Abstract
Background:
Studies have labelled chronic kidney disease (CKD) among the adult population in urban Bangladesh. To address knowledge gaps on CKD, we aimed to generate data on prevalence, health and nutrition of CKD individuals living in rural and peri-urban Bangladesh.
Methods:
Participants were recruited from the Mirzapur Demographic Surveillance System by age-stratified random sampling. We screened participants by measuring serum creatinine and urine albumin to creatinine ratio, and collected socio-demographic, lifestyles and health information (phase I). After 3 months (phase II), we repeated the urine and blood tests as per the Kidney Disease Outcomes Quality Initiative guidelines. The glomerular filtration rate was calculated using the CKD Epidemiology Collaboration equation.
Results:
Among 928 participants, 872 completed the study. In phase I, probable CKD cases were 281 (32.2%); in phase II, confirmed cases were 192 (22.0%) (stage 1, 4.0%; stage 2, 11.8%; stage 3, 5.5%; stage 4, 0.6%; stage 5, 0.1%). In multivariable analysis, associated factors for prevalent CKD included aged ⩾60 years [adjusted odds ratio (aOR) 5.02; 95% confidence interval (CI) 1.85–13.65], hypertension (aOR 3.08; 95% CI 2.07–4.59), diabetes (aOR 2.52; 95% CI 1.60–3.96), presence of red blood cell in urine (aOR 3.20; 95% CI 1.71–5.98) and anemia (aOR 2.50; 95% CI 1.63–3.84).
Conclusions:
This is the first ever research on CKD prevalence in rural and peri-urban Bangladesh and recorded about 22%, which is higher than urban settings. Monitoring systems are needed to evaluate the overall burden and to mitigate risk factors with an emphasis on the rural and peri-urban population.
Keywords: Bangladesh, chronic kidney disease, health and nutritional status, prevalence, rural and peri-urban
Introduction
Globally, the prevalence of chronic kidney disease (CKD) is increasing and is documented as a rising public health threat for the future. 1 CKD is responsible for at least 2.4 million deaths each year 2 and the overall mortality rate has increased by 41.5% between 1990 and 2017. 3 Diabetes, hypertension and obesity are the world’s leading risk factors for CKD. 4 Proper management and mitigation of the risks associated with CKD are of critical importance as they have been reported to prolong the progression to end-stage renal disease (ESRD) and other related health consequences. 5 Approximately 3 million patients worldwide are currently receiving renal replacement therapy, which is only half of the total case load, and this figure is projected to rise to between 5 and 10 million by 2030. 6 The treatment costs of CKD and its complications differ and therefore are not affordable in many parts of the world.7,8 In low and middle-income countries (LMICs), most patients with kidney failure have limited access to life-saving dialysis and kidney transplantation. 9 Early detection of CKD and reduction of risk factors, therefore, would be the most pragmatic strategy to prevent premature mortality and morbidity in those countries.
The prevalence of CKD is increasing among the general and disadvantaged population in LMICs, 10 and community-based screening services to assess the prevalence and identify the related factors can be a crucial intervention for early case detection. A few studies on CKD have been conducted in Bangladesh, and most of them were hospital based among urban and slum populations,11–13 which measured the prevalence of CKD as ranging at 16–18%. Although 65% of the Bangladeshi population reside in rural areas and they are in greater need due to lack of diagnostic facilities, only one study has been conducted in rural Bangladesh. This study, however, contains several methodological flaws in which the study population and the study procedure were not clearly mentioned. 14 Taking this previous study as the point of departure, we designed and carried out the population-based screening and early assessment of CKD project to document the prevalence and determine factors associated with CKD in rural and peri-urban Bangladesh.
Methods
Study site
This cross-sectional epidemiological research was performed in the Mirzapur sub-district of Tangail, located approximately 60 km northwest of Dhaka, the capital city of Bangladesh from January to June 2020. It has a mixed (rural and peri-urban) population of 407,781 (men 50%, women 50%); men are mostly engaged in agriculture, whereas women are mostly busy with household chores. 15 Mirzapur has 13 unions (the smallest administrative unit with an average population size of 25,000) and 219 villages. The Mirzapur Demographic Surveillance System (DSS), established in 2007 consists of approximately 300,000 individuals from 10 unions of Mirzapur who are visited every 4 months for updating demographic information on births, deaths, and profile of the population. 16
Study population
The study participants were stratified into three age groups (18–30 years, 31–45 years, and 46 years and older) and were selected randomly with the assistance of the DSS database of Mirzapur field site. Adult individuals of either sex, aged 18 years and older who are residents of the DSS area, have been living steadily in the locality for at least 5 years and have given written informed consent to participate in the study were considered for enrolment in the study. However, individuals hospitalised at the time of enrolment and having any known serious illness with questionable prognosis that is, malignancy, mental illness, congenital disease and physical disability (if they have evidence for getting treatments) were excluded from the study. Among eight unions in Mirzapur that comprise the DSS area, three unions (Mirzapur, Bhatgram and Gorai) nearest to our sentinel health facility, Kumudini Hospital, were selected purposively due to budgetary constraints and time limitations (Supplemental Figure 1).
Sample size calculation
The study participants were stratified into different age groups and selected randomly with the assistance of the DSS database. Sample size estimation was calculated based on an estimation of the prevalence of CKD in urban Dhaka, Bangladesh; in which the proportion of CKD among adults was 6.4%, 9.4%, 29.3% among ages 18–30 years, 31–45 years and 46 years and above, respectively. 13 The sample size was calculated using the following formula: n = (z)2 p (1−p)/d2, where n = sample size; z = level of confidence according to the standard normal distribution (level of confidence was chosen at 95%, z = 1.96); d = tolerated margin of error (d = 0.04); and p = estimated proportion of the population according to the prevalence of CKD valid among different age groups. The cumulative sample size was 844 (18–30 years 143, 31–45 years 204, 46 years and above 497). The final sample size was 928, considering a 10% attrition rate because of out-migration, absence, non-response, and refusal for the invasive blood specimen collection procedure.
