Abstract
Background:
There are limited comparisons of first- and second-generation EGFR tyrosine kinase inhibitors (TKIs) in large, real-world cohorts of non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutations.
Methods:
Patients with advanced NSCLC (N = 612) with common EGFR mutations receiving first-line gefitinib/erlotinib and afatinib were grouped and propensity-score matched. Progression-free survival (PFS), overall survival (OS) and secondary T790M mutations were analyzed.
Results:
The gefitinib/erlotinib and afatinib groups each contained 206 patients after matching. Compared with gefitinib/erlotinib, patients receiving afatinib achieved longer median PFS (16.3 versus 14.2 months; log-rank test p = 0.020) and had a lower risk of progression [hazard ratio (HR) 0.73 (95% confidence interval (CI), 0.57–0.94); p = 0.017]. Median OS (37.3 versus 34.2 months; log-rank test p = 0.500) and reduction in risk of death [HR 0.89 (95% CI, 0.65–1.23); p = 0.476] did not differ significantly between groups. T790M positivity was significantly higher in the gefitinib/erlotinib than afatinib group (70.9% versus 44.6%, p < 0.001). Multivariate analysis demonstrated that afatinib was independently associated with lower T790M positivity [odds ratio (OR) 0.27 (95% CI, 0.14–0.53); p < 0.001], whereas ⩾12 months PFS after EGFR-TKI treatment [OR 3.00 (95% CI, 1.56–5.98); p = 0.001] and brain metastasis [OR 2.12 (95% CI, 1.08–4.26); p = 0.030] were associated with higher T790M positivity. Sequential third-generation EGFR-TKI treatment was administered to 63 patients, in whom median OS after the second–third-generation and first–third-generation EGFR-TKI sequences were 38.8 and 29.1 months, respectively.
Conclusion:
Compared with gefitinib/erlotinib, afatinib had a higher treatment efficacy and a lower secondary T790M positivity in a large, real-world cohort of Asian patients with EGFR-mutated NSCLC.
Keywords: afatinib, EGFR mutation, erlotinib, gefitinib, NSCLC, real world
Background
Approximately 50% of Asian patients with advanced non-small cell lung cancer (NSCLC) harbor EGFR mutations.1,2 Treatment with EGFR-targeting agents, the first- and second-generation EGFR-tyrosine kinase inhibitors (TKIs), has greatly improved the survival outcomes of patients of EGFR mutation-positive NSCLC.3,4
The first-generation EGFR-TKIs gefitinib and erlotinib, which are reversible inhibitors that bind to EGFR/ErbB1 non-covalently, 5 have demonstrated superior efficacies compared with platinum-based chemotherapy in numerous randomized controlled trials.6–9 Afatinib, a second-generation EGFR-TKI that exhibits a broader spectrum of ErbB family suppression and irreversibly and covalently binds to ErbB receptors, 10 is also an approved front-line treatment for EGFR mutation-positive NSCLC.11,12
Although afatinib possesses a broader capacity to suppress ErbB than gefitinib and erlotinib, the degree of translation to clinically relevant effectiveness tends to be somewhat moderate. In the previous head-to-head comparative LUX-Lung 7 trial, 13 afatinib versus gefitinib led to a statistically significant risk reduction, whereas the benefits in terms of median and 1-year progression-free survival (PFS) were less clinically meaningful. Overall survival (OS), though not a primary endpoint, was not statistically different between the afatinib and gefitinib treatment arms. 13 In a similar study comparing another second-generation EGFR-TKI, dacomitinib, with gefitinib, higher PFS and OS were observed in the dacomitinib group; however, this study involved a highly selected cohort of patients with NSCLC without brain metastasis. 14 With regard to acquired drug resistance, the threonine-to-methionine substitution at amino acid position 790 in exon 20 (T790M) of EGFR underlies the major mechanism of resistance to first- and second-generation EGFR-TKI treatment. The frequency of the EGFR T790M mutation is approximately 50% for patients acquiring resistance to gefitinib or erlotinib, whereas the rate of the T790M mutation in patients receiving afatinib was reported to be lower in other studies.15–19 However, direct comparisons of the incidence of the T790M mutation in large cohorts of patients who underwent first- and second-generation EGFR-TKI treatment are lacking.
Given that patients enrolled to clinical trials do not usually reflect the whole picture of patients with NSCLC in daily practice, analyses generated from real-world patients represent a valuable source of knowledge that supplements randomized controlled studies. 20 Limited comparisons of first- and second-generation EGFR-TKIs have been conducted in large, real-world cohorts of Asian patients with NSCLC. The present study aimed to analyze the treatment efficacy of EGFR-TKIs and acquired T790M resistance in a large cohort of Asian patients with EGFR-mutated NSCLC from a single institution electronic database.
Methods
Patients and treatment
In the present study, we analyzed patients diagnosed with advanced NSCLC with common EGFR mutations (exon 19 deletion or L858R) who received front-line EGFR-TKI treatment, including gefitinib, erlotinib or afatinib. These three drugs were reimbursed by Taiwan’s National Health Insurance for first-line treatment of advanced EGFR-mutated NSCLC between 2011 and 2014. Therefore, eligible patients treated between January 2014 and December 2019 were retrospectively included. All patients received EGFR-TKI treatment at a daily dose of 250 mg gefitinib, 150 mg erlotinib or 40 mg afatinib; patients who received EGFR-TKI treatment for less than one week were excluded. PFS was defined as the interval between the date of starting EGFR-TKI treatment and the date of radiologically or clinically determined progression or death. Treatment responses, including complete response (CR), partial response (PR), stable disease, and progressive disease, were evaluated according to the Response Evaluation Criteria in Solid Tumors (version 1.1). This study assessed data from the Chang Gung Research Database and the study protocol was approved by the Ethics Committee of Chang Gung Memorial Hospital (No. 201901341A3).
