Abstract
In recent years, a rising number of studies have confirmed that microRNA (miRNA) plays a prominent role in the early diagnosis and prognostic value assessment of cardiovascular diseases. The current study was conducted to examine the expression of miR-675-3p in atherosclerosis (AS) patients and to evaluate its clinical diagnosis and prognostic value. 110 AS patients and 70 healthy controls were included in the study. Serum miR-675-3p levels were detected by quantitative real-time PCR (qRT-PCR). The clinical diagnostic significance of serum miR-675-3p in AS patients were investigated by the receiver operating characteristic (ROC) curve. The correlation between miRNA and carotid intima-media thickness (CIMT) was analyzed by the Spearman correlation coefficient. The prognostic significance of serum miR-675-3p was evaluated by the Kaplan-Meier method and Cox regression analysis. The patient’s serum miR-675-3p was significantly increased than the healthy individuals (P < 0.05). An increase of carotid intima-media thickness (CIMT) was positively correlated with the promotion of serum miR-675-3p levels. The area under the ROC curve (AUC) was 0.918, with high sensitivity and specificity. miR-675-3p is a key independent predictor of cardiovascular adverse events in AS patients (HR = 5.375, 95%CI = 1.590-18.170, P = 0.007), and patients with elevated miR-675-3p were more likely to have cardiovascular adverse events (log-rank P = 0.030). Increased miR-675-3p can be used as a potential marker for the diagnosis of AS, and was associated with the poor prognosis of AS.
Keywords: miR-675-3p, asymptomatic AS, diagnosis, prognosis
Introduction
Atherosclerosis (AS) is a cardiovascular disease caused by the thickening and hardening of arterial walls caused by the accumulation of cells, cholesterol, and extracellular matrix. 1 Epidemiological studies have shown that smoking, hypercholesterolemia, hypertension, and diabetes mellitus are major risk factors for AS and related disease progression. 2 Although significant prognosis has been made in lifestyle improvement and drug treatment, AS is still the main cause of morbidity and mortality in developed countries. 3 At the same time, the occurrence of AS is the main pathological basis of other cardiovascular and cerebrovascular diseases. Therefore, there is an urgent need to develop new and reliable diagnostic or prognostic biomarkers and treatment options to reduce the burden of AS treatment.
miRNAs are short non-coding RNAs that are involved in the post-transcriptional regulation of gene expression and play key roles in many biological processes. 4 Previous research has evidence that miRNAs are stably expressed in peripheral blood circulation and exhibit tolerance to pH, temperatures, and store time, showing good physiological characteristics. 5 Besides, other studies have shown that the changes of miRNA can be detected in different cardiovascular diseases, and its expression is conducive to the early diagnosis and prognosis assessment of cardiovascular diseases such as AS. 5 For example, miR-574-5p is a new candidate target for severe coronary artery disease. 6 Plasma miR-29c is a feasible marker for the early identification of AS. 7 As one of the miRNAs, miR-675-3p can regulate colorectal cancer, esophageal squamous cell carcinoma, and skeletal muscle differentiation and regeneration. 8 –10 Recently. Yi et al confirmed 19 miRNAs that are abnormally expressed during lipid droplet formation, including miR-675-3p. 11 Besides, Li et al in 2018 studied atherosclerotic diabetic rat tissues and found 9 differentially expressed miRNAs, including miR-675-3p. 12 What’s more, elevated miR-675-3p is associated with hallmarks of heart failure. 13 As the first exon located in H19, miR-675 is derived from the conserved transcript of lncRNA H19 and can produce 2 kinds of function miRNA, namely miR-675-3p and miR-675-5p. 14 In addition, miR-675 regulated by H19 was initially proved to be involved in placental growth before birth. Currently, more and more studies have confirmed that miR-675-3p has different tissue sources and multiple functions. 15 Although miR-675-3p has been reported in adipogenesis, atherosclerotic diabetic rat, and complication heart failure, the expression pattern, clinical diagnosis, and predictive value of miR-675-3p in AS patients remain unclear.
In the present work, the serum miR-675-3p expression level and clinical diagnosis, and prognostic value of asymptomatic AS patients were evaluated, in order to provide a theoretical basis for early screening and intervention of AS.