Enrolment of study participants
Community healthcare workers (CHWs), trained in interview techniques and anthropometric and blood pressure (BP) measurement, made home visits and performed interviews and physical examinations after getting written informed consent. Interviews were performed by administering a field-tested structured questionnaire and information was obtained on age, gender, marital status, occupation, educational background, income/month, smoking status, participants’s current medical history, past medical history, sleeping hours, and medical history of family (up to the third generation). Physical examinations were performed to measure BP, pulse, height, weight, waist circumference, hip circumference. The participants were then advised to visit Kumudini Hospital laboratory for their study-related investigations.
Sample collection of study participants
First assessment during initial visit: at Kumudini Hospital laboratory, blood samples were collected to measure serum creatinine, serum albumin, hemoglobin (Hb), fasting blood glucose (FBG), total cholesterol (TC), high density lipoprotein (HDL) cholesterol, and triglyceride (TG); and urine specimens were collected to measure the albumin to creatinine ratio (ACR) and routine microscopic examination. Serum creatinine was measured using the Jaffe colorimetric method of Siemens Dimension EXL 200/Siemens Dimension RXL Max. ACR was calculated using the urine albumin concentration/urine creatinine concentration equation. Urinary albumin was assessed using a nephelometric method, and urinary creatinine was measured using a Flex reagent cartridge principle based on Jaffe reaction. Tests were carried out using Siemens Dimension EXL 200/Siemens Dimension RXL Max.
Second assessment (3 months after first assessment): after the first assessment, we evaluated the participants’ CKD status following the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation and estimated their glomerular filtration rate (GFR). Those participants who had an estimated glomerular filtration rate (eGFR) below 60 ml/min/1.73 m2 and/or ACR ⩾30 mg/g were considered for the second assessment. CHWs made phone calls and advised them to visit the hospital laboratory after 3 months of their first assessment. Blood samples were collected to measure serum creatinine, and urine specimens were also collected to measure ACR. After completing investigations, the CKD status of the participants was confirmed following the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF/KDOQI) guidelines. 17
Definitions
Chronic kidney disease
The CKD-EPI equation was used to estimate the GFR, and the NKF/KDOQI guidelines were used to define and stage the CKD. A participant was diagnosed as a case of CKD if (s)he had eGFR below 60 ml/min/1.73 m2 or had albuminuria (ACR ⩾30 mg/g) for more than 3 months. 17
Stages of CKD
Stage 1 (eGFR ⩾90 mL/min/1.73 m2 and ACR ⩾30 mg/g); stage 2 (eGFR 60–89 mL/min/1.73 m2 and ACR ⩾30 mg/g); stage 3 (eGFR 30–59 mL/min/1.73 m2 regardless of ACR); stage 4 (eGFR 15–29 mL/min/1.73 m2 regardless of ACR); and stage 5 (eGFR < 15 mL/min/1.73 m2 regardless of ACR).17,18
Hypertension
Participants who currently require antihypertensive therapy to control their BP or those with a systolic blood pressure (SBP) of ⩾140 mmHg and/or diastolic blood pressure (DBP) of ⩾90 mmHg at screening were considered to have hypertension. 19
Diabetes
Participants with diabetes had a history of diabetes or fasting blood glucose >7 mmol/L [according to the World Health Organization (WHO)].
Obesity, overweight and underweight
[(Weight in kilograms)/(square of the height in meters)] was used to calculate the body mass index (BMI). BMI ⩾ 30 was defined as obese, those between 25 and 29.9 were considered as overweight, and those <18.5 were considered underweight (according to WHO).
Abdominal obesity
Abdominal obesity was a waist circumference of ⩾94 cm in men and ⩾80 cm in women (according to WHO).
Hypercholesterolemia and hypertriglyceridemia and low HDL-cholesterol
Serum total cholesterol >200 mg/dL, serum triglyceride >150 mg/dL and serum HDL-cholesterol <40 mg/dL at screening was considered as hypercholesterolemia, hypertriglyceridemia and low HDL-cholesterol, respectively. 20
Anemia
Anemia was a blood hemoglobin level <13 g/dL and <12 g/dL for men and women, respectively (according to WHO).
Undernutrition
Mid upper arm circumference (MUAC) <25 cm for men and <24 cm for women was considered as undernutrition in developing countries. 21
Income (<$100/month)
Income was a monthly earnings from households <8400 Bangladeshi Taka ($1 = 84 Bangladeshi Taka).
Illiterate
Participants without formal education were regarded as illiterate.
Present tobacco smoker
Participants who currently smoked any form of bidi or cigarette were considered present tobacco smokers.
Present smokeless tobacco user
Participants who currently consumed any form of smokeless tobacco product were considered smokeless tobacco users. 22
Data analysis
Statistical analysis was performed using the SPSS v. 20.0 (IBM Co., Armonk, NY, USA). In the descriptive analysis, continuous variables were expressed as mean ± standard deviation (SD) and categorical variables were expressed as count (percentages). The outcome variable was the prevalence of CKD and exposure variables included age [18–30 years (reference), 31–45 years, 46–59 years and ⩾60 years], sex [male (reference), female], education [literate (reference), illiterate], occupation [housewife, farmer, other than housewife and farmer (reference)], marital status [married, widowed, other than married and widowed (reference)], monthly income [⩾USD 100, t-test or Mann–Whitney U test was performed for continuous variables after checking the data distribution. For categorical variables, we used the Pearson chi-square test to see the associations between outcome and exposure variables. Variables with p-values less than 0.05 were simultaneously included into the multivariate logistic model and adjusted odds ratios (aORs) and 95% confidence intervals (CIs) were estimated. Multivariate logistic regression analysis was also performed when non-CKD was the reference group (non-CKD = 0, CKD = 1) to measure the aORs. aORs were calculated to assess the association between the outcome and exposure variables. Odds ratios (ORs) also indicated the strength of association. In addition to ORs, their 95% CIs were also estimated. A probability of less than 0.05 was considered significant.
Ethical statement
The Research Review Committee and the Ethical Review Committee of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) approved this study (registration no. 19081).
At the time of enrolment, written consents were taken from the study participants. They were assured about the non-disclosure of information collected from them. However, without revealing the name or identification, they were also informed about the use of data for evaluation and use of findings to enhance patient care activities, as well as publication.
