Abstract
Purpose
The status of human papillomavirus (HPV) infection in pregnant and non-pregnant women in China remains unclear. This study aimed to compare the prevalence and genotype distributions of HPV between pregnant and non-pregnant women in China.
Patients and Methods
A case-control study was conducted of pregnant women during the second trimester and age-matched non-pregnant women attending the Fujian Maternity and Child Health Hospital between January 1, 2017 and December 31, 2018. Participants underwent cervical cytology testing and HPV genotyping. The genotyping test was able to identify 14 high-risk HPV (HR-HPV), four possible HR-HPV, and five low-risk HPV (LR-HPV) types. Further colposcopy and a cervical biopsy were performed if indicated. The primary outcomes were HPV prevalence and genotype distribution.
Results
In total, 1077 pregnant and 1077 non-pregnant women were enrolled. Compared with non-pregnant women, pregnant women had a higher prevalence of HPV (24.2% vs 14.8%), HR-HPV (20.2% vs 11.7%), and LR-HPV (8% vs 4.5%) infection. In pregnant women, the most prevalent HPV genotypes were HPV-52 (6.0%), -16 (3.5%), -58 (2.6%), -53 (2.5%), and -51 (2.5%), while in non-pregnant women the most prevalent genotypes were HPV-52 (3.6%), -81 (1.9%), -51 (1.8%), -68 (1.4%), and -16 (1.3%). In women aged ≥35 years, HR-HPV (P=0.002) and LR-HPV (P=0.001) prevalence were significantly higher in pregnant women. However, in women aged <35 years, only HR-HPV prevalence was higher in pregnant women. Pregnant and non-pregnant women with HPV-16 and HPV-58 infection had a high prevalence of high-grade squamous intra-epithelial lesions (HSIL) (HPV-16: P<0.001 and P=0.005, HPV-58: P=0.043 and P=0.005); but with other HR-HPV genotypes, only non-pregnant women had an increased HSIL prevalence.
Conclusion
In China, the HPV prevalence is higher in pregnant women than that in non-pregnant women and is also age- and genotype-dependent. HPV-infected pregnant women aged ≥35 years and those with HPV-16 should be closely monitored to enable rapid clinical intervention.
Keywords: human papillomavirus, genotype, squamous intraepithelial lesions, cervical cytology, China, pregnant
Introduction
Cervical cancer is one of the three most frequently occurring cancers in women. There were approximately 570,000 new cases worldwide, with more than 310,000 deaths in 2018.1 In China, there are approximately 100,000 newly diagnosed cervical cancer cases per year, with age-standardized morbidity and mortality rates of 7.5 and 3.4 per 100,000 women per year, respectively.2
In recent years, most evidence suggests that the incidence and mortality of cervical cancer are increasing in young women.3 Cervical lesions are common in pregnant women; therefore, routine cervical cancer screening is very important in this specific population.4 However, it is difficult to screen and diagnose cervical lesions in pregnant women for two main reasons:1) Pregnancy changes the shape of the cervix, making it more difficult to collect specimens, and specimen collection requires extra staff;and 2) some women refuse to allow sample collection because of concern about the risk of miscarriage and premature delivery, particularly among women who have experienced vaginal bleeding during pregnancy.
The progression of cervical intraepithelial neoplasia grade 3 or worse (CIN3+) lesions requires persistent infection with high-risk human papillomavirus (HR-HPV) types.5 More than 200 different genotypes of HPV have been identified. Our previous research showed that HPV-16, -18, -58, -59, and -33 were the five most prevalent genotypes in cervical cancer and that HPV-16, -18, -59, -45, and -33 were the five highest risk genotypes for cervical cancer.6 Our previous research also showed that HPV-52 was the most prevalent genotype in younger women and HPV-16 was the most prevalent genotype in older women.6 The geographic distribution of HPV genotypes varies. HPV-16 and HPV-18 are the two most common cervical cancer-related genotypes in North America and Oceania.7 The most prevalent types in China in decreasing order are, HPV-16, -52, -58, -43, -18, -53, -33, and -51.6 Although few studies have revealed increased persistence of HPV infection in pregnant women, few studies have examined HPV infection prevalence in pregnant women.8–10
The factor facilitating higher HPV infection in pregnant women compared with non-pregnant women is based on the immunosuppressive role of hormones. High levels of steroid hormones inhibit the function of cell-mediated immunity, which is necessary for the clearance of HPV infection.11 Pregnancy is associated with mild immunosuppression and the response mediated by natural killer cells and type 1 helper T cells is reduced.12 Sillman and Sedlis13 found that immunosuppressed women have a higher incidence of cervical cancer, and HPV infection appears to be more prevalent in pregnant women.14 Few studies have compared the distribution of HPV genotypes between pregnant and non-pregnant women, and the sample sizes in such studies are small; thus knowledge of the distribution of HPV genotypes in pregnant women is limited. Therefore, there is need to determine the prevalence of HPV infection genotype distribution in pregnant women compared to non-pregnant women. Young women are the main population receiving cervical cancer vaccines. In China, 2-valent and 4-valent HPV vaccines are more widely used than the 9-valent vaccine, so the prevalence of HPV-16 and -18 infection may decrease and the prevalence of other HR-HPV types may increase in the future.It is thus very important to determine the prevalence of specific types of HPV among young women in China, and to compare the prevalence of specific types in pregnant and non-pregnant women.
