Abstract
Apathy is prevalent in dementia, such as behavioral variant frontotemporal dementia (bvFTD), primary progressive aphasia (PPA), and Alzheimer disease (AD). As a multidimensional construct, it can be assessed and subsumed under a Dimensional Apathy Framework. A consistent apathy profile in bvFTD and PPA has yet to be established. The aim was to explore apathy profiles and awareness in bvFTD, PPA, and AD. A total of 12 patients with bvFTD, 12 patients with PPA, 28 patients with AD, and 20 matched controls, as well as their informants/carers, were recruited. All participants completed the Dimensional Apathy Scale (DAS), assessing executive, emotional, and initiation apathy subtypes, a 1-dimensional apathy measure, depression measure, and functional and cognitive screens. Apathy subtype awareness was determined through DAS informant/carer and self-rating discrepancy. Apathy profile comparison showed patients with bvFTD had significantly higher emotional apathy than patients with AD (P < .01) and significantly higher apathy over all subtypes than patients with PPA (Ps < .05). Additionally, patients with bvFTD had significantly lower awareness for emotional apathy (P < .01) when compared to patients with AD and PPA. All patient groups had significant global apathy over all subtypes compared to controls. The emergent apathy profile for bvFTD seems to be emotional apathy (indifference or emotional/affective neutrality), with lower self-awareness in this subtype. Further, lower self-awareness for executive apathy (lack of motivation for planning, organization, or attention) differentiates bvFTD from PPA. Future research should investigate the cognitive and neural correlates as well as the practical impact of apathy subtypes.
Keywords: Alzheimer disease, behavioral variant frontotemporal dementia, primary progressive aphasia, frontotemporal dementia, apathy, awareness, insight
Introduction
Apathy as a lack of motivation is frequently observed in dementia, occurring in up to 90% of patients with frontotemporal dementia (FTD) 1 and Alzheimer disease (AD). 2 Frontotemporal dementia is an umbrella term for behavioral variant frontotemporal dementia (bvFTD) and primary progressive aphasia, which can be further subdivided into semantic dementia (SD), progressive nonfluent aphasia (PNFA), and logopenic variant primary progressive aphasia (lvPPA). In terms of FTD, research has shown that patients with bvFTD have higher levels of apathy compared to patients with primary progressive aphasia. 3,4 The impact of demotivation is widespread in these diseases, being associated with problems in activities of daily living, decreased quality of life, and increased caregiver burden. 5 -8
Apathy is composed of different subtypes, 9 -11 with certain multidimensional models focusing on cortical and subcortical brain network dysfunction. Levy and Dubois proposed a prefrontal cortex-basal ganglia neuroanatomical apathy model composed of auto-activation apathy (eg, impairments of self-generation), cognitive apathy/inertia (eg, impairment of goal management, use of strategy, and planning), and emotional apathy (eg, impairment of emotional processing). 10,11 Apathy subtypes can further be subsumed under the Dimensional Apathy Framework, which is a cumulative model taking into account previous subtypes of apathy inclusive of the Levy and Dubois model. 12 This is a 3-dimensional model of apathy comprising executive, emotional, and initiation apathy subtypes with self-awareness or insight interacting with each subtype. Executive apathy is a lack of motivation toward planning, organization, or attention; emotional apathy is an indifference, emotional/affective neutrality, blunting, or flatness; and initiation apathy is lack of motivation for self-generation of thought or actions. While several tools measure elements of this framework, 12 the Dimensional Apathy Scale (DAS) 13 directly measures these subtypes. Previous research has shown different profiles of apathy in motor neurone disease 14,15 and Parkinson disease. 16,17 Additionally, the apathy profile in AD has been characterized by increased executive, emotional, and initiation subtypes, with decreased awareness, or insight, restricted to executive and initiation subtypes. 18 More recent research using the DAS has found differing apathy profiles with higher emotional apathy in bvFTD when compared to AD and higher executive apathy in AD when compared to bvFTD. 19 However, the profile of apathy and self-awareness of demotivation has not been explored in PPA and bvFTD.
Other research using different tools, such as the apathy subscale questions of the Neuropsychiatric Inventory, 20 found that certain characteristics of apathy differentiate FTD from AD, where FTD showed a decreased emotional output, lack of initiative, or lack of interest toward friends or family. 21,22 More recently, when compared to patients with AD, bvFTD has been observed to have decreased self-awareness relating to apathy as well as increased apathy in emotional domains on the Lille Apathy Rating Scale (LARS). 23 Another study looking at apathy characteristics derived from various functional (Disability Assessment for Dementia Scale) 24 and behavioral scales (Cambridge Behaviour Inventory–Revised) 25 found prominent affective-emotional apathy characteristics (ie, the inability to use emotional context for guidance of behavior) in bvFTD, while both AD and bvFTD displayed cognitive apathy characteristics (ie, demotivation for participation in goal-directed behavior). 26 However, these aforementioned tools were designed as general behavior measures, therefore being nonspecific to apathy subtypes with only a few multidimensional apathy tools, for example, DAS 13 and LARS, 27 currently validated for use in dementia. To build upon this research, it is timely to determine the apathy profile based on a structured framework such as the Dimensional Apathy Framework and using multidimensional apathy tools such as the DAS, within dementia diagnosis of bvFTD, PPA, and AD.
The aim was to explore the apathy profile and awareness of apathy subtypes in bvFTD and PPA in comparison to AD and determine any relationships to cognitive functioning and activities of daily living.
