroxide, phenol, filling of the lesion with PMMA (Fig. 4), the use of a high-speed burr or the use of electrocautery [22], [24]. Even local MRI-guided percutaneous cryoablation has been used as adjuvant therapy [48]. Nevertheless, the benefit of the different adjuvants is still questionable. Kececi et al.[49] showed no significant effect of adjuvant phenol or of the use of a high-speed burr on local recurrence after intralesional curettage of ABCs in a study with 85 patients. Even Grahneis et al. [31] could not confirm a significant benefit of adjuvant phenolization in intralesional curettage in a similar study with 65 patients. On the other hand, Garg et al. [22], [50] described a significant superiority of the combined use of a high-speed burr and electrocautery compared to simple curettage concerning local recurrence rate in a small study with 12 patients with an ABC of the spine.
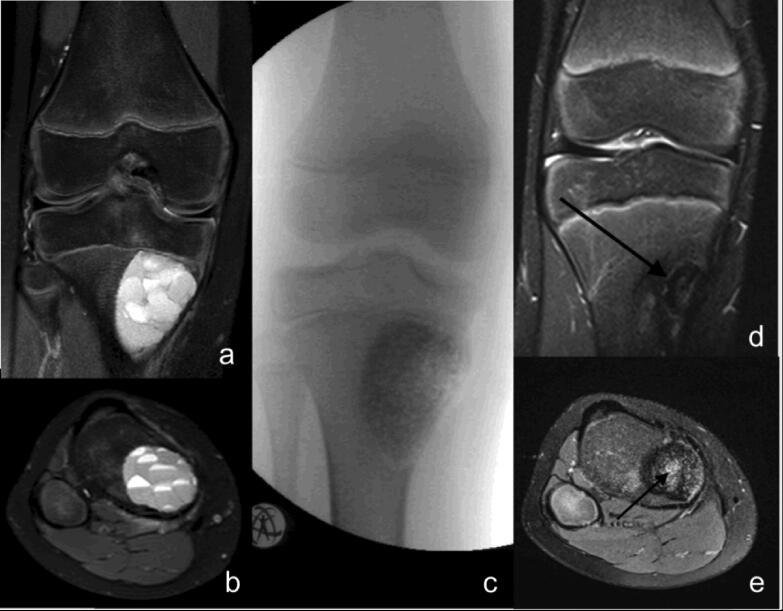
Fig. 3.
ABC of the proximal tibia of a 12 years old boy – preoperative MRI scans with typical fluid-fluid levels (a + b), intraoperative fluoroscopy after curettage and defect reconstruction with bone substitute (c) and progressive osseous integration of the bone substitute (d + e; arrow).
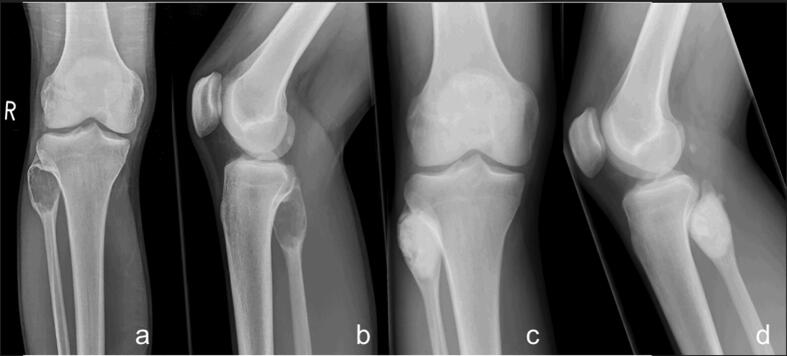
Fig. 4.
ABC of the proximal fibula of an 18 years old patient – preoperative radiographs (a + b) and after curettage and reconstruction with PMMA (c + d).
A minimally invasive treatment is the percutaneous intralesional injection of different agents. Nowadays one of the most common agents is the sclerosant “polidocanol” (Fig. 5). Since the 1960 s polidocanol was used as endovenous sclerosing agent to varicose veins [25]. Sclerosants damage the endothelium and start a coagulation cascade which ends in thrombosis [25]. The use of sclerosants in ABCs is based on the above- mentioned theory of a hemorrhagic lesion of the bone as relevant factor of the pathogeneses [34], [51]. There are numerous authors who postulate the excellent therapeutic effect of sclerosants on ABCs [20], [25], [31], [44], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64] (table 1). Rastogi et al. [52] reported a cure rate of 97% in a study of 72 patients with primary ABCs and sequential polidocanol instillations; Jasper et al. [64] described a cure rate of 83% in a comparative study of 70 patients. Trends toward increased risk for treatment failure in this study were age younger than 5, epiphyseal plate involvement, and lower leg lesions.
