Abstract
Cryopreserved haematopoietic progenitor cells are used to restore autologous haematopoiesis after high dose chemotherapy. Although the cells are routinely stored for a long period, concerns remain about the maximum storage time and the possible negative effect of storage on their potency. We evaluated the effect of cryopreservation on the quality of peripheral stem cell grafts stored for a short (3 months) and a long (10 years) period and we compared it to native products.The viability of CD34+ cells remained unaffected during storage, the apoptotic cells were represented up to 10% and did not differ between groups. The clonogenic activity measured by ATP production has decreased with the length of storage (ATP/cell 1.28 nM in native vs. 0.63 in long term stored products, P < 0.05). Only borderline changes without statistical significance were detected when examining mitochondrial and aldehyde dehydrogenase metabolic activity and intracellular pH, showing their good preservation during cell storage. Our experience demonstrates that cryostorage has no major negative effect on stem cell quality and potency, and therefore autologous stem cells can be stored safely for an extended period of at least 10 years. On the other hand, long term storage for 10 years and longer may lead to mild reduction of clonogenic capacity. When a sufficient dose of stem cells is infused, these changes will not have a clinical impact. However, in products stored beyond 10 years, especially when a low number of CD34+ cells is available, the quality of stem cell graft should be verified before infusion using the appropriate potency assays.
Keywords: haematopoietic stem cell, cryopreservation, storage, viability, potency, ALDH, pH, mitochondrial activity
Introduction
A high-dose chemotherapy followed by an autologous stem cell transplant represents a standard treatment approach in chemosensitive lymphoid neoplasms. Peripheral blood stem cells (PBSCs) are a preferable stem cell source used for autologous stem cell support, where, in most cases, mobilization of PBSCs with G-CSF (alone or combined with chemotherapy) generates sufficient stem cell numbers for further use. The leukapheresis products must be cryopreserved and stored in liquid nitrogen until their subsequent reinfusion. The success of the whole transplant depends on the PBSC graft’s ability to reconstitute haematopoiesis following multiple procedural steps, including stem cell collection, processing, freezing, storage, transportation, and finally thawing and infusion.
The majority of stem cell products are infused within months of harvesting; however, whenever multiple transplants are required the need for long-term storage arises. A second autologous transplant can be a suitable option for multiple myeloma patients who have relapsed after previous high-dose chemotherapy, especially if the response to the first transplant was long-lasting 1 . A similar multiple transplant approach can be useful in Hodgkin lymphomas, where the tandem autologous stem cell transplant is proposed as an option to improve the outcome in high-risk patients 2 . In all cases, all grafts are collected before the first transplant as stem cell harvest at the time of relapse may be challenging due to the advanced disease stage and increased cumulative stem cell toxicity. Moreover, the risk of poor mobilization increases with each course of chemotherapy, which may ultimately reduce the stem cell pool within the bone marrow.
Autologous PBSCs are cryopreserved and stored in the liquid or vapor phase of nitrogen until thawing and reinfusion. A conventional cryopreservation method involves controlled-rate freezing and 5%–10% dimethyl sulfoxide (DMSO) as the cryoprotectant 3 . For decades, DMSO is a verified additive for long-term bone marrow or peripheral blood stem cell storage protecting cells from the formation of ice crystals and resulting cellular damage. Under these conditions, haematopoietic stem cell (HPC) products can be stored for extended periods ranging from months to years.
Quality control of stem cell grafts typically considers nucleated cells, where CD34+ cell counts and viability are key functional markers. The CD34 antigen is used as a surrogate marker for haematopoietic stem cell identification, and CD34+ cell enumeration is an accepted method stem cell content quantification. Standardized methods for CD34+ cell analysis, such as ISHAGE based protocols, offer low intra- and inter-laboratory variability, thus allowing comparison of different stem cell grafts, mobilizations, or cryopreservation protocols 4 .
Viability characterization relies on indirect measurements and hence questions remain as to its ability to capture functional integrity and potency of CD34+ cells following long-term storage. Dead cells are identified and excluded from analysis in the CD34+ assay using viability dye. Membrane integrity viability measurements, however, correlate poorly with the post-thaw cell condition and function. The dye exclusion method only detects the latest stages of cell death, whereas freezing and thawing could induce more subtle functional impairment in cells that are still living. In other words, the viable CD34+ count used in standard quality control criteria for thawed grafts does not guarantee that PBSC function and potency are retained. Indeed, cases of engraftment failure despite high post-thaw viable CD34+ counts point to a problem of false assurance of stem cell function check after cryopreservation 5 . Increasing the reinfusion dose to at least 2 × 106/kg CD34+ as measured before freezing circumvents this problem 6 but not without its pitfalls. A higher dose of 4–5 × 106/kg is associated with faster engraftment of neutrophils causing reduction of risk of infections 7 . The CD34+ cell counting can be combined with the CFU assay to further evaluate the functional capacity of the whole graft and its individual cell subsets. Significant losses in total nucleated cells, cell viability, and colony-forming units (CFU) may be caused by cryopreservation and thawing 8,9 .
Long-term stability and post-thaw potency of haematopoietic stem cell grafts remain less explored. Only sparse data is available for grafts stored for over a decade. Several studies were performed on cord blood (CB) grafts confirming storage of up to 10–15 years does not significantly decrease stem cell recovery 10,11 . Studies dedicated to peripheral blood stem cells are more limited, most considering only relatively short storage periods 12 –14 . Only a couple of studies address storage periods beyond 10 years 15,16 . Another limitation of many studies is the lack of functional stem cell assay in the analysis. Here in we report the effect of cryopreservation on the quality and activity of PBSC samples stored for a short (3 months) and a long (10 years) period in comparison to native samples. The goal of the study is to determine whether autologous PBSCs retain the quality and potency after long-term cryostorage and hence can be stored safely for an extended period.
Methods
Samples Preparation
Three different groups of PBSC samples were subjected to in vitro analyses and compared. The native non-cryopreserved samples (N) were processed immediately after the completion of apheresis (n = 12); the short-term storage samples (STC) were analysed after 3 months (2.5–3.4) of liquid nitrogen storage (n = 12); and long-term storage samples (LTC) were stored for 10 years (9.0–10.8; n = 20). The study scheme is showed in Fig. S1.