It has been reported that 10–70% of dysplasia cases diagnosed during pregnancy regress spontaneously and sometimes disappear postpartum,15–17 while persistence in the severity of cervical neoplasia is reported in 25–47% of cases,15,16,18 and progression in 3–30% of cases. The purpose of screening during pregnancy is to find cervical cancer. Screening during pregnancy does not pose a threat to pregnancy and maternal and child outcomes.19 This study aimed to determine and compare the HPV prevalence and genotype distributions between pregnant and non-pregnant women who underwent HPV genotyping and cytological testing.
Materials and Methods
Study Population and Design
The study was authorized by the Ethics Committees of the Fujian Maternity and Child Health Hospital (2014–045) and was conducted in accordance with the Declaration of Helsinki. All ethical and professional considerations were followed throughout the study to maintain confidentiality of the participants data. The participants screened for participation in this study included 1345 pregnant women who underwent a routine pregnancy check-up, and another 11,252 non-pregnant women. The participants were recruited between January 1, 2017 and December 31, 2018.Among pregnant women, the inclusion criteria were: 1) confirmed pregnancy; 2) in the second trimester (gestational age 14–27 weeks) of pregnancy; and 3) provided informed consent and were willing to be followed-up. The exclusion criteria were: 1) history of a malignancy; 2) history of cervical lesions or cervical surgery; 3) severe autoimmune disease (such as systemic lupus erythematosus, rheumatoid arthritis and scleroderma); 4) pregnancy complications (such as preeclampsia, eclampsia, gestational diabetes mellitus); 5) gestational age of ≤13 or ≥28 weeks; and 6) refusal to sign informed consent and women who did not undergo cervical cytology and HPV testing. We collected a specimen of cervical exfoliated cells from each eligible patient attending the Fujian Maternity and Child Health Hospital for cervical cytology and HPV genotyping. “Pregnant women” refers to women in the second trimester (14–27 weeks), and “non-pregnant women” refers to age-matched non-pregnant women who were screened within 1 month of the matched pregnant woman, after excluding the unqualified population, we use random number table method for random sampling to avoid the influence of subjective factors. Those who were recruited into the “pregnant women” group were not eligible to be included in the “non-pregnant women” group subsequently.
We performed a sample size calculation according to two independent design data calculation formulas:
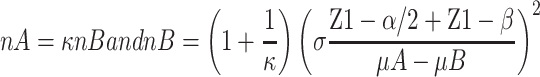 |
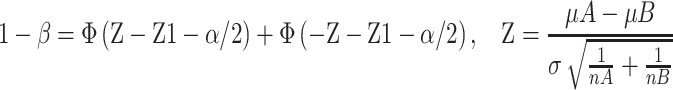 .
. 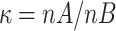 is the matching ratio, σ is standard deviation, Ф is the standard Normal distribution function. The result we got was that each group needed 762 cases at least. The sample size calculated was enough, and the power was 0.8.
is the matching ratio, σ is standard deviation, Ф is the standard Normal distribution function. The result we got was that each group needed 762 cases at least. The sample size calculated was enough, and the power was 0.8.
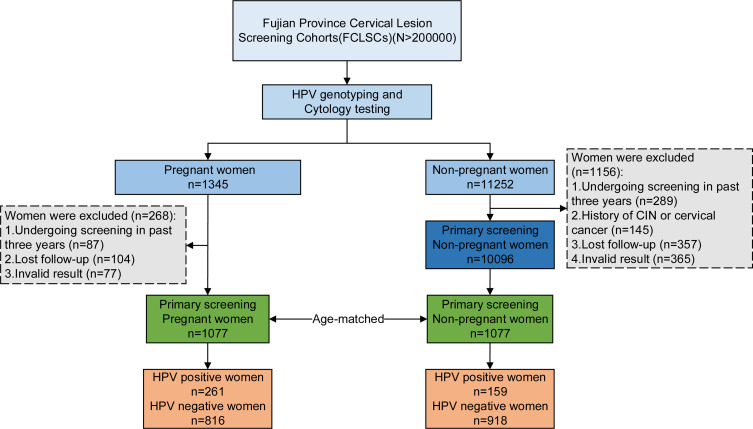
From both pregnant and non-pregnant women, experienced gynecologists collected a specimen of cervical exfoliated cells, which were tested for cytological abnormalities using liquid-based thin-layer cytology (TCT) and tested for HPV genotypes. The cytology testing and HPV genotyping were performed using the same sample. If the sample was HR-HPV-16/18 positive; other (non-16/18) HR-HPV positive with abnormal cervical cytology; or had HSIL/atypical glandular cells (AGC)/atypical squamous cells-cannot exclude HSIL (ASC-H) on cervical cytology, further colposcopy and cervical biopsy were performed. (Figure 1).
Figure 1.
Flow diagram of this study.
Abbreviation: CIN, cervical intraepithelial neoplasia.
Cytology
All the cervical specimens were examined by two experienced cytopathologists, who were blinded and who evaluated the results independently. In case of different results, the original cytological samples were re-read by a third pathologist until a consistent diagnosis was obtained.