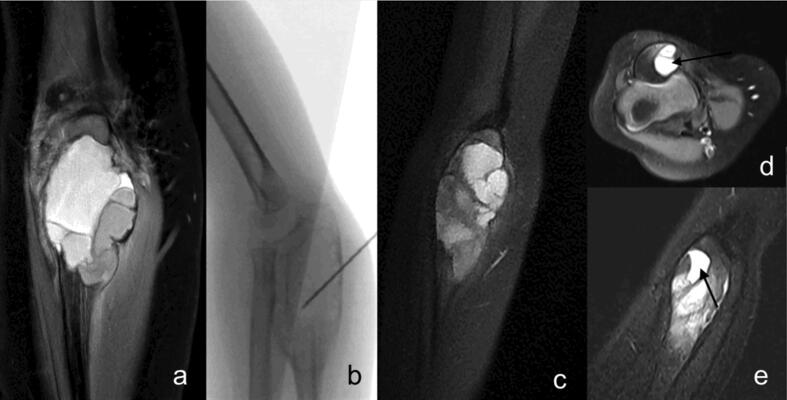
Fig. 5.
ABC of the proximal ulna of a 3 years old girl – initial MRI scan (a); fluoroscopy of instillation of polidocanol (b); progressive resolution of the cyst (after 3 instillations) with residual cystic elements and scattered fluid–fluid levels (c); healing grade I according to Rastogi et al. with complete resolution of fluid–fluid levels (d + e; arrow).
Table 1.
Results of treatment of aneurysmal bone cysts with slerotherapy.
| Study | Year | Sclerosant | Number of patients | Mean number of instillations | Number of patients with one injection | Number of patients with multiple injections | Complete healing | Partial healing | Recurrence/pesistent disease requiring further treatment |
|---|---|---|---|---|---|---|---|---|---|
| Guibaud et al. [54] | 1998 | Ethibloc | 16 | (1–3) | 10 | 6 | 13 | 2 | 1 |
| Garg et al. [50] | 2000 | Ethibloc | 10 | 1,3 (1–2) | 7 | 3 | 7 | 3 | 0 |
| Falappa et al. [55] | 2002 | Ethibloc | 13 | 2,4 (1–4) | 4 | 9 | 13 | 0 | 0 |
| Adamsbaum et al. [44] | 2003 | Ethibloc | 17 | (1–3) | 14 | 3 | 14 | 0 | 3 |
| Dubois et al. [56] | 2003 | Ethibloc | 17 | 1,7 (1–5) | 8 | 9 | 16 | 1 | 0 |
| Topouchian et al. [57] | 2004 | Ethibloc | 15 | 1,4 (1–3) | 11 | 4 | 9 | 2 | 4 |
| de Gauzy et al. [58] | 2005 | Ethibloc | 12 | 1,1 (1–2) | 11 | 1 | 6 | 3 | 3 |
| Rastogi et al. [52] | 2006 | Polidocanol | 72 | 3 (1–5) | 10 | 62 | 0 | 70 | 2 |
| George et al. [59] | 2009 | Ethibloc | 33 | 1,2 (1–2) | 25 | 8 | 18 | 11 | 2 |
| Varshney et al. [51] | 2010 | Polidocanol | 45 | 2,3 (1–3) | 14 | 31 | 44 | 0 | 3 |
| Lambot-Juhan et al. [60] | 2012 | absolute alcohol | 29 | 1,8 (1–4) | 13 | 16 | 17 | 9 | 3 |
| Brosjo et al. [25] | 2013 | Polidocanol | 38 | 4 (1–11) | 5 | 33 | 37 | 0 | 1 |
| Batisse et al. [20] | 2016 | Ethibloc(6), absolute alcohol (2) absolute alcohol gel (2) Aethoxi-sclerol (9) | 19 | 1,2 (1–2) | 15 | 4 | 11 | 2 | 0 |
| Ulici et al. [61] | 2018 | Ethanol 96% | 17 | – | – | – | 17 | 0 | 0 |
| Grahneis et al. [31] | 2019 | Polidocanol | 3 | – | – | – | 0 | 1 | 2 |
| Puri et al. [53] | 2020 | Polidocanol | 56 | 2 (1–5) | 24 | 32 | 42 | 0 | 13 |
| Marie-Hardy et al. [62] | 2020 | alcohol-based | 55 | 1,7 (1–4) | – | – | – | – | 1 |
| Deventer et al. [65] | 2021 | Polidocanol | 32 | 5,7 (1–12) | 0 | 32 | 3 | 19 | 10 |
| Puthoor et al. [63] | 2021 | Polidocanol | 31 | – | – | – | 31 | 0 | 0 |
| Jasper et al. [64] | 2021 | Polidocanol | 70 | 1,83 (1–5) | 28 | 30 | 58 | – | 12 |
In a prospective study of Varshney et al. [51] 94 patients with a primary ABC were treated with intralesional curettage or sequential percutaneous instillation of polidocanol. The authors could not find significant differences for recurrence rates between the two treatment groups. Deventer at al. [65] did not find either a significant difference between the intralesional curettage and the sequential instillation of polidocanol in the treatment of primary ABCs in a study with 74 patients. The authors relativized the outstanding results of former studies by Rastogi et al. [52] curing a primary ABC by a single instillation. The average number of instillations in the above mentioned study of Deventer et al. [65] was 5.7. In two cases (6.3%) a healing disorder occurred as relevant side effect after instillation of polidocanol. Potential complications named in literature are hypopigmentation [66], necrosis of the skin at the side of injection, osteomyelitis, allergic reactions and anesthetic complications [67]. Jasper et al. described [64] only one case of an allergic reaction to polidocanol in a study of 70 patients with a primary ABC. Polidocanol is relatively contraindicated for pregnant patients in the first trimenon, for patients with asthma, with heart disease and preexisting wound healing disorders [67].