Statistical analysis
The Mann–Whitney test was used to determine the significance of the differences in continuous variables between the two groups and Fisher exact test was used to evaluate categorical variables. Kaplan–Meier survival curves were generated using the R package survival, and hazard ratios (HRs) were calculated using the Cox regression model. Propensity-score matched analysis was employed to balance the clinical characteristics of the treatment groups. Briefly, the afatinib and gefitinib/erlotinib groups served as the dependent variables, and the covariates included age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS), smoking status, EGFR mutation subtypes and brain metastasis. Paired patients treated with afatinib or gefitinib/erlotinib with equivalent propensity scores were selected in a 1:1 manner using the R package MatchIt. All reported p-values are two sided; p < 0.05 was considered statistically significant. Data were also analyzed using SPSS (version 10.1; SPSS, Chicago, IL, USA).
Results
Baseline patient characteristics
Of the 612 patients with treatment-naïve, EGFR mutation-positive NSCLC, 120 (19.6%) received gefitinib, 147 (24.0%) received erlotinib and 345 (56.4%) received afatinib as the first-line treatment. The baseline characteristics of the patients in the first-generation (gefitinib/erlotinib) and second-generation groups were compared. Some clinical features were similar between the two groups, including smoking status, disease stage and the presence of liver metastasis; however, most variables were significantly different between the groups (Table 1). In real-world practice, patients who received afatinib treatment were more likely to be younger, male, have better ECOG performance status, have EGFR exon 19del mutations and were less likely to have brain metastasis compared with patients who received gefitinib/erlotinb.
Table 1.
Overall patient characteristics.
| Total (%) | Gefitinib/erlotinib | Afatinib | p-value | |
|---|---|---|---|---|
| N = 612 | n = 267 | n = 345 | ||
| Age ⩾65 years | 350 (57.2) | 193 (72.2) | 157 (45.5) | <0.001 |
| ECOG PS 0–1 | 510 (83.3) | 194 (72.7) | 316 (91.6) | <0.001 |
| Gender | ||||
| Male | 232 (37.9) | 91 (34.1) | 141 (40.9) | 0.093 |
| Current/ex-smoker | 134 (21.9) | 62 (23.2) | 72 (20.9) | 0.492 |
| Histology | ||||
| Adenocarcinoma | 604 (98.7) | 264 (98.9) | 340 (98.6) | 1.000 |
| Others | 8 (1.3) | 3 (1.1) | 5 (1.4) | |
| EGFR mutation | ||||
| L858R | 328 (53.6) | 162 (60.7) | 166 (48.1) | 0.002 |
| 19deletion | 284 (46.4) | 105 (39.3) | 179 (51.9) | |
| Disease stage | ||||
| IIIB | 39 (6.4) | 17 (6.4) | 22 (6.4) | 1.000 |
| IV | 573 (93.6) | 250 (93.6) | 323 (93.6) | |
| Site of metastasis | ||||
| Brain | 211 (34.4) | 113 (42.3) | 98 (28.4) | <0.001 |
| Liver | 73 (11.9) | 33 (12.4) | 40 (11.6) | 0.801 |
ECOG PS, Eastern Cooperative Oncology Group performance status.
Cox regression survival analyses for all patients
Multivariate Cox regression analyses were performed to determine the independent factors that impact PFS and OS in the 612 study participants. An ECOG PS 0-1 [HR 0.59 (95% confidence interval (CI), 0.45–0.78); p < 0.001] and afatinib treatment [HR 0.78 (95% CI, 0.63–0.98); p = 0.033; Table 2] were predictive of better PFS. In contrast, brain metastasis [HR 1.35 (95% CI, 1.08–1.68); p = 0.008] and male gender [HR 1.32 (95% CI, 1.02–1.70); p = 0.033; Table 2] were associated with lower PFS.
Table 2.
Cox regression analysis of progression-free survival and overall survival.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Progression free survival | ||||||
| Age ⩾65 years | 1.01 | 0.83–1.24 | 0.894 | – | – | – |
| ECOG PS 0, 1 | 0.49 | 0.38–0.63 | <0.001 | 0.59 | 0.45–0.78 | <0.001 |
| Male | 1.24 | 1.01–1.53 | 0.038 | 1.32 | 1.02–1.70 | 0.033 |
| Current/ex-smoker | 1.30 | 1.02–1.64 | 0.031 | 1.14 | 0.86–1.51 | 0.380 |
| EGFR L858R | 1.20 | 0.98–1.47 | 0.090 | – | – | – |
| Afatinib treatment | 0.68 | 0.55–0.83 | <0.001 | 0.78 | 0.63–0.98 | 0.032 |
| Brain metastasis | 1.53 | 1.23–1.89 | <0.001 | 1.35 | 1.08–1.68 | 0.008 |
| Liver metastasis | 1.54 | 1.15–2.06 | 0.004 | 1.31 | 0.96–1.79 | 0.085 |
| Overall survival | ||||||
| Age ⩾65 years | 1.20 | 0.92–1.56 | 0.186 | – | – | – |
| ECOG PS 0, 1 | 0.35 | 0.26–0.48 | <0.001 | 0.39 | 0.28–0.55 | <0.001 |
| Male | 1.12 | 0.86–1.47 | 0.401 | – | – | – |
| Current/ex-smoker | 1.11 | 0.82–1.51 | 0.487 | – | – | – |
| EGFR L858R | 1.32 | 1.01–1.72 | 0.044 | 1.36 | 1.04–1.80 | 0.027 |
| Afatinib treatment | 0.73 | 0.56–0.95 | 0.021 | 1.01 | 0.75–1.35 | 0.961 |
| Brain metastasis | 1.52 | 1.16–2.00 | 0.002 | 1.35 | 1.02–1.78 | 0.034 |
| Liver metastasis | 1.72 | 1.20–2.46 | 0.003 | 1.45 | 0.99–2.11 | 0.053 |
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio.