PBSC collection and freezing
PBSCs from patients with different malignant diseases (non-Hodgkin, Hodgkin lymphoma, and multiple myeloma) were collected on an apheresis device. All enrolled patients signed informed consent before performing apheresis. Large volume procedures were performed, processing from 3 to 4 times the patient blood volume in order to collect the amount of CD34+ cells required for reinfusion. Prior to collection, patients were mobilized with a combination of chemotherapy (cyclophosphamide 2–4 g/m2 or disease-specific salvage regimens) and granulocyte colony-stimulating factor (filgrastim 10 μg/kg/day). Target doses of CD34+ cells were set to ≥ 3.0 × 106/kg (lymphoma patients) or to ≥ 4.0 × 106/kg (myeloma patients, tandem transplant) of the recipient. All apheresis products were cryopreserved on the same day.
Before freezing, the cell count was adjusted with autologous plasma to a maximum concentration of 200 × 106/l. Cryobags (OriGen Biomedical, USA) with cells were placed on cooling plates, followed by the addition of the cryopreservation medium containing 20% dimethyl sulfoxide (DMSO, Wak-Chemie, Germany), 5% human serum albumin (HSA - albunorm, Octopharma, Belgium), and phosphate-buffered saline (PBS, local source) in a 1: 1 ratio resulting in 10% DMSO concentration final. Small 1 ml samples were drawn from the cryobag, divided into cryotubes (Nunc, USA), and frozen together with the bags for subsequent quality control and/or research. All stem cell processing was done in a cleanroom facility under GMP conditions. Sealed cryobags were inserted into metal cassettes and subjected to the controlled rate freezing in a programmable freezing machine (IceCube 1810, Sy-lab, Austria). After reaching the temperature −140°C, cells were transferred into liquid nitrogen for long-term storage. Identical processing, freezing, and storage procedures were used for all bags and samples.
PBSC Samples Thawing
Prior to testing for differentiation potential, flow cytometry, mitochondrial activity, viability/apoptosis/necrosis, metabolic activity, and intracellular pH, PBSC samples (representative samples in cryotubes stored under same conditions as the cell products) were thawed according to optimized protocol reducing the clumping of neutrophils. Aliquots were removed from liquid nitrogen, immediately transferred to a pre-warmed 37°C water bath, and thawed approximately for 3 min. Then 1 ml of pre-warmed IMDM (Gibco, Scotland) medium and 150 UI of DNAse (for clamping reduction; Roche, Germany) were added (drop-by-drop), and cells were resuspended by gentle agitation. The final cell suspension was transferred into 10 ml of pre-warmed IMDM. The leukocyte concentration was determined on a haematology analyser.
Analysis of viability, Necrosis, and Apoptosis
A combination of 7-Aminoactinomycin D (7-AAD) and Annexin V was used for the evaluation of viable, apoptotic, and necrotic cells. 7-AAD penetrates through the cell membrane of dead cells only. Membrane phosphatidylserine (PS) translocation from the inner to the surface side occurs in dying cells. Annexin V is characterized by its high affinity to PS. Flow cytometry allows recognition of living cells (Annexin V negative/7AAD negative), apoptotic cells (Annexin V positive/7AAD negative), and dead cells (positive for both markers).
Primary antibodies Annexin V—FITC (Exbio, Czech republic), CD45—Krome orange, CD34—PE, and 7AAD marker (all Immunotech, USA) were added to 100 µl of cell suspension (concentration 5 × 105 cell in 1 ml Annexin Binding buffer) and incubated for 15 min in the dark (room temperature). Unbound antibodies were washed away with 4 ml PBS at 350g/5 min. The cell pellet was resuspended in 5 ml Annexin binding buffer, and samples were immediately measured on BD FACS Canto II flow cytometer. Analysis was performed using the software FlowJo (Treestar, USA). Because granulocytes are susceptible to disruption, viability of all leukocytes (with granulocytes) and mononuclear cells was analysed separately.
Mitochondrial Potential
JC-1 (5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazolylcarbocyanine iodide), a lipophilic cationic dye assay (Life Technologies, USA) was used to detect changes in the mitochondrial membrane. This dye exhibits mitochondrial potential indicated by a bright red and green fluorescence emission in healthy cells. A decrease in green fluorescence indicates mitochondrial depolarization and cell damage. Cells were resuspended in 1 ml of PBS at the final concentration 1 × 106/ml. JC-1 solution (10 µl at the concentration of 200 μM) was added to the cell suspension, and cells were incubated at 37°C for 20 min. CCCP (2 μl; Carbonyl cyanide m-chlorophenyl hydrazone), a disruptor of the mitochondrial membrane potential, was used in the control tube. Washing with PBS and centrifugation (350g/5 min) followed the incubation period. The supernatant was removed, and the pellet was resuspended in 100 µl PBS. Staining of membrane antigens CD45 and CD34 was performed by incubating with the antibodies anti-CD34-PE and anti-CD45-Krome Orange (both Immunotech, USA) for 15 min at room temperature in the dark. After washing, the suspension was immediately measured on BD FACSCanto II flow cytometer (BD Bioscience, USA). The analysis was performed in the software FlowJo.
Metabolic Potential
High aldehyde dehydrogenase (ALDH) activity is typical for healthy haematopoietic precursor cells. The ALDEFLOUR™ assay (STEMCELL Technologies, USA) uses substrate BIDOPY™ aminoacetaldehyde (BAAA), which diffuses into the cells where it is converted to BODIPY® - aminoacetate (BAA), causing the cells to express high levels of ALDH becoming brightly fluorescent when measured on a flow cytometer. Cells were diluted to the concentration 1 × 106 cells/ml in PBS. The erythrocytes were lysed, and the cell suspension was washed with PBS. BAAA (5 μl) was added and half of the suspension was transferred to a control tube containing a specific inhibitor of ALDH, diethylaminobenzaldehyde (DEAB). After 30 min incubation at 37°C, cells were washed with PBS (1500 RPM, 5 min) and the pellet was resuspended in 100 µl Aldefluor Assay buffer. Antibodies CD45-Krome Orange and CD34-PE were added for identification of haematopoietic cells, and further washing with PBS at 1500 RPM/5 min followed. Finally, the cells were resuspended in 300 µl of Aldefluor Assay buffer and immediately measured on BD FACSCanto II flow cytometer. The analysis was done with the software FlowJo.