Results
Demographic characteristics
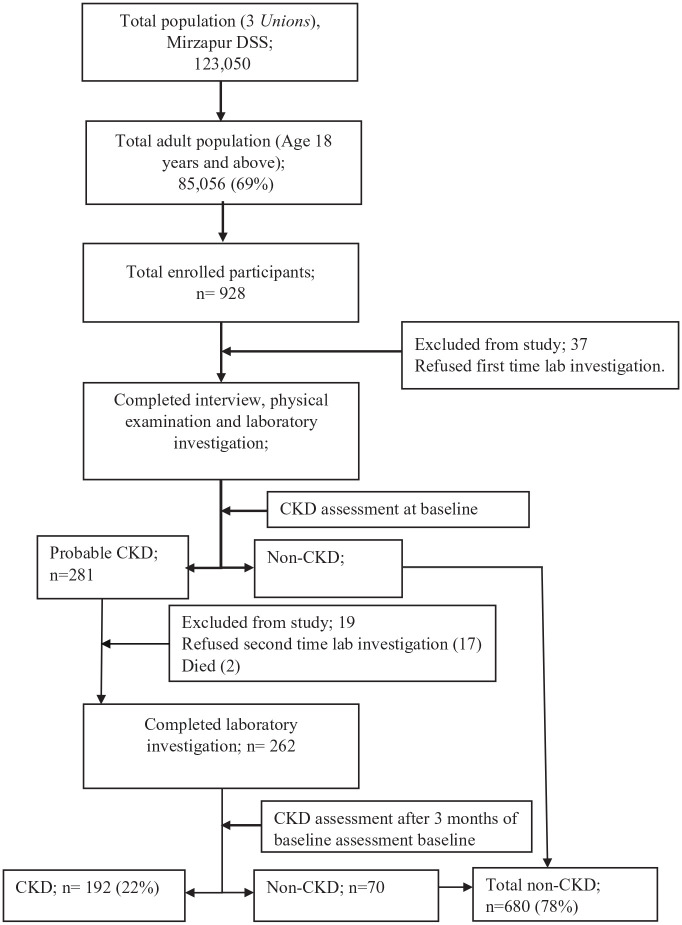
A total of 928 patients were enrolled from the DSS. Out of them 872 (94.0%) completed the study procedure and were included in this study (Figure 1).
Figure 1.
Study population of the community-based screening for chronic kidney diseases in rural and peri-urban Bangladesh.
DSS, demographic surveillance system; CKD, chronic kidney disease.
Overall, the mean ± SD of age was 48.2 ± 16.4, the mean ± SD of BMI was 23.5 ± 4.1 and the mean ± SD of waist circumference was 82.3 ± 11.5. The majority of the population were women (56.2%), married (79.0%), illiterate (36.2%) and housewives (49.0%). The minority of them were current smokers (19.6%) and smokeless tobacco users (28.9%). The remaining demographic, clinical and laboratory findings are also summarized in Table 1.
Table 1.
Demographic characteristics and risk factors data between CKD and non-CKD participants (t-test and multivariate logistic regression was performed).
| Variable | All n = 872 (%) | CKD n = 192 (%) | Non-CKD n = 680 (%) | Unadjusted OR (95% CI), p-value | Adjusted OR (95% CI), p-value |
|---|---|---|---|---|---|
| Age in years (mean ± SD) | 48.2 ± 16.4 | 58.9 ± 16.0 | 45.2 ± 15.2 | – | – |
| 18–30 | 143 (16.4) | 7 (3.6) | 136 (20.0) | – | – |
| 31–45 | 217 (24.9) | 32 (16.7) | 185 (27.2) | 0.53 (0.35–0.81)* | 1.49 (0.58–3.81) |
| 46–59 | 319 (36.6) | 64 (33.3) | 255 (37.5) | 0.83 (0.59–1.17) | 1.63 (0.63–4.20) |
| 60 and older | 193 (22.1) | 89 (46.4) | 104 (15.3) | 4.78 (3.36–6.80)* | 5.02 (1.85–13.65)* |
| Gender | |||||
| Female | 490 (56.2) | 119 (62.0) | 371 (54.6) | 1.36 (0.98–1.88) | – |
| Literacy | |||||
| Illiterate | 316 (36.2) | 86 (44.8) | 230 (33.8) | 1.59 (1.15–2.20)* | 1.08 (0.69–1.68) |
| Occupation | |||||
| Housewife | 427 (49.0) | 109 (56.8) | 318 (46.8) | 1.49 (1.08–2.06)* | 1.03 (0.65–1.61) |
| Farmer | 78 (8.9) | 14 (7.3) | 64 (9.4) | 0.76 (0.41–1.38) | 0.56 (0.26–1.17) |
| Marital status | |||||
| Married | 689 (79.0) | 143 (74.5) | 546 (80.3) | 0.72 (0.49–1.04) | 1.82 (0.61–5.45) |
| Widowed | 104 (11.9) | 44 (22.9) | 60 (8.8) | 3.07 (2.00–4.71)* | 2.86 (0.85–9.57) |
| Income (<$100/month) | 136 (15.6) | 36 (18.8) | 100 (14.7) | 1.34 (0.88–2.04) | – |
| Sleeping duration (<7 h/24 h) | 242 (27.8) | 78 (40.6) | 164 (24.1) | 2.15 (1.53–3.01)* | 1.20 (0.80–1.81) |
| Present tobacco smoker | 171 (19.6) | 29 (15.1) | 142 (20.9) | 0.67 (0.43–1.04) | – |
| Present smokeless tobacco user | 252 (28.9) | 71 (37.0) | 181 (26.6) | 1.62 (1.15–2.27)* | 0.86 (0.55–1.32) |
| Hypertension | 355 (40.7) | 133 (69.3) | 222 (32.6) | 4.65 (3.29–6.57)* | 3.08 (2.07–4.59)* |
| Fasting blood glucose (mmol/L) (mean ± SD) | 6.2 ± 2.2 | 7.1 ± 3.1 | 6.0 ± 1.7 | – | – |
| Diabetes | 147 (16.9) | 61 (31.8) | 86 (12.6) | 3.22 (2.20–4.70)* | 2.52 (1.60–3.96)* |
| BMI (mean ± SD) | 23.5 ± 4.1 | 24.0 ± 4.1 | 23.3 ± 4.0 | – | – |
| Underweight (BMI <18.5) | 91 (10.4) | 19 (9.9) | 72 (10.6) | 0.93 (0.54–1.58) | 1.11 (0.55–2.22) |
| Overweight/Obese (BMI ⩾25) | 303 (34.7) | 81 (42.2) | 222 (32.6) | 1.50 (1.08–2.09)* | 1.67 (0.98–2.83) |
| Waist circumference (cm) (mean ± SD) | 82.3 ± 11.5 | 85.0 ± 11.7 | 81.6 ± 11.3 | – | – |