Methods
Participants
A total of 12 patients with PPA, 12 patients with bvFTD, and 28 patients with AD, as well as their carers/relatives/close friends, were recruited from a Specialist Early Onset Dementia Research Clinic (the Edinburgh Cognitive Diagnosis Audit Research and Treatment Register; CDC-DART), at the Anne Rowling Regenerative Neurology Clinic, University of Edinburgh. The PPA patient group was composed of 9 patients with lvPPA, 2 patients with PNFA, and 1 patient with SD. All patients fulfilled consensus clinical diagnostic criteria for each disease. 28 -30 Diagnoses were made following multidisciplinary clinical assessments (neurology, psychiatry, neuropsychology), which included neuropsychological assessment of domains such as executive, language, memory, and visuospatial functioning and behavior. Cerebrospinal fluid biomarkers and neuroimaging were incorporated where appropriate to support the diagnostic process. Twenty healthy controls and their informants were recruited from the University of Edinburgh Departmental Volunteer Panel. Exclusion criteria for participants were severe diabetes, epilepsy, alcohol/substance-related disorders, severe head injury (that required intensive care hospitalization), traumatic brain injury (inclusive of subarachnoid hemorrhage), and other present or past significant comorbid medical illness (such as stroke, psychiatric disease, etc). Controls were not specifically assessed for cognitive impairment (ie, using Addenbrooke’s Cognitive Examination III [ACE-III] or other measures) in the present study, although were excluded if information on the University of Edinburgh Departmental Volunteer Panel database indicated cognitive impairment.
Ethical approval was obtained from the National Health Service South East Scotland Research Ethics Committee 02 and the School Philosophy, Psychology and Language Sciences Ethical Committee. All patient, control, informant, and carer participants gave informed consent following the Declaration of Helsinki.
Procedures
Patients (and their carers/relatives/close friends) and controls (and their informants) were asked to complete measures of apathy and depression. Carers/relatives/close friends and informants completed apathy, depression, and activities of daily living measures about their observations of the patients and controls, so as to account for problems with awareness or insight.
Measures
The DAS 13,14 was used to assess multidimensional apathy through 3 subscales: executive apathy, emotional apathy, and initiation apathy. It is composed of 24 items which are scored on a 4-point Likert response scale. Each 8-item subscale has a minimum of 0 (least apathy) and maximum of 24 (most apathy). The total score can range from 0 to 72. The DAS has been validated for use in dementia. 18 Previously published cutoffs were used for each subscale. 14 The cutoff of ≥14 was used for the presence of executive apathy, ≥15 was used for the presence of emotional apathy, and ≥16 was used for the presence of initiation apathy. Both self-rated and informant/carer-rated DAS data were collected.
The Apathy Evaluations Scale (AES) 31 was used as a gold standard to assess 1-dimensional apathy. It is composed of 18 items which are scored on a 4-point Likert response scale. The scale ranges from a minimum of 0 (least apathy) to a maximum of 72 (most apathy). The AES has been validated in dementia, and an abnormality cutoff of >41.5 (carer-rated version) was used. 32 The informant-rated version was utilized.
The Geriatric Depression Scale–Short Form (GDS-15) 33 was used to screen for depression. It is a 15-item scale that is scored dichotomously (yes/no). The results range from a minimum of 0 (not depressed) to a maximum of 15 (most depressed). The cutoff of >6 was used for the presence of depressive symptoms. 34 The informant-rated version was utilized.
Please see supplementary materials for correlations between the AES, DAS, and GDS-15 in the patient sample.
The Lawton Instrumental Activities of Daily Living (LIADL) 35 assessment was used to assess functional independence of the patients. It is an 8-item carer-rated assessment, with total scores ranging from 0 (low function, dependent) to 8 (high function, independent).
The ACE-III 36 and the Edinburgh Cognitive and Behavioural ALS Screen (ECAS) 37 were used to examine global cognitive functioning and behavior change of patients.
Statistical Analysis
R software 38 and SPSS statistics were used to perform all analysis. Shapiro-Wilk tests were used to examine distribution of the data to determine the use of parametric or nonparametric analysis. Descriptive data (clinical and demographic variables) were compared using analysis of variance (ANOVA), with follow-up post hoc t test. Informant/carer-rated versions of AES and GDS-15 were used for comparison. Gender distribution was compared using χ2.
A 4 × 3 mixed ANOVA was used to compare groups (bvFTD vs PPA/lvPPA only vs AD vs control) on each informant/carer-rated DAS subscale (executive vs emotional vs initiation) with post hoc t tests (Holm correction). Additionally, a further 4 × 3 mixed ANOVA was used to compare groups (bvFTD vs PPA/lvPPA only vs AD vs control) on awareness discrepancy on different DAS subscales (executive vs emotional vs initiation) with post hoc t tests (Holm correction). Awareness discrepancy on apathy subtypes was determined by calculating the difference between informant/carer-rated DAS scores and self-rated DAS scores. Power was calculated using the partial eta squared ( ) and Cohen d. Chi-square analysis was used for comparison of frequency of apathy impairment (number of participants above cutoffs) for each patient group. The subsampled lvPPA-only group (n = 9) was used in addition to the whole PPA group (n = 12) for additional analysis. Correlational analysis was conducted using Spearman rho (Holm corrected).
Results
Descriptive
The most common carer or informant relationship to patients and controls was spouse (71%), followed by other relative (21%) and other (8%), such as close friends. Table 1 shows there was no significant difference between patient groups (bvFTD, PPA, and AD) and controls on age, years of education, and gender distribution (see Table 1).
Table 1.