Materials and Methods
Subject’ Recruitment
The present survey was approved by the Medical Ethics Committee of Binzhou Medical University Hospital, and all subjects signed informed consent. 110 asymptomatic AS patients admitted to Binzhou Medical University Hospital from June 2010 to June 2014 were selected to be included in this study. The asymptomatic AS were identified based on the carotid intima-media thickness (CIMT) >0.7 mm in the previous studies. 16,17 Patients with heart disease, stroke, heart failure, angina, transient ischemic time, hypertension, renal insufficiency, and other cardiovascular diseases were excluded from the subjects. 70 healthy people of similar age were selected from the health examination center as the control group. Physical examination was performed on all subjects and basic information was recorded. Measurement of carotid intima-media thickness (CIMT) of subjects was also performed using the ATL HDI 3000 ultrasound system. The upper limb venous blood of the subjects was collected after 12 h of fasting. After centrifugation, the upper serum was separated and stored at −80°C.
Fluorescence Quantitative Polymerase Chain Reaction (qRT-PCR)
Serum samples of the subject’s total RNA were extracted by TRIzol reagent. According to the manufacturer’s instructions of the miRNA cDNA Synthesis Kit (CWBiotech), the RNA extracted was synthesized the first strand of complementary DNA. The qRT-PCR reaction was performed in 7500 FAST RT-PCR system (Thermo Fisher Scientific, Inc) as required by the miRcute Plus miRNA qPCR Kit (SYBR Green). 2− ΔΔCt 18 method to calculate the relative expression level of miR-675-3p in standardized to the endogenous controls U6 small nuclear RNA. 19 The primer sequence was used in the qRT-PCR were as follows: miR-675-3p forward 5′-GCCGAGCATCTTACCGGACGT-3′ and reverse 5′-CTCAACTGGTGTCGTGGA-3′; U6 forward 5′-AACGCTTCACGAATTTGCGT-3′ and reverse 5′-CTCAACTGGTGTCGTGGA-3′. The thermocycling condition used were as follows: 95°C for 10 min followed by 40 cycles of 94°C for 15 sec, 55°C for 30 sec and 70°C for 30 sec. 20
Follow-Up Plan and Content
According to the definition of cardiovascular end events in the 2014 American College of Cardiology/American Heart Association clinical Trial as the endpoints of this study. 21 Hospitalization or death due to events such as myocardial infarction, stroke, transient, ischemic attack, coronary intervention (including stent thrombosis), peripheral vascular intervention, and unstable angina was defined as cardiovascular endpoints. 21 The follow-up time of this study was 5 years. Participants’ cardiovascular endpoints events were recorded during telephone follow-up.
Statistical Analysis
Statistical calculations of experimental data were performed using GraphPad Prism 7.0 and SPSS 23.0 software. The expression level of miRNAs in control and AS patients were compared by unpaired two-tailed student tests. The ROC curve to investigate the diagnostic significance of serum miR-675-3p in AS patients. The correlation between miRNA and CIMT was analyzed by the Spearman correlation coefficient. Kaplan-Meier and log-rank methods detect the ability of miR-675-3p to predict adverse cardiovascular events in patients. The prognostic significance of miR-675-3p in AS was measured by multivariate Cox regression analysis. The level of statistical significance was set at P < 0.05.
Results
Clinical Indicators of the Patients Involved
The clinicopathological characteristics of the subjects were counted in Table 1. Studies have found that C-reactive protein, a marker of inflammation, was generally elevated in AS patients (P < 0.05). There was no statistical significance in age, gender, BMI, total cholesterol, and other indicators between the 2 groups (P > 0.05).
Table 1.
Demographic and Clinical Characteristics Between Groups.
| Features | Healthy controls (n = 70) | AS patients (n = 110) | P value |
|---|---|---|---|
| Age (years) | 54.61 ± 8.60 | 53.33 ± 5.70 | 0.208 |
| Gender (male/female) | 35/35 | 58/52 | 0.761 |
| BMI (kg/m 2 ) | 25.73 ± 1.71 | 26.01 ± 1.52 | 0.252 |
| Total cholesterol (mg/dl) | 198. 91 ± 13.26 | 200.15 ± 14.10 | 0.557 |
| HDL-C (mg/dl) | 45.92 ± 3.52 | 46.82 ± 2.62 | 0.069 |
| LDL-C (mg/dl) | 122.00 ± 12.36 | 125.09 ± 12.76 | 0.110 |
| Triglyceride (mg/dl) | 174.64 ± 16.61 | 179.42 ± 20.54 | 0.088 |
| SBP (mm Hg) | 138.77 ± 8.34 | 141.19 ± 8.92 | 0.071 |
| DBP (mm Hg) | 82.48 ± 6.95 | 83.55 ± 6.30 | 0.062 |
| CRP (mg/l) | 0.81 ± 0.20 | 2.72 ± 0.60 | 0.000 |
Abbreviations: AS, atherosclerosis; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; CRP, C-reactive protein.