The instillation of doxycycline seems to be another possible treatment option [68]. Woon et al. [68] treated seven patients with a primary ABC by instillation of doxycycline. The authors describe a sufficient treatment success after a single instillation in three cases, three further patients needed further instillations, one patient was converted to a surgical treatment. Shiels et al. [69] reported a low recurrence rate of 6% within the first 18 months of follow-up after instillation of doxycycline in a retrospective case series of 16 patients with a primary ABC. As relevant side effect focal skin necrosis occurred in one case only due to an extravasation of doxycycline. Larger, especially prospective studies of percutaneous instillation of doxycycline are necessary to verify the persistent treatment success. Comparative studies between the instillation of doxycycline and polidocanol could verify the effectiveness of both substances regarding the treatment of primary ABCs.
Embolization of ABCs can be used as adjuvant or single therapy when surgical resection is difficult or connected with probable risk of complications. Henrichs et al. reported the successful use of embolization in ABCs in the sacrum as single therapy [70].
There are also reports in literature about a conservative treatment of ABCs with Denosumab or bisphosphonates [45], [71], [72]. Denosumab can be used in cases when surgical procedures and/or embolization are not successful or possible. Denosumab has been approved for use in osteoporosis, multiple myeloma, skeletal metastasis and recently giant cell tumor of the bone [71]. It is a monoclonal antibody which blocks Receptor Activator of NF-κB Ligand (RANKL), a ligand that binds to Receptor Activator of NF-κB (RANK). The use of Denosumab as a rescue therapy is also described by Lange et al. who used denosumab in two cases of ABC of the spine where embolization failed [73].
A related therapeutic approach is the application of bisphosphonates. Bisphosphonates principally were used for the therapy of osteoporosis, inhibiting bone resorption by increasing osteoclast apoptosis [45]. Several studies showed an antitumoral effect of bisphosphonates in metastatic bone disease [45]. Nowadays bisphosphonates established successfully in the management of unresectable, symptomatic bone tumours [45]. Kieser et al. [45] demonstrated the favorable response of ABCs to bisphosphonates. Like Cornelis et al. [74] the authors reported about the reduction of symptoms and the progressive ossification of the lesion [45].
3. Conclusion
Clinical symptoms and the radiographic appearance of different cystic bone lesions can be similar; however, the diagnostic pathway and the treatment options are significantly different.
Asymptomatic unfractured SBCs of the upper extremity do not need any treatment. However, if the fracture is completely dislocated, joint affecting, unstable or open, surgical treatment is advisable. In SBCs affecting the lower extremity surgical treatment is recommended in most cases with pathological fracture to regain the ability to mobilize timely and to prevent secondary axis deviations.
In case of an ABC a biopsy is compulsory to verify the diagnosis and to exclude relevant differential diagnoses. Sequential instillations of polidocanol are the most commonly recommended treatment option. Nevertheless, several instillations can be necessary. In a considerable number of cases, a conversion to intralesional curettage can be necessary.
4. Type of study
Review of the literature.
Funding
No financial support was received for this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.V. R., Über die Bildung von Knochencysten, Monatsberichte der Königlich Preussischen Akademie der Wissenschaften. (1876) 369–438.