HPV Genotyping
The isolated and extracted DNA was amplified in a 24 µL reaction system with the L1 shared HPV PGMY09/PGMY11 primer, and 5 µL of the extracted HPV DNA was used as control. The amplified products were tested by hybridization and reverse dot blot (RDB) on a nylon membrane fixed with 23 genotype probes. PCR-RDB (Yaneng Biotech, Shenzhen, China) assay was used to identify 23 genotypes, including 14 HR-HPV genotypes (HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, and -68), 4 possible HR-HPV genotypes (-53, -66,-73, -82, and -83) and five low-risk (LR)-HPV genotypes (HPV-6, -11, -42, -43, and -81).
Histology
Further colposcopy and biopsy were performed according to the 2012/2015 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors, in the following three situations: 1) HR-HPV16/18 positive; 2) other HR-HPV types (non-16/18) positive and abnormal cervical cytology; 3) HSIL/AGC/ASC-H in cervical cytology. When the diagnosis showed HSIL or worse, then the affected pregnant women did not underwent a loop electrosurgical excision procedure cone biopsy or conization by a cold knife during pregnancy unless cervical invasive cancer is diagnosed. Specimens were fixed with 10% formalin and embedded in paraffin routinely. Then, hematoxylin and eosin stain was used to dye the 4-μm-thick histological sections by the standard method. The Bethesda System20 was used to classify and examine cervical biopsy specimens.
Statistical Analysis
IBM SPSS Statistics for Windows, version 24.0 (IBM, Corp., Armonk, NY, USA) was used for the data analysis.The chi-square test and Fisher’s exact test were used to compare the results between the two groups.The mean and standard deviation were calculated in the categorical variables. The values and percentages were also accessed.Two-sided P values <0.05 were regarded as statistically significant.
Results
This study included 1077 pregnant and 1077 non-pregnant women (mean age, 30.0 ± 4.25 years for each group). This study excluding 268 pregnant women and 1156 non-pregnant women who did not meet the inclusion criteria. There were no statistically significant differences between the groups in age, age at first sexual intercourse, age at marriage, smoking history, drinking history, and degree of education (Table 1). There were statistically significant differences between the groups with respect to the number of pregnancies and the cytology results (both P<0.001, Table 1). A total of 23 HPV genotypes (14 HR-HPV, four possible HR-HPV and five LR-HPV genotypes) were detected (Table 2). In the pregnant group, the overall HPV prevalence was 24.2% (261/1077), while the prevalence of single HPV types, multiple HPV types, HR-HPV, and LR-HPV were 16.2% (174/1077); 8.1% (87/1077); 20.2% (218/1077); and 8.0% (86/1077), respectively. In the non-pregnant group, the overall HPV prevalence was 14.8% (159/1077), while the prevalence of single HPV type, multiple HPV types, HR-HPV, and LR-HPV were 11.6% (125/1077); 3.2% (34/1077); 11.7% (126/1077); and 4.5% (49/1077), respectively (Table 2).In the comparisons of HPV prevalence in pregnant and non-pregnant women, the differences in the overall HPV prevalence, the prevalence of single-type, multiple types, HR-HPV, and LR-HPV were statistically significant. (P<0.001, P=0.002, P<0.001, P<0.001, P=0.001, respectively).
Table 1.
Characteristics for Women with Pregnant or Non-Pregnant (N=2154)
| Variables | Pregnant Women (n=1077) | Non-Pregnant Women (n=1077) | P value |
|---|---|---|---|
| Age (mean ± SD) | 30.0±4.25 | 30.0±4.25 | 1.000 |
| Age of first sexual intercourse (mean ± SD) | 17.0±5.17 | 17.1±6.03 | 0.255 |
| Age of marriage (mean ± SD) | 26.29±2.94 | 26.12±2.79 | 0.174 |
| Pregnancy Times | |||
| ≤2 | 782 | 961 | <0.001 |
| >2 | 295 | 116 | |
| Smoking history | |||
| Yes-At least once a week | 15 | 22 | 0.246 |
| No | 1062 | 1055 | |
| Drinking history | |||
| Yes--At least once a week | 109 | 128 | 0.191 |
| No | 968 | 949 | |
| Degree of education | |||
| <Higher education | 280 | 273 | 0.730 |
| ≥Higher education | 797 | 804 | |
| Cytology | |||
| Normal | 822 | 1015 | <0.001 |
| Abnormal | 255 | 62 |
Abbreviation: SD, standard deviation.
Table 2.