Serum miR-675-3p Is Upregulation in AS Patients
To clarify the expression pattern of miR-675-3p in AS, we first detected the level of serum miR-675-3p in subjects. qRT-PCR reactions confirmed that serum miR-675-3p was markedly increased in AS patients compared with the control group (P < 0.001, Figure 1). The results prompt that the dysregulation of miR-675-3p may be involved in the progression of AS.
Figure 1.
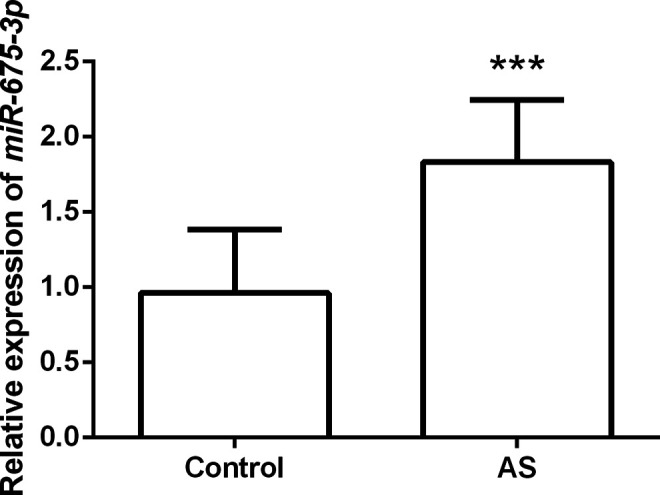
Serum miR-675-3p expression levels in AS patients and controls. Compared with control, serum miR-675-3p expression in AS patients was significantly increased, *** P < 0.001.
Serum miR-675-3p Is Positively Correlated With the CIMT Value
A noninvasive approach before the onset of symptoms of AS can reduce morbidity and mortality. Elevated CIMT is considered to be a subclinical index of AS independent of age, gender, and cardiovascular risk factors, and is widely used in the early detection of AS. 22,23 In this study, the mean CIMT of AS patients was 0.90 ± 0.09 mm. Spearman correlation coefficient analysis detected that the increase of serum levels of miR-675-3p was markedly correlated with the CIMT (r = 0.701, P < 0.001, Figure 2).
Figure 2.
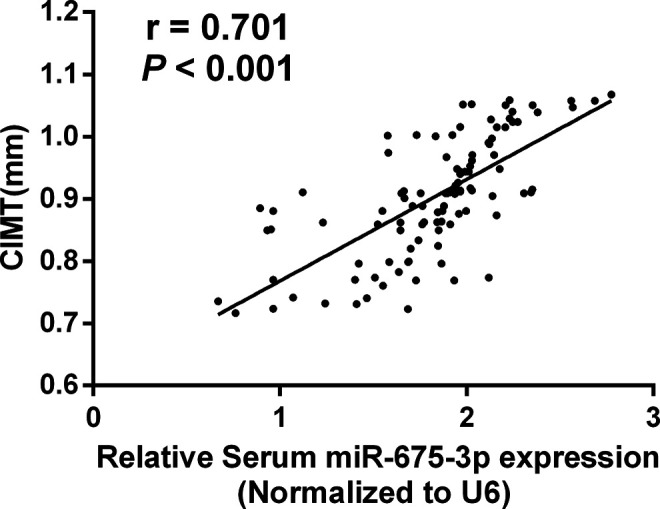
Correlation between serum miR-675-3p levels and CIMT value. Serum miR-675-3p were significantly positively correlated with CIMT in AS patients, r = 0.701, P < 0.001.
Diagnostic Potential of Serum miR-675-3p in AS Patients
Because of the positive correlation between serum miR-675-3p and CIMT, we further studied the diagnostic potential of miR-675-3p in AS patients by receiver operating characteristic curve (ROC) analysis. As shown in Figure 3, when the cut-off value is 1.368, the AUC of serum miR-675-3p was 0.918, sensitivity was 88.60%, and specificity was 89.09%. Studies have shown that miR-675-3p has a high diagnostic value and can clearly distinguish AS patients from healthy people.