- 2.Zhang K., Wang Z., Zhang Z. Comparison of curettage and bone grafting combined with elastic intramedullary nailing vs curettage and bone grafting in the treatment of long bone cysts in children. Medicine (Baltimore) 2019;98(25) doi: 10.1097/MD.0000000000016152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang P., Zhu N., Du L., Zheng J., Hu S., Xu B. Treatment of simple bone cysts of the humerus by intramedullary nailing and steroid injection. BMC Musculoskelet. Disord. 2020;21(1):70. doi: 10.1186/s12891-020-3054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traub F., Eberhardt O., Fernandez F.F., Wirth T. Solitary bone cyst: a comparison of treatment options with special reference to their long-term outcome. BMC Musculoskelet. Disord. 2016;17:162. doi: 10.1186/s12891-016-1012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erol B., Onay T., Topkar O.M., Tokyay A., Aydemir A.N., Okay E. A comparative study for the treatment of simple bone cysts of the humerus: open curettage and bone grafting either without instrumentation or with intramedullary nailing. J. Pediatr. Orthop. B. 2017;26(1):5–13. doi: 10.1097/BPB.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 6.Neer C.S., Francis K.C., Marcove R.C., Terz J., Carbonara P.N. Treatment of unicameral bone cyst. A follow-up study of one hundred seventy-five cases. J. Bone Joint Surg. Am. 1966;48(4):731–745. [PubMed] [Google Scholar]
- 7.Mascard E., Gomez-Brouchet A., Lambot K. Bone cysts: unicameral and aneurysmal bone cyst. Orthop. Traumatol. Surg. Res. 2015;101(1):S119–S127. doi: 10.1016/j.otsr.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 8.Docquier P.-L., Delloye C. Treatment of simple bone cysts with aspiration and a single bone marrow injection. J. Pediatr. Orthop. 2003;23(6):766–773. doi: 10.1097/00004694-200311000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson S.E., Chundamala J., Yandow S., Wright J.G. Treatment for unicameral bone cysts in long bones: an evidence based review. Orthop. Rev. (Pavia) 2010;2(1):13. doi: 10.4081/or.2010.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komiya S., Minamitani K., Sasaguri Y., Hashimoto S., Morimatsu M., Inoue A. Simple bone cyst. Treatment by trepanation and studies on bone resorptive factors in cyst fluid with a theory of its pathogenesis. Clin. Orthop. Relat. Res. 1993;287:204–211. doi: 10.1097/00003086-199302000-00032. [DOI] [PubMed] [Google Scholar]
- 11.Shindell R., Huurman W.W., Lippiello L., Connolly J.F. Prostaglandin levels in unicameral bone cysts treated by intralesional steroid injection. J. Pediatr. Orthop. 1989;9(5):516–519. doi: 10.1097/01241398-198909010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Ambacher T., Maurer F., Weise K. Spontaneous healing of a juvenile bone cyst of the tibia after pathological fracture. Unfallchirurg. 1999;102(12):972–974. doi: 10.1007/s001130050512. [DOI] [PubMed] [Google Scholar]
- 13.Hardes J., Schultheiss M., Gosheger G., Schulte M. The juvenile bone cyst: treatment with continuous decompression using cannulated screws. Orthopade. 2009;38(3):256–262. doi: 10.1007/s00132-009-1407-9. [DOI] [PubMed] [Google Scholar]
- 14.Arazi M., Senaran H., Memik R., Kapicioglu S. Minimally invasive treatment of simple bone cysts with percutaneous autogenous bone marrow injection. Orthopedics. 2005;28(2):108–112. doi: 10.3928/0147-7447-20050201-09. [DOI] [PubMed] [Google Scholar]
- 15.Zhao J.G., Ding N., Huang W.J., Wang J., Shang J., Zhang P. Interventions for treating simple bone cysts in the long bones of children. Cochrane Database Syst. Rev. 2014;9:CD010847. doi: 10.1002/14651858.CD010847.pub2. [DOI] [PubMed] [Google Scholar]
- 16.O.H. Freyschmidt J, Knochentumoren. Klinik, Radiologie, Pathologie, 2. Aufl. Springer, Berlin Heidelberg New York, 1988, S 738–754.