Measurement of pH in Cells
The cell suspension was diluted to a concentration of 1 × 106/ml in EBSS buffer. Next, the BCECF-AM solution (Sigma Aldrich, USA) was added at the final concentration of 10 μM. The suspension was incubated for 25 minutes at 37°C in a water bath. After incubation and washing with EBSS the samples were analysed using a FC500 flow cytometer (Beckman Coulter, USA). Fluorescence was measured at 525 nm and 610 nm, and the pH value was determined using a calibration curve.
Differentiation Potential
Differentiation potential was evaluated using the HALO 96-PCAeq kit (Hemogenix, USA). This assay was intended as a replacement of the standard CFU assay. The test is based on a chemical reaction of ATP with luciferin (in the presence of luciferase), leading to the production of a bioluminescence signal. Bioluminescence is measured on the plate reader, and the concentration of ATP is calculated using a standard curve. Cell suspension containing 3–5 × 106 leukocytes first was lysed with ammonium chloride (5 ml) to remove the red blood cell and then washed and resuspended in 1 ml IMDM medium. Leukocyte concentration was measured, and samples were diluted to the final concentration 0.75 × 106 cells/ml. 100 µl of suspension was transferred to 900 µl of HALO® Culture Master Mix. Cells were seeded at the concentration of 7500 cells/well in six replicates and cultivated for six days in 5% CO2 and 37°C. Following the culture period, the ATP Enumeration Reagent (100 μl) was added to each well. The plate was incubated for 10 min at room temperature in the dark. The bioluminescence was measured on SYNERGY HT reader (BioTek, USA). A standard curve was done for each measurement.
Statistical Analysis
We used STATISTICA software (StatSoft, Czech Republic) for the statistical evaluation of the data. Non-parametric Mann–Whitney U test was chosen to determine statistical differences between groups at P ≤ 0.05.
Results
Determination of Viability, Necrosis, and Apoptosis
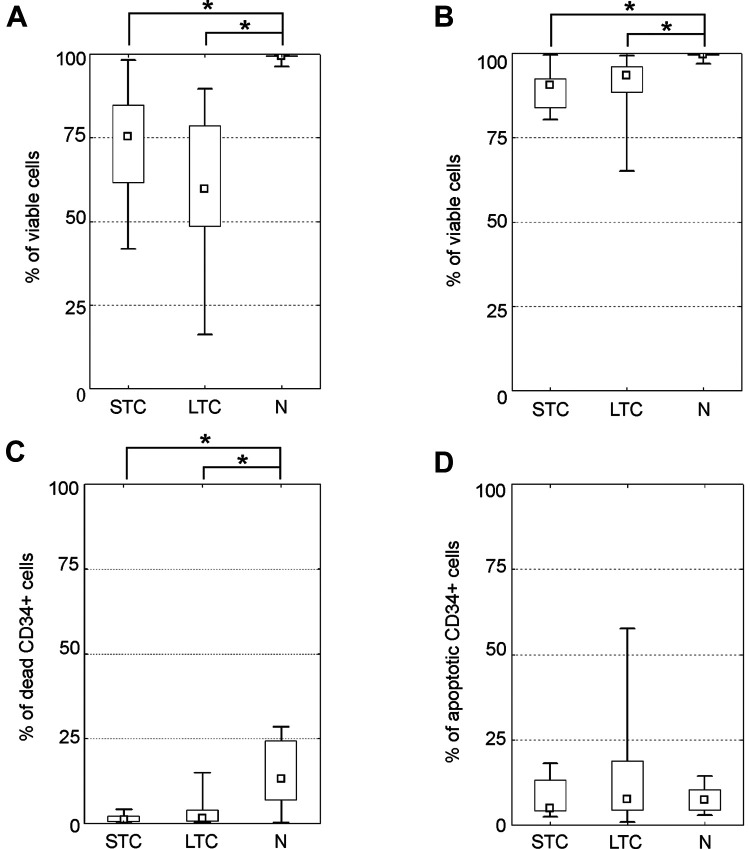
7-Aminoactinomycin (7-AAD) was used to determine viable (negative) and dead (positive) cells. Apoptotic cells were determined by Annexin V expression on the surface of 7-AAD negative cells (see Supplementary Fig. S2). Both groups of cryopreserved cells showed lower leukocyte/total nucleated (TNC) and mononuclear cell (MNC) viability (see Fig. 1A, B). Total leukocyte viability was the lowest in the LTC group (median = 68%). The STC group had 80% viability. The N group had the highest viability of almost 100%. Differences between the N group and either the STC or LTC group were statistically significant, P < 0.05. A minimal difference in mononuclear cell viability was observed between cryopreserved (STC resp. LTC) and native groups (medians: 91% resp. 94% vs. 99%, P < 0.05). The viability of CD34+ cells remained above 95% in both cryopreserved subgroups (medians: 99% and 98%, ns) and was surprisingly higher than in the native products (median = 87%, P < 0.05; Fig. 1C). Apoptotic CD34+ cell populations were identified in all subgroups but were slightly more represented in the LTC (median = 8%) and the N (median = 7%) groups as compared to the STC group (median = 5%). However, this difference was not statistically significant (see Fig. 1D).
Figure 1.
Determination of viability, necrosis and apoptosis. Detection of viable and apoptotic cells with 7-Aminoactinomycin/Annexin V. (A) A total leukocyte viability in groups; (B) mononuclear cells (MNC) viability, (C) proportion of dead CD34+ cells, (D) percentage of apoptotic cells within the CD34+ cell population. (median; box: 25%–75%; quantiles; * significancy P ≤ 0.05).
Evaluation of Mitochondrial Potential
Fluorescent dye JC-1 with bright red and green fluorescence was used to compare mitochondrial potential between the three subgroups (see Supplementary Fig. S3). Only minimal and not statistically significant differences between groups were observed irrespective of storage length. The mitochondrial activity in SCT, LCT, and N subgroups measured as a percentage of cells with bright green fluorescence reached 60%, 65%, and 64% (medians, ns); for details, see Fig. 2.
Figure 2.