Clinical and Demographic Variables for Patients and Controls.a
| lvPPA-only (n = 9) | PPA (n = 12) | bvFTD (n = 12) | AD (n = 28) | Control (n = 20) | F/χ2 value | P value | |
|---|---|---|---|---|---|---|---|
| Age, mean (SD) | 62.8 (7.5) | 63.2 (6.7) | 61.0 (11.9) | 62.5 (5.6) | 64.9 (9.6) | F = 0.603 | n.s. |
| Gender (male/female) | 6/3 | 7/5 | 8/4 | 16/12 | 12/8 | χ2 = 0.328 | n.s. |
| Years of education, mean (SD) | 17.0 (4.8)b | 15.6 (4.7)c | 12.2 (3.7)c | 13.4 (3.0)d | 14.7 (2.7) | F = 2.451 | n.s. |
| AES, mean (SD)/72 | 42.2 (13.1) | 41.3 (12.1) | 55.3 (9.5) | 42.9 (9.3) | 27.7 (5.9) | F = 24.828 | <.001 |
| GDS-15, mean (SD)/15 | 11.7 (5.4) | 5.9 (5.5) | 5.0 (2.3 | 6.5 (4.3) | 1.9 (2.0) | F = 6.524 | <.001 |
| Age onset, mean (SD) | 61.0 (6.0)e | 58.3 (6.9)f | 52.1 (11.9)g | 57.9 (6.3)h | F = 2.035 | n.s. | |
| Disease duration, median (IQR) | 4 (1.5)e | 5 (1.75)f | 5 (7)g | 5 (4)h | F = 2.102 | n.s. | |
| ACE-III total, mean (SD)/100 | 62.9 (26.0) | 66.3 (24.2)i | 71.4 (14.5)c | 65.2 (15.8) | F = 0.472 | n.s. | |
| ECAS cognitive total, mean (SD)/136 | 61.8 (35.9) | 67.2 (34.9) | 72.3 (22.0)g | 72.5 (21.1)j | F = 0.157 | n.s. | |
| ECAS behavior domain, median (IQR)/5 | 3 (3) | 2 (3.5)c | 5 (3)g | 2 (1.5)j | F = 6.305 | <.01 | |
| LIADL total, mean (SD)/8 | 6.1 (1.4) | 6.5 (1.4) | 3.2 (1.5) | 5.1 (2.0) | F = 10.949 | <.001 |
Abbreviations: ACE-III, Addenbrooke’s Cognitive Examination III; AD, Alzheimer’s disease; AES, Apathy Evaluation Scale; bvFTD, behavioral variant frontotemporal dementia; ECAS, Edinburgh Cognitive and Behavioural ALS Screen; GDS-15, Geriatric Depression Scale–Short Form; IQR, interquartile range; LIADL, Lawton Instrumental Activities of Daily Living; lvPPA, logopenic variant primary progressive aphasia; n.s., not significant; PPA, primary progressive aphasia.
a Comparison is between PPA, bvFTD, AD, and controls. The lvPPA-only group is a subsample from the PPA group.
b n = 8.
c n = 11.
d n = 22.
e n = 7.
f n=10.
g n = 9.
h n = 27.
i n = 12.
j n = 15.
In comparing bvFTD, PPA, and AD groups on clinical variables, there was no significant difference between age of onset and disease duration (see Table 1). Post hoc tests showed that all patient groups were significantly more apathetic on the AES than controls, PPA versus controls, t(30) = −4.269, P < .001; bvFTD versus control, t(30) = −10.277, P < .001, AD versus control, t(46) = −6.434, P < .001. Post hoc tests showed patients with bvFTD were significantly more apathetic on the AES than AD, t(38) = −3.862, P < .001, and PPA, t(22) = 3.202, P < .01, with no significant difference between PPA and AD; 57.1% of patients with AD (n = 16), 83.3% of patients with bvFTD (n = 10), and 66.7% of patients with PPA (n = 8) were above cutoff on the AES, but this was not significantly different; 66.7% of the patients with lvPPA (n = 6), 50.0% of the patients with PNFA (n = 1), and the patient with SD were apathetic based on the AES. No controls were above cutoffs for apathy, based on the AES.
In terms of depression, post hoc tests showed patient groups were significantly more depressed than controls, PPA versus controls, t(30) = −3.563, P < .01, bvFTD versus control, t(30) = −3.370, P < .01, AD versus control, t (46) = −4.498, P < .001. There was no significant difference between patients on depression levels; 21.4% of patients with AD (n = 6), 25.0% of patients with bvFTD (n = 3), and 25.0% of patients with PPA (n = 3) showed above cutoff depressive symptoms on the GDS, but this was not significant; 44.4% of patients with lvPPA (n = 4) were above cutoff for depression based on the GDS-15. The patient with SD and none of the patients with PNFA were above cutoff for depression, based on the GDS-15. No controls were above cutoffs for depression, based on the GDS-15.
Further, there was a significant difference in the LIADL between all patient groups, with patients with bvFTD being significantly more functionally impaired than both AD, t(38) = 3.037, P < .01, and PPA, t(22) = −5.606, P < .001, as well as AD being significantly more functionally impaired than PPA, t(38) = −2.220, P < .05. However, there were no significant correlations between AES and LAIDL in any patient groups, showing no relationship between 1-dimensional apathy and function. There were no significant correlations between the AES and cognitive functioning (ACE-III and ECAS). Additionally, there was a significant difference in the ECAS behavior domains. Behavioral variant frontotemporal dementia had significantly more behavior change than AD, t(22) = −3.773, P < .01, and PPA, t(18) = 2.569, P < .05.