Prevalence of Different HPV Genotypes in Pregnant and Non-Pregnant Women (N=2154)
| Variate | Pregnant Women (n=1077) | Non-Pregnant Women (n=1077) | P value |
|---|---|---|---|
| HPV | 24.2% (261/1077) | 14.8% (159/1077) | <0.001 |
| Single-type infectiona | 16.2% (174/1077) | 11.6% (125/1077) | 0.002 |
| Multi-type infectionsb | 8.1% (87/1077) | 3.2% (34/1077) | <0.001 |
| HR-HPV | 20.2% (218/1077) | 11.7% (126/1077) | <0.001 |
| HPV16 | 3.5% (38/1077) | 1.3% (14/1077) | 0.001 |
| HPV18 | 1.9% (21/1077) | 0.6% (6/1077) | 0.004 |
| HPV31 | 0.6% (6/1077) | 0.5% (5/1077) | 0.762 |
| HPV33 | 1.2% (13/1077) | 0.5% (5/1077) | 0.058 |
| HPV35 | 0.3% (3/1077) | 0.1% (1/1077) | 0.617 |
| HPV39 | 1.0% (11/1077) | 0.9% (10/1077) | 0.826 |
| HPV45 | 0.3% (3/1077) | 0.1% (1/1077) | 0.617 |
| HPV51 | 2.5% (27/1077) | 1.8% (19/1077) | 0.233 |
| HPV52 | 6.0% (65/1077) | 3.6% (39/1077) | 0.009 |
| HPV56 | 1.2% (13/1077) | 0.7% (8/1077) | 0.273 |
| HPV58 | 2.6% (28/1077) | 1.3% (14/1077) | 0.029 |
| HPV59 | 1.4% (15/1077) | 0.8% (9/1077) | 0.218 |
| HPV66 | 1.3% (14/1077) | 0.6% (6/1077) | 0.072 |
| HPV68 | 2.1% (23/1077) | 1.4% (15/1077) | 0.190 |
| LR-HPV | 8.0% (86/1077) | 4.5% (49/1077) | 0.001 |
| HPV6 | 1.1% (12/1077) | 0.2% (2/1077) | 0.007 |
| HPV11 | 0.8% (9/1077) | 0.2% (2/1077) | 0.034 |
| HPV42 | 1.5% (16/1077) | 0.6% (7/1077) | 0.059 |
| HPV43 | 0.9% (10/1077) | 0.4% (4/1077) | 0.108 |
| HPV53 | 2.5% (27/1077) | 1.1% (12/1077) | 0.015 |
| HPV73 | 0.2% (2/1077) | 0.3% (3/1077) | 0.999 |
| HPV81 | 1.6% (17/1077) | 1.9% (20/1077) | 0.619 |
| HPV82 | 0.1% (1/1077) | 0.0% (0/1077) | 0.999 |
| HPV83 | 0.1% (1/1077) | 0.3% (3/1077) | 0.617 |
Notes: aSuffer from a single human papillomavirus genotype infection; bsuffer from at least two or more genotypes of human papillomavirus infection.
Abbreviations: HPV, human papillomavirus, including types HPV-6, -11, -16, -18, -31, -33, -35, -39, -42, -43, -45, -51, -52, -53, -56, -58, -59, -66, -68, -73, -81, -82, -83; HR-HPV, high-risk human papillomavirus, including types HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, -66, -68; LR-HPV, low-risk human papillomavirus, including types HPV-6, -11, -42, -43, -53, -73, -81, -82, -83.
In HPV-infected pregnant women, the three most prevalent genotypes, HPV-52, -16, and -58, accounted for 6.0% (65/1077), 3.5% (38/1077), and 2.6% (28/1077) of all positive cases (including co-infection), respectively.In the HPV-infected non-pregnant women, the three most prevalent genotypes, HPV-52, -81, and -51, accounted for 3.6% (39/1077), 1.9% (20/1077), and 1.8% (19/1077) of all positive cases (including co-infection), respectively (Table 2). The differences in prevalence of HPV genotypes HPV-16, -18, -52, -58, -6, -11, and -53 between pregnant and non-pregnant women were statistically significant.
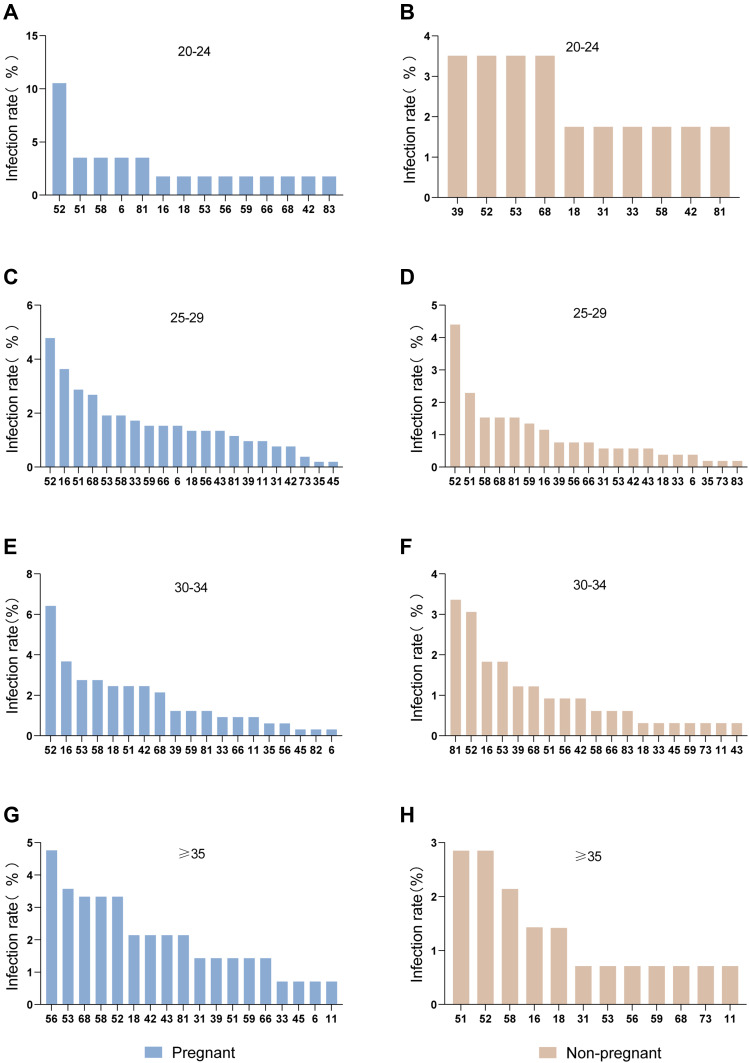
In the pregnant women, the most prevalent HPV genotype was HPV-56 in those aged ≥35 years, and HPV-52 in those aged <35 years. In the non-pregnant women, the most prevalent HPV genotype varied by age (Figure 2). In women aged ≥35 years, the differences in the overall HPV prevalence, and the prevalence of single-type, multiple types, HR-HPV, LR-HPV between pregnant and non-pregnant women were statistically significant. In women aged 30–34 years, the overall HPV prevalence and the prevalence of single-type, multiple types, and HR-HPV infection rate of pregnant women were significantly higher than in non-pregnant women. In women aged 25–29 years, the overall HPV prevalence and the prevalence of multiple types, HR-HPV, and LR-HPV were significantly higher in pregnant than in non-pregnant women. In women aged <25 years, the HPV prevalence did not differ significantly by pregnancy status (Table 3).