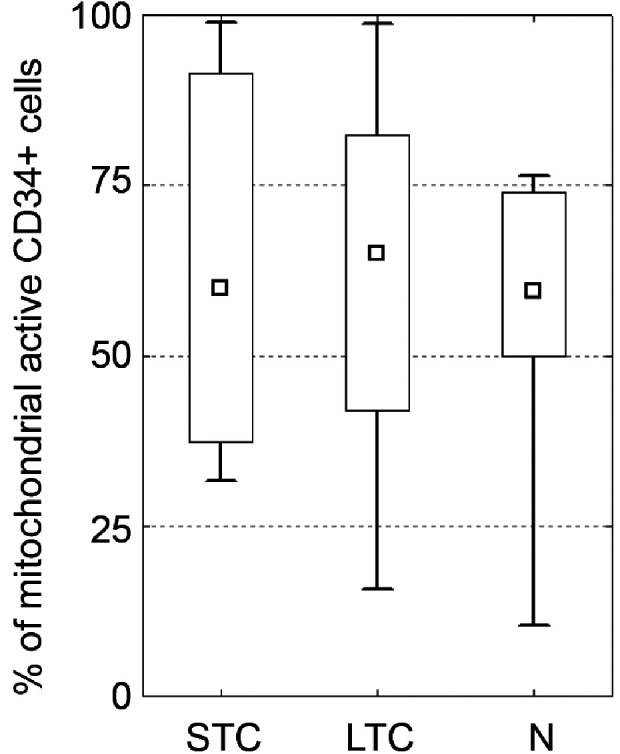
Evaluation of mitochondrial potential. Detection of mitochondrial potential using the JC-1 assay. Percentage of green cells indicates the health of mitochondria in groups. There is no significant difference between native and both groups of cryopreserved cells. (median; box: 25%–75%; quantiles; * significancy P ≤ 0.05).
Testing of Metabolic Activity
Aldehyde dehydrogenase (ALDH) activity was used to determine metabolically viable cryopreserved and native haematopoietic stem cells (see Supplementary Fig. S4). The same numbers of CD34+ cells with high metabolic activity (high ALDH expression) were recorded in the native and short time cryopreserved cells (86% and 87%, median). Slightly lower activity was measured in the LTC group (81% median), see Fig. 3. The differences are not statistically significant.
Figure 3.
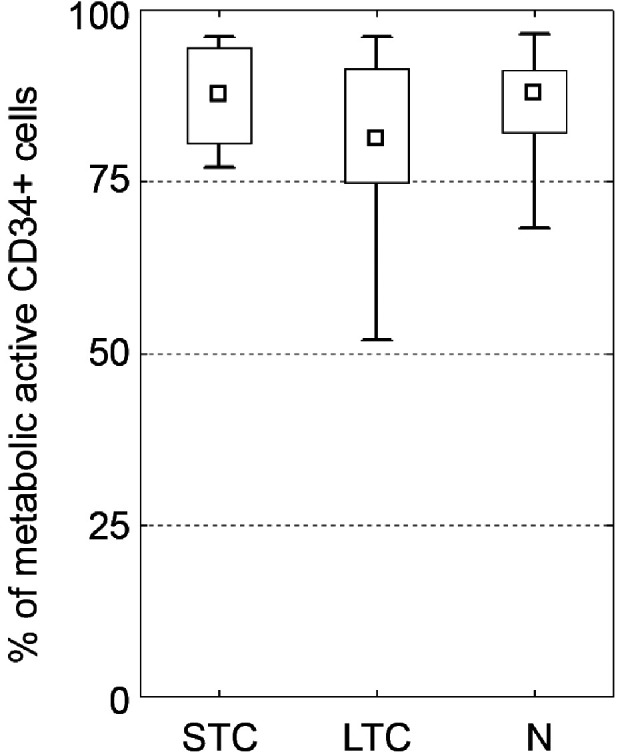
Testing of metabolic activity. ALDH expression by CD34+ cells showing metabolic activity of cells. No difference between groups was observed. (median; box: 25%–75%; quantiles; * significancy P ≤ 0.05).
Differentiation Potential
Bioluminescence assay was used for evaluation of haematopoietic stem cells differentiation potential. A considerable degree of heterogeneity was seen within all tested subgroups. The level of ATP/cell measured in the N group was 1.28 nM (range 0.33–4.9) and was higher in comparison to 1.03 nM measured in the STC group (range 0.15–1.84, ns) and 0.63 nM measured in the LTC group (range 0.16–4.9, P < 0.05). A downward trend of ATP concentration was observed with the N group having the highest concentration and the LTC group having the lowest concentration, see Fig. 4.
Figure 4.
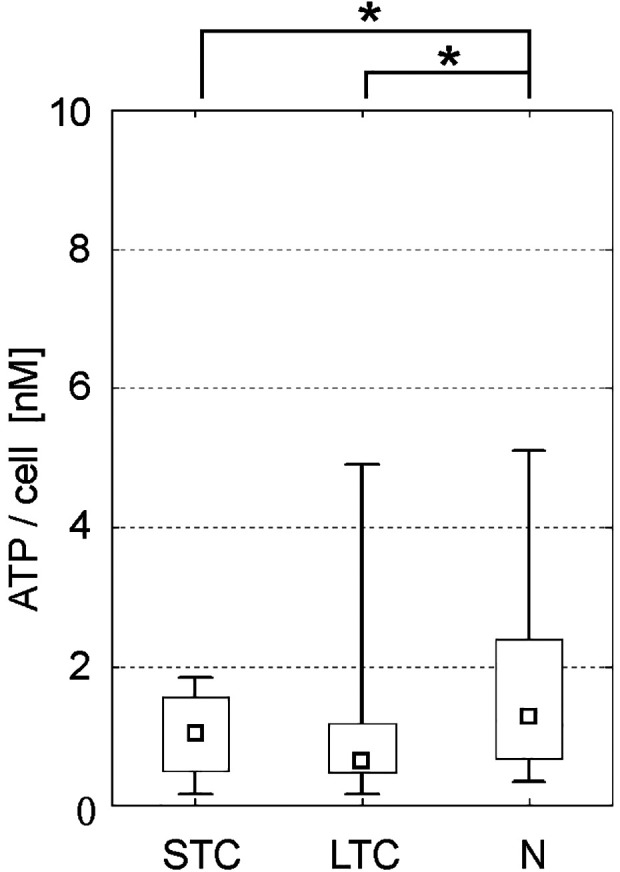
Analysis of clonogenic potential. The level of ATP/cell measured after activation by HALO® Culture Master Mix. All groups showed potential to differentiate/proliferate; however, long-term storage slightly decreased the clonogenic potential when compared to native cells. (median; box: 25%–75%; quantiles; * significancy P ≤ 0.05).