Apathy Profile Comparison
Using previously published DAS subscale cutoff scores 14 to examine the frequency of impairment, 75.0% patients with bvFTD (n = 9) were impaired on emotional apathy, which was significantly higher, χ2 (2, n = 52) = 8.73, P < .05, when compared to 25.0% of patients with AD (n = 7) and 41.7% of patients with PPA (n = 5). There was no significant difference in frequency of impairment on executive apathy between bvFTD (83.3%, n = 10), PPA (41.7%, n = 5), and AD (50.0%, n = 14). There was no significant difference in frequency of impairment on initiation apathy between bvFTD (83.3%, n = 10), PPA (50.0%, n = 6), and AD (67.9%, n = 19). Subdividing the PPA group, the patient with SD, 50.0% of the patients with PNFA (n = 1), and 44.4% of the patients with lvPPA (n = 4) were impaired on initiation apathy. The SD patient, both patients with PNFA and 22.2% of the patients with lvPPA (n = 2) were impaired on emotional apathy. The patient with SD and 33.3% of the patients with lvPPA (n = 3) were impaired on executive apathy, with the patients with PNFA being unimpaired.
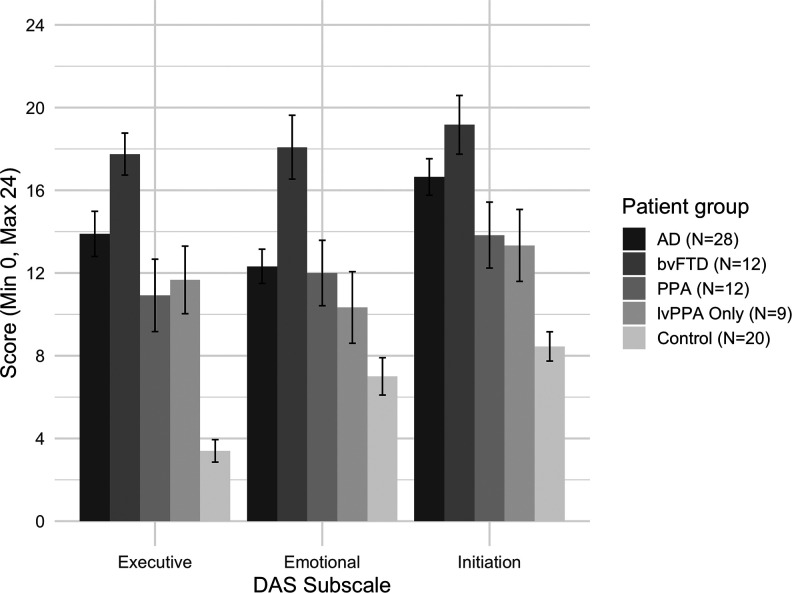
Figure 1 presents the comparison between patient groups (bvFTD vs PPA vs AD vs controls) on the informant/carer-rated DAS subscales. There was a significant main effect for group, F 3,68 = 33.357, P < .001, = 0.595, main effect of DAS subscale, F 2,136 = 11.548, P < .05, = 0.145, and significant group versus DAS subscale interaction, F 6,136 = 2.373, P < .05, = 0.095), showing overall differential apathy profile (DAS subscale scores) between and within patient groups. Intergroup post hoc tests showed that only patients with bvFTD had significantly higher emotional apathy than patients with AD, t(38) = −3.562, P < .01, d = 1.23, with no difference on executive (d = 0.74) and initiation (d = 0.53) apathy. Furthermore, patients with bvFTD had significantly higher apathy over all apathy subtypes when compared to patients with PPA, executive: t(22) = 3.375, P < .01, d = 1.23; emotional: t(22) = 2.752, P < .05, d = 1.02; initiation: t(22) = 2.499, P < .05, d = 1.02. There was no significant difference between patients with AD and PPA on DAS subscales (executive: d = 0.51; emotional: d = 0.07; initiation: d = 0.56). When compared to controls, global apathy over all subtypes was observed in patients with bvFTD, executive: t(30) = −13.640, p < .001, d = 4.98, emotional: t(30) = −6.650, P < .001, d = 2.43, initiation: t(30) = −7.523, P < .001, d = 2.74; in patients with PPA, executive: t(30) = −4.955, P < .001, d = 1.81, emotional: t(30) = −2.965, P < .05, d = 1.08, initiation: t(30) = −3.519, P < .01, d = 1.28; and patients with AD, executive: t(46) = −7.628, P < .001, d = 2.23, emotional: t(46) = −4.279, P < .001, d = 1.25, initiation: t(46) = −6.790, P < .001, d = 1.99.
Figure 1.
Apathy subtype profile (informant/carer ratings) for AD, bvFTD, PPA (including the lvPPA-only group), and controls. Higher score indicates higher apathy. Standard error bars shown. Note: The lvPPA-only group is a subsample from the PPA group. AD indicates Alzheimer disease; bvFTD, behavioral variant frontotemporal dementia; lvPPA, logopenic variant primary progressive aphasia; PPA, primary progressive aphasia.
Analysis using the lvPPA-only group (in place of the PPA group) showed similar pattern of apathy profile results, with a significant main effect for group, F 3,65 = 34.724, P < .001, = 0.616, main effect of DAS subscale, F 2,130 = 10.564, P < .05, = 0.140, and significant group versus DAS subscale interaction, F 6,136 = 2.771, P < .05, = 0.113, showing overall differential apathy profiles (DAS subscale scores) between and within patient groups. Post hoc tests showed that lvPPA only had significantly higher executive apathy than controls, t(30) = −6.130, P < .05, d = 2.46, with no differences on emotional (d = 0.76) and initiation apathy (d = 1.26). Patients with bvFTD had significantly higher apathy than lvPPA over all DAS subtypes, executive: t(19) = 3.319, P < .01, d = 1.46, emotional: t(19) = 3.325, P < .01, d = 1.46, initiation, t(19) = 2.622, P < .05, d = 1.16. There was no significant difference between patients with lvPPA and AD on DAS subscales (executive: d = 0.40; emotional: d = 0.43; initiation: d = 0.69). In terms of function, there were no significant correlations between any DAS subscales and the ECAS, ACE-III, or LIADL.