An ECOG PS 0–1 [HR 0.39 (95% CI, 0.28–0.55); p < 0.001; Table 2] was an independent predictor for better OS, whereas the EGFR L858R mutation [HR 1.36 (95% CI, 1.04–1.80); p = 0.027], brain metastasis [HR 1.35 (95% CI, 1.02–1.78); p = 0.034] and liver metastasis [HR 1.45 (95% CI, 0.99–2.11); p = 0.053; Table 2] were associated with poorer OS.
Treatment outcomes of EGFR-TKIs in the propensity-score matched patients
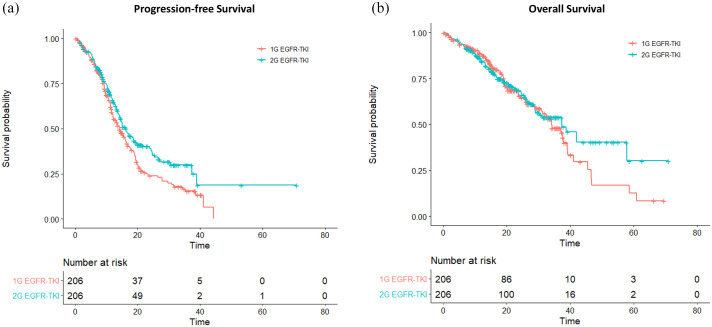
Propensity-score matching was implemented in a 1:1 manner for the first- and second-generation EGFR-TKI groups. A 412-patient propensity-score matched cohort was obtained, in which each of the two groups contained 206 patients with balanced clinical profiles (Table 3). The median follow-up duration was 24.1 months and 26.6 months in the gefitinib/erlotinib and afatinib groups, respectively. By the end of follow-up, 134 (65.0%) disease progression or death events were observed in the gefitinib/erlotinib group and 110 (53.4%) events were noted in the afatinib group. Compared with gefitinib or erlotinib, patients receiving afatinib treatment achieved longer median PFS (16.3 versus 14.2 months; log-rank test, p = 0.020), had a lower reduction in the risk of disease progression [HR 0.73 (95% CI, 0.57–0.94); p = 0.017] and had a higher 24-month PFS rate [40.4% (95% CI, 33.3–49.1%) versus 24.8% (95% CI, 18.6–33.1%); Figure 1(a)].
Table 3.
Patient characteristics of the propensity-score matched cohort.
| Total (%) | Gefitinib/erlotinib | Afatinib | p-value | |
|---|---|---|---|---|
| N = 412 | n = 206 | n = 206 | ||
| Age ⩾65 years | 271 (65.8) | 138 (66.9) | 133 (64.5) | 0.678 |
| ECOG PS 0–1 | 364 (88.3) | 179 (86.9) | 185 (89.8) | 0.443 |
| Gender | ||||
| Male | 147 (35.7) | 73 (35.4) | 74 (35.9) | 1.000 |
| Current/ex-smoker | 85 (20.6) | 47 (22.8) | 38 (18.4) | 0.330 |
| Histology | ||||
| Adenocarcinoma | 405 (98.3) | 203 (98.5) | 202 (98.1) | 1.000 |
| Others | 7 (1.7) | 3 (1.5) | 4 (1.9) | |
| EGFR mutation | ||||
| L858R | 240 (58.3) | 118 (57.3) | 122 (59.2) | 0.764 |
| 19deletion | 172 (41.7) | 88 (42.7) | 84 (40.8) | |
| Disease stage | ||||
| IIIB | 30 (7.3) | 15 (7.3) | 15 (7.3) | 1.000 |
| IV | 382 (92.7) | 191 (92.7) | 191 (92.7) | |
| Site of metastasis | ||||
| Brain | 152 (36.9) | 79 (38.3) | 73 (35.4) | 0.610 |
| Liver | 53 (12.9) | 25 (12.1) | 28 (13.6) | 0.769 |
ECOG PS, Eastern Cooperative Oncology Group performance status.
Figure 1.
(a) Progression-free survival and (b) overall survival for the first- (1G) and second-generation (2G) EGFR-TKI groups.
TKI, tyrosine kinase inhibitor.
Similarly, improved objective responses were noted in patients who received second-generation versus first-generation EGFR-TKI (68.4% versus 74.8%, p = 0.190). The CR and PR were 0.50% and 67.9%, respectively, in the gefitinib/erlotinib group. In the afatinib group, the CR and PR were 1.9% and 72.9%, respectively. The median OS (37.3 versus 34.2 months; log-rank test, p = 0.500) and reduction in the risk of death [HR 0.89 (95% CI, 0.65–1.23); p = 0.476] did not differ significantly between the second- and first-generation EGFR-TKI groups. The 24-month OS rate was also similar between the two groups [67.8% (95% CI, 61.0–75.4%) versus 65.5% (95% CI, 58.01–74.0%); Figure 1(b)].