Figure 3.
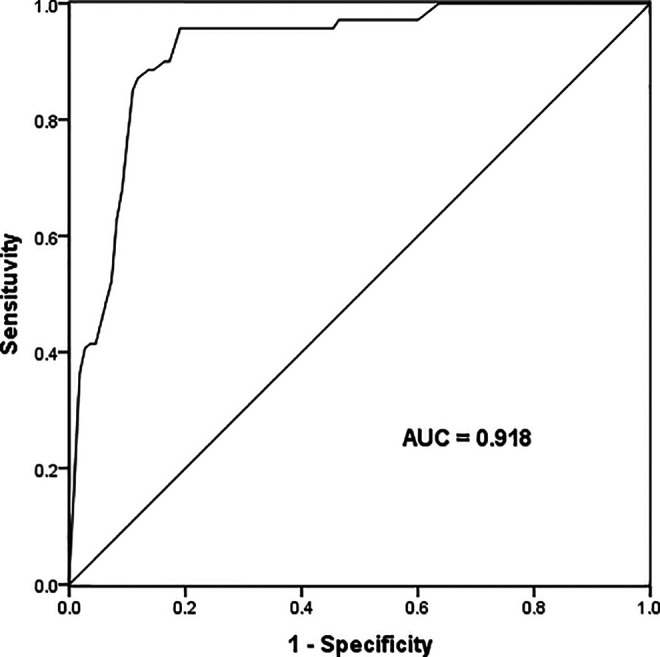
The potential diagnostic significance of miR-675-3p was evaluated by Receiver Operating Characteristic (ROC) curves. When the cut-off value is 1.368, the AUC of serum miR-675-3p was 0.918, sensitivity was 88.60%, and specificity was 89.09%.
Patients With High Serum miR-675-3p Have a Poor Prognosis
Then we analyzed the prognostic effect of serum miR-675-3p levels on the survival rate of cardiovascular adverse events. Based on the mean levels of miR-675-3p (1.832 ± 0.413), the patients were divided into the miR-675-3p high expression group and the low expression group. Survival curves were drawn based on cardiovascular endpoints during the 5-year follow-up. The results showed that compared with the low expression of miR-675-3p, patients with high levels of miR-675-3p had a markedly higher probability of cardiovascular adverse events (log-rank P = 0.030, Figure 4). Besides, to further study the prognostic significance of serum miR-675-3p in AS, multivariate Cox regression analysis verified the independent predictive value of miR-675-3p for the occurrence of cardiovascular adverse events in AS patients (P < 0.05, Table 2).
Figure 4.
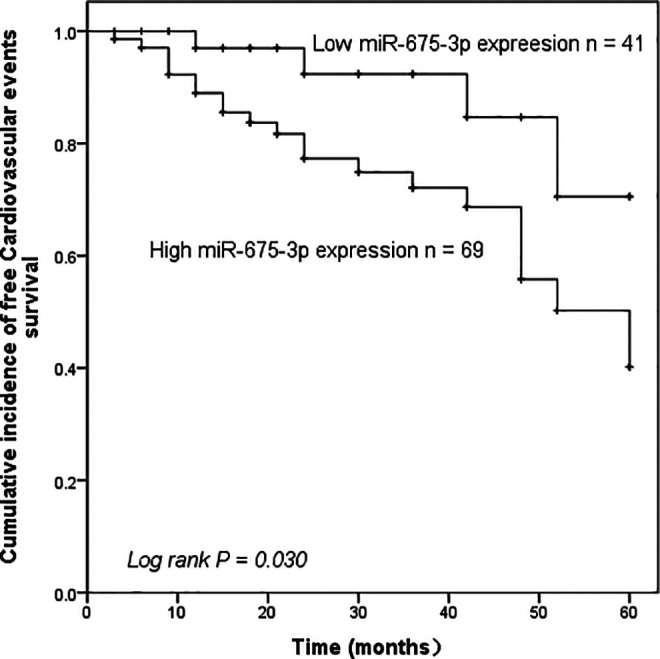
Kaplan-Meier analysis was used to calculate the predictive potential of miR-675-3p for cardiovascular adverse events in asymptomatic AS patients. Patients with high miR-675-3p expression are more likely to have cardiovascular adverse events, log-rank P = 0.030.