Apathy Subtype Awareness
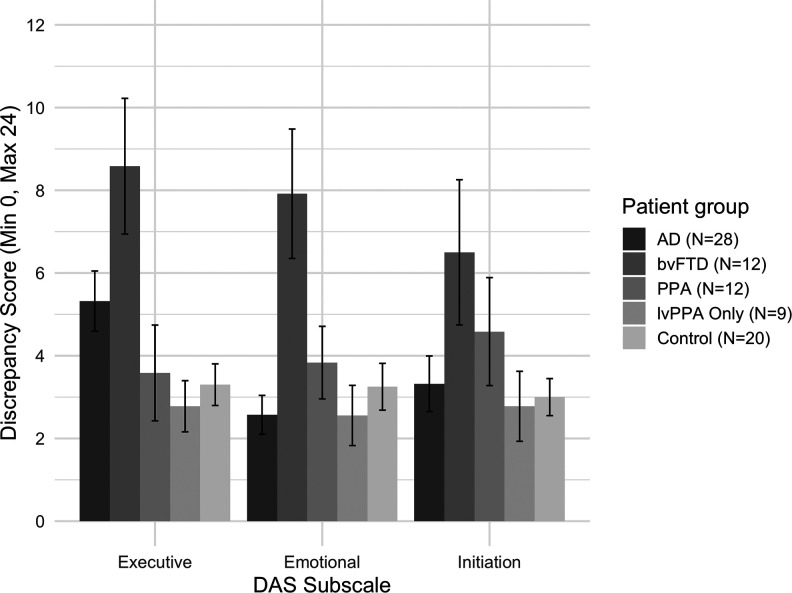
There was only a significant main effect for group, F 3,68 = 6.505, P < .01, = 0.223, showing an overall difference in the awareness discrepancy score between groups (see Figure 2). Intergroup post hoc tests showed that bvFTD were found to have significantly less awareness for emotional apathy when compared to patients with AD, t(38) = −4.315, P < .001, d = 1.49, and patients with PPA, t(22) = 2.277, P < .05, d = 0.93. There was no significant difference for initiation apathy awareness between bvFTD and AD (d = 0.72) or PPA (d = 0.36). Additionally, patients with bvFTD were observed to only have significantly less awareness of executive apathy when compared to patients with PPA, t(22) = 2.491, P < .05, d = 1.02. When compared to controls, only bvFTD had significantly less awareness over all apathy subtypes, executive: t(30) = −3.731, P < .01, d = 1.31, emotional: t(30) = −3.320, P < .01, d = 1.21; initiation: t(30) = −2.389, P < .05, d = 0.83. There was no significant difference between PPA and controls on apathy subtype awareness (executive: d = 1.81; emotional: d = 1.08; initiation: d = 1.29). There was no significant difference between AD and controls on apathy subtype awareness (executive: d = 0.62; emotional: d = 0.27; initiation: d = 0.11).
Figure 2.
Apathy subtype awareness profile (difference between self-ratings and informant/carer ratings) for AD, bvFTD, PPA (including the lvPPA-only group), and controls. Higher discrepancy score indicates less awareness. Standard error bars shown. Note: The lvPPA-only group is a subsample from the PPA group. AD indicates Alzheimer disease; bvFTD, behavioral variant frontotemporal dementia; lvPPA, logopenic variant primary progressive aphasia; PPA, primary progressive aphasia.
Analysis using the lvPPA-only group (in place of the PPA group) showed a main effect of group, F 3,65 = 8.356, P < .001, = 0.278, showing a between-group difference on DAS subscales. Post hoc tests showed that bvFTD had significantly less awareness compared to lvPPA for executive, t(19) = −2.934, P < .05, d = 1.29, and emotional, t(19) = −2.789, P < .05, d = 1.23, apathy, with no difference on initiation apathy (d = 0.76). There were no differences between lvPPA and AD on apathy subtype awareness (executive: d = 0.73; emotional: d = 0.01; initiation: d = 0.16). There were no significant differences between lvPPA and controls on apathy subtype awareness (executive: d = 0.24; emotional: d = 0.29; initiation: d = 0.10).
Discussion
The findings show that it is important to understand apathy profiles in different dementia subtypes. Apathy subtype profiles using the DAS can be used to differentiate bvFTD from PPA and AD. Specifically, emotional apathy (as indifference, emotional/affective neutrality, blunting or flatness) was the distinguishing apathy subtype for bvFTD compared to other dementias. Further, bvFTD showed less awareness of emotional apathy overall. In comparison to PPA, patients with bvFTD showed global apathy over all subtypes, additionally supplemented by less awareness of executive apathy (lack of motivation for planning, organizing, and attention) and less awareness of emotional apathy. While bvFTD showed most apathy overall, global apathy was observed in all dementia diagnosis, when compared to controls. All these results are further supported by a similar pattern of difference on the 1-dimensional apathy measure (AES), with bvFTD displaying the most apathy compared to other dementias (PPA and AD) and controls. This suggests that interdementia comparison using the DAS allows for breaking down components of apathy and may hold more value in identifying specific apathy subtype profiles.