Subgroup analysis of PFS and OS
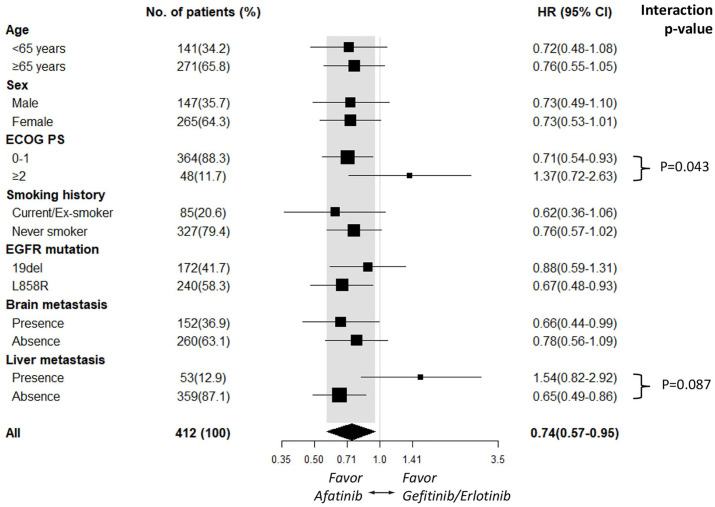
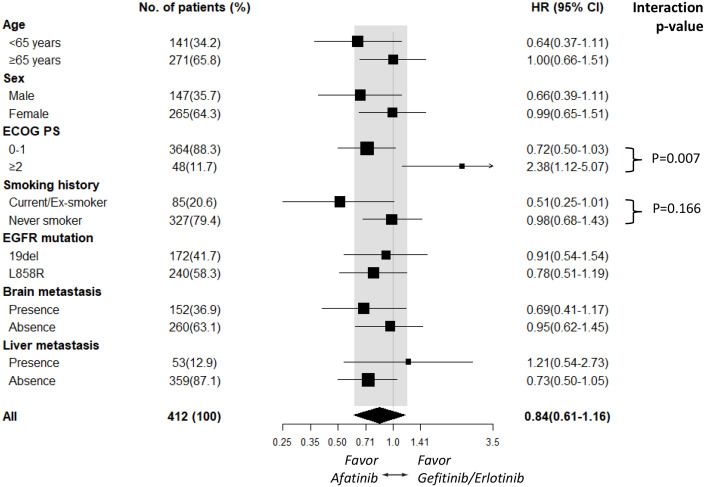
Subgroup analyses of PFS based on clinical characteristics suggested that afatinib improved PFS compared with gefitinib/erlotinib across most subgroups. In contrast, the subgroups of patients with an ECOG PS ⩾2 [HR 1.37 (95% CI, 0.72–2.63); p = 0.337] and who had liver metastasis [HR 1.54 (95% CI, 0.82–2.92); p = 0.181; Figure 2] tended to receive more benefit from gefitinib/erlotinib treatment. The PFS benefit associated with first- and second-generation EGFR-TKI treatment tended to be affected significantly by ECOG PS status (interaction, p = 0.043) and marginally affected by liver metastasis status (interaction, p = 0.087; Figure 2). Although OS did not differ significantly between the second- and first-generation EGFR-TKI groups, subgroup analyses of OS demonstrated that patients who had a smoking history [HR 0.51 (95% CI, 0.25–1.01); p = 0.054] tended to receive more benefit from afatinib treatment, whereas patients with an ECOG PS ⩾2 [HR 2.38 (95% CI, 1.12–5.07); p = 0.024; Figure 3] tended to receive more benefit from gefitinib/erlotinib treatment. The OS benefit associated with first- and second-generation EGFR-TKI treatment tended to be significantly affected by ECOG PS status (interaction, p = 0.007) and marginally affected by smoking status (interaction, p = 0.166; Figure 3).
Figure 2.
Subgroup analysis of progression-free survival for the afatinib and gefitinib/erlotinib groups.
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio.
Figure 3.
Subgroup analysis of overall survival for the afatinib and gefitinib/erlotinib groups.
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio.
Acquired EGFR T790M mutation and survival outcomes
Among all study participants, 373 (60.9%) patients had experienced disease progression by the end of follow-up. One hundred and ninety-one (31.2%) patients underwent a tissue and/or liquid biopsy for diagnosis of the EGFR T790M mutation; 106 (55.5%) patients were confirmed as T790M-positive. The T790M positive rate was significantly higher in the gefitinib/erlotinib group compared with the afatinib group (70.9% versus 44.6%, p < 0.001).
The clinical factors associated with T790M positivity were assessed by logistic regression in all patients. Multivariate analysis demonstrated that afatinib treatment was predictive of a lower T790M positive rate [odds ratio (OR) 0.27 (95% CI, 0.14–0.53); p < 0.001]. In contrast, ⩾12 months PFS after EGFR-TKI treatment [OR 3.00 (95% CI, 1.56–5.98); p = 0.001] and brain metastasis [OR 2.12 (95% CI, 1.08–4.26); p = 0.030; Table 4] were associated with a higher incidence of T790M positivity. Patients who had a secondary T790M mutation tended to achieve longer OS compared with patients who were T790M-negative (Supplemental material Figure 1 online).
Table 4.
Logistic regression analysis of the factors associated with T790M positivity.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variables | Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value |
| Age ⩾65 years | 0.92 (0.52–1.64) | 0.782 | – | – |
| Male | 0.84 (0.47–1.50) | 0.565 | – | – |
| ECOG PS 0–1 | 0.66 (0.27–1.56) | 0.361 | – | – |
| Current/ex-smoker | 0.86 (0.46–1.63) | 0.646 | – | – |
| EGFR exon 19del | 1.56 (0.88–2.79) | 0.132 | 1.62 (0.85–3.10) | 0.145 |
| Brain metastasis | 2.10 (1.15–3.91) | 0.018 | 2.12 (1.08–4.26) | 0.030 |
| Liver metastasis | 1.52 (0.65–3.77) | 0.348 | – | – |
| Afatinib treatment | 0.33 (0.18–0.61) | <0.001 | 0.27 (0.14–0.53) | <0.001 |
| PFS ⩾12 months | 1.99 (1.12–3.58) | 0.019 | 3.00 (1.56–5.98) | 0.001 |
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; PFS, progression-free survival.