Figure 2.
Age-related distribution of HPV infection in pregnant and non-pregnant women in China.
Notes: HPV genotypes infection rates of age groups in (A) 20–24, (C) 25–29, (E) 30–34, (G) ≥35 in pregnant women; HPV genotypes infection rates of age groups in (B) 20–24, (D) 25–29, (F) 30–34, (H) ≥35 in non-pregnant women.
Abbreviation: HPV, human papillomavirus.
Table 3.
Prevalence of HPV in Pregnant and Non-Pregnant Women After Age Stratification. (N=2154)
| Age | Pregnant Women (n=1077) | Non-Pregnant Women (n=1077) | P value |
|---|---|---|---|
| <25 years, n=57 | |||
| HPV | 28.1% (16/57) | 14.0% (8/57) | 0.066 |
| HR-HPV | 21.1% (12/57) | 12.3% (7/57) | 0.209 |
| LR-HPV | 10.5% (6/57) | 7.0% (4/57) | 0.508 |
| Single-type infectiona | 21.1% (12/57) | 5.3% (3/57) | 0.013 |
| Multi-type infectionsb | 10.5% (6/57) | 8.8% (5/57) | 0.751 |
| 25–29 year, n=523 | |||
| HPV | 21.6% (113/523) | 16.1% (84/523) | 0.022 |
| HR-HPV | 18.7% (98/523) | 13.4% (70/523) | 0.018 |
| LR-HPV | 7.1% (37/523) | 3.6 (19/523) | 0.013 |
| Single-type infectiona | 12.2% (64/523) | 12.8% (67/523) | 0.779 |
| Multi-type infectionsb | 7.1% (37/523) | 3.3% (17/523) | 0.005 |
| 30–34 years, n=327 | |||
| HPV | 24.7% (81/327) | 14.4% (47/327) | 0.001 |
| HR-HPV | 20.8% (68/327) | 9.5% (31/327) | <0.001 |
| LR-HPV | 7.6% (25/327) | 7.0% (23/327) | 0.764 |
| Single-type infectiona | 18.0% (59/327) | 11.3% (37/327) | 0.015 |
| Multi-type infectionsb | 6.7% (22/327) | 3.1% (10/327) | 0.030 |
| ≥35 years, n=170 | |||
| HPV | 30.0% (51/170) | 11.8% (20/170) | <0.001 |
| HR-HPV | 23.5% (40/170) | 10.6% (18/170) | 0.002 |
| LR-HPV | 10.6% (18/170) | 1.8% (3/170) | 0.001 |
| Single-type infectiona | 22.9% (39/170) | 10.6% (18/170) | 0.002 |
| Multi-type infectionsb | 7.1% (12/170) | 1.2% (2/170) | 0.006 |
Notes: aSuffer from a single human papillomavirus genotype infection; bsuffer from at least two or more genotypes of human papillomavirus infection.
Abbreviations: HPV, human papillomavirus, including types HPV-6, -11, -16, -18, -31, -33, -35, -39, -42, -43, -45, -51, -52, -53, -56, -58, -59, -66, -68, -73, -81, -82, -83; HR-HPV, high-risk human papillomavirus, including types HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, -66, -68; LR-HPV, low-risk human papillomavirus, including types HPV-6, -11, -42, -43, -53, -73, -81, -82, -83.