With 75% of bvFTD patients displaying emotional apathy based on previously published cutoffs 14 , this showcases the prominence of this subtype relative to controls and other dementias. This is further supported by previous research using specific and nonspecific apathy subtype measures, showing these emotional apathy characteristics are key in bvFTD. 19,21 -23,26 Previous research using the LARS showed bvFTD displayed greater impairment of emotional apathy and self-awareness domains in comparison with AD. 23 Emotional apathy could indeed be said to overlap contextually with loss of sympathy and empathy, which is a defining feature of bvFTD. 28 Further, patients with bvFTD have been observed to have impairments in emotional recognition and social cognition. 39 -42 In bvFTD, empathy and social cognition deficits were associated with atrophy to orbitofrontal areas, medial prefrontal cortex, and amygdala. 43 -45 These areas overlap with the emotional-affective apathy subtype 10,11 which is akin to the emotional apathy subtype of the Dimensional Apathy Framework. 12 The cognitive-neuroanatomical-motivational overlap for emotional apathy could be explained by impairment in discrete processes of behavioral/emotional self-regulation, which mediate motivational, emotional, and social aspects of behaviour. 12,46 The high degree of conceptual overlap between empathy, social cognition, and emotional apathy points toward a need for further comprehensive examination of the mechanistic relationship between these factors.
Within dementia syndromes, lower awareness of emotional apathy may be distinguishing characteristic for bvFTD and that an additional lower executive apathy awareness differentiates bvFTD from PPA. This study overall reaffirms that awareness of apathy subtypes is a key factor for defining apathy subtype profiles for different dementias. Previous research has shown widespread loss of insight relative to other cognitive and behavioral symptoms, inclusive of emotional insight, 47 -49 which may be an extension of the emotional apathy reduction in self-awareness. As such, awareness of apathy could be used to diagnostically differentiate dementia syndromes, particularly bvFTD from AD and PPA, and clinicians could therefore work with families/caregivers to improve understanding of this. Through measuring this by the discrepancy between self-ratings and informant/carer ratings on DAS apathy subtypes, a more representative view of awareness and impairments associated with it can be produced. Our finding is supported by previous research showing bvFTD patient’s self-awareness deficit in combination with emotional apathy differed from patients with AD, albeit originally being assessed by individual questions rather than a discrepancy score. 23 As such self-awareness through individual questions may be paradoxical as answering questions about oneself implies a certain level of awareness. This is further compounded by apathy being associated with anosognosia, 50 further influencing self-ratings. Of note, there was no significant difference between dementia syndromes on initiation apathy awareness or in scores on the initiation apathy subscale. This could be accounted for by the lack of differentiation of dementia syndromes on the initiation apathy profile scores, which has been previously observed when comparing bvFTD and AD. 19 How apathy subtype awareness changes as disease progresses, and its interaction with cognitive functioning should be further explored, with an aim to understand the practical impact of these subtypes.
While this provides a foundation for apathy profile research in FTD, this study would merit larger scale replication. Additionally, while imaging biomarkers or cerebrospinal biomarkers were used to support diagnosis, there were no specific data available, which would be beneficial for understanding apathy profiles. Furthermore, while patients with PPA were observed to have global apathy relative to controls, there were no differences in comparison to AD. This could be accounted for by the majority of the PPA group being composed of lvPPA, which overlap with AD pathology. 51 Based on frequency of impairment on the DAS, lvPPA group had a mixed apathy profile, with a lower occurrence of emotional apathy, which could be accounted for by the lack of difference in relation to this subtype when compared to controls. The 1 patient with SD showed global apathy over all subtypes (executive, emotional, and initiation). Both the patients with PNFA showed emotional apathy (with one showing additional initiation apathy) and no executive apathy. However, due to small sample size of PPA group, future larger scale research should aim to elucidate apathy profiles of PNFA, SD, and lvPPA patient groups. Further, the lack of association between cognitive functioning, activities of daily living, and apathy (AES) is contraindicative of findings from previous research. 7,8 Previous research has found that certain deficits in emotional recognition are associated with emotional apathy, and deficits in intrinsic response generation are associated with initiation apathy in motor neuron disease. 52 Additional research should also explore the underlying cognitive processes and their association with particular apathy subtypes in dementia. Further, due to sample size constraints, it was not feasible to explore the impact of apathy subtypes on these practical variables. Future research should explore the practical elements of living with specific apathy profiles in various dementia syndromes to build on functional elements of the Dimensional Apathy Framework.
To conclude, while patients with bvFTD displayed the highest levels of apathy over all subtypes, Emotional apathy seems to be consistently characteristic in terms of bvFTD, when compared to AD, PPA, and controls. Further to this, supplementary decreased awareness for apathy subtypes were observed to be variable in dementia syndromes, with patients with bvFTD displaying less awareness of their emotional apathy and also less awareness of executive apathy (compared only to PPA). This shows the robust application of the Dimensional Apathy Framework within dementia for differentiating apathy subtype profiles. It supports the importance of routine evaluation to further clinical understanding of motivation in neurodegenerative disease. Future research should utilize the Dimensional Apathy Framework to explore neural, as well as cognitive and functional, correlates of apathy subtypes and their practical impact in dementia and other neurodegenerative diseases. This will help inform person-centered interventions through better profiling and therefore mediation or management of demotivational problems.
Supplemental Material
Supplemetary_Materials for Multidimensional Apathy in Behavioral Variant Frontotemporal Dementia, Primary Progressive Aphasia, and Alzheimer Disease by Ratko Radakovic, Shuna Colville, Denise Cranley, John M. Starr, Suvankar Pal and Sharon Abrahams in Journal of Geriatric Psychiatry and Neurology
Acknowledgments
The authors would like to thank all the participants and their families for taking part in the study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Anne Rowling Regenerative Neurology Clinic, Alzheimer Scotland Dementia Research Centre, University of Edinburgh, and Motor Neurone Disease Scotland.
