Abstract
Background
Nonsteroidal anti-inflammatory drugs (NSAIDs) have been discouraged for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections, fearing that they could increase the risk of infection or the severity of SARS-CoV-2.
Methods
Original studies providing information on exposure to NSAIDs and coronavirus disease 2019 (COVID-19) outcomes were retrieved and were included in a descriptive analysis and a meta-analysis with Cochrane Revue Manager (REVMAN 5.4), using inverse variance odds ratio (OR) with random- or fixed-effects models.
Results
Of 92,853 papers mentioning COVID-19, 266 mentioned NSAIDs and 61 mentioned ibuprofen; 19 papers had analysable data. Three papers described NSAID exposure and the risk of SARS-CoV-2 positivity, five papers described the risk of hospital admission in positive patients, 10 papers described death, and six papers described severe composite outcomes. Five papers studied exposure to ibuprofen and death. Using random-effects models, there was no excess risk of SARS-CoV-2 positivity (OR 0.86, 95% confidence interval [CI] 0.71–1.05). In SARS-CoV-2-positive patients, exposure to NSAIDs was not associated with excess risk of hospital admission (OR 0.90, 95% CI 0.80–1.17), death (OR 0.88, 95% CI 0.80–0.98), or severe outcomes (OR 1.14, 95% CI 0.90–1.44). With ibuprofen, there was no increased risk of death (OR 0.94, 95% CI 0.78–1.13). Using a fixed-effect model did not modify the results, nor did the sensitivity analyses.
Conclusion
The theoretical risks of NSAIDs or ibuprofen in SARS-CoV-2 infection are not confirmed by observational data.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-021-01089-5.
Key Points
| Exposure to nonsteroidal anti-inflammatory drugs (NSAIDs) was not associated with increased risk of testing positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), hospital admission in SARS-CoV-2-positive (coronavirus disease 2019 [COVID-19]) patients, or severe COVID-19 or death with enough power to confidently exclude a significant risk. |
| There is no reason not to use NSAIDs to alleviate the symptoms of SARS-CoV-2 infection if needed. |
Introduction
Based on anecdotal reports that severe coronavirus disease 2019 (COVID-19) cases had been exposed to ibuprofen (nonsteroidal anti-inflammatory drugs [NSAIDs]) [1], and on theoretical bases such as upregulation of angiotensin-converting enzyme (ACE) 2, a target of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2, 3], in April 2020 French authorities warned against the use of ibuprofen, available over the counter (OTC), along with other NSAIDs, in patients with COVID-19 symptoms [1, 4]. One year after this warning, it seemed opportune to collate the evidence regarding the possible risks associated with the use of NSAIDs in COVID-19. The questions addressed were whether exposure to NSAIDs could increase the risk of testing positive to SARS-CoV-2, and whether, in such patients, infection by SARS-CoV-2 could lead to COVID-19, resulting in more severe disease as manifested by hospital admission, admission to intensive care or mechanical ventilation, or death. This did not relate to a hypothetical effect of NSAIDs on the immune response to vaccination [5].
In the absence of clinical trials of NSAIDs in COVID-19, the present review was of observational studies only, regardless of their study design and patient populations.
Methods
We conducted a review and meta-analysis of publications mentioning the use of NSAIDs and COVID-19 outcomes, identified from the PubMed MEDLINE database using EndNote 20 (Clarivate Analytics, Philadelphia, PA, USA), any mention of COVID or SARS-CoV-2 in the title or any field and any mention of NSAIDs or ibuprofen in the title or any field, and published in 2020 or 2021 (last searched on 1 March 2021). Papers mentioning risk factors for the occurrence of COVID-19 or its outcomes were scanned for the presence of NSAIDs among the factors reported. Only studies reporting NSAIDs or ibuprofen and reporting original research were retained, either published or provided as preprints. Opinion papers and editorials were scanned for relevant references. Papers were selected based on their title and abstract, followed by careful analysis of the whole text to ascertain the presence of useful data. Such data included number of exposed and control participants, number of events, and measures of association (relative risk [RR], hazard ratios [HRs], or odds ratios [ORs] according to the study design), with confidence intervals (CIs), and including methods used for adjustment or matching. Original papers were abstracted and tabulated. Effect estimates associated with relevant events and exposures were then included in a meta-analysis using the Cochrane RevMan 5.4 software (Review Manager [RevMan; computer program]. Version 5.4. The Cochrane Collaboration, 2020), applying inverse variance ORs in random effects models for each outcome of interest. Log(OR) and standard error (SE) were recomputed from ORs and CIs. A fixed-effects model was also tested. Outcomes of interest were the positivity of tests for SARS-CoV-2 in general or selected populations (e.g. patients with rheumatoid arthritis or other rheumatic diseases); hospital admission in patients with positive tests; fatal outcomes in such patients, whether admitted to hospital or not; and other outcomes, such as composite outcomes indicating severe infection when individual outcomes were not specified, or the need for intensive care. Papers mentioning exposure to ibuprofen and death were examined separately. Outcomes were taken as is from the publications without attempting re-ascertainment.