| Abdominal obesity (waist circumference, men ⩾94 cm, women ⩾80 cm) | 341 (39.1) | 95 (49.5) | 246 (36.2) | 1.73 (1.25–2.39)* | 0.78 (0.45–1.34) |
| MUAC (cm) (mean ± SD) | 27.23 ± 3.27 | 27.24 ± 3.37 | 27.22 ± 3.24 | – | – |
| Undernutrition (MUAC, men <25 cm, women <24 cm) | 168 (19.3) | 34 (17.7) | 134 (19.7) | 0.88 (0.58–1.33) | – |
| Hemoglobin (g/dL) (mean ± SD) | 13.2 ± 1.5 | 12.8 ± 1.5 | 13.3 ± 1.5 | – | – |
| Anemia (hemoglobin, men <13 g/dL, women <12 g/dL) | 197 (22.6) | 70 (36.5) | 127 (18.7) | 2.50 (1.76–3.55)* | 2.50 (1.63–3.84)* |
| Presence of RBCs in urine | 63 (7.2) | 29 (15.1) | 34 (5.0) | 3.38 (2.00–5.71)* | 3.20 (1.71–5.98)* |
| Serum albumin (g/dL) (mean ± SD) | 3.8 ± 0.3 | 3.7 ± 0.3 | 3.9 ± 0.3 | – | – |
| Low serum albumin (albumin <3.5 g/dL) | 56 (6.4) | 22 (11.5) | 34 (5.0) | 2.46 (1.40–4.31)* | 1.11 (0.55–2.22) |
| Serum cholesterol (mg/dL) (mean ± SD) | 171.8 ± 37.2 | 179.7 ± 37.2 | 169.5 ± 36.8 | – | – |
| Hypercholesterolemia (total cholesterol >200 mg/dL) | 187 (21.4) | 49 (25.5) | 138 (20.3) | 1.34 (0.92–1.96) | – |
| Serum HDL-cholesterol (mg/dL) (mean ± SD) | 46.4 ± 12.1 | 47.1 ± 12.9 | 46.3 ± 11.9 | – | – |
| Low HDL-cholesterol (HDL-cholesterol <40 mg/dL) | 287 (32.9) | 61 (31.8) | 226 (33.2) | 0.93 (0.66–1.32) | – |
| Serum triglyceride (mg/dL) (mean ± SD) | 140.8 ± 99.8 | 163.1 ± 113.7 | 134.5 ± 94.6 | – | – |
| Hypertriglyceridemia (triglyceride >150 mg/dL) | 286 (32.8) | 83 (43.2) | 203 (29.9) | 1.79 (1.29–2.49)* | 1.23 (0.82–1.85) |
| History of heart disease | 40 (4.6) | 18 (9.4) | 22 (3.2) | 3.09 (1.62–5.90)* | 1.66 (0.76–3.66) |
| History of stroke | 31 (3.6) | 11 (5.7) | 20 (2.9) | 2.00 (0.94–4.26) | – |
| Family history of diabetes | 232 (26.6) | 54 (28.1) | 178 (26.2) | 1.10 (0.77–1.58) | – |
| Family history of hypertension | 339 (38.9) | 80 (41.7) | 259 (38.1) | 1.16 (0.84–1.61) | – |
Dependent variable: Presence of CKD when non-CKD was considered as reference group (non-CKD = 0, CKD = 1).
Reference categories for independent variable were: (18–30 years, male sex, literate, other than housewife and farmer, other than married and widowed, income.
p < 0.05.
BMI, body mass index; CKD, chronic kidney disease; HDL, high-density lipoprotein; MUAC, mid-upper arm circumference; OR, odds ratio; RBC, red blood cell; SD, standard deviation.
CKD prevalence
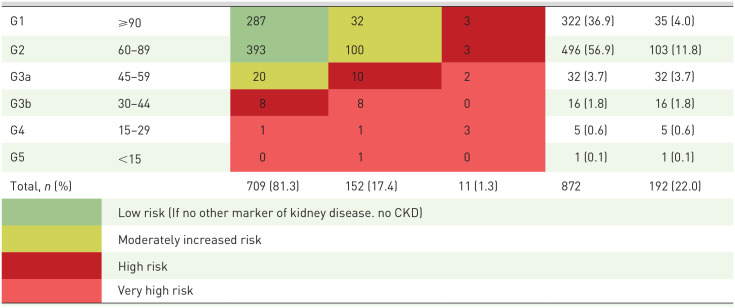
The overall prevalence of CKD was 22.0% (192 cases of 872 screened participants); of them the prevalence of CKD was 4.0% in stage 1, 11.8% in stage 2, 5.5% in stage 3, 0·6% in stage 4, and 0·1% in stage 5 (Table 2).
Table 2.
Overall distribution of study participants based on repeated estimated glomerular filtration rate and albumin to creatinine ratio in rural and peri-urban Bangladesh.
| Chronic kidney disease (CKD) stages | Estimated glomerular filtration rate (ml/min/1.73 m2) | Albumin to creatinine ratio (ACR) | Total n (%) | *CKD prevalence, n (%) | ||
|---|---|---|---|---|---|---|
| A1 (<30 mg/g) | A2 (30–300 mg/g) | A3 (>300 mg/g) | ||||
| ||||||
CKD: estimated glomerular filtration rate below 60 ml/min/1.73 m2 and/or ACR ⩾30 mg/g.