Outcomes of sequential third-generation EGFR-TKI or chemotherapy
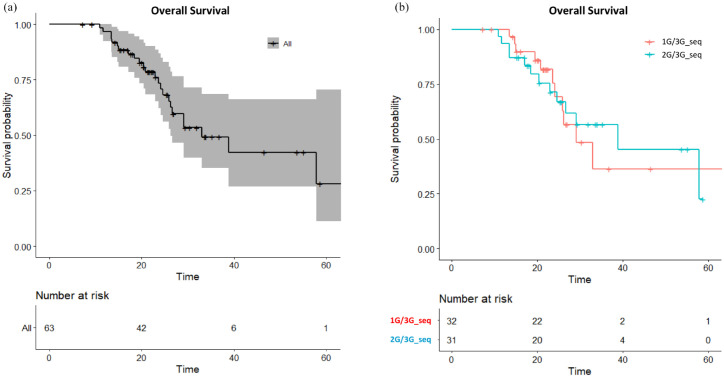
Sixty-three (59.4%) out of the 106 patients with secondary T790M mutations received a sequential third-generation EGFR-TKI treatment; these patients had a median OS of 33.0 months (95% CI, 26.2 months to not reach) and 24-month OS rate of 73.5% [95% CI, 62.2–86.8%; Figure 4(a)]. The PFS of third-generation EGFR-TKI was similar between the first-line gefitinib/erlotinib and the afatinib groups (Supplemental Figure 2). Patients who received a second–third-generation EGFR-TKI sequence and first–third-generation EGFR-TKI sequence demonstrated median OS of 38.8 versus 29.1 months and 24-month OS rates of 71.3% (95% CI, 56.2–90.6%) and 75.4% [95% CI, 59.5–95.7%; Figure 4(b)], respectively. On the other hand, T790M-positive patients who otherwise received a subsequent chemotherapy had equivalent outcomes to those who received a third-generation EGFR-TKI (Supplemental Figure 3). On the contrary, T790M-negative patients received significantly more benefit from subsequent chemotherapy than subsequent third-generation EGFR-TKI treatment (Supplemental Figure 4).
Figure 4.
(a) Overall survival for all T790M-positive patients receiving sequential third-generation EGFR-TKI treatment and (b) overall survival for the first–third-generation sequence (1G/3G_seq) and second–third-generation sequence (2G/3G_seq) groups.
TKI, tyrosine kinase inhibitor.
Discussion
The present analysis of a large Asian cohort of patients with EGFR-mutated NSCLC treated by first- and second-generation EGFR-TKIs demonstrates that afatinib significantly reduced the risk of disease progression compared with gefitinib/erlotinib in the front-line setting. Moreover, patients who received afatinib (versus gefitinib/erlotinib) upon disease progression had a lower frequency of acquired T790M mutations.
Previous real-world comparisons of gefitinib/erlotinib and afatinib treatment were frequently affected by common clinical characteristic biases introduced by physicians’ practices.21–23 As afatinib generally presents a higher toxicity profile than gefitinib/erlotinib, the prescription is usually biased to patients who are younger, have better performance status and sometimes patients with an EGFR 19del mutation. Most studies addressed these confounding factors by regression adjustment, while the original PFS and OS rates were not directly interpretable. 21 The first-generation EGFR-TKI groups were usually included between 2008 and 2012, earlier than the afatinib groups as this drug was only approved during 2014 and 2015 in most countries.22–24 This factor introduces post-progression treatment bias, because the patients in the gefitinib/erlotinib groups who developed secondary T790M resistance before 2015 rarely subsequently received the third-generation EGFR-TKI, which was initially approved for treatment of EGFR T790M mutated-NSCLC in late 2015. While some of these real-world cohorts enrolled substantial numbers of patients, the sample sizes of the afatinib groups were usually much smaller than those of the first-generation EGFR-TKI groups.22–24 This unequal sample size generally moderates the post-hoc statistical power. To address these limitations, the present study uniformly included patients treated from 2014 onwards for both the first- and second-generation EGFR-TKI groups to ensure an equal likelihood of exposure to the subsequent third-generation EGFR-TKI treatment, and balanced the sample size of the two groups using a propensity-score matched cohort to enable direct comparisons of the survival curves and a reasonable post-hoc statistical power of 70%.
In agreement with the randomized LUX-Lung 7 trial, 13 the present study demonstrated that afatinib leads to superior PFS compared with first-generation EGFR-TKI treatment, whereas subgroup analysis revealed that the superior efficacy of afatinib was not observed in patients with poorer ECOG PS status. Though our sample size was underpowered to conclude an OS benefit, OS did not significantly differ between the first- and second-generation EGFR-TKI groups and the magnitude of risk reduction observed for afatinib treatment was similar to the finding of the LUX-Lung 7 study. Notably, in subgroup analysis of OS, afatinib treatment was significantly associated with poorer OS compared with gefitinib/erlotinib in patients with an ECOG PS ⩾2. This real-world finding complements the knowledge from randomized clinical trials and suggests that the tolerability of afatinib treatment should be particularly considered in patients who are more severely ill. Additionally, an interesting finding was noted in the patients in the smoking subgroup in the present study. Multiple previous analyses suggested that cigarette smoking impaired the response to gefitinib and erlotinib treatment in patients with EGFR-mutated NSCLC.25–27 In our analysis, these patients tended to receive more benefit from afatinib treatment, thus afatinib may be potentially a preferred choice over gefitinib/erlotinib in this patient group.