Timings of exposure were considered indiscriminately, without taking into account recent or longer exposure, considering the small numbers of papers; these are described in the main results table. The papers included mostly cohort studies in population databases, or ad hoc studies in patients, exposed or control populations being adjusted, or matched, including those with propensity scores. The data provided by the papers regarding ORs, HRs, or RRs were used as reported. Standard errors were recomputed from the reported CIs. There was no specific attempt to quantify risk of bias in these observational studies, which were all considered as potentially biased. Individual study data are provided in Table 2, while the recomputed SE in the forest plots are shown in Figs. 1, 2, 3, 4 and 5. Computation of the meta-analytical results included the standard statistics provided by the software, as described in Figs. 1, 2, 3, 4 and 5, including measures of heterogeneity.
Table 2.
SARS-CoV-2 risks in NSAIDs users
| Study (reference) | Subjects | Exposure | Timing | Outcome of interest | Control | NSAIDs/Ibuprofen | OR, HR, RRa | ||
|---|---|---|---|---|---|---|---|---|---|
| Number | Outcomes | Number exposed | Exposed outcomes | ||||||
| (a) SARS-CoV-2 positivity | |||||||||
| Chandan et al. [12] | OA | NSAIDs vs. codamol | COVID+ | 8595 | 76 | 8595 | 63 | 0.79 [0.57–1.11] | |
| Chang et al. [11] | General population | NSAIDs | <90 days | COVID+ | 26,600 | 992 | 1948 | 58 | 0.89 [0.65–1.10] |
| Costantino et al. [13] | Rheumatic diseases | NSAIDs | Current | COVID+ | 655 | 45 | 313 | 16 | 0.92 [0.50–1.69] |
| (b) Hospital admissions | |||||||||
| Abu Esba et al. [14] | C+ | NSAIDs vs. non-NSAIDs | Current | Hospital | 357 | 62 | 40 | 9 | 1.27 [0.55–2.95] |
| Chang et al. [11] | COVID+ (C+) | NSAIDs | < 90 days | Hospital | 843 | 177 | 58 | 17 | 1.00 [0.48–2.10] |
| Gianfrancesco et al. [15] | RA | NSAIDs | Prior | Hospital | 600 | 277 | 111 | 39 | 0.64 [0.39–1.06] |
| Imam et al. [16] | C+ | NSAIDs | Prior | Hospital | 2040 | 1305 | 714 | 466 | 1.00 [0.85–1.20] |
| Lund et al. [17] | C+ | NSAIDs | < 30 days | Hospital | 896 | 175 | 224 | 50 | 1.16 [0.87–1.53] |
| (c) Deaths | |||||||||
| Abu Esba et al. [14] | C+ | NSAIDs vs. non-NSAIDs | Current | Death | 357 | 11 | 17 | 1 | 5.81 [0.43–77.8] |
| Alamdari et al. [18] | Hospitalised C+ | NSAIDs | Prior | Death | 459 | 63 | 37 | 6 | 1.18 [0.48–2.91 |
| Bruce et al. [19] | Hospitalised C+ | NSAIDs | Prior | Death | 1222 | 358 | 54 | 14 | 0.89 [0.52–1.53] |
| Chandan et al. [12] | Matched cohort (THIN) | NSAIDs vs. paracetamol | Current | Death | 8595 | 76 | 8595 | 63 | 0.85 [0.61–1.20]. |
| Drake et al. [24] | Hospitalised C+ | NSAIDs | 14 days | Death | 4205 | 1324 | 4205 | 1273 | 0.95 [0.84–1.07] |
| Gupta et al. [20] | ICU patients | NSAIDs | Previous | Death | 2215 | 784 | 191 | 61 | 0.89 [0.66–1.20] |