- 17.Rapp M., Svoboda D., Wessel L.M., Kaiser M.M. Elastic Stable Intramedullary Nailing (ESIN), Orthoss(R) and Gravitational Platelet Separation-System (GPS(R)): an effective method of treatment for pathologic fractures of bone cysts in children. BMC Musculoskelet. Disord. 2011;12:45. doi: 10.1186/1471-2474-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakamoto A., Matsuda S., Yoshida T., Iwamoto Y. Clinical outcome following surgical intervention for a solitary bone cyst: emphasis on treatment by curettage and steroid injection. J. Orthop. Sci. 2010;15(4):553–559. doi: 10.1007/s00776-010-1485-x. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe H.L., Lichtenstein L. Solitary unicameral bone cyst: with emphasis on the roentgen picture, the pathologic appearance and the pathogenesis. Arch. Surg. 1942;44(6):1004–1025. [Google Scholar]
- 20.Batisse F., Schmitt A., Vendeuvre T., Herbreteau D., Bonnard C. Aneurysmal bone cyst: A 19-case series managed by percutaneous sclerotherapy. Orthop. Traumatol. Surg. Res. 2016;102(2):213–216. doi: 10.1016/j.otsr.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser C.L., Yeung C.M., Raskin K.A., Lozano-Calderon S.A. Aneurysmal bone cyst of the clavicle: a series of 13 cases. J. Shoulder Elbow Surg. 2019;28(1):71–76. doi: 10.1016/j.jse.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 22.Tsagozis P., Brosjo O. Current strategies for the treatment of aneurysmal bone cysts. Orthop. Rev. (Pavia) 2015;7(4):6182. doi: 10.4081/or.2015.6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dormans J.P., Hanna B.G., Johnston D.R., Khurana J.S. Surgical treatment and recurrence rate of aneurysmal bone cysts in children. Clin. Orthop. Relat. Res. 2004;421:205–211. doi: 10.1097/01.blo.0000126336.46604.e1. [DOI] [PubMed] [Google Scholar]
- 24.Noordin S., Ahmad T., Umer M., Allana S., Hilal K., Uddin N., Hashmi P. Aneurysmal bone cyst of the pelvis and extremities. Int. J. Surg. Oncol. 2019;4(3) [Google Scholar]
- 25.Brosjö O., Pechon P., Hesla A., Tsagozis P., Bauer H. Sclerotherapy with polidocanol for treatment of aneurysmal bone cysts. Acta Orthop. 2013;84(5):502–505. doi: 10.3109/17453674.2013.850013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leithner A., Windhager R., Lang S., Haas O.A., Kainberger F., Kotz R. Aneurysmal bone cyst. A population based epidemiologic study and literature review. Clin. Orthop. Relat. Res. 1999;363:176–179. doi: 10.1097/00003086-199906000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Blackburn P.R., Davila J.I., Jackson R.A., Fadra N., Atiq M.A., Pitel B.A., Nair A.A., VanDeWalker T.J., Hessler M.G., Hovel S.K., Wehrs R.N., Fritchie K.J., Jenkins R.B., Halling K.C., Geiersbach K.B. RNA sequencing identifies a novel USP9X-USP6 promoter swap gene fusion in a primary aneurysmal bone cyst. Genes Chromosom. Cancer. 2019;58(8):589–594. doi: 10.1002/gcc.22742. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira A.M., Chou M.M. USP6-induced neoplasms: the biologic spectrum of aneurysmal bone cyst and nodular fasciitis. Hum. Pathol. 2014;45(1):1–11. doi: 10.1016/j.humpath.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Song W., Suurmeijer A.J.H., Bollen S.M., Cleton-Jansen A.M., Bovee J., Kroon H.M. Soft tissue aneurysmal bone cyst: six new cases with imaging details, molecular pathology, and review of the literature. Skeletal Radiol. 2019;48(7):1059–1067. doi: 10.1007/s00256-018-3135-x. [DOI] [PubMed] [Google Scholar]
- 30.Ghanem I., Nicolas N., Rizkallah M., Slaba S. Sclerotherapy using Surgiflo and alcohol: a new alternative for the treatment of aneurysmal bone cysts. J. Child. Orthop. 2017;11(6):448–454. doi: 10.1302/1863-2548.11.170106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grahneis F., Klein A., Baur-Melnyk A., Knösel T., Birkenmaier C., Jansson V., Dürr H.R. Aneurysmal bone cyst: a review of 65 patients. J. Bone Oncol. 2019;18:100255. doi: 10.1016/j.jbo.2019.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Docquier P.-L., Delloye C., Galant C. Histology can be predictive of the clinical course of a primary aneurysmal bone cyst. Arch. Orthop. Trauma Surg. 2010;130(4):481–487. doi: 10.1007/s00402-009-0887-8. [DOI] [PubMed] [Google Scholar]