Measurement of pH in Cells
Detection of intracellular pH (ipH) revealed with borderline statistical significance lower values for samples stored for extended periods (7.18; 7.10–7.45) as compared to native (7.33; 7.30–7.35; P = 0.05) and briefly stored products (7.31; 7.18–7.50; ns). For details see Fig. 5.
Figure 5.
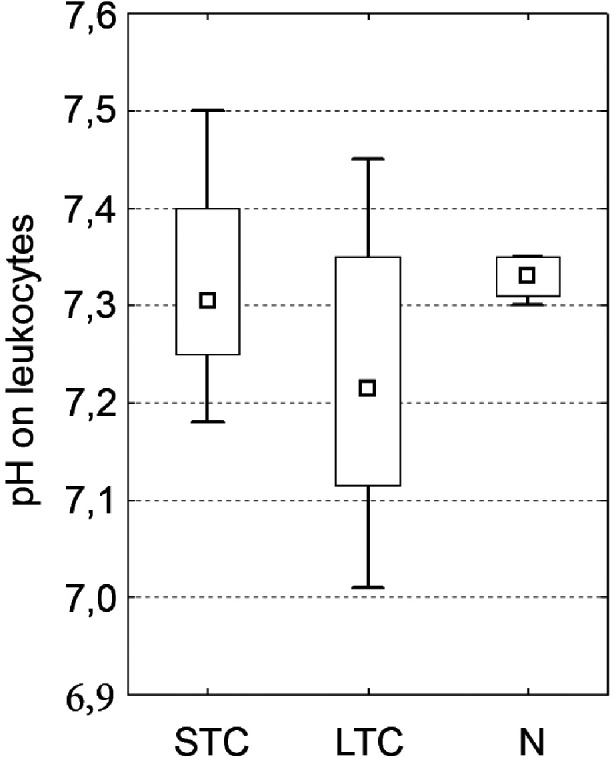
Intracellular pH detection There was a trend for lower intracellular pH (ipH) in the LTC group in comparison to the N subgroup and the STC subgroup but without statistical significancy. (median; box: 25%–75%; quantiles; * significancy P ≤ 0.05).
Discussion
High-dose chemotherapy followed by autologous stem cell transplantation represents an effective treatment for several haematological malignancies. Despite the significant improvements in the therapeutic protocols, many patients still require re-transplantation. The need for a second transplant often occurs years later and opens the question of the use of the long-term cryopreserved cells or to collect a new graft. In this presented study, we show that the PBSCs cryopreserved even for 10 years can still be safely used for the transplantation.
The quality of the graft is assessed by several key parameters that include, in particular, WBC and stem cell dose, viability, sterility, and in some cases CFU count. The dose of CD34+ cells affects the engraftment rate 17,18 , and some reports also suggest a relationship between the stem cell dose and survival parameters. Therefore, a sufficient stem cell dose is a prerequisite for a successful stem cell transplant. The analysis of CD34+ cells by flow cytometry is the most common method for quantification of haematopoietic stem cells in apheresis products prior to cryopreservation. The method is well standardized and achieves a low level of inter-laboratory variability 19 . On the contrary, there is no clear consensus regarding product quality control after thawing. Post-thaw analysis of stem cell products may cause multiple problems. Cryopreservation can lead to a significant loss of viable CD34+ cells and hence infused CD34+ cell numbers may be substantially lower than those obtained at the time of CD34+ cell harvesting 20,21 . Moreover, flow cytometry analysis can be complicated by changes in leukocyte subpopulations, the presence of cellular debris, or post-cryopreservation processing 22 . This can cause over/underestimation of CD34+ content in thawed grafts. Indeed, several studies have reported post-thaw recoveries of CD34+ or CFU greater than 100% 23 .
The capability of CD34+ cells to be stored for more than 10 years is proven in cord blood units and reviewed extensively 24 –27 , but these results are not directly comparable with PBSC, where the CD34+ cells are being mobilized from their natural environment and cryopreserved accompanied with highly activated neutrophils. Our study showed that the viability of leukocytes declined with long-term storage (68% after 10 years); however, the viability of mononuclear and CD34+ cells was retained with <10% decrease in comparison to native products. Similar data were shown by Abbruzzese et al. who reported that the viability of CD34+ cells measured after 5 weeks or after 10 years of cryopreservation remains without change (97.2 vs. 95.9%) 28 . Almost the same was obtained by Veeraputhiran et al. showing no difference of viability after > 9 years 8 . On the contrary, Liseth et al. found a decrease of CD34+ cell viability to 73% after 5 years of liquid nitrogen storage. The CD34+ loss does not impact the safety of PBSC products as the engraftment kinetics are equivalent for products stored for 1.5, 42 29 , and 60 months 30 .
The standard 7AAD viability analysis only detects necrotic and late apoptotic cells that have altered membrane permeability and early apoptotic cells are not captured. A significant number of CD34+ Annexin V+ events can be found within the 7AAD negative population, suggesting many of these viable cells may undergo apoptosis or have a limited functional potential after reinfusion. By analysing our samples together, the mean percentage of viable cells was 94.4% using 7-AAD alone and 83% when also using Annexin V staining, the difference was more pronounced in long-term cryopreserved cells (about 15%). An earlier study showed even a greater difference of about 30% in the percentage of dead cells when using 7-AAD alone as compared to 7-AAD in combination with an apoptotic marker 31 . Increased numbers of apoptotic MNCs in the thawed product may be associated with lower CFU count post-thaw and delayed neutrophil recovery after transplant 32 . Therefore, the standard CD34+ enumeration method implementing the dye exclusion viability test does not completely reflect cell potency after thawing. Furthermore, some authors suggested that CFU recovery is superior even relative to Annexin V assessment 33 .