| Hwang et al. [21] | Hospitalised | NSAIDs | Prior | Death | 103 | 26 | 5 | 2 | 1.52 [0.29–8.63] |
| Imam et al. [22] | Hospitalised C+ | NSAIDs | Prior | Death | 1305 | 200 | 466 | 38 | 0.55 [0.39–0.78] |
| Lund et al. [17] | C+ | NSAIDs | < 30 days | Death | 896 | 55 | 224 | 14 | 1.02 [0.56–2.30] |
| Park et al. [10] | C+ | NSAIDs vs. paracetamol | 14 days | Death | 397 | 12 | 397 | 16 | 1.33 [0.67–2.88] |
| Sahai et al. [30] | Hospitalised C+ | NSAIDs | < 90 days | Death | 444 | 32 | 444 | 31 | 0.97 [0.58–1.62] |
| Wong et al. [23] | General population | NSAIDs | < 4 months | Death | 1,924,095 | 611 | 535,519 | 218 | 0.95 [0.80–1.13] |
| Wong et al. [23] | RA | NSAIDs | < 4 months | Death | 1,525,421 | 2441 | 175,631 | 123 | 0.79 [0.65–0.94] |
| (d) Ibuprofen and death | |||||||||
| Abu Esba et al. [14] | C+ | IBU vs. non-NSAID | Current | Death | 357 | 11 | 40 | 1 | 0.63 [0.07–5.44] |
| Drake et al. [24] | C+ | IBU vs. non-NSAID | Current | Death | 721 | NA | 721 | NA | 0.90 [0.71–1.13] |
| Drake et al. [24] | C+ | IBU vs. other NSAID | Current | Death | 908 | NA | 908 | NA | 0.82 [0.66–1.03] |
| Rinott et al. [26]b | C+ | IBU | < 7 days | Death | 403 | 12 | 87 | 3 | 1.13 [0.49–2.63] |
| Wong et al. [23] | General population | IBU | < 4 months | Death | 1,924,095 | 611 | 50,603 | 46 | 1.23 [0.90–1.67] |
| Wong et al. [23] | RA | IBU | < 4 months | Death | 1,535,421 | 2441 | 21,893 | 25 | 0.83 [0.59–1.25 |
| (e) Severe outcomes (ICU, ventilation or death) | |||||||||
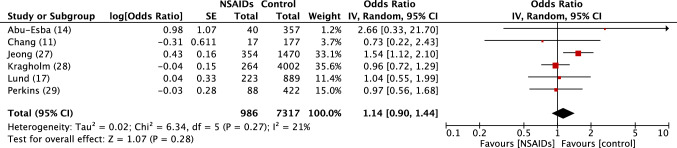
| Abu Esba et al. [14] | C+ | IBU vs. non-NSAID | Current | Composite | 357 | 40 | 2.69 [0.33–22.0] | ||
| Chang et al. [11] | Hospitalised C+ | NSAIDs | < 90 days | Severe COVID | 177 | 56 | 17 | 5 | 0.73 [0.22–2.20] |
| Jeong et al. [27]b | Hospitalised C+ | NSAIDs | < 7 days | Composite | 1470 | 52 | 354 | 22 | 1.54 [1.13–2.11] |
| Kragholm et al. [28] | C+ | IBU | < 90 days | Composite | 4002 | 646 | 264 | 42 | 0.96 [0.72–1.23] |
| Lund et al. [17] | C+ | NSAIDs | < 30 days | ICU admission | 889 | 175 | 223 | 11 | 1.04 [0.54–2.02] |
| Lund et al. [17]* | C+ | NSAIDs | < 30 days | Severe | 891 | 35 | 224 | 10 | 1.14 [0.56–2.30] |
| Perkins et al. [29] | C+ | NSAIDs | Current | Composite | 422 | 89 | 88 | 18 | 0.97 [0.56–1.69] |
C+ patients positive to SARS-CoV-2 tests, NSAIDs nonsteroidal anti-inflammatory drugs, IBU ibuprofen, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, OR odds ratio, HR hazard ratio, RR relative risk, OA osteoarthritis, COVID coronavirus disease, RA rheumatoid arthritis, ICU intensive care unit, NA not available
aMeasure of association: RR, OR, or HR, adjusted as provided by the authors
bNo deaths occurred in patients who received only ibuprofen (see Rinott et al. [26]) or only NSAIDs (see Jeong et al. [27])
*Lund et al. include several outcomes
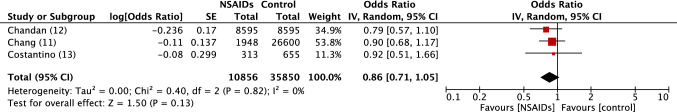
Fig. 1.