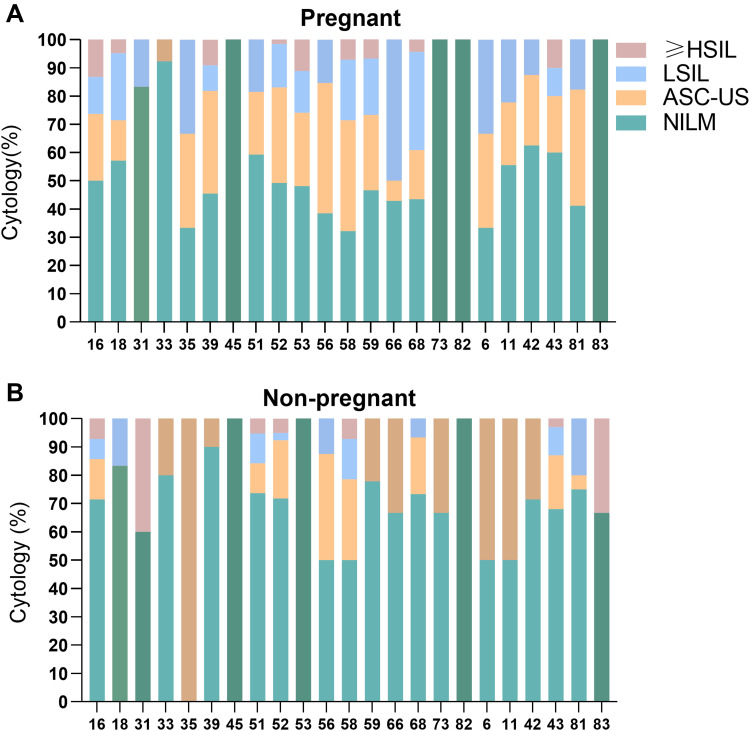
Among pregnant women, those with HSIL or worse on cervical cytology had a higher prevalence of HPV-16 and -18 than those with lower grade lesions. Among non-pregnant women, those with HSIL or worse on cervical cytology had a higher prevalence of HPV-16, -18, -31, -33 -52 and -58 than those with lower grade lesions (Table 4). In pregnant women infected with HPV-16, -53 and -43, the prevalence of HSIL or worse was 13.16%, 11.11%, and 10%, respectively. In non-pregnant women infected with HPV-31, -83, -58 and -16, the prevalence of HSIL or worse was 40%, 33.33%, 7.14%, 7.14%, respectively (Figure 3).
Table 4.
Distribution of Different HR-HPV Genotypes According to Cytological Diagnosis in Women with Pregnant or Non-Pregnant. (N=2154)
| Variate | Pregnant Women (n=1077) | Non-Pregnant Women (n=1077) | ||||
|---|---|---|---|---|---|---|
| NILM/ASC-US/LSIL (n=1064) | ≥HSILa (n=13) | P value | NILM/ASC-US/LSIL (n=1068) | ≥HSILa (n=9) | P value | |
| HPV | 23.5% (250) | 84.6% (11) | <0.001 | 14.2% (152) | 77.8% (7) | <0.001 |
| Single-type infectionb | 15.7% (167) | 53.8% (7) | <0.001 | 11.4% (121) | 44.4% (4) | 0.010 |
| Multi-type infectionsc | 7.8% (83) | 30.8% (4) | 0.012 | 3.0% (32) | 22.2% (2) | 0.030 |
| HR-HPV | 19.5% (207) | 84.6% (11) | <0.001 | 11.1% (119) | 77.8% (7) | <0.001 |
| HPV16 | 3.1% (33) | 38.5% (5) | <0.001 | 1.2% (12) | 22.2% (2) | 0.005 |
| HPV18 | 1.9% (20) | 7.7% (1) | 0.227 | 0.6% (5) | 11.1% (1) | 0.049 |
| HPV31 | 0.5% (5) | 7.7% (1) | 0.070 | 0.3% (3) | 22.2% (2) | 0.001 |
| HPV33 | 1.1% (12) | 7.7% (1) | 0.147 | 0.4% (4) | 11.1% (1) | 0.041 |
| HPV52 | 6.0% (64) | 7.7% (1) | 0.557 | 3.5% (37) | 22.2% (2) | 0.039 |
| HPV58 | 2.4% (26) | 15.4% (2) | 0.043 | 1.2% (12) | 22.2% (2) | 0.005 |
| Other Typesd | 9.4% (100) | 23.1% (3) | 0.233 | 6.4% (68) | 11.1% (1) | 0.450 |
Notes: aHigh grade squamous intraepithelial lesion or worse; bsuffer from a single human papillomavirus genotype infection; csuffer from at least two or more genotypes of human papillomavirus infection; dincluding types HPV-35, -39, -45, -51, -56, -59, -66, -68.
Abbreviations: HR-HPV, high-risk human papillomavirus, including types HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, -66, -68; NILM/ASC-US/LSIL, negative for intraepithelial lesion or malignancy/atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesions; ≥HSIL, high grade squamous intraepithelial lesion or worse.
Figure 3.
Cervical cytologic pathology in pregnant and non-pregnant women with different genotypes of HPV infection.
Notes: (A) Cervical cytologic pathology in pregnant women with different genotypes; (B) cervical cytologic pathology in non-pregnant women with different genotypes.
Abbreviations: NILM, intraepithelial lesion or malignancy; LSIL, low-grade squamous intraepithelial lesion; ASC-US, atypical squamous cells of undetermined significance; ≥HSIL, high grade squamous intraepithelial lesion or worse.
Discussion
Our study revealed that there were statistically significant differences between pregnant and non-pregnant women regarding the overall, single-type, multiple type, HR-HPV, and LR-HPV prevalence. The five most prevalent HPV genotypes among pregnant women were HPV-52, -16, -58, -53, and -51, while the five most prevalent HPV genotypes among non-pregnant women were HPV-52, -81, -51, -68, and -16 in China. Furthermore, HPV-16 had the second highest prevalence in pregnant women, and the fifth highest prevalence in non-pregnant women. In addition to being associated with an increased risk of cervical cancer, HPV-16 may be associated with adverse pregnancy outcomes,21 which underscores the importance of monitoring of HPV infection in pregnant women.