ORCID iD: Ratko Radakovic  https://orcid.org/0000-0001-9425-3639
https://orcid.org/0000-0001-9425-3639
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Mendez MF, Lauterbach EC, Sampson SM. ANPA Committee on Research. An evidence-based review of the psychopathology of frontotemporal dementia: a report of the ANPA Committee on Research. J Neuropsychiatry Clin Neurosci. 2000;20(2);130–149. [DOI] [PubMed] [Google Scholar]
- 2. Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46(1):130–135. [DOI] [PubMed] [Google Scholar]
- 3. Marczinski CA, Davidson W, Kertesz A. A longitudinal study of behavior in frontotemporal dementia and primary progressive aphasia. Cogn Behav Neuro. 2004;17(4):185–190. [PubMed] [Google Scholar]
- 4. Banks SJ, Weintraub S. Neuropsychiatric symptoms in behavioral variant frontotemporal dementia and primary progressive aphasia. J Geriatr Psychiatry Neuro. 2008;21(2);133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaufer DI, Cummings JL, Christine D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the neuropsychiatric inventory caregiver distress scale. J Am Geriatr Soc. 1998;46(2):210–215. [DOI] [PubMed] [Google Scholar]
- 6. Appelhof B, Bakker C, Zwijsen SA, et al. The determinants of quality of life of nursing home residents with young-onset dementia and the differences between dementia subtypes. Dement Geriatr Cogn Disord. 2017;43(5-6):320–329. [DOI] [PubMed] [Google Scholar]
- 7. O’Connor CM, Romero RL, Clemson L, et al. Behavioral-variant frontotemporal dementia distinct phenotypes with unique functional profiles. Neurology. 2017;89(6):570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yassuda MS, da Silva TBL, O’Connor CM, et al. Apathy and functional disability in behavioral variant frontotemporal dementia. Neurol Clin Pract. 2018;8(2):120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robert PH, Onyike CU, Leentjens AFG, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98–104. [DOI] [PubMed] [Google Scholar]
- 10. Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex–basal ganglia circuits. Cerb Cortex. 2006;16(7):916–928. [DOI] [PubMed] [Google Scholar]
- 11. Levy R. Apathy: a pathology of goal-directed behaviour. A new concept of the clinic and pathophysiology of apathy. Rev Neurol (Paris). 2012;168(8):585–597. [DOI] [PubMed] [Google Scholar]
- 12. Radakovic R, Abrahams S. Multidimensional apathy: evidence from neurodegenerative disease. Curr Opin Behav Sci. 2018;22:42–49. [Google Scholar]
- 13. Radakovic R, Abrahams S. Developing a new apathy measurement scale: Dimensional Apathy Scale. Psychiatry Res. 2014;219(3):658–663. [DOI] [PubMed] [Google Scholar]
- 14. Radakovic R, Stephenson L, Colville S, Swingler R, Chandran S, Abrahams S. Multidimensional apathy in ALS: validation of the Dimensional Apathy Scale. J Neurol Neurosurg Psychiatry. 2016;87(6):663–669. [DOI] [PubMed] [Google Scholar]
- 15. Santangelo G, Siciliano M, Trojano L, et al. Apathy in amyotrophic lateral sclerosis: insights from Dimensional Apathy Scale. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(5-6):434–442. [DOI] [PubMed] [Google Scholar]
- 16. Radakovic R, Davenport R, Starr JM, Abrahams S. Apathy dimensions in Parkinson’s disease. Int J Geriatr Psychiatry. 2018;33(1):151–158. [DOI] [PubMed] [Google Scholar]
- 17. D’Iorio A, Vitale C, Piscopo F, et al. Impact of anxiety, apathy and reduced functional autonomy on perceived quality of life in Parkinson’s disease. Parkinsonism Relat Disord. 2017;43:114–117. [DOI] [PubMed] [Google Scholar]
- 18. Radakovic R, Starr JM, Abrahams S. A novel assessment and profiling of multidimensional apathy in Alzheimer’s disease. J Alzheimer’s Dis. 2017;60(1):57–67. [DOI] [PubMed] [Google Scholar]
- 19. Wei G, Irish M, Hodges JR, Piguet O, Kumfor F. Disease-specific profiles of apathy in Alzheimer’s disease and behavioural-variant frontotemporal dementia differ across the disease course. J Neurol. 2020;267(4):1086–1096. In press. [DOI] [PubMed] [Google Scholar]
- 20. Cummings JL, Mega M, Gray K, Thompson SR, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2308. [DOI] [PubMed] [Google Scholar]
- 21. Chow TW, Binns MA, Cummings JL, et al. Apathy symptom profile and behavioral associations in frontotemporal dementia vs dementia of Alzheimer type. Arch Neurol. 2009;66(7):888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quaranta D, Marra C, Rossi C, Gainotti G, Masullo C. Different apathy profile in behavioral variant of frontotemporal dementia and Alzheimer’s disease: a preliminary investigation. Curr Gerontol Geriatr Res. 2012;2012:719250. doi:10.1155/2012/719250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matarrubia MF, Matías Guiu JA, Cabrera Martín MN, et al. Different apathy clinical profile and neural correlates in behavioral variant frontotemporal dementia and Alzheimer’s disease. Int J Geriatr Psychiatry. 2018;33(1):141–150. [DOI] [PubMed] [Google Scholar]
- 24. Gélinas I, Gauthier L, McIntyre M. Development of a functional measure for persons with Alzheimer’s disease: the Disability Assessment for Dementia. Am J Occup Ther. 1999;53(5):471-481. [DOI] [PubMed] [Google Scholar]
- 25. Wear HJ, Wedderburn CJ, Mioshi E, et al. The Cambridge Behavioural Inventory Revised. Dementia Neuropsychologia. 2008;2(2):102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kumfor F, Zhen A, Hodges JR, Piguet O, Irish M. Apathy in Alzheimer’s disease and frontotemporal dementia: distinct clinical profiles and neural correlates. Cortex. 2018;103:350–359. [DOI] [PubMed] [Google Scholar]