In this work, multivariate analysis demonstrated that the EGFR L858R mutation and brain and liver metastasis were prognostic factors for poor OS. This finding is consistent with some observational studies which reported that liver metastasis is independently associated with shorter OS.28,29 The role of the EGFR L858R mutation as a negative surrogate of OS was previously reported in a number of clinical trials. Significantly lower OS was found in L858R patients compared with 19del patients in the EURTAC trial, 30 and poorer OS was also reported for L858R patients in multiple randomized trials.12,14,31 Recent studies that found EGFR patients with the L858R mutation are more likely to harbor concomitant genomic alterations that enhance tumor complexity and impair the sensitivity to gefitinib and erlotinib.32,33 In the LUX-Lung 7 trial, afatinib provided a higher objective response rate in patients with the L858R mutation than gefitinib (66% versus 42%), though this finding was not observed in patients with 19del mutations (73% versus 66%). 13 Interestingly, the subgroup analysis in this work demonstrated that the PFS benefit of afatinib was also more pronounced in patients with L858R than patients with 19del.
Some clinical factors were identified to impact the development of secondary T790M in this analysis. Compared with gefitinib/erlotinib, afatinib treatment was associated with a lower T790M-positive rate (70.9% versus 44.6%). However, this rate is higher than the rates reported for small cohorts of afatinib-treated patients, which exhibited a T790M-positive rate of approximately 25–35%.15–18 The slightly higher T790M yield of both the gefitinib/erlotinib and afatinib groups in the present study might be related to the addition of a liquid biopsy when a tissue biopsy was unavailable or T790M-negative. Moreover, ⩾12 months PFS after EGFR-TKI treatment also predicted a higher rate of development of a secondary T790M mutation. Previous studies suggested that longer PFS after EGFR-TKI treatment is associated with fewer concurrent mutations in EGFR-mutated tumors,32–34 thus progressive enrichment of the T790M variant during chronic exposure to EGFR-TKIs may account for the resistance mechanism at a later time. Notably, brain metastasis at baseline was also identified as a predictive factor. Several earlier studies demonstrated that EGFR-mutated tumors had a higher tendency for brain metastasis compared with EGFR-wild type tumors.35,36 Whether the higher rate of T790M mutations in patients with brain metastasis at baseline is due to the original tumor possessing a higher volume of EGFR mutation versus other co-mutation clones remains to be investigated.
A recent global observational study that focused on the efficacy of the second–third-generation EGFR-TKI sequence demonstrated median OS of 37.6 months and 44.8 months in the overall cohort and in Asian patients, respectively. 37 In the present study, the subset of T790M-positive patients who received a third-generation EGFR-TKI had median OS of 38.8 months and 29.1 months in the second-third and first-third sequence groups, respectively. A hypothesis that involves differential clonal selection pressure between first- and second-generation EGFR-TKIs has been formulated to explain the clinical effects of sequential EGFR-TKI treatment. 38 Recently, the introduction of the third-generation EGFR-TKI as front-line treatment, the FLAURA study, 39 demonstrated median OS of 38.6 months. The present study revealed that patients who were allowed to receive a sequential second-third-generation EGFR-TKI treatment had a comparable outcome; however, a longer observational period and data maturity are required to further assess this finding. The first inherent limitation of the present study is its retrospective nature. Second, the first-generation EGFR-TKI group included two drugs in order to increase the sample size, at the expense of increasing heterogeneity. However, this heterogeneity is likely to be acceptable as a previous meta-analysis of more than 17,000 patients suggested that the efficacy of gefitinib and erlotinib are comparable. 40 Third, the recent advance of third-generation EGFR-TKIs also challenges the role of afatinib as a front-line treatment. Interestingly, a recent study of a Japanese cohort demonstrated that afatinib resulted in better OS compared with osimertinib, 41 though this issue remains largely unsettled.
In conclusion, this study assessed a large, real-world cohort of Asian patients with EGFR-mutated NSCLC and was properly adjusted for clinical biases to compare the outcomes of first- and second-generation EGFR-TKIs. The results demonstrate that afatinib has a more favorable efficacy and leads to a lower incidence of secondary T790M mutations and suggests that clinical profiles including the ECOG PS and smoking status may potentially inform the selection of EGFR-TKIs.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211035710 for First- or second-generation epidermal growth factor receptor tyrosine kinase inhibitors in a large, real-world cohort of patients with non-small cell lung cancer by Allen Chung-Cheng Huang, Chi-Hsien Huang, Jia-Shiuan Ju, Tzu-Hsuan Chiu, Pi-Hung Tung, Chin-Chou Wang, Chien-Ying Liu, Fu-Tsai Chung, Yueh-Fu Fang, Yi-Ke Guo, Chih-Hsi Scott Kuo and Cheng-Ta Yang in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank for the contributions of research assistants Ms. Yen-Wen Wang and Ms. Yu-Chi Chiang to this study.
Footnotes
Author contributions: ACH wrote and CSK revised the manuscript; CSK, CCW, CYL and CTY were responsible for study conception and design; CHH, JSJ, THC, PHT and ACH collected the data; CSK, CYL, FTC, YFF and CTY provided study materials and patients; CSK and YKG analyzed and interpreted the data; all authors read and approved the final manuscript.
Availability of data and materials: The datasets generated and/or analyzed during the current study are not publicly available due to local regulations related to medical confidentiality, but are available from the corresponding author on reasonable request.
Conflict of interest statement: CS-K received speaker honoraria from AstraZeneca, Boehringer Ingelheim, Roche, Pfizer, Eli Lilly, Novartis, OnO Pharma, Chugai, Merck and Guardant Health. CS-K provided consultation for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck, Chugai, Takeda and Novartis. None of the other authors has any conflict of interest to disclose.
Ethics statement: The study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki. The Ethics Committee of Chang Gung Memorial Hospital approved the study (No. 201901341A3) and granted permission for access to the Chang Gung Research Database; written informed consent was provided by all study participants.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study received funding support from Chang Gung Medical Foundation CORPG3J0332; CORPG3J0333.