Forest plot of observational studies of positivity to SARS-COV2 in persons exposed or not to NSAIDs. Inverse variance odds ratios with a random-effects model. SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, NSAIDs Non-steroidal anti-inflammatory drugs, IV inverse variance, CI confidence interval, SE standard error, df degrees of freedom
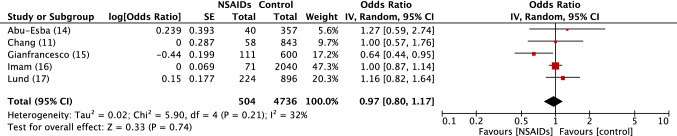
Fig. 2.
Forest plot of observational studies of Hospital admission in SARS-COV2 positive patients exposed or not to NSAIDs. Inverse variance odds ratios with a random-effects model. SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, NSAIDs Non-steroidal anti-inflammatory drugs, IV inverse variance, CI confidence interval, SE standard error, df degrees of freedom
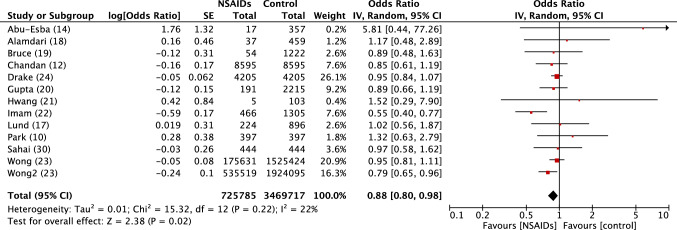
Fig. 3.
Forest plot of observational studies of death related to SARS-COV2 in persons exposed or not to NSAIDs. Inverse variance odds ratios with a random-effects model. SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, NSAIDs Non-steroidal anti-inflammatory drugs, IV inverse variance, CI confidence interval, SE standard error, df degrees of freedom
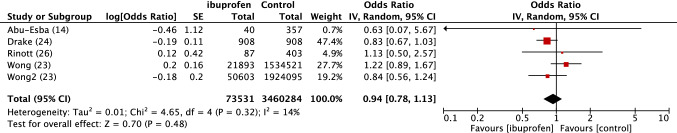
Fig. 4.
Forest plot of observational studies of death in SARS-COV2 patients exposed or not to ibuprofen. Inverse variance odds ratios with a random-effects model. SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, NSAIDs Non-steroidal anti-inflammatory drugs, IV inverse variance, CI confidence interval, SE standard error, df degrees of freedom
Fig. 5.
Forest plot of observational studies of severe outcomes to SARS-COV2 in persons exposed or not to NSAIDs. See table 2 for references to studies. Inverse variance odds ratios with a random-effects model. SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, NSAIDs Non-steroidal anti-inflammatory drugs, IV inverse variance, CI confidence interval, SE standard error, df degrees of freedom
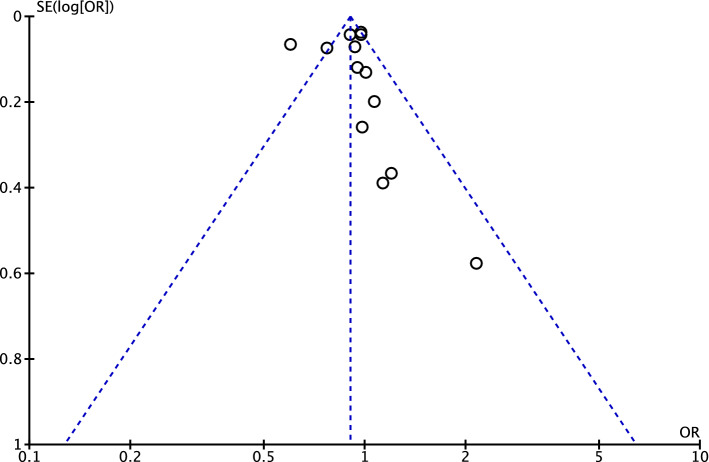
A funnel plot was generated for death.