Table 2.
Multivariate Cox Regression Analysis for the Overall Survival of AS Patients.
| Parameters | Multivariate analysis | ||
|---|---|---|---|
| Hazard ratio | 95% CI | P value | |
| miR-675-3p | 5.375 | 1.590-18.170 | 0.007 |
| Age (years) | 0.615 | 0.240-1.579 | 0.312 |
| Gender (male/female) | 0.894 | 0.374-2.136 | 0.801 |
| BMI (kg/m2) | 0.543 | 0.218-1.354 | 0.190 |
| Total cholesterol (mg/dl) | 0.711 | 0.281-1.800 | 0.472 |
| HDL-C (mg/dl) | 00.910 | 0.376-2.202 | 0.835 |
| LDL-C (mg/dl) | 0.773 | 0.339-1.763 | 0.541 |
| Triglyceride (mg/dl) | 0.676 | 0.269-1.704 | 0.407 |
| SBP (mm Hg) | 0.752 | 0.312-1.813 | 0.526 |
| DBP (mm Hg) | 0.451 | 0.182-1.117 | 0.085 |
| CRP (mg/l) | 2.728 | 1.054-7.064 | 0.039 |
Abbreviations: AS, atherosclerosis; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; CRP, C-reactive protein.
Discussion
AS is a chronic inflammatory disease related to the systemic arterial wall, which can lead to myocardial infarction, ischemic stroke, peripheral artery disease, and other cardiovascular diseases, with high morbidity and mortality. 24 Several studies have reported that inflammation is involved in all stages of AS formation, including early AS, plaque instability, thrombosis, and cardiovascular events. 25 CRP, a nonspecific marker of inflammation, is a strong predictor of future cardiovascular events. 26 In the present study, we found that the patient’s CRP concentration was significantly increased, which is consistent with the results of existing studies.
Previous studies have revealed key signaling and molecular pathways involved in the initiation and progression of AS. 27,28 As an important physiological process regulator of diseases, miRNAs, such as cell adhesion, proliferation, lipid uptake and efflux, and inflammatory response, has identified new therapeutic targets. 29,30 Besides, miRNAs can be detected outside the cell, including in circulating blood, urine, and other body fluids, and their expression is relatively stable, which improves their potential as biomarkers for diagnosis, prognosis, or response to cardiovascular treatment. 31 For example, aberrant expression of plasma miR-33a in AS patients can be used as a candidate biomarker for AS. 32 miR-374 is significantly elevated in patients and is a potential diagnostic marker for AS. 33
Our current research confirmed that miR-675-3p was generally upregulated in AS patients. In 2018, Li et al conducted a miRNA microarray analysis on diabetic atherosclerotic rats and identified 9 differentially expressed genes including miR-675-3p. 12 Liu et al confirmed that miR-675-3p was markedly up-regulated in human heart failure patients, and it also increased in the myocardial hypertrophy mice model induced by aortic constriction. 13 Our results are consistent with those of common complications of AS. So far as we know, this is the first study to detect the expression level of miR-675-3p in AS patients, and the study suggests that the dysregulation of miR-675-3p may play a major part in the progression of AS.
The incidence of cardiovascular events was generally associated with CIMT. Moreover, the increase in CIMT is recognized as a subclinical indicator of AS. 34 In the present survey, it was confirmed that the serum miR-675-3p level was markedly positively correlated with the CIMT. Furthermore, the present investigation also evaluated the diagnostic potential of miR-675-3p in AS through the ROC curve. The results indicate that miR-675-3p elevation is a new potential diagnostic biomarker for AS, which can significantly distinguish AS patients from healthy personnel.
Next, to further study the clinical prognostic value of serum miR-675-3p levels in AS. We analyzed the adverse cardiovascular events of patients based on the 5-year follow-up records. The results showed that patients with high expression of miR-675-3p had a poor prognosis, and we proved that miR-675-3p was an independent prognostic factor for cardiovascular adverse events in AS patients by multivariate Cox regression analysis. Present investigation indicates that miR-675-3p elevation is a new prognostic marker for AS.