Mitochondria contribute to the haematopoietic stem cell condition by a complex network of catabolic and anabolic pathways suggesting loss of mitochondrial activity can predict further cell damage. The organelle generates ATP by the mitochondrial ATP synthase activity and utilizes acetyl-CoA as a source of reduced equivalents to produce NADH 34 . Mitochondrial activity of HSC is linked to haematopoietic function and fate-decision making. Cells with higher mitochondrial potential (MP) have a higher differential capacity than self-renewing cells with low MP 35 . Mitochondrial damage caused by cryopreservation is widely studied in reproductive biology and freezing effects on mitochondria are also described for hepatocytes 36 or mesenchymal stromal cells 37 . Our analysis showed no differences between native and short- or long-term stored products. Detection of metabolic activity haematopoietic stem cells by the ALDH assay also failed to demonstrate any significant effects associated with extended cryopreservation time. Putting together, the mitochondrial activity and metabolic functions of stem cells seem to be well preserved even after long-term storage.
In addition to the quantification of CD34+ cells, determination of clonogenic potential represents another standard test for the evaluation of graft quality. Some authors showed decreased CFUs in PBSCs or cord blood units after cryopreservation and thawing 38,39 . Regardless, CFU recoveries remain high and do not change significantly with storage time. Winter et al. found similar CFU recoveries for grafts stored for less than 1 month and for those stored for 14 years 39 . Vosganian et al. also did not reveal any decrease in clonogenic activity of PBSCs following 10 years of cryopreservation. However, in samples stored over 10 years, activity decreased significantly 40 . Published data confirms the functional progenitor cell fraction within PBSC grafts is retained for at least 14 years. Our data from equivalent potency assay (ATP detection) indicate that long-term cryopreservation can affect the quality of the autologous PBSCs and that storage for 10 years or longer may result in some loss of clonogenic and differentiation capacity.
Regulation of intracellular pH is an essential cellular function that maintains intracellular homeostasis. Proteins, including those within the cell membrane and enzymes involved in energy metabolism, are very sensitive to changes in intracellular pH (ipH). Cells have complex systems that maintain constant ipH in the presence of metabolic and environmental changes. We found only a borderline decrease in ipH that correlated with MNC viability following long-term storage. Minor ipH changes cannot be ruled out during long-term storage and are probably caused by cell damage and loss of function. Experimental study of Xu et al. on chondrocytes showed that exposure of cells to DMSO results in a decrease of ipH followed by a recovery upon cryoprotectant removal. The ipH changes also correlated with membrane integrity and cell volume 41 .
Our complex analysis of the cryopreserved PBSC samples demonstrates that stem cells can retain their quality for up to 10 years in cryostorage. In long-term cryopreservation (∼10 years), there are no major changes in haematopoietic stem cell properties or function. Based on this study, the expiration time for grafts stored in our tissue bank was set to 10 years.
The study also confirmed that, to support high-dose chemotherapy, only minimal graft quality control post thawing is necessary when validated and standardized methods for stem cell freezing, storing, and application are used and when sufficient doses of CD34+ cells are collected. However, PBSC grafts may deteriorate with extended storage and a decrease in stem cell clonogenic activity may be observed after periods of over 10 years. Post-freeze estimation may be necessary when transplantation is considered for patients with grafts in long-term storage, especially when a low number of CD34+ cells was collected, for example, due to poor mobilization. The quality of aged grafts should be confirmed using the appropriate graft potency assays (CFU assay or ATP assay as the equivalent method).
Supplemental Material
Supplemental Material, sj-docx-1-cll-10.1177_09636897211036004 for Long-Term Cryopreservation Does Not Affect Quality of Peripheral Blood Stem Cell Grafts: A Comparative Study of Native, Short-Term and Long-Term Cryopreserved Haematopoietic Stem Cells by Daniel Lysak, Michaela Brychtová, Martin Leba, Miroslava Čedíková, Daniel Georgiev, Pavel Jindra, Tomáš Vlas and Monika Holubova in Cell Transplantation
Footnotes
Ethical Approval: The informed consents were obtained from all patients in accordance with the good clinical practice. Whereas the study was carried out to improve the quality of long-term storage of transplants and was not primarily experimental work, no specific approval was required by the institutional ethics committee.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the national ethical standards (respecting the Helsinki declaration), good clinical practice (GCP) and good laboratory practice (GLP).
Statement of Informed Consent: All patients enrolled in the autologous stem cell transplant program have agreed and signed informed consent to perform apheresis procedure and to use the remaining cryopreserved cellular material and data from collections for scientific purposes (including publishing of anonymous data).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the grant of Ministry of Health of the Czech Republic - Conceptual Development of Research Organization (Faculty Hospital in Pilsen - FNPl, 00669806) and by the grant SVV–2020-2022 No 260 540.