Results
Literature Search
Of 92,853 papers identified in the Pubmed MEDLINE database that cited COVID-19 in the title (Table 1), 14 also mentioned NSAIDs in the title and 21 also mentioned ibuprofen; 266 included NSAIDs in all fields (title, keywords, abstract) and 61 included ibuprofen; and 2292 included mortality in the title and 11,616 included mortality in all fields. Of the latter, 141 included risk factors in the title and 114 included hospital admission. Overall, 18 papers with evaluable data were retained (Table 2). ‘Other’ papers were editorials or opinion papers. Two papers mentioning reviews in the title were identified: one related to ibuprofen and acute bacterial or viral respiratory lower tract infections [6], while the other was a narrative review of the issues involved [7]. We found no other systematic reviews on this topic, while some papers of potential interest provided no usable data [8, 9].
Table 1.
Literature search strategy and results (last updated 10 May 2021)
| Search strategy | |
|---|---|
| COVID-19 (Title) | 92,853 |
| And (Title) | |
| NSAIDs | 14 |
| Ibuprofen | 21 |
| And (All fields) | |
| NSAIDs | 266 |
| Ibuprofen | 61 |
| COVID (All fields) | |
| And (all fields) | |
| NSAIDs | 393 |
| Ibuprofen | 79 |
| COVID (title) | |
| And | |
| Mortality (All fields) | 11,616 |
| Mortality (title) | 2292 |
| Mortality (title) and risk factors (title) | 141 |
| COVID (Title) | |
| And | |
| Hospital admission (title) | 114 |
| Risk factors (title) | 5 |
| COVID (Title) and NSAIDs (all fields) and death (all fields) | 23 |
COVID-19 coronavirus disease 2019, NSAIDs non-steroidal anti-inflammatory drugs
In ClinicalTrials.gov, 22 studies cited COVID and NSAIDs, a few of which might be of interest when completed or published, i.e. one observational study of OTC medication and COVID (NCT04500639), results expected in March 2023; one randomised clinical trial (LIBERATE, NCT04334629), results expected in September 2021; and an observational study of the impact of exposure to NSAIDs on the outcomes of COVID-19 patients in the intensive care unit (ICU) [NCT04757792], indicated as completed in February 2021 but no results have been given as yet and no publications were found. One clinical trial of naproxen (ENACOVID, NCT04325633) was terminated due to the lack of recruitment.
The Cochrane library provided another previously unidentified paper; in Korea, Park et al. [10] identified patients positive for COVID-19, and described outcomes (death), in cohorts of patients who were prescribed NSAIDs or paracetamol within 14 days prior to a positive test [10].
Three papers reported on SARS-CoV-2 positivity with NSAID exposure in the general population [11], in patients with osteoarthritis [12], and in patients with rheumatic diseases (Table 2a) [13].
Five papers reported on hospital admissions in patients positive to COVID-19 exposed to NSAIDs (Table 2b) [11, 14–17].
Ten papers described death in NSAID users [12, 14, 17–24], with various exposure periods, including current or acute exposure [12, 14, 25], or within 30 days [17] or 4 months [23].
Other studies indicate prior [16, 18, 19, 21] or previous use [20]. Abu Esba et al. also included both acute and chronic users, but only acute users were included [14]. Wong et al. included two patient populations, in OpenSAFELY: the general population and patients with rheumatic diseases, and two exposures, relating to all NSAIDs and specifically ibuprofen.
Studies that provided different populations, such as OpenSAFELY, were treated as separate studies for each population. There were no duplicate patients (Wong et al. [23]).
Four papers studied death in ibuprofen users, for current exposures [14, 24], exposures of < 7 days [26] or exposures of < 4 months, in rheumatoid arthritis, or in the general population [23] (Table 2d). The paper by Wong et al., in either the general population or the rheumatoid arthritis population, was considered as two different studies [23].
In addition, six papers studied the association of exposure to ibuprofen or NSAIDs and severe COVID-19, a composite of admission to intensive care, artificial ventilation, and death [11, 14, 17, 27–29]. Jeong et al., Kragholm et al., and Perkins et al. only provided results for the composite outcome [27–29]. Jeong et al. [27] and Rinott et al. [26] provided data on patients using only NSAIDs or ibuprofen, compared with paracetamol, and provided death frequency but not the size of the exposure groups or measures of association. In both cases, there were no deaths with ibuprofen or NSAIDs alone, compared with 3–4% with paracetamol.