- 33.Martinez V., Sissons H.A. Aneurysmal bone cyst. A review of 123 cases including primary lesions and those secondary to other bone pathology. Cancer. 1988;61(11):2291–2304. doi: 10.1002/1097-0142(19880601)61:11<2291::aid-cncr2820611125>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 34.Lichtenstein L. Aneurysmal bone cyst; observations on fifty cases. J. Bone Joint Surg. Am. 1957;39(4):873–882. [PubMed] [Google Scholar]
- 35.Biesecker J.L., Marcove R.C., Huvos A.G., Miké V. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer. 1970;26(3):615–625. doi: 10.1002/1097-0142(197009)26:3<615::aid-cncr2820260319>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 36.Panoutsakopoulos G., Pandis N., Kyriazoglou I., Gustafson P., Mertens F., Mandahl N. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosom. Cancer. 1999;26(3):265–266. doi: 10.1002/(sici)1098-2264(199911)26:3<265::aid-gcc12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L., Hwang S., Benayed R., Zhu G.G., Mullaney K.A., Rios K.M., Sukhadia P.Y., Agaram N., Zhang Y., Bridge J.A., Healey J.H., Athanasian E.A., Hameed M. Myositis ossificans-like soft tissue aneurysmal bone cyst: a clinical, radiological, and pathological study of seven cases with COL1A1-USP6 fusion and a novel ANGPTL2-USP6 fusion. Mod. Pathol. 2020;33(8):1492–1504. doi: 10.1038/s41379-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliveira A.M., Perez-Atayde A.R., Inwards C.Y., Medeiros F., Derr V., Hsi B.-L., Gebhardt M.C., Rosenberg A.E., Fletcher J.A. USP6 and CDH11 oncogenes identify the neoplastic cell in primary aneurysmal bone cysts and are absent in so-called secondary aneurysmal bone cysts. Am. J. Pathol. 2004;165(5):1773–1780. doi: 10.1016/S0002-9440(10)63432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliveira A.M., Hsi B.L., Weremowicz S., Rosenberg A.E., Dal Cin P., Joseph N., Bridge J.A., Perez-Atayde A.R., Fletcher J.A. USP6 (Tre2) fusion oncogenes in aneurysmal bone cyst. Cancer Res. 2004;64(6):1920–1923. doi: 10.1158/0008-5472.can-03-2827. [DOI] [PubMed] [Google Scholar]
- 40.Šekoranja D., Zupan A., Mavčič B., Martinčič D., Salapura V., Snoj Ž., Limpel Novak A.K., Pižem J. Novel ASAP1-USP6, FAT1-USP6, SAR1A-USP6, and TNC-USP6 fusions in primary aneurysmal bone cyst. Genes Chromosom. Cancer. 2020;59(6):357–365. doi: 10.1002/gcc.22836. [DOI] [PubMed] [Google Scholar]
- 41.Oliveira A.M., Chou M.M. The TRE17/USP6 oncogene: a riddle wrapped in a mystery inside an enigma. Front. Biosci. (Schol Ed) 2012;4:321–334. doi: 10.2741/s271. [DOI] [PubMed] [Google Scholar]
- 42.Guseva N.V., Jaber O., Tanas M.R., Stence A.A., Sompallae R., Schade J., Fillman A.N., Miller B.J., Bossler A.D., Ma D. Anchored multiplex PCR for targeted next-generation sequencing reveals recurrent and novel USP6 fusions and upregulation of USP6 expression in aneurysmal bone cyst. Genes Chromosom. Cancer. 2017;56(4):266–277. doi: 10.1002/gcc.22432. [DOI] [PubMed] [Google Scholar]
- 43.Mohaidat Z.M., Al-Gharaibeh S.R., Aljararhih O.N., Nusairat M.T., Al-Omari A.A. Challenges in the diagnosis and treatment of aneurysmal bone cyst in patients with unusual features. Adv. Orthop. 2019;2019:2905671. doi: 10.1155/2019/2905671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adamsbaum C., Mascard E., Guinebretiere J.M., Kalifa G., Dubousset J. Intralesional Ethibloc injections in primary aneurysmal bone cysts: an efficient and safe treatment. Skeletal Radiol. 2003;32(10):559–566. doi: 10.1007/s00256-003-0653-x. [DOI] [PubMed] [Google Scholar]
- 45.Kieser D.C., Mazas S., Cawley D.T., Fujishiro T., Tavolaro C., Boissiere L., Obeid I., Pointillart V., Vital J.-M., Gille O. Bisphosphonate therapy for spinal aneurysmal bone cysts. Eur. Spine J. 2018;27(4):851–858. doi: 10.1007/s00586-018-5470-y. [DOI] [PubMed] [Google Scholar]