Our previous research revealed the distribution of HPV genotypes among women in China.6 The most prevalent HPV genotypes, in order of decreasing frequency, were HPV-16, -52, -58, -43, -18, -53, -33, and -51. In women with NILM or LSIL, the most prevalent genotype was HPV-52. The results of this study confirm the universal contribution of the eight most common HPV types (-16, -18, -31, -33, -35, -45, -52, and -58) to invasive cervical cancer and the predominant role of types 16, -18, and -45 in cervical adenocarcinoma.7 The incidence of HSIL increases with age. In younger women, the most prevalent HPV genotype was HPV-52, while in older women, the most prevalent genotype was HPV-16, which is the genotype with the highest cancer risk.6 Little is known about the relative distribution of HPV genotypes among pregnant and non-pregnant women in China; while the results of studies conducted in other countries are inconsistent.9,10,14,22
Previous studies had shown an increasing risk of HSIL with increasing age.6,23 This study found statistically significant differences in the prevalence of HPV infection according to pregnancy status in women aged ≥35 years. This result may be related to increasing susceptibility to infection with increasing age. In unscreened populations, the peak risk of invasive cervical cancer occurs earlier than for most other adult cancers, peaking or reaching a plateau between 35 and 55 years of age.24 The prevalence of HPV infection in this study reflects this trend. Notably, among women aged 25–29 years, the prevalence of HPV did differ significantly according to pregnancy status, except with single-type HPV infection; among women aged 30–34 years the prevalence of HPV infection did differ significantly according to pregnancy status except with LR-HPV genotypes; among women aged ≥35 years the prevalence of HR-HPV and LR-HPV genotypes differed significantly according to pregnancy status; while among woman aged <35 years, the prevalence of HR-HPV infection differed significantly by pregnancy status. A previous meta-analysis revealed that pregnant women younger than 25 years were more likely to contract HPV.25 The tendency of differences in HR-HPV prevalence to be greater than differences in LR-HPV prevalence according to pregnancy status gradually became less marked after 38 years of age.26 In the pregnant women, the genotype with the highest prevalence in those aged ≥35 years was HPV-56, whereas, in the other age groups, HPV-52 was the most prevalent genotype. In the non-pregnant women, HPV-52 was the first or second most prevalent genotype in every age group. Among all the patients with abnormal cytology, the most frequent HPV genotype was HPV-16, as was reported previously.27 Additionally, in patients infected with HPV-16, the majority of lesions were ≥HSIL.
Limitations
Our research still have some limitations: it was not a prospective study and we did not collect HPV data on pregnant women before pregnancy, so some pregnant women may have acquired the HPV infection before pregnancy. Most of the participants were from southern China, and so they may not be representative of the entire population of women in China.
Conclusion
HPV infection rates in pregnant and non-pregnant women showed statistically significant differences, which were age- and genotype-dependent. Pregnant women need to be closely monitored for HPV infection, especially those aged ≥35 years or HPV16 positive, so that prompt clinical interventions can be undertaken postpartum unless there is a suspicion of malignancy that necessitates earlier intervention. Further studies with larger sample sizes are needed to compare HPV prevalence between pregnant and non-pregnant women aged <25 years, and to explore the pattern of HPV infections during the gestational period.
Acknowledgments
We thank all the people who were included in this study and the staff who provided assistance for this study.
Funding Statement
This study was funded by the Fujian Provincial Natural Science Foundation of China (grant no. 2017J01232); Fujian Maternity and Child Health Hospital Natural Science Foundation (grant no. YCXM18–18); Fujian Provincial Health and Education Joint Project (grant no. 2019-WJ-05) and Fujian Provincial Maternity and Child Health Hospital Fund (grant no. 13-27); Fujian Provincial Health Commission Innovation Project (grant no. 2019-CX-7).
Abbreviations
ASC-US, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; HR-HPV, high-risk human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LEEP, loop electrosurgical excision procedure; LR-HPV, low-risk HPV; LSIL, low-grade squamous intraepithelial lesion; NILM, intraepithelial lesion or malignancy; PCR-RDB, polymerase chain reaction - reverse dot blot; TCT, tested liquid-based thin-layer cytology; AGC, atypical glandular cells; ASC-H, atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesion.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Erratum: global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;70(4):313. doi: 10.3322/caac.21609 [DOI] [PubMed] [Google Scholar]