ORCID iD: Chih-Hsi Scott Kuo  https://orcid.org/0000-0003-3309-937X
https://orcid.org/0000-0003-3309-937X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Allen Chung-Cheng Huang, Division of Thoracic Oncology, Department of Thoracic Medicine, Chang Gung Memorial Hospital, Chang Gung University, College of Medicine, Gueishan; Thoracic Oncology Unit, Chang Gung Memorial Hospital Cancer Center.
Chi-Hsien Huang, Division of Thoracic Oncology, Department of Thoracic Medicine, Chang Gung Memorial Hospital, Chang Gung University, College of Medicine, Gueishan.
Jia-Shiuan Ju, Division of Thoracic Oncology, Department of Thoracic Medicine, Chang Gung Memorial Hospital, Chang Gung University, College of Medicine, Gueishan; Thoracic Oncology Unit, Chang Gung Memorial Hospital Cancer Center.
Tzu-Hsuan Chiu, Division of Thoracic Oncology, Department of Thoracic Medicine, Chang Gung Memorial Hospital, Chang Gung University, College of Medicine, Gueishan.
Pi-Hung Tung, Division of Thoracic Oncology, Department of Thoracic Medicine, Chang Gung Memorial Hospital, Chang Gung University, College of Medicine, Gueishan.
Chin-Chou Wang, Division of Pulmonary & Critical Care Medicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Niaosung.
Chien-Ying Liu, Division of Thoracic Oncology, Department of Thoracic Medicine, Chang Gung Memorial Hospital, Chang Gung University, College of Medicine, Gueishan; Thoracic Oncology Unit, Chang Gung Memorial Hospital Cancer Center.
Fu-Tsai Chung, Division of Thoracic Oncology, Department of Thoracic Medicine, Chang Gung Memorial Hospital, Chang Gung University, College of Medicine, Gueishan; Thoracic Oncology Unit, Chang Gung Memorial Hospital Cancer Center.
Yueh-Fu Fang, Division of Thoracic Oncology, Department of Thoracic Medicine, Chang Gung Memorial Hospital, Chang Gung University, College of Medicine, Gueishan.
Yi-Ke Guo, Data Science Institute, Department of Computing, Imperial College London, London, UK.
Chih-Hsi Scott Kuo, Division of Thoracic Oncology, Department of Thoracic Medicine, Chang Gung Memorial Hospital, Chang Gung University, College of Medicine, No 199, Tun-Hwa Nr Rd, Taipei, Gueishan, 333; Thoracic Oncology Unit, Chang Gung Memorial Hospital Cancer Center; Data Science Institute, Department of Computing, Imperial College London, London, UK.
Cheng-Ta Yang, Division of Thoracic Oncology, Department of Thoracic Medicine, Chang Gung Memorial Hospital, Chang Gung University, College of Medicine, Taiyuan; Thoracic Oncology Unit, Chang Gung Memorial Hospital Cancer Center.
References
- 1. Wang R, Wang L, Li Y, et al. FGFR1/3 tyrosine kinase fusions define a unique molecular subtype of non-small cell lung cancer. Clin Cancer Res 2014; 20: 4107–4114. [DOI] [PubMed] [Google Scholar]
- 2. Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014; 9: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rennert G, Gottfried M, Rennert H, et al. Long term follow-up of EGFR mutated NSCLC cases. Transl Oncol 2021; 14: 100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin JJ, Cardarella S, Lydon C, et al. Five-year survival in EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs. J Thorac Oncol 2016; 11: 556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharma SV, Bell DW, Settleman, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007; 7: 169–181. [DOI] [PubMed] [Google Scholar]
- 6. Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013; 24: 54–59. [DOI] [PubMed] [Google Scholar]
- 7. Mok TS, Wu YL, Thongprasert, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–957. [DOI] [PubMed] [Google Scholar]
- 8. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–246. [DOI] [PubMed] [Google Scholar]
- 9. Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015; 26: 1883–1889. [DOI] [PubMed] [Google Scholar]
- 10. Nelson V, Ziehr J, Agulnik M, et al. Afatinib: emerging next-generation tyrosine kinase inhibitor for NSCLC. Onco Targets Ther 2013; 6: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014; 15: 213–222. [DOI] [PubMed] [Google Scholar]
- 12. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–3334. [DOI] [PubMed] [Google Scholar]
- 13. Paz-Ares L, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017; 28: 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017; 18: 1454–1466. [DOI] [PubMed] [Google Scholar]
- 15. Campo M, Gerber D, Gainor JF, et al. Acquired resistance to first-line afatinib and the challenges of prearranged progression biopsies. J Thorac Oncol 2016; 11: 2022–2026. [DOI] [PubMed] [Google Scholar]
- 16. Nosaki K, Satouchi M, Kurata T, et al. Re-biopsy status among non-small cell lung cancer patients in Japan: a retrospective study. Lung Cancer 2016; 101: 1–8. [DOI] [PubMed] [Google Scholar]
- 17. Liang SK, Hsieh MS, Lee MR, et al. Real-world experience of afatinib as a first-line therapy for advanced EGFR mutation-positive lung adenocarcinoma. Oncotarget 2017; 8: 90430–90443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang YH, Hsu KH, Tseng JS, et al. The association of acquired T790M mutation with clinical characteristics after resistance to first-line epidermal growth factor receptor tyrosine kinase inhibitor in lung adenocarcinoma. Cancer Res Treat 2018; 50: 1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuo CS, Huang CH, Liu CY, et al. Prior EGFR-TKI treatment in EGFR-mutated NSCLC affects the allele frequency fraction of acquired T790M and the subsequent efficacy of osimertinib. Target Oncol 2019; 14: 433–440. [DOI] [PubMed] [Google Scholar]