- 27. Matarrubia MF, Matías Guiu JA, Ramos TM, et al. Validation of the lille’s apathy rating scale in very mild to moderate dementia. Am J Geriatr Psychiatry. 2016;24(7):517–527. [DOI] [PubMed] [Google Scholar]
- 28. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(9);2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gorno Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimers Dement. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38(2):143–162. [DOI] [PubMed] [Google Scholar]
- 32. Clarke D, van Reekum R, Simard M, Streiner D, Freedman M, Conn D. Apathy in dementia: an examination of the psychometric properties of the Apathy Evaluation Scale. J Neuropsychiatry Clin Neurosci. 2007;19(1):57–64. [DOI] [PubMed] [Google Scholar]
- 33. Brown LM, Schinka JA. Development and initial validation of a 15-item informant version of the Geriatric Depression Scale. Int J Geriatr Psychiatry. 2005;20(10):911–918. [DOI] [PubMed] [Google Scholar]
- 34. Wancata J, Alexandrowicz R, Marquart B, Weiss M, Friedrich F. The criterion validity of the Geriatric Depression Scale: a systematic review. Acta Psychiatr Scan. 2006;114(6):398–410. [DOI] [PubMed] [Google Scholar]
- 35. Lawton MP, Brody EM. Assessment of older people: self- maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 36. Hsieh S, Schubert S, Hoon C, Mioshi E, Hodges JR. Validation of the Addenbrooke’s Cognitive Examination III in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2013;36(3-4):242–250. [DOI] [PubMed] [Google Scholar]
- 37. Abrahams S, Newton J, Niven E, Foley J, Bak TH. Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(1-2):9–14. [DOI] [PubMed] [Google Scholar]
- 38. Core Team R. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 39. Henry JD, Phillips LH, Von Hippel C. A meta-analytic review of theory of mind difficulties in behavioural-variant frontotemporal dementia. Neuropsychologia. 2014;56:53–62. [DOI] [PubMed] [Google Scholar]
- 40. Bora E, Velakoulis D, Walterfang M. Meta-analysis of facial emotion recognition in behavioral variant frontotemporal dementia: comparison with Alzheimer disease and healthy controls. J Geriatr Psychiatry Neuro. 2016;29(4):205–211. [DOI] [PubMed] [Google Scholar]
- 41. Hutchings R, Palermo R, Piguet O, Kumfor F. Disrupted face processing in frontotemporal dementia: a review of the clinical and neuroanatomical evidence. Neuropsychol Rev. 2017;27(1):18–30. [DOI] [PubMed] [Google Scholar]
- 42. Van den Stock J, Kumfor F. Behavioural variant frontotemporal dementia: at the interface of interoception, emotion and social cognition? Cortex. 2019;115:335–340. [DOI] [PubMed] [Google Scholar]
- 43. Bertoux M, Volle E, Funkiewiez A, de Souza LC, Leclercq D, Dubois B. Social cognition and emotional assessment (SEA) is a marker of medial and orbital frontal functions: a voxel-based morphometry study in behavioral variant of frontotemporal degeneration. J Int Neuropsychol Soc. 2012;18(6):972–985. [DOI] [PubMed] [Google Scholar]
- 44. Baez S, Morales JP, Slachevsky A, et al. Orbitofrontal and limbic signatures of empathic concern and intentional harm in the behavioral variant frontotemporal dementia. Cortex. 2016;75:20–32. [DOI] [PubMed] [Google Scholar]
- 45. Dermody N, Wong S, Ahmed R, Piguet O, Hodges JR, Irish M. Uncovering the neural bases of cognitive and affective empathy deficits in Alzheimer’s disease and the behavioral-variant of frontotemporal dementia. J Alzheimer’s Dis. 2016;53(3):801–816. [DOI] [PubMed] [Google Scholar]
- 46. Stuss DT. Functions of the frontal lobes: relation to executive functions. J int neuropsychol Soc. 2011;17(5):759–765. [DOI] [PubMed] [Google Scholar]
- 47. Hornberger M, Yew B, Gilardoni S, et al. Ventromedial-frontopolar prefrontal cortex atrophy correlates with insight loss in frontotemporal dementia and Alzheimer’s disease. Hum Brain Mapp. 2014;35(2):616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosen HJ, Oscar A, Jessica Z, Arthur PS, John N, Bruce LM. Metacognition in the behavioral variant of frontotemporal dementia and Alzheimer’s disease. Neuropsychology. 2014;28(3):436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mendez MF, Shapira JS. Loss of emotional insight in behavioral variant frontotemporal dementia or “frontal anosodiaphoria”. Conscious cogn. 2011;20(4):1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Starkstein SE, Brockman S, Bruce D, Petracca G. Anosognosia is a significant predictor of apathy in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2010;22(4):378–383. [DOI] [PubMed] [Google Scholar]
- 51. Rohrer JD, Rossor MN, Warren JD. Alzheimer’s pathology in primary progressive aphasia. Neurobiol Aging. 2012;33(4):744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Radakovic R, Stephenson L, Newton J, et al. Multidimensional apathy and executive dysfunction in amyotrophic lateral sclerosis. Cortex. 2017;94:142–151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemetary_Materials for Multidimensional Apathy in Behavioral Variant Frontotemporal Dementia, Primary Progressive Aphasia, and Alzheimer Disease by Ratko Radakovic, Shuna Colville, Denise Cranley, John M. Starr, Suvankar Pal and Sharon Abrahams in Journal of Geriatric Psychiatry and Neurology