Study authors were contacted to check the status of NCT04325633 (from ClinicalTrials.gov), a clinical trial of naproxen. Furthermore, an attempt was made to obtain data from Jeong regarding the number of patients receiving ibuprofen alone but this was unsuccessful [27], and to verify an apparent inversion of columns in the study by Drake et al. [24], and the number of cases in the study by Sahai et al. [30].
No non-English-language papers were identified (see Table 2 for a summary of the studies).
Meta-analysis
Results of the meta-analysis are shown in Figs. 1, 2, 3, 4, 5 and 6 and electronic supplementary Figs. e1–e6. No association was found between the selected outcomes and prior exposure to NSAIDs in the random effect models. The heterogeneity was generally low (I2 from 0% to 32%, all p > 0.05).
Fig. 6.
Funnel plot for studies of the association of exposure to NSAIDs and risk of death in users of NSAIDs with COVID-19. NSAIDs nonsteroidal anti-inflammatory drugs, COVID-19 coronavirus disease 2019
There was no excess risk of SARS-CoV-2 positivity in patients exposed to NSAIDs or ibuprofen (OR 0.86, 95% CI 0.80–1.05) (Fig. 1). In patients positive to SARS-CoV-2, exposure to NSAIDs was not associated with an increased risk of being admitted to hospital (OR 0.97, 95% CI 0.80–1.17) (Fig. 2), dying after exposure to NSAIDs (OR 0.88, 95% CI 0.80–0.98) (Fig. 3) or ibuprofen (OR 0.94, 95% CI 0.78–1.13), or having severe outcomes (OR 1.14, 95% CI 0.90–1.44) (Fig. 5).
The funnel plot for the studies of death as an outcome is provided. It shows a lack of small studies showing lower or no risk with NSAIDs (Fig. 6).
Sensitivity Analyses
Fixed-effects models did not change the results (see electronic supplementary Figs. e1–5).
COVID+: OR 0.86, 95% CI 0.71–1.05; I2 = 0% (electronic supplementary Fig. e1).
Hospital admission: OR 0.98, 95% CI 0.88–1.10; I2 = 32% (electronic supplementary Fig. e2).
Death: OR 0.90, 95% CI 0.83–0.97; I2 = 22% (electronic supplementary Fig. e3).
Death with ibuprofen: OR 0.92, 95% CI 0.79–1.08; I2 = 14% (electronic supplementary Fig. e4).
Severe or composite outcome: OR 1.15, 95% CI 0.95–1.38; I2 = 21% (electronic supplementary Fig. e5).
Removing the largest study (Wong et al. [23]) did not significantly change the outcome (OR 0.89, 95% CI 0.77–1.02; I2 = 23%) [not shown].
For most outcomes, the upper limit of the 95% CI of the meta-analysis OR was below 1.25, generally considered as indicating equivalence and enabling to confidently reject an increased risk of NSAIDs or ibuprofen, on Covid-19 positivity, hospitalisation or death, at least in the circumstances covered by these studies.
Discussion
We found no indication to date of any negative association on the use of NSAIDS, including ibuprofen, with SARS-CoV-2 infection or its outcomes.
The reasons for the recommendation against the use of ibuprofen to relieve pain (especially headache) and fever during the SARS-CoV-2 infection included a suspicion that ibuprofen might increase the risk of acquiring SARS-CoV-2 infection through ACE-2 upregulation [31] and a risk of greater severity of the disease, including an increasing risk of death, theoretical arguments that were extended to all NSAIDs [1, 4, 32].
The relevance of the upregulation of ACE-2 in the occurrence or severity of COVID-19 is disputed [33, 34]. Previous use of ACE inhibitors or angiotensin receptor blockers did not affect the risk of contracting SARS-CoV-2 [35–39]. ACE-2 upregulation might limit the severity of COVID-19 infection [40]. A lower death rate in patients using ACE inhibitors has been described [37]. The finding that ibuprofen might upregulate ACE-2 came from a single animal experiment in diabetic rats [31]; however, ibuprofen was found to neither affect expression of ACE-2 in human cell lines or mouse tissues nor SARS-CoV-2 entry or replication [41].