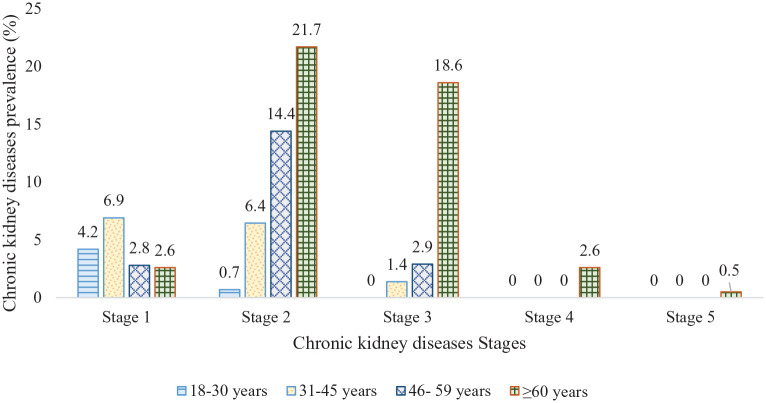
However, the age-specific prevalence of CKD was 4.9% (stage 1: 4.2%, stage 2: 0.7%), 14.7% (stage 1: 6.9%, stage 2: 6.4%, stage 3: 1.4%), 20.1% (stage 1: 2.8%, stage 2: 14.4%, stage 3: 2.9%) and 46.0% (stage 1: 2.6%, stage 2: 21.7%, stage 3: 18.6%, stage 4: 2.6%, stage 5: 0.5%) among the age groups 18–30 years, 31–45 years, 46–59 years and ⩾60 years, respectively (Figure 2).
Figure 2.
Prevalence of chronic kidney diseases by ages and stages (stages 1–5) in rural and peri-urban Bangladesh.
Factors associated with CKD
In the univariate analysis, aged 31–45 years (OR 0.53; 95% CI 0.35–0.81), aged ⩾60 years (OR 4.78; 95% CI 3.36–0.80), illiterate (OR 1.59; 95% CI 1.15–2.20), housewife (OR 1.49; 95% CI 1.08–2.06), widowed (OR 3.07; 95% CI 2.00–4.71), sleeping duration less than 7 h/24 h (OR 2.15; 95% CI 1.53–3.01), present smokeless tobacco user (OR 1.62; 95% CI 1.15–2.27), hypertension (OR 4.65; 95% CI 3.29–6.57), diabetes (OR 3.22; 95% CI 2.20–4.70), overweight or obesity (OR 1.50; 95% CI 1.08–2.09), abdominal obesity (OR 1.73; 95% CI 1.25–2.39) anemia (OR 2.50; 95% CI 1.76–3.55), presence of RBCs in the urine (OR 3.38; 95% CI 2.00–5.71), low serum albumin (OR 2.46; 95% CI 1.40–4.31), hypertriglyceridemia (OR 1.79; 95% CI 1.29–2.49), and history of heart disease (OR 3.09; 95% CI 1.62–5.90) were associated with CKD. However, there was no significant association between CKD and aged 46–59 years, female gender, farmer, married, income (<$100/month), present tobacco smoker, underweight, undernutrition, hypercholesterolemia, low HDL-cholesterol, history of stroke; and history of diabetes and hypertension in the family (Table 1).
The multivariate logistic regression analysis revealed that aged ⩾60 years (aOR 5.02; 95% CI 1.85–13.65), hypertension (aOR 3.08; 95% CI 2.07–4.59), diabetes (aOR 2.52; 95% CI 1.60–3.96), anemia (aOR 2.50; 95% CI 1.63–3.84) and presence of RBCs in the urine (aOR 3.20; 95% CI 1.71–5.98) were associated with CKD (Table 1).
In the physical measurement and laboratory findings, age (years) [CKD (mean ± SD) versus non-CKD (mean ± SD); 58.9 ± 16.0 versus 45.2 ± 15.2], BMI (kg/m2) (24.0 ± 4.1 versus 23.3 ± 4.0), waist circumference (cm) (85.0 ± 11.7 versus 81.6 ± 11.3), FBG (mmol/L) (7.1 ± 3.1 versus 6.0 ± 1.7), serum cholesterol (mg/dL) (179.7 ± 37.2 versus 169.5 ± 36.8), serum triglyceride (mg/dL) (163.1 ± 113.7 versus 134.5 ± 94.6) were significantly higher; conversely, hemoglobin (g/dL) (12.8 ± 1.5 versus 13.3 ± 1.5) and serum albumin (g/dL) (3.7 ± 0.3 versus 3.9 ± 0.3) were significantly lower among CKD participants compared to non-CKD participants (Table 1).
Discussion
The main objective of this study was to determine the prevalence and factors associated with CKD among adults in rural and peri-urban Bangladesh. The overall CKD prevalence documented in our study (22%) is higher than the pooled prevalence (17.3%) in Bangladesh. 23 However, the prevalence of CKD is rising in Bangladesh 24 and the exact figure varies from study to study based on the defining criteria and study settings. These studies are mostly from urban Dhaka, Bangladesh; and the majority of these studies have not repeated serum creatinine and urine ACR measurements over 3 months to define CKD,11,13 as suggested by the KDIGO guidelines. 17 This is critical because we identified 281 participants initially with probable CKD (Supplemental Table 1) and, on retesting after 3 months, almost 27% proved to be non-CKD individuals. This could be due to false-positive findings or the resolution of acute kidney injury. 25
The overall CKD prevalence identified in our study is higher than the prevalence of the global (9%), 3 developed (United States 11%, 26 Japan 13% 27 ) and developing (Pakistan 21.2%, 28 India 10.2% 28 ) countries. Our study also ascertained a higher prevalence of CKD in stages 1–2 (15.82% versus global 5.0%, India 11.3%) and stage 3 (5.50% versus global 3.90%, 3 India 4.3% 28 ). The increasing trend of non-communicable diseases (e.g. diabetes, hypertension, stroke), unhealthy lifestyle and dietary behaviors, lack of physical exercise may potentially be the key reasons behind our findings. In addition, a higher prevalence of CKD among the age group ⩾60 years (46.4% versus United States 39.4%, 29 India 40.6) 30 may be the explanation of the higher prevalence of CKD in our current study. Other causes may include heterogeneity in the methodological approach, inclusion criteria and size of the study sample that were able to capture the diversity as much as possible. Although the prevalence of CKD was remarkably high in our study, the participants had a low awareness about their kidney status. Only 6.8% of participants were conscious of being a CKD patient prior to the study, which is lower than that of the neighboring country India (7.9%). 31 This also reflects the lack of provision of healthcare and diagnostic facilities available to this population.