- 2.Pan XF, Zhao ZM, Sun J, et al. Acceptability and correlates of primary and secondary prevention of cervical cancer among medical students in southwest China: implications for cancer education. PLoS One. 2014;9(10):e110353. doi: 10.1371/journal.pone.0110353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castanon A, Leung VM, Landy R, et al. Characteristics and screening history of women diagnosed with cervical cancer aged 20–29 years. Br J Cancer. 2013;109(1):35–41. doi: 10.1038/bjc.2013.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benoit L, Mir O, Vialard F, Berveiller P. Cancer during pregnancy: a review of preclinical and clinical transplacental transfer of anticancer agents. Cancers (Basel). 2021;13(6):1238. doi: 10.3390/cancers13061238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anhang R, Goodman A, Goldie SJ. HPV communication: review of existing research and recommendations for patient education. CA Cancer J Clin. 2004;54(5):248–259. doi: 10.3322/canjclin.54.5.248 [DOI] [PubMed] [Google Scholar]
- 6.Sun P, Song Y, Ruan G, et al. Clinical validation of the PCR-reverse dot blot human papillomavirus genotyping test in cervical lesions from Chinese women in the Fujian province: a hospital-based population study. J Gynecol Oncol. 2017;28(5):e50. doi: 10.3802/jgo.2017.28.e50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Sanjose S, Quint WG, Alemany L, et al.; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–1056. doi: 10.1016/S1470-2045(10)70230-8 [DOI] [PubMed] [Google Scholar]
- 8.Hernández-Girón C, Smith JS, Lorincz A, Lazcano E, Hernández-Avila M, Salmerón J. High-risk human papillomavirus detection and related risk factors among pregnant and nonpregnant women in Mexico. Sex Transm Dis. 2005;32(10):613–618. doi: 10.1097/01.olq.0000179888.47309.db [DOI] [PubMed] [Google Scholar]
- 9.Castellsagué X, Drudis T, Cañadas MP, et al. Human Papillomavirus (HPV) infection in pregnant women and mother-to-child transmission of genital HPV genotypes: a prospective study in Spain. BMC Infect Dis. 2009;9:74. doi: 10.1186/1471-2334-9-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fife KH, Katz BP, Brizendine EJ, Brown DR. Cervical human papillomavirus deoxyribonucleic acid persists throughout pregnancy and decreases in the postpartum period. Am J Obstet Gynecol. 1999;180(5):1110–1114. doi: 10.1016/S0002-9378(99)70602-2 [DOI] [PubMed] [Google Scholar]
- 11.Banura C, Franceschi S, van Doorn LJ, et al. Prevalence, incidence and clearance of human papillomavirus infection among young primiparous pregnant women in Kampala, Uganda. Int J Cancer. 2008;123(9):2180–2187. doi: 10.1002/ijc.23762 [DOI] [PubMed] [Google Scholar]
- 12.Weinberg ED. Pregnancy-associated depression of cellmediated immunity. Rev Infect Dis. 1984;6:814–831. doi: 10.1093/clinids/6.6.814 [DOI] [PubMed] [Google Scholar]
- 13.Sillman F, Sedlis A. Anogenital papillomavirus infection and neoplasia in immunodeficient women. Obstet Gynecol Clin North Am. 1987;14:537. doi: 10.1016/S0889-8545(21)00070-X [DOI] [PubMed] [Google Scholar]
- 14.Nobbenhuis MA, Helmerhorst TJ, van den Brule AJ, et al. High-risk human papillomavirus clearance in pregnant women: trends for lower clearance during pregnancy with a catch-up postpartum. Br J Cancer. 2002;87(1):75–80. doi: 10.1038/sj.bjc.6600367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palle C, Bangsboll S, Andreasson B. Cervical intraepithelial neoplasia in pregnancy. Acta Obstet Gynecol Scand. 2000;79:306–310. [PubMed] [Google Scholar]
- 16.Vlahos G, Rodolakis A, Diakomanolis E, et al. Conservative management of cervical intraepithelial neoplasia (CIN (2-3)) in pregnant women. Gynecol Obstet Invest. 2002;54(2):78–81. doi: 10.1159/000067715 [DOI] [PubMed] [Google Scholar]
- 17.Coppola A, Sorosky J, Casper R, Anderson B, Buller RE. The clinical course of cervical carcinoma in situ diagnosed during pregnancy. Gynecol Oncol. 1997;67:162–165. doi: 10.1006/gyno.1997.4856 [DOI] [PubMed] [Google Scholar]
- 18.Yost NP, Santoso JT, Mcintire DD, Iliya FA. Postpartum regression rates of antepartum cervical intraepithelial neoplasia II and III lesions. Obstet Gynecol. 1999;93:359–362. doi: 10.1016/s0029-7844(98)00483-9 [DOI] [PubMed] [Google Scholar]
- 19.Van Calsteren K, Vergote I, Amant F. Cervical neoplasia during pregnancy: diagnosis, management and prognosis. Best Pract Res Clin Obstet Gynaecol. 2005;19:611–630. doi: 10.1016/j.bpobgyn.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 20.Massad LS, Collins YC, Meyer PM. Biopsy correlates of abnormal cervical cytology classified using the Bethesda system. Gynecol Oncol. 2001;82(3):516~522. doi: 10.1006/gyno.2001.6323 [DOI] [PubMed] [Google Scholar]
- 21.Bosch FX, Burchell AN, Schiffman M, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26(Suppl 10):K1–16. doi: 10.1016/j.vaccine.2008.05.064 [DOI] [PubMed] [Google Scholar]
- 22.Niyibizi J, Zanré N, Mayrand MH, Trottier H. Association between maternal human papillomavirus infection and adverse pregnancy outcomes: systematic Review and Meta-Analysis. J Infect Dis. 2020;221(12):1925–1937. doi: 10.1093/infdis/jiaa054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0 [DOI] [PubMed] [Google Scholar]
- 24.Gustafsson L, Pontén J, Zack M, Adami HO. International incidence rates of invasive cervical cancer after introduction of cytological screening. Cancer Causes Control. 1997;8(5):755–763. doi: 10.1023/A:1018435522475 [DOI] [PubMed] [Google Scholar]
- 25.Liu P, Xu L, Sun Y, Wang Z. The prevalence and risk of human papillomavirus infection in pregnant women. Epidemiol Infect. 2014;142(8):1567–1578. doi: 10.1017/S0950268814000636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nygård M, Hansen BT, Kjaer SK, et al. Human papillomavirus genotype-specific risks for cervical intraepithelial lesions. Hum Vaccin Immunother. 2021;17(4):972–981. doi: 10.1080/21645515.2020.1814097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1157–1164. doi: 10.1158/1055-9965.EPI-04-0812 [DOI] [PubMed] [Google Scholar]