- 46.Farsetti P., Tudisco C., Rosa M., Pentimalli G., Ippolito E. Aneurysmal bone cyst. Long-term follow-up of 20 cases. Arch. Orthop. Trauma Surg. 1990;109(4):221–223. doi: 10.1007/BF00453145. [DOI] [PubMed] [Google Scholar]
- 47.Flont P., Kolacinska-Flont M., Niedzielski K. A comparison of cyst wall curettage and en bloc excision in the treatment of aneurysmal bone cysts. World J. Surg. Oncol. 2013;11:109. doi: 10.1186/1477-7819-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fritz J., Sonnow L., Morris C.D. Adjuvant MRI-guided percutaneous cryoablation treatment for aneurysmal bone cyst. Skeletal Radiol. 2019;48(7):1149–1153. doi: 10.1007/s00256-018-3115-1. [DOI] [PubMed] [Google Scholar]
- 49.Kececi B., Kucuk L., Isayev A., Sabah D. Effect of adjuvant therapies on recurrence in aneurysmal bone cysts. Acta Orthop. Traumatol. Turc. 2014;48(5):500–506. doi: 10.3944/AOTT.2014.14.0020. [DOI] [PubMed] [Google Scholar]
- 50.Garg S., Mehta S., Dormans J.P. Modern surgical treatment of primary aneurysmal bone cyst of the spine in children and adolescents. J. Pediatr. Orthop. 2005;25(3):387–392. doi: 10.1097/01.bpo.0000152910.16045.ee. [DOI] [PubMed] [Google Scholar]
- 51.Varshney M.K., Rastogi S., Khan S.A., Trikha V. Is sclerotherapy better than intralesional excision for treating aneurysmal bone cysts? Clin. Orthop. Relat. Res. 2010;468(6):1649–1659. doi: 10.1007/s11999-009-1144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rastogi S., Varshney M.K., Trikha V., Khan S.A., Choudhury B., Safaya R. Treatment of aneurysmal bone cysts with percutaneous sclerotherapy using polidocanol. A review of 72 cases with long-term follow-up. J. Bone Joint Surg. Br. 2006;88-B(9):1212–1216. doi: 10.1302/0301-620X.88B9.17829. [DOI] [PubMed] [Google Scholar]
- 53.Puri A., Hegde P., Gulia A., Parikh M. Primary aneurysmal bone cysts. Bone Joint J. 2020;102-B(2):186–190. doi: 10.1302/0301-620X.102B2.BJJ-2019-1083.R1. [DOI] [PubMed] [Google Scholar]
- 54.Guibaud L., Herbreteau D., Dubois J., Stempfle N., Berard J., Pracros J.P., Merland J.J. Aneurysmal bone cysts: percutaneous embolization with an alcoholic solution of zein–series of 18 cases. Radiology. 1998;208(2):369–373. doi: 10.1148/radiology.208.2.9680561. [DOI] [PubMed] [Google Scholar]
- 55.Falappa P., Fassari F.M., Fanelli A., Genovese E., Ascani E., Crostelli M., Salsano V., Montanaro A., Di Lazzaro A., Serra F. Aneurysmal bone cysts: treatment with direct percutaneous Ethibloc injection: long-term results. Cardiovasc. Intervent. Radiol. 2002;25(4):282–290. doi: 10.1007/s00270-001-0062-2. [DOI] [PubMed] [Google Scholar]
- 56.Dubois J., Chigot V., Grimard G., Isler M., Garel L. Sclerotherapy in aneurysmal bone cysts in children: a review of 17 cases. Pediatr. Radiol. 2003;33(6):365–372. doi: 10.1007/s00247-003-0899-4. [DOI] [PubMed] [Google Scholar]
- 57.Topouchian V., Mazda K., Hamze B., Laredo J.D., Pennecot G.F. Aneurysmal bone cysts in children: complications of fibrosing agent injection. Radiology. 2004;232(2):522–526. doi: 10.1148/radiol.2322031157. [DOI] [PubMed] [Google Scholar]
- 58.de Gauzy J.S., Abid A., Accadbled F., Knorr G., Darodes P., Cahuzac J.P. Percutaneous Ethibloc injection in the treatment of primary aneurysmal bone cysts. J. Pediatr. Orthop. B. 2005;14(5):367–370. doi: 10.1097/01202412-200509000-00010. [DOI] [PubMed] [Google Scholar]
- 59.George H.L., Unnikrishnan P.N., Garg N.K., Sampath J.S., Bass A., Bruce C.E. Long-term follow-up of Ethibloc injection in aneurysmal bone cysts. J. Pediatr. Orthop. B. 2009;18(6):375–380. doi: 10.1097/BPB.0b013e32832f724c. [DOI] [PubMed] [Google Scholar]
- 60.Lambot-Juhan K., Pannier S., Grevent D., Pejin Z., Breton S., Berteloot L., Emond-Gonsard S., Boddaert N., Glorion C., Brunelle F. Primary aneurysmal bone cysts in children: percutaneous sclerotherapy with absolute alcohol and proposal of a vascular classification. Pediatr. Radiol. 2012;42(5):599–605. doi: 10.1007/s00247-011-2312-z. [DOI] [PubMed] [Google Scholar]