- 20. Katkade VB, Sanders KN, Zou KH. Real world data: an opportunity to supplement existing evidence for the use of long-established medicines in health care decision making. J Multidiscip Healthc 2018; 11: 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin YT, Chen JS, Liao WY, et al. Clinical outcomes and secondary Epidermal Growth Factor Receptor (EGFR) T790M mutation among first-line gefitinib, erlotinib and afatinib-treated non-small cell lung cancer patients with activating EGFR mutations. Int J Cancer 2019; 144: 2887–2896. [DOI] [PubMed] [Google Scholar]
- 22. Ito K, Murotani K, Kubo A, et al. Propensity score analysis of overall survival between first- and second-generation EGFR-TKIs using real-world data. Cancer Sci 2020; 111: 3705–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pluzanski A, Krzakowski M, Kowalski D, et al. Real-world clinical outcomes of first-generation and second-generation epidermal growth factor receptor tyrosine kinase inhibitors in a large cohort of European non-small-cell lung cancer patients. ESMO Open 2020; 5: e001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Su VY, Yang KY, Huang TY, et al. The efficacy of first-line tyrosine kinase inhibitors combined with co-medications in Asian patients with EGFR mutation non-small cell lung cancer. Sci Rep 2020; 10: 14965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu M, Zhou C, Zheng J. Cigarette smoking impairs the response of EGFR-TKIs therapy in lung adenocarcinoma patients by promoting EGFR signaling and epithelial-mesenchymal transition. Am J Transl Res 2015; 7: 2026–2035. [PMC free article] [PubMed] [Google Scholar]
- 26. Kim MH, Kim HR, Cho BC, et al. Impact of cigarette smoking on response to epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors in lung adenocarcinoma with activating EGFR mutations. Lung Cancer 2014; 84: 196–202. [DOI] [PubMed] [Google Scholar]
- 27. Mitchell P, Mok T, Barraclough H, et al. Smoking history as a predictive factor of treatment response in advanced non-small-cell lung cancer: a systematic review. Clin Lung Cancer 2012; 13: 239–251. [DOI] [PubMed] [Google Scholar]
- 28. Yao ZH, Liao WY, Ho CC, et al. Real-world data on prognostic factors for overall survival in EGFR mutation-positive advanced non-small cell lung cancer patients treated with first-line gefitinib. Oncologist 2017; 22: 1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu KL, Tsai MJ, Yang CJ, et al. Liver metastasis predicts poorer prognosis in stage IV lung adenocarcinoma patients receiving first-line gefitinib. Lung Cancer 2015; 88: 187–194. [DOI] [PubMed] [Google Scholar]
- 30. Karachaliou N, Mayo-de las Casas C, Queralt C, et al. Association of EGFR L858R mutation in circulating free DNA with survival in the EURTAC trial. JAMA Oncol 2015; 1: 149–157. [DOI] [PubMed] [Google Scholar]
- 31. Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16: 141–151. [DOI] [PubMed] [Google Scholar]
- 32. Hong S, Gao F, Fu S, et al. Concomitant genetic alterations with response to treatment and epidermal growth factor receptor tyrosine kinase inhibitors in patients with EGFR-mutant advanced non-small cell lung cancer. JAMA Oncol 2018; 4: 739–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barnet MB, O’Toole S, Horvath LG, et al. EGFR-co-mutated advanced NSCLC and response to EGFR tyrosine kinase inhibitors. J Thorac Oncol 2017; 12: 585–590. [DOI] [PubMed] [Google Scholar]
- 34. Yu HA, Suzawa K, Jordan E, et al. Concurrent alterations in EGFR-mutant lung cancers associated with resistance to EGFR kinase inhibitors and characterization of MTOR as a mediator of resistance. Clin Cancer Res 2018; 24: 3108–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shin DY, Na II, Kim CH, et al. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol 2014; 9: 195–199. [DOI] [PubMed] [Google Scholar]
- 36. Fujimoto D, Ueda H, Shimizu R, et al. Features and prognostic impact of distant metastasis in patients with stage IV lung adenocarcinoma harboring EGFR mutations: importance of bone metastasis. Clin Exp Metastasis 2014; 31: 543–551. [DOI] [PubMed] [Google Scholar]
- 37. Hochmair MJ, Morabito A, Hao D, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: final analysis of the GioTag study. Future Oncol 2020; 16: 2799–2808. [DOI] [PubMed] [Google Scholar]
- 38. Kohsaka S, Nagano M, Ueno T, et al. A method of high-throughput functional evaluation of EGFR gene variants of unknown significance in cancer. Sci Transl Med 2017; 9: eaan6566. [DOI] [PubMed] [Google Scholar]
- 39. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 2020; 382: 41–50. [DOI] [PubMed] [Google Scholar]
- 40. Yang Z, Hackshaw A, Feng Q, et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer 2017; 140: 2805–2819. [DOI] [PubMed] [Google Scholar]
- 41. Ito K, Morise M, Wakuda K, et al. A multicenter cohort study of osimertinib compared with afatinib as first-line treatment for EGFR-mutated non-small-cell lung cancer from practical dataset: CJLSG1903. ESMO Open 2021; 6: 100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211035710 for First- or second-generation epidermal growth factor receptor tyrosine kinase inhibitors in a large, real-world cohort of patients with non-small cell lung cancer by Allen Chung-Cheng Huang, Chi-Hsien Huang, Jia-Shiuan Ju, Tzu-Hsuan Chiu, Pi-Hung Tung, Chin-Chou Wang, Chien-Ying Liu, Fu-Tsai Chung, Yueh-Fu Fang, Yi-Ke Guo, Chih-Hsi Scott Kuo and Cheng-Ta Yang in Therapeutic Advances in Medical Oncology