We did not find an increased risk of SARS-CoV-2 infection in the general population or in specific populations exposed to NSAIDs or ibuprofen. Furthermore, we did not find an increased risk of hospital admission or severe COVID-19 in users of ibuprofen or NSAIDs, and did not find an increased risk of death from COVID-19 in patients exposed to NSAIDs or ibuprofen. In fact, the fixed-effects model actually showed a lower risk of death in patients exposed to NSAIDs, which was suggested in experimental or theoretical papers [5, 41–43].
The funnel plot suggests publication bias in favour of small studies reporting a higher risk of death with NSAIDs; small studies finding no effect or protection with NSAIDs or ibuprofen seem to be missing.
The use of ibuprofen to relieve symptoms of SARS-CoV-2 infection such as fever or pain is expected, as is finding severe cases exposed to ibuprofen or other NSAIDs. Ibuprofen is effective in relieving pain [44–46]. The description of the cases involved in the initial warning did not generally discuss exposure to other analgesics such as paracetamol [1]. Furthermore, some of these anecdotal reports did not ascertain the diagnosis of SARS infection or actual ibuprofen use. A suggested worsening of bacterial infections with ibuprofen or other NSAIDs would not be relevant to severe COVID-19, which is not related to bacterial superinfection, as confirmed by the lack of benefit of azithromycin [47, 48], but to acute cytokine storm and inflammation, which NSAIDs might reduce or avoid [5, 42, 43]. Our results also indicate a possibly lower risk of death in COVID-19 patients exposed to NSAIDs, consistent with the role of inflammation and the cytokine storm in severe COVID-19.
In addition, two studies found that when ibuprofen or NSAIDs were used alone in patients with COVID-19, there were no deaths, compared with 3 or 4% with paracetamol used alone [26, 27], as had been found in acute soft tissue infection in varicella [49]. Two studies comparing the acute use of ibuprofen or NSAIDs during COVID-19 infection with paracetamol combinations or with non-NSAIDs users found nonsignificantly lower mortality rates in the NSAIDs users [12, 14]. Some researchers have also suggested that by depleting glutathione, paracetamol might also worsen the outcome of COVID-19 [50–53].
The limits of this meta-analysis are related to the observational nature of the studies, which preclude firm conclusions on causality; however, that would apply more were we to affirm a difference, which is not the case here. The upper limits of the CIs for the association of NSAIDs or ibuprofen with the selected outcomes are included within the usual equivalence limits (0.80–1.25). Therefore, what we describe is not just lack of evidence of risk but evidence of lack of risk.
Another typical limit of such systematic reviews is publishing bias. Indeed, there is indication of some publishing bias in studies reporting death, with excess reporting of increased death with NSAIDs, which would bias the results against our findings, rather than the opposite.
Conclusion
We concur with the regulatory agencies, scientific societies and the World Health Organization, which concluded their analyses by stating “There is currently no evidence that the acute use of NSAIDs causes an increased risk of developing COVID-19 or of developing a more severe COVID-19 disease” [54, 55]. In fact there is evidence that it does not cause an increased risk. A regulatory decision based on anecdotal reports and irrelevant experimental data may well have deprived patients of an effective drug to control pain and fever.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflict of interest
Nicholas Moore has provided expert advice to pharmaceutical companies and regulators regarding the risks associated with low-dose NSAIDs and other analgesics, over the last 30 + years. Bordeaux PharmacoEpi conducts postmarketing safety or efficacy studies funded by industry at the request of regulatory authorities, in addition to publicly funded studies, none of which relate to the topic reported in this article. These studies are listed in the European PAS register (www.encepp.EU). Pauline Bosco-Levy, Nicolas Thurin, Patrick Blin, and Cécile Droz-Perroteau are employees of Bordeaux PharmacoEpi and their salaries are generated by post-authorisation studies requested, or not, by regulatory authorities. They have no competing interests within the topic of this study.
Ethics approval
Not applicable for observational studies or meta-analyses.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Data transparency
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study. All data used for the meta-analysis are provided in the tables and figures, based on publicly available data.
Code availability
Not applicable.
Author contributions
Initial idea and overall supervision: NM. Related search: NM, NT, CDP, PB. Data collection: NM, PBL, NT. Validation and statistics: NM, NT, PBL, PB, CD. There were no disagreements between authors and all authors read and approved the final version.