- 61.Ulici A., Florea D.-C., Carp M., Ladaru A., Tevanov I. Treatment of the aneurysmal bone cyst by percutaneous intracystic sclerotherapy using ethanol ninety five percent in children. Int. Orthop. 2018;42(6):1413–1419. doi: 10.1007/s00264-018-3841-y. [DOI] [PubMed] [Google Scholar]
- 62.Marie-Hardy L., El Sayed L., Alves A., Brunelle F., Ouchrif Y., Naggara O., Breton S., Mascard E., Glorion C., Pannier S. Percutaneous alcohol-based sclerotherapy in aneurysmal bone cyst in children and adolescents. Orthop. Traumatol. Surg. Res. 2020;106(7):1313–1318. doi: 10.1016/j.otsr.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 63.Puthoor D., Francis L., Ismail R. Is sclerotherapy with polidocanol a better treatment option for aneurysmal bone cyst compared to conventional curettage and bone grafting? J. Orthop. 2021;25:265–270. doi: 10.1016/j.jor.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jasper J., van der Heijden L., van Rijswijk C.S.P., van de Sande M.A.J. Efficacy of Sclerotherapy With Polidocanol (Ethoxysclerol) in Primary Aneurysmal Bone Cysts in Children and Adolescents. J. Pediatr. Orthop. 2021 doi: 10.1097/BPO.0000000000001839. [DOI] [PubMed] [Google Scholar]
- 65.N. Deventer, M. Schulze, G. Gosheger, M. de Vaal, N. Deventer, Primary aneurysmal bone cyst and its recent treatment options: a comparative review of 74 cases, Cancers (Basel) 13(10) (2021). [DOI] [PMC free article] [PubMed]
- 66.Georgiev M. Postsclerotherapy hyperpigmentations. Chromated glycerin as a screen for patients at risk (a retrospective study) J. Dermatol. Surg. Oncol. 1993;19(7):649–652. doi: 10.1111/j.1524-4725.1993.tb00405.x. [DOI] [PubMed] [Google Scholar]
- 67.Guex J.J. Indications for the sclerosing agent polidocanol (aetoxisclerol dexo, aethoxisklerol kreussler) J. Dermatol. Surg. Oncol. 1993;19(10):959–961. doi: 10.1111/j.1524-4725.1993.tb00985.x. [DOI] [PubMed] [Google Scholar]
- 68.Woon J.T.K., Hoon D., Graydon A., Flint M., Doyle A.J. Aneurysmal bone cyst treated with percutaneous doxycycline: is a single treatment sufficient? Skeletal Radiol. 2019;48(5):765–771. doi: 10.1007/s00256-019-03188-y. [DOI] [PubMed] [Google Scholar]
- 69.Shiels W.E., 2nd, Beebe A.C., Mayerson J.L. Percutaneous doxycycline treatment of juxtaphyseal aneurysmal bone cysts. J. Pediatr. Orthop. 2016;36(2):205–212. doi: 10.1097/BPO.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 70.Henrichs M.P., Beck L., Gosheger G., Streitbuerger A., Koehler M., Heindel W., Hardes J., Vieth V. Selective arterial embolisation of aneurysmal bone cysts of the sacrum: a promising alternative to surgery. Rofo. 2016;188(1):53–59. doi: 10.1055/s-0041-106069. [DOI] [PubMed] [Google Scholar]
- 71.Kurucu N., Akyuz C., Ergen F.B., Yalcin B., Kosemehmetoglu K., Ayvaz M., Varan A., Aydin B., Kutluk T. Denosumab treatment in aneurysmal bone cyst: evaluation of nine cases. Pediatr. Blood Cancer. 2018;65(4):e26926. doi: 10.1002/pbc.v65.410.1002/pbc.26926. [DOI] [PubMed] [Google Scholar]
- 72.Kulkarni A.G., Patel A. Denosumab: a potential new treatment option for recurrent Aneurysmal Bone Cyst of the spine. SICOT J. 2019;5:10. doi: 10.1051/sicotj/2019007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lange T., Stehling C., Frohlich B., Klingenhofer M., Kunkel P., Schneppenheim R., Escherich G., Gosheger G., Hardes J., Jurgens H., Schulte T.L. Denosumab: a potential new and innovative treatment option for aneurysmal bone cysts. Eur. Spine J. 2013;22(6):1417–1422. doi: 10.1007/s00586-013-2715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cornelis F., Truchetet M.E., Amoretti N., Verdier D., Fournier C., Pillet O., Gille O., Hauger O. Bisphosphonate therapy for unresectable symptomatic benign bone tumors: a long-term prospective study of tolerance and efficacy. Bone. 2014;58:11–16. doi: 10.1016/j.bone.2013.10.004. [DOI] [PubMed] [Google Scholar]