Our study reported higher odds among the diabetes and hypertensive individuals, which is consistent with several previous studies;31,32 and might partially contribute to the higher prevalence of CKD in our sample population. The prevalence of diabetes and hypertension has dramatically increased in Bangladesh over the past two decades33,34 and particular attention should be paid to manage these factors in order to prevent or slow down CKD.
In the course of CKD, anemia can occur early and may worsen in advanced stages. 35 We found that anemia is substantially high among CKD patients, which is expected and has also been reported in other studies.36–38 The presence of RBCs in the urine is also documented as a strong factor associated with CKD in our research, which may be attributed to kidney pathology that blocks the urinary tract and triggers decreased kidney function. 39
Although CKD can develop at any time, we found that increasing age particularly aged ⩾60 years and older is a risk and lower age (18–45 years) is protective against developing CKD. After the age of 40 years, kidney filtration starts to decline; 40 and in addition to the natural aging of the kidneys, in the elderly, certain diseases including diabetes, high BP, and heart disease that affect the kidneys to become more prevalent to CKD. For the elderly population, eGFR up to 45 ml/min/1.73 m2 is acceptable without proteinuria or primary kidney disease diagnosis, 41 and eGFR less than 45 ml/min/1.73 m2 with or without proteinuria are associated with a high risk of mortality. 42 Therefore, it will be prudent to screen the elderly population to gather further evidence. Moreover, we observed that lower serum albumin levels are independently correlated with decreased renal function among our participants, which has also been reported in other studies, particularly in the elderly population, 43 Further study is required to understand the biological mechanisms that relate low serum albumin to decreased renal function.
An interesting finding from our research was that the widows were more likely to develop CKD. We do not have a ready explanation for this finding; however, evidence from Bangladesh suggests that 12% of widows live alone. They are more at risk of poverty because of the absence of a male earner. In addition, they suffer from social isolation, and higher mortality and morbidity including stress that shortens the duration of their sleeping hours at night, thus increasing their susceptibility to CKD. A high prevalence of CKD is observed among the illiterate and housewives. In Bangladesh, rural and peri-urban residents have a lower rate of literacy, particularly among housewives and the elderly population. 44 This group is largely ignorant of their unhealthy lifestyle and dietary patterns which may contribute to the development of risk factors for CKD such as dyslipidemia, overweight or obesity. Dyslipidemia is associated with a decrease in GFR; thus the lipid profile depends on the degree of renal function and the degree of proteinuria. 45 Our study demonstrated hypertriglyceridemia is a risk factor for CKD, but hypercholesterolemia and low HDL-cholesterol showed no relevant relation. CKD has been found to be more prevalent in the general overweight or obese and abdominal obese population. Overweight and obesity are reported to increase kidney disease, particularly by causing endocrine dysfunction. 46 Another important lifestyle factor associated with the development of CKD documented in our study is sleeping less than 7 h per day which can be explained by the regulation of kidney function by the sleep–wake cycle 47 and the relationship between melatonin secretion and sleeping hours. 48 Further studies are needed to establish the mechanism to explain our observations.
Although several studies documented a strong relationship between smoking and CKD,49,50 we have not recognized any significant association with CKD. Instead, we found that smokeless tobacco use is a distinct associated factor of CKD, even though we have not found sufficient literature to reinforce this issue. In Taiwan, a study showed a greater prevalence of CKD among betel nut users than the prevalence of CKD among non-betel nut users. 51 Tobacco compounds hinder the formation of vascular endothelial cells and induce endothelial dysfunction and might be responsible for the development of CKD. Surprisingly, the use of smokeless tobacco is culturally accepted in Bangladesh, and there are more smokeless tobacco consumers than smokers (27% versus 23%). 22 There is a need for public health awareness along with the implementation of a national tobacco control act to curb smokeless tobacco.
Limitations and strengths
We used the CKD-EPI equation that was created on the basis of the population of the United States, most of which were whites and blacks with diverse characteristics. So, this equation would be less valid in the context of citizens in Bangladesh. In addition, we were unable to retest 19 of the 281 participants who screened positive in phase I. Two of these 19 participants had died, and others refused laboratory investigation. They may have suffered from kidney disease; however, during screening they denied any history of kidney disease. Moreover, the cross-sectional design, purposive inclusion of three unions, observation only based on Mirzapur sub-district and assessment of undernutrition status using only the MUAC measurement are the main limitations of our study. The main strengths of this analysis include the unbiased age-stratified sampling method to enrol patients, use of an accredited laboratory, use of standardised equipment and trained workforce.
Implications of all the available evidence
The evidence presented in our study will help to strengthen public health strategies to reduce the prevalence of CKD and slowing down the progression of the disease, particularly in the rural and per-urban settings of the developing countries. Strategies include the development of a CKD screening programme based on a nationally established equation to confirm diagnosis, awareness through health education, proper management of CKD risk factors such as diabetes, hypertension, the presence of RBCs in the urine and special care for the elderly population.
Conclusion
Our research shows that patients who reside in rural and peri-urban settings have a high prevalence of CKD, in which diabetes, hypertension, anemia, old age, the presence of RBCs in the urine, hypertriglyceridemia, obesity or overweight, widowed and smokeless tobacco use have a significant role. Therefore, the scale-up of preventive and therapeutic steps to manage these major risk factors may help to reduce CKD-related ramifications.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223211035281 for Community-based screening to determine the prevalence, health and nutritional status of patients with CKD in rural and peri-urban Bangladesh by Mohammad Habibur Rahman Sarker, Michiko Moriyama, Harun Ur Rashid, Mohammod Jobayer Chisti, Md Moshiur Rahman, Sumon Kumar Das, Aftab Uddin, Samir Kumar Saha, Shams El Arifeen, Tahmeed Ahmed and ASG Faruque in Therapeutic Advances in Chronic Disease
Supplemental material, sj-tiff-2-taj-10.1177_20406223211035281 for Community-based screening to determine the prevalence, health and nutritional status of patients with CKD in rural and peri-urban Bangladesh by Mohammad Habibur Rahman Sarker, Michiko Moriyama, Harun Ur Rashid, Mohammod Jobayer Chisti, Md Moshiur Rahman, Sumon Kumar Das, Aftab Uddin, Samir Kumar Saha, Shams El Arifeen, Tahmeed Ahmed and ASG Faruque in Therapeutic Advances in Chronic Disease
Acknowledgments
The authors are thankful to the study participants and CHWs (Ayesha Akter, Sompa Bakali, Shikha Akter, Shanta Islam, Tanni Sikder, Hazera Begum, Hiramon Akter, Afrin Sultana) for their generous support. They are grateful to Yuko Ito for her enormous support, Yasmin Jahan for her assistance in CHW recruitment and Ashir Ahmed (Kyushu University, Japan) for providing the Portable Health Clinic device. They would like to express their sincere gratitude to Soroar Hossain Khan for his data analysis assistance and all the laboratory staff for their invaluable support in laboratory investigations.