ORCID iD: Monika Holubova  https://orcid.org/0000-0002-5096-9659
https://orcid.org/0000-0002-5096-9659
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Martino M, Recchia AG, Fedele R, Neri S, Vincelli ID, Moscato T, Gentile M, Morabito F. The role of tandem stem cell transplantation for multiple myeloma patients. Expert Opin Biol Ther. 2016;16(4):515–534. [DOI] [PubMed] [Google Scholar]
- 2. Lewalle P, Wittnebel S. Risk-adapted transplant strategies for high-risk Hodgkin lymphoma: are we there? Curr Opin Oncol. 2016;28(5):390–397. [DOI] [PubMed] [Google Scholar]
- 3. Windrum P, Morris TCM, Drake MB, Niederwieser D, Ruutu T, EBMT Chronic Leukaemia Working Party Complications Subcommittee. Variation in dimethyl sulfoxide use in stem cell transplantation: a survey of EBMT centres. Bone Marrow Transplant. 2005;36(7):601–603. [DOI] [PubMed] [Google Scholar]
- 4. Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5(3):213–226. [DOI] [PubMed] [Google Scholar]
- 5. Morgenstern DA, Ahsan G, Brocklesby M, Ings S, Balsa C, Veys P, Brock P, Anderson J, Amrolia P, Goulden N, Cale CM, et al. Post-thaw viability of cryopreserved peripheral blood stem cells (PBSC) does not guarantee functional activity: important implications for quality assurance of stem cell transplant programmes. Br J Haematol. 2016;174(6):942–951. [DOI] [PubMed] [Google Scholar]
- 6. Abrahamsen JF, Wentzel-Larsen T, Bruserud O. Autologous transplantation: the viable transplanted CD34+ cell dose measured post-thaw does not predict engraftment kinetics better than the total CD34+ cell dose measured pre-freeze in patients that receive more than 2 × 10(6) CD34+ cells/kg. Cytotherapy. 2004;6(4):356–362. [DOI] [PubMed] [Google Scholar]
- 7. Lanza F, Mangianti S, Accorsi P, Lombardini L, Martino M, Saccardi R, Vassanelli A, Ostuni A, Ciceri F. Manipulation, and cryopreservation of autologous peripheral blood stem cell products in Italy: A survey by GITMO, SIDEM and GIIMA societies. Transfus. Apher Sci. 2020;59(2):102753. [DOI] [PubMed] [Google Scholar]
- 8. Veeraputhiran M, Theus JW, Pesek G, Barlogie B, Cottler-Fox M. Viability and engraftment of hematopoietic progenitor cells after long-term cryopreservation: effect of diagnosis and percentage dimethyl sulfoxide concentration. Cytotherapy. 2010;12(6):764–766. [DOI] [PubMed] [Google Scholar]
- 9. Humpe A, Riggert J, Vehmeyer K, Troff C, Hiddemann W, Köhler M, Wörmann B. Comparison of CD34+ cell numbers and colony growth before and after cryopreservation of peripheral blood progenitor and stem cell harvests: influence of prior chemotherapy. Transfusion (Paris). 1997;37(10):1050–1057. [DOI] [PubMed] [Google Scholar]
- 10. Broxmeyer HE, Srour EF, Hangoc G, Cooper S, Anderson SA, Bodine DM. High-efficiency recovery of functional hematopoietic progenitor and stem cells from human cord blood cryopreserved for 15 years. Proc Natl Acad Sci U S A. 2003;100(2):645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mugishima H, Harada K, Chin M, Suzuki T, Takagi K, Hayakawa S, Sato K, Klein JP, Gale RP. Effects of long-term cryopreservation on hematopoietic progenitor cells in umbilical cord blood. Bone Marrow Transplant. 1999;23(4):395–396. [DOI] [PubMed] [Google Scholar]
- 12. Detry G, Calvet L, Straetmans N, Cabrespine A, Ravoet C, Bay JO, Petre H, Paillard C, Husson B, Merlin E, Boon-Falleur L, et al. Impact of uncontrolled freezing and long-term storage of peripheral blood stem cells at - 80 °C on haematopoietic recovery after autologous transplantation. Report from two centres. Bone Marrow Transplant. 2014;49(6):780–785. [DOI] [PubMed] [Google Scholar]
- 13. Ayello J, Semidei-Pomales M, Preti R, Hesdorffer C, Reiss RF. Effects of long-term storage at -90 degrees C of bone marrow and PBPC on cell recovery, viability, and clonogenic potential. J Hematother. 1998;7(4):385–390. [DOI] [PubMed] [Google Scholar]
- 14. Valeri CR, Pivacek LE. Effects of the temperature, the duration of frozen storage, and the freezing container on in vitro measurements in human peripheral blood mononuclear cells. Transfusion (Paris). 1996;36(4):303–308. [DOI] [PubMed] [Google Scholar]
- 15. Fernyhough LJ, Buchan VA, McArthur LT, Hock BD. Relative recovery of haematopoietic stem cell products after cryogenic storage of up to 19 years. Bone Marrow Transplant. 2013;48(1):32–35. [DOI] [PubMed] [Google Scholar]
- 16. Donnenberg AD, Koch EK, Griffin DL, Stanczak HM, Kiss JE, Carlos TM, Buchbarker DM, Yeager AM. Viability of cryopreserved BM progenitor cells stored for more than a decade. Cytotherapy. 2002;4(2):157–163. [DOI] [PubMed] [Google Scholar]
- 17. Weaver CH, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L, West W. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86(10):3961–3969. [PubMed] [Google Scholar]
- 18. Ergene U, Cağirgan S, Pehlivan M, Yilmaz M, Tombuloğlu M. Factors influencing engraftment in autologous peripheral hematopoetic stem cell transplantation (PBSCT). Transfus Apher Sci. 2007;36(1):23–29. [DOI] [PubMed] [Google Scholar]
- 19. Levering WHBM, Preijers FWMB, van Wieringen WN, Kraan J, van Beers WAM, Sintnicolaas K, van Rhenen DJ, Gratama JW. Flow cytometric CD34+ stem cell enumeration: lessons from nine years’ external quality assessment within the Benelux countries. Cytometry B Clin Cytom. 2007;72(3):178–188. [DOI] [PubMed] [Google Scholar]
- 20. Castelhano MV, Reis-Alves SC, Vigorito AC, Rocha FF, Pereira-Cunha FG, De Souza CA, Lorand-Metze I. Quantifying loss of CD34+ cells collected by apheresis after processing for freezing and post-thaw. Transfus Apher Sci. 2013;48(2):241–246. [DOI] [PubMed] [Google Scholar]
- 21. D’Rozario J, Parisotto R, Stapleton J, Gidley A, Owen D. Pre infusion, post thaw CD34+ peripheral blood stem cell enumeration as a predictor of haematopoietic engraftment in autologous haematopoietic cell transplantation. Transfus Apher Sci. 2014;50(3):443–450. [DOI] [PubMed] [Google Scholar]