This study verified that miR-675-3p is highly expressed in AS patients. Elevated miR-675-3p may be a potential diagnostic biomarker for asymptomatic AS, and indicates a poor prognosis, providing a theoretical basis for early screening and intervention of AS.
Footnotes
Authors’ Note: The present survey was approved by the Medical Ethics Committee of Binzhou Medical University Hospital, and all subjects signed informed consent. Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Science and Technology Program of Binzhou Medical University [NO. BY2018KJ06] and Natural Science Foundation of Shandong Province [NO. ZR2020QH104].
ORCID iD: Deyong Du  https://orcid.org/0000-0002-3341-9743
https://orcid.org/0000-0002-3341-9743
References
- 1. Fledderus J, Vanchin B, Rots MG. The endothelium as a target for anti-atherogenic therapy: a focus on the epigenetic enzymes EZH2 and SIRT1. J Pers Med. 2021;11(2):103. doi:10.3390/jpm11020103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Howard G, Wagenknecht LE, Burke GL, et al. Cigarette smoking and progression of atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) study. JAMA. 1998;279(2):119–124. doi:10.1001/jama.279.2.119 [DOI] [PubMed] [Google Scholar]
- 3. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi:10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- 4. Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141(4):1202–1207. doi:10.1016/j.jaci.2017.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu J, Chen Z, Wang Y, et al. Several circulating miRNAs related to hyperlipidemia and atherosclerotic cardiovascular diseases. Lipids Health Dis. 2019;18(1):104. doi:10.1186/s12944-019-1046-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lai Z, Lin P, Weng X, et al. MicroRNA-574-5p promotes cell growth of vascular smooth muscle cells in the progression of coronary artery disease. Biomed Pharmacother. 2018;97:162–167. doi:10.1016/j.biopha.2017.10.062 [DOI] [PubMed] [Google Scholar]
- 7. Huang YQ, Li J, Huang C. Plasma MicroRNA-29c levels are associated with carotid intima-media thickness and is a potential biomarker for the early detection of atherosclerosis. Cell Physiol Biochem. 2018;50(2):452–459. doi:10.1159/000494158 [DOI] [PubMed] [Google Scholar]
- 8. Yang X, Lou Y, Wang M. miR675 promotes colorectal cancer cell growth dependent on tumor suppressor DMTF1. Mol Med Rep. 2019;19(3):1481–1490. doi:10.3892/mmr.2018.9780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xiao Q, Chen T, Wu Y, et al. MicroRNA6753p promotes esophageal squamous cell cancer cell migration and invasion. Mol Med Rep. 2018;18(4):3631–3640. doi:10.3892/mmr.2018.9372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dey BK, Pfeifer K, Dutta A. The H19 long noncoding RNA gives rise to microRNAs miR-675-3p and miR-675-5p to promote skeletal muscle differentiation and regeneration. Genes Dev. 2014;28(5):491–501. doi:10.1101/gad.234419.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yi X, Liu J, Wu P. The key microRNA on lipid droplet formation during adipogenesis from human mesenchymal stem cells. J Cell Physiol. 2020;235(1):328–338. doi:10.1002/jcp.28972 [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Xiao L, Li J, et al. MicroRNA profiling of diabetic atherosclerosis in a rat model. Eur J Med Res. 2018;23(1):55. doi:10.1186/s40001-018-0354-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu L, An X, Li Z, et al. The H19 long noncoding RNA is a novel negative regulator of cardiomyocyte hypertrophy. Cardiovasc Res. 2016;111(1):56–65. doi:10.1093/cvr/cvw078 [DOI] [PubMed] [Google Scholar]