Footnotes
Author contributions: MHRS, MM and ASGF designed the study; MHRS, MM and ASGF performed the main statistical analysis; HUR, SKS, SKD, MMR, SEA, AU, TA and MJC provided assistance for the main statistical analysis and data interpretation; MM, MMR, MJC, SKS, AU, SEA, TA, ASGF and MHRS provided material support during the study; and all authors participated in drafting and reviewing the manuscript, and approved the final version of the manuscript to be published.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research project was funded by the Grants-in-Aid for Scientific Research Program (KAKENHI), Japan, grant number Kiban-B # 18H03113.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Mohammad Habibur Rahman Sarker  https://orcid.org/0000-0001-9614-0806
https://orcid.org/0000-0001-9614-0806
Contributor Information
Mohammad Habibur Rahman Sarker, Graduate School of Biomedical and Health Sciences, Hiroshima University, 1-2-3, Kasumi, Minami-ku, Hiroshima, 734-8553, Japan; International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), Dhaka, Bangladesh.
Michiko Moriyama, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan.
Harun Ur Rashid, Kidney Foundation Hospital and Research Institute, Dhaka, Bangladesh.
Mohammod Jobayer Chisti, International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), Dhaka, Bangladesh.
Md Moshiur Rahman, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan.
Sumon Kumar Das, Menzies – School of Health Research, Charles Darwin University, Darwin, Australia.
Aftab Uddin, International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), Dhaka, Bangladesh.
Samir Kumar Saha, Dhaka Shishu Hospital, Dhaka, Bangladesh; Child Health Research Foundation, Dhaka, Bangladesh.
Shams El Arifeen, International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), Dhaka, Bangladesh.
Tahmeed Ahmed, International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), Dhaka, Bangladesh.
ASG Faruque, International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), Dhaka, Bangladesh.
References
- 1. Luyckx VA, Tuttle KR, Garcia-Garcia G, et al. Reducing major risk factors for chronic kidney disease. Kidney Int Suppl 2011 2017; 7: 71–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luyckx VA, Tonelli M, Stanifer JW. The global burden of kidney disease and the sustainable development goals. Bull WHO 2018; 96: 414–422D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020; 395: 709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hall ME, do Carmo JM, da Silva AA, et al. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis 2014; 7: 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wouters OJ, O’Donoghue DJ, Ritchie J, et al. Early chronic kidney disease: diagnosis, management and models of care. Nat Rev Nephrol 2015; 11: 491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015; 385: 1975–1982. [DOI] [PubMed] [Google Scholar]
- 7. Luyckx VA, Naicker S, McKee M. Equity and economics of kidney disease in sub-Saharan Africa. Lancet 2013; 382: 103–104. [DOI] [PubMed] [Google Scholar]
- 8. Levin A, Tonelli M, Bonventre J, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 2017; 390: 1888–1917. [DOI] [PubMed] [Google Scholar]
- 9. Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013; 382: 260–272. [DOI] [PubMed] [Google Scholar]
- 10. Abraham G, Varughese S, Thandavan T, et al. Chronic kidney disease hotspots in developing countries in South Asia. Clin Kidney J 2016; 9: 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anand S, Khanam MA, Saquib J, et al. High prevalence of chronic kidney disease in a community survey of urban Bangladeshis: a cross-sectional study. Global Health 2014; 10: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huda MN, Alam KS, Harun UR. Prevalence of chronic kidney disease and its association with risk factors in disadvantageous population. Int J Nephrol 2012; 2012: 267329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fatema K, Abedin Z, Mansur A, et al. Screening for chronic kidney diseases among an adult population. Saudi J Kidney Dis Transpl 2013; 24: 534–541. [DOI] [PubMed] [Google Scholar]
- 14. Hasan M, Kashem M, Rahman M, et al. Prevalence of chronic kidney disease (CKD) and identification of associated risk factors among rural population by mass screening. Community Based Med J 2013; 1: 20–26. [Google Scholar]
- 15. Saha SK, Ahmed ZB, Modak JK, et al. Group B Streptococcus among pregnant women and newborns in Mirzapur, Bangladesh: colonization, vertical transmission, and serotype distribution. J Clin Microbiol 2017; 55: 2406–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Das SK, Nasrin D, Ahmed S, et al. Health care-seeking behavior for childhood diarrhea in Mirzapur, rural Bangladesh. Am J Trop Med Hyg 2013; 89: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014; 63: 713–735. [DOI] [PubMed] [Google Scholar]
- 18. Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease: a systematic review and meta-analysis. PLoS One 2016; 11: e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fottrell E, Ahmed N, Shaha SK, et al. Distribution of diabetes, hypertension and non-communicable disease risk factors among adults in rural Bangladesh: a cross-sectional survey. BMJ Glob Health 2018; 3: e000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP), Expert Panel On Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 21. Sultana T, Karim MN, Ahmed T, et al. Assessment of under nutrition of Bangladeshi adults using anthropometry: can body mass index be replaced by mid-upper-arm circumference? PLoS One 2015; 10: e0121456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huque R, Zaman MM, Huq SM, et al. Smokeless tobacco and public health in Bangladesh. Indian J Public Health 2017; 61: S18–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hasan M, Sutradhar I, Gupta RD, et al. Prevalence of chronic kidney disease in South Asia: a systematic review. BMC Nephrol 2018; 19: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Das SK, Afsana SM, Elahi SB, et al. Renal insufficiency among urban populations in Bangladesh: a decade of laboratory-based observations. PLoS One 2019; 14: e0214568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferguson R, Leatherman S, Fiore M, et al. Prevalence and risk factors for CKD in the general population of Southwestern Nicaragua. J Am Soc Nephrol 2020; 31: 1585–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003; 41: 1–12. [DOI] [PubMed] [Google Scholar]