- 22. Reich-Slotky R, Colovai AI, Semidei-Pomales M, Patel N, Cairo M, Jhang J, Schwartz J. Determining post-thaw CD34+ cell dose of cryopreserved haematopoietic progenitor cells demonstrates high recovery and confirms their integrity. Vox Sang. 2008;94(4):351–357. [DOI] [PubMed] [Google Scholar]
- 23. Itoh T, Minegishi M, Fushimi J, Takahashi H, Kudo Y, Suzuki A, Narita A, Sato Y, Akagi K, Wada Y, Saito A, et al. A simple controlled-rate freezing method without a rate-controlled programmed freezer provides optimal conditions for both large-scale and small-scale cryopreservation of umbilical cord blood cells. Transfusion (Paris). 2003;43(9):1303–1308. [DOI] [PubMed] [Google Scholar]
- 24. Kurita N, Frassoni F, Chiba S, Podestà M. Impact of length of cryopreservation and origin of cord blood units on hematologic recovery following cord blood transplantation. Bone Marrow Transplant. 2015;50(6):818–821. [DOI] [PubMed] [Google Scholar]
- 25. Kim G-H, Kwak J, Kim SH, Kim HJ, Hong HK, Jin HJ, Choi SJ, Oh W, Um S. High integrity and fidelity of long-term cryopreserved umbilical cord blood for transplantation. J Clin Med. 2021;10(2):293. Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7830419/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mitchell R, Wagner JE, Brunstein CG, Cao Q, McKenna DH, Lund TC, Verneris MR. Impact of long-term cryopreservation on single umbilical cord blood transplantation outcomes. Biol Blood Marrow Transplant. 2015;21(1):50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arutyunyan I, Fatkhudinov T, Sukhikh G. Umbilical cord tissue cryopreservation: a short review. Stem Cell Res Ther. 2018;9(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abbruzzese L, Agostini F, Durante C, Toffola RT, Rupolo M, Rossi FM, Lleshi A, Zanolin S, Michieli M, Mazzucato M. Long term cryopreservation in 5% DMSO maintains unchanged CD34(+) cells viability and allows satisfactory hematological engraftment after peripheral blood stem cell transplantation. Vox Sang. 2013;105(1):77–80. [DOI] [PubMed] [Google Scholar]
- 29. Liseth K, Ersvær E, Abrahamsen JF, Nesthus I, Ryningen A, Bruserud Ø. Long-term cryopreservation of autologous stem cell grafts: a clinical and experimental study of hematopoietic and immunocompetent cells. Transfusion (Paris). 2009;49(8):1709–1719. [DOI] [PubMed] [Google Scholar]
- 30. Lisenko K, Pavel P, Kriegsmann M, Bruckner T, Hillengass J, Goldschmidt H, Witzens-Harig M, Ho AD, Wuchter P. Storage duration of autologous stem cell preparations has no impact on hematopoietic recovery after transplantation. biol. blood marrow transplant. Blood Marrow Transplant. 2017;23(4):684–690. [DOI] [PubMed] [Google Scholar]
- 31. de Boer F, Dräger AM, Pinedo HM, Kessler FL, van der Wall E, Jonkhoff AR, van der Lelie J, Huijgens PC, Ossenkoppele GJ, Schuurhuis GJ. Extensive early apoptosis in frozen-thawed CD34-positive stem cells decreases threshold doses for haematological recovery after autologous peripheral blood progenitor cell transplantation. Bone Marrow Transplant. 2002;29(3):249–255. [DOI] [PubMed] [Google Scholar]
- 32. Wu L, Al-Hejazi A, Filion L, Ben R, Halpenny M, Yang L, Giulivi A, Allan DS. Increased apoptosis in cryopreserved autologous hematopoietic progenitor cells collected by apheresis and delayed neutrophil recovery after transplantation: a nested case-control study. Cytotherapy. 2012;14(2):205–214. [DOI] [PubMed] [Google Scholar]
- 33. Duggleby RC, Querol S, Davy RC, Fry LJ, Gibson DA, Horton RBV, Mahmood SN, Gomez SG, Madrigal JA. Flow cytometry assessment of apoptotic CD34+ cells by annexin V labeling may improve prediction of cord blood potency for engraftment. Transfusion (Paris). 2012;52(3):549–559. [DOI] [PubMed] [Google Scholar]
- 34. Bonora M, Pinton P, Ito K. Mitochondrial control of hematopoietic stem cell balance and hematopoiesis. Front Biol. 2015;10(2):117–124. [Google Scholar]
- 35. Vannini N, Girotra M, Naveiras O, Nikitin G, Campos V, Giger S, Roch A, Auwerx J, Lutolf MP. Specification of haematopoietic stem cell fate via modulation of mitochondrial activity. Nat Commun. 2016;7:13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stéphenne X, Najimi M, Ngoc DK, Smets F, Hue L, Guigas B, Sokal EM. Cryopreservation of human hepatocytes alters the mitochondrial respiratory chain complex 1. Cell Transplant. 2007;16(4):409–419. [DOI] [PubMed] [Google Scholar]
- 37. Xu X, Liu Y, Cui Z, Wei Y, Zhang L. Effects of osmotic and cold shock on adherent human mesenchymal stem cells during cryopreservation. J Biotechnol. 2012;162(2–3):224–231. [DOI] [PubMed] [Google Scholar]
- 38. Yamamoto S, Ikeda H, Toyama D, Hayashi M, Akiyama K, Suzuki M, Tanaka Y, Watanabe T, Fujimoto Y, Hosaki I, Nishihira H, et al. Quality of long-term cryopreserved umbilical cord blood units for hematopoietic cell transplantation. Int J Hematol. 2011;93(1):99–105. [DOI] [PubMed] [Google Scholar]
- 39. Winter JM, Jacobson P, Bullough B, Christensen AP, Boyer M, Reems J-A. Long-term effects of cryopreservation on clinically prepared hematopoietic progenitor cell products. Cytotherapy. 2014;16(7):965–975. [DOI] [PubMed] [Google Scholar]
- 40. Vosganian GS, Waalen J, Kim K, Jhatakia S, Schram E, Lee T, Riddell D, Mason JR. Effects of long-term cryopreservation on peripheral blood progenitor cells. Cytotherapy. 2012;14(10):1228–1234. [DOI] [PubMed] [Google Scholar]
- 41. Xu X, Cui ZF, Wilkins RJ, Urban JPG. Intracellular pH changes in isolated bovine articular chondrocytes during the loading and removal of cryoprotective agents. Cryobiology. 2003;46(2):161–173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-docx-1-cll-10.1177_09636897211036004 for Long-Term Cryopreservation Does Not Affect Quality of Peripheral Blood Stem Cell Grafts: A Comparative Study of Native, Short-Term and Long-Term Cryopreserved Haematopoietic Stem Cells by Daniel Lysak, Michaela Brychtová, Martin Leba, Miroslava Čedíková, Daniel Georgiev, Pavel Jindra, Tomáš Vlas and Monika Holubova in Cell Transplantation