- 14. Wang J, Sun J, Yang F. The role of long non-coding RNA H19 in breast cancer. Oncol Lett. 2020;19(1):7–16. doi:10.3892/ol.2019.11093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang J, Ye C, Xiong H, et al. Dysregulation of long non-coding RNA in breast cancer: an overview of mechanism and clinical implication. Oncotarget. 2017;8(3):5508–5522. doi:10.18632/oncotarget.12537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rajasuriar R, Kong YY, Nadarajah R, et al. The CD14 C-260 T Single Nucleotide Polymorphism (SNP) modulates monocyte/macrophage activation in treated HIV-infected individuals. J Transl Med. 2015;13:30. doi:10.1186/s12967-015-0391-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yong YK, Shankar EM, Westhorpe CLV, et al. Genetic polymorphisms in the CD14 gene are associated with monocyte activation and carotid intima-media thickness in HIV-infected patients on antiretroviral therapy. Medicine (Baltimore). 2016;95(31):e4477. doi:10.1097/MD.0000000000004477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 19. Podkalicka P, Mucha O, Bronisz-Budzynska I, et al. Lack of miR-378 attenuates muscular dystrophy in mdx mice. JCI Insight. 2020;5(11):e135576. doi:10.1172/jci.insight.135576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao D, Hao L, Zhao Z. Long non-coding RNA PART1 promotes intervertebral disc degeneration through regulating the miR93/MMP2 pathway in nucleus pulposus cells. Int J Mol Med. 2020;46(1):289–299. doi:10.3892/ijmm.2020.4580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA Key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol. 2015;66(4):403–469. doi:10.1016/j.jacc.2014.12.018 [DOI] [PubMed] [Google Scholar]
- 22. Nezu T, Hosomi N, Aoki S. Carotid intima-media thickness for atherosclerosis. J Atheroscler Thromb. 2016;23(1):18–31. doi:10.5551/jat.31989 [DOI] [PubMed] [Google Scholar]
- 23. Pang Y, Sang Y, Ballew SH, et al. Carotid intima-media thickness and incident ESRD: the Atherosclerosis Risk in Communities (ARIC) study. Clin J Am Soc Nephrol. 2016;11(7):1197–1205. doi:10.2215/CJN.11951115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jeong HS, Kim JY, Lee SH, et al. Synergy of circulating miR-212 with markers for cardiovascular risks to enhance estimation of atherosclerosis presence. PLoS One. 2017;12(5):e0177809. doi:10.1371/journal.pone.0177809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Swastini DA, Wiryanthini IAD, Ariastuti NLP. Atherosclerosis prediction with high sensitivity C-reactive protein (hs-CRP) and related risk factor in patient with dyslipidemia. Open Access Maced J Med Sci. 2019;7(22):3887–3890. doi:10.3889/oamjms.2019.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soeki T, Sata M. Inflammatory biomarkers and atherosclerosis. Int Heart J. 2016;57(2):134–139. doi:10.1536/ihj.15-346 [DOI] [PubMed] [Google Scholar]
- 27. Zhu K, Meng Q, Zhang Z, et al. Aryl hydrocarbon receptor pathway: role, regulation and intervention in atherosclerosis therapy (review). Mol Med Rep. 2019;20(6):4763–4773. doi:10.3892/mmr.2019.10748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang H, Gong X, Ni S. C1q/TNF-related protein-9 attenuates atherosclerosis through AMPK-NLRP3 inflammasome singling pathway. Int Immunopharmacol. 2019;77:105934. doi:10.1016/j.intimp.2019.105934 [DOI] [PubMed] [Google Scholar]
- 29. Wu Y, Zhang F, Lu R, et al. Functional lncRNA-miRNA-mRNA networks in rabbit carotid atherosclerosis. Aging (Albany NY). 2020;12(3):2798–2813. doi:10.18632/aging.102778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang F, Chen Q, Wang W. Hepatocyte-derived extracellular vesicles promote endothelial inflammation and atherogenesis via microRNA-1. J Hepatol. 2020;72(1):156–166. doi:10.1016/j.jhep.2019.09.014 [DOI] [PubMed] [Google Scholar]
- 31. Song R, Hu XQ, Zhang L. Mitochondrial MiRNA in cardiovascular function and disease. Cells. 2019;8(12):1475. doi:10.3390/cells8121475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim SH, Kim GJ, Umemura T. Aberrant expression of plasma microRNA-33a in an atherosclerosis-risk group. Mol Biol Rep. 2017;44(1):79–88. doi:10.1007/s11033-016-4082-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang W, Ma F, Zhang H. MicroRNA-374 is a potential diagnostic biomarker for atherosclerosis and regulates the proliferation and migration of vascular smooth muscle cells. Cardiovasc Diagn Ther. 2020;10(4):687–694. doi:10.21037/cdt-20-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gobbi CA, Asbert P, Alba PB, et al. Subclinical markers of atherosclerosis and cardiovascular risk factors in early arthritis. Rev Fac Cien Med Univ Nac Cordoba. 2019;76(3):174–179. doi:10.31053/1853.0605.v76.n3.21610 [DOI] [PubMed] [Google Scholar]