- 27. Imai E, Horio M, Watanabe T, et al. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol 2009; 13: 621–630. [DOI] [PubMed] [Google Scholar]
- 28. Varma PP. Prevalence of chronic kidney disease in India: where are we heading? Indian J Nephrol 2015; 25: 133–135. [PMC free article] [PubMed] [Google Scholar]
- 29. Mallappallil M, Friedman EA, Delano BG, et al. Chronic kidney disease in the elderly: evaluation and management. Clin Pract (Lond Engl) 2014; 11: 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rai PK, Rai P, Bhat RG, et al. Chronic kidney disease among middle-aged and elderly population: a cross-sectional screening in a hospital camp in Varanasi, India. Saudi J Kidney Dis Transpl 2019; 30: 795–802. [DOI] [PubMed] [Google Scholar]
- 31. Singh AK, Farag YM, Mittal BV, et al. Epidemiology and risk factors of chronic kidney disease in India: results from the SEEK (Screening and Early Evaluation of Kidney Disease) study. BMC Nephrol 2013; 14: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cao Y, Li W, Yang G, et al. Diabetes and hypertension have become leading causes of CKD in Chinese elderly patients: a comparison between 1990–1991 and 2009–2010. Int Urol Nephrol 2012; 44: 1269–1276. [DOI] [PubMed] [Google Scholar]
- 33. Biswas T, Islam A, Rawal LB, et al. Increasing prevalence of diabetes in Bangladesh: a scoping review. Public Health 2016; 138: 4–11. [DOI] [PubMed] [Google Scholar]
- 34. Chowdhury MZI, Rahman M, Akter T, et al. Hypertension prevalence and its trend in Bangladesh: evidence from a systematic review and meta-analysis. Clin Hypertens 2020; 26: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dowling TC. Prevalence, etiology, and consequences of anemia and clinical and economic benefits of anemia correction in patients with chronic kidney disease: an overview. Am J Health Syst Pharm 2007; 64: S3–S7. quiz S23–S25. 2007/07/13. [DOI] [PubMed] [Google Scholar]
- 36. McClellan W, Aronoff SL, Bolton WK, et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin 2004; 20: 1501–1510. [DOI] [PubMed] [Google Scholar]
- 37. Astor BC, Muntner P, Levin A, et al. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 2002; 162: 1401–1408. [DOI] [PubMed] [Google Scholar]
- 38. Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 2002; 13: 504–510. [DOI] [PubMed] [Google Scholar]
- 39. Orlandi PF, Fujii N, Roy J, et al. Hematuria as a risk factor for progression of chronic kidney disease and death: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. BMC Nephrol 2018; 19: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc 2009; 120: 419–428. [PMC free article] [PubMed] [Google Scholar]
- 41. Jonsson AJ, Lund SH, Eriksen BO, et al. The prevalence of chronic kidney disease in Iceland according to KDIGO criteria and age-adapted estimated glomerular filtration rate thresholds. Kidney Int 2020; 98: 1286–1295. [DOI] [PubMed] [Google Scholar]
- 42. Delanaye P, Jager KJ, Bökenkamp A, et al. CKD: a call for an age-adapted definition. J Am Soc Nephrol 2019; 30: 1785–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guligowska A, Corsonello A, Pigłowska M, et al. Association between kidney function, nutritional status and anthropometric measures in older people: the screening for CKD among Older People across Europe (SCOPE) study. BMC Geriatr 2020; 20: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hurt LS, Ronsmans C, Saha S. Effects of education and other socioeconomic factors on middle age mortality in rural Bangladesh. J Epidemiol Community Health 2004; 58: 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mikolasevic I, Žutelija M, Mavrinac V, et al. Dyslipidemia in patients with chronic kidney disease: etiology and management. Int J Nephrol Renovasc Dis 2017; 10: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cao X, Zhou J, Yuan H, et al. Chronic kidney disease among overweight and obesity with and without metabolic syndrome in an urban Chinese cohort. BMC Nephrol 2015; 16: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park S, Lee S, Kim Y, et al. Short or long sleep duration and CKD: a Mendelian randomization study. J Am Soc Nephrol 2020; 31: 2937–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Russcher M, Koch B, Nagtegaal E, et al. The role of melatonin treatment in chronic kidney disease. Front Biosci (Landmark edition) 2012; 17: 2644–2656. [DOI] [PubMed] [Google Scholar]
- 49. Yacoub R, Habib H, Lahdo A, et al. Association between smoking and chronic kidney disease: a case control study. BMC Public Health 2010; 10: 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xia J, Wang L, Ma Z, et al. Cigarette smoking and chronic kidney disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Nephrol Dial Transplant 2017; 32: 475–487. [DOI] [PubMed] [Google Scholar]
- 51. Wang M, Yu SY, Lv ZT, et al. Betel nut chewing and the risk of chronic kidney disease: evidence from a meta-analysis. Int Urol Nephrol 2018; 50: 1097–1104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223211035281 for Community-based screening to determine the prevalence, health and nutritional status of patients with CKD in rural and peri-urban Bangladesh by Mohammad Habibur Rahman Sarker, Michiko Moriyama, Harun Ur Rashid, Mohammod Jobayer Chisti, Md Moshiur Rahman, Sumon Kumar Das, Aftab Uddin, Samir Kumar Saha, Shams El Arifeen, Tahmeed Ahmed and ASG Faruque in Therapeutic Advances in Chronic Disease
Supplemental material, sj-tiff-2-taj-10.1177_20406223211035281 for Community-based screening to determine the prevalence, health and nutritional status of patients with CKD in rural and peri-urban Bangladesh by Mohammad Habibur Rahman Sarker, Michiko Moriyama, Harun Ur Rashid, Mohammod Jobayer Chisti, Md Moshiur Rahman, Sumon Kumar Das, Aftab Uddin, Samir Kumar Saha, Shams El Arifeen, Tahmeed Ahmed and ASG Faruque in Therapeutic Advances in Chronic Disease