References
- 1.Micallef J, Soeiro T, Jonville-Bera AP, French Society of Pharmacology T Non-steroidal anti-inflammatory drugs, pharmacology, and COVID-19 infection. Therapie. 2020;75(4):355–362. doi: 10.1016/j.therap.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore N, Carleton B, Blin P, Bosco-Levy P, Droz C. Does ibuprofen worsen COVID-19? Drug Saf. 2020;43(7):611–614. doi: 10.1007/s40264-020-00953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen JS, Alfajaro MM, Chow RD, Wei J, Filler RB, Eisenbarth SC, et al. Non-steroidal anti-inflammatory drugs dampen the cytokine and antibody response to SARS-CoV-2 infection. J Virol. 2021;95(7):e00014–21. doi: 10.1128/JVI.00014-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaja R, Chan JSK, Ferreira P, Harky A, Rogers LJ, Gashaw HH, et al. The COVID-19 ibuprofen controversy: a systematic review of NSAIDs in adult acute lower respiratory tract infections. Br J Clin Pharmacol. 2021;87(3):776–785. doi: 10.1111/bcp.14514. [DOI] [PubMed] [Google Scholar]
- 7.Pergolizzi JV, Jr, Varrassi G, Magnusson P, LeQuang JA, Paladini A, Taylor R, et al. COVID-19 and NSAIDS: a narrative review of knowns and unknowns. Pain Ther. 2020;9(2):353–358. doi: 10.1007/s40122-020-00173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reese JT, Coleman B, Chan L, Blau H, Callahan TJ, Cappelletti L, et al. Cyclooxygenase inhibitor use is associated with increased COVID-19 severity. medRxiv. 2021. 10.1101/2021.04.13.21255438.
- 9.Samimagham HR, Arabi M, Hooshyar D, KazemiJahromi M. The association of non-steroidal anti-inflammatory drugs with COVID-19 severity and mortality. Arch Clin Infect Dis. 2020;15(4):e106847. doi: 10.5812/archcid.106847. [DOI] [Google Scholar]
- 10.Park J, Lee SH, You SC, Kim J, Yang K. Non-steroidal anti-inflammatory agent use may not be associated with mortality of coronavirus disease 19. Sci Rep. 2021;11(1):5087. doi: 10.1038/s41598-021-84539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang TS, Ding Y, Freund MK, Johnson R, Schwarz T, Yabu JM, et al. Prior diagnoses and medications as risk factors for COVID-19 in a Los Angeles Health System. medRxiv. 2020. 10.1101/2020.07.03.20145581.
- 12.Chandan JS, Zemedikun DT, Thayakaran R, Byne N, Dhalla S, Acosta-Mena D, et al. Non-steroidal anti-inflammatory drugs and susceptibility to COVID-19. Arthritis Rheumatol. 2021;73(5):731–739. doi: 10.1002/art.41593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costantino F, Bahier L, Tarancon LC, Leboime A, Vidal F, Bessalah L, et al. COVID-19 in French patients with chronic inflammatory rheumatic diseases: clinical features, risk factors and treatment adherence. Jt Bone Spine. 2021;88(1):105095. doi: 10.1016/j.jbspin.2020.105095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abu Esba LC, Alqahtani RA, Thomas A, Shamas N, Alswaidan L, Mardawi G. Ibuprofen and NSAID use in COVID-19 infected patients is not associated with worse outcomes: a prospective cohort study. Infect Dis Ther. 2021;10(1):253–268. doi: 10.1007/s40121-020-00363-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imam Z, Odish F, Armstrong J, Elassar H, Dokter J, Langnas E, et al. Independent correlates of hospitalization in 2040 patients with COVID-19 at a large hospital system in Michigan, United States. J Gen Intern Med. 2020;35(8):2516–2517. doi: 10.1007/s11606-020-05937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lund LC, Kristensen KB, Reilev M, Christensen S, Thomsen RW, Christiansen CF, et al. Adverse outcomes and mortality in users of non-steroidal anti-inflammatory drugs who tested positive for SARS-CoV-2: a Danish nationwide cohort study. PLoS Med. 2020;17(9):e1003308. doi: 10.1371/journal.pmed.1003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alamdari NM, Afaghi S, Rahimi FS, Tarki FE, Tavana S, Zali A, et al. Mortality risk factors among hospitalized COVID-19 patients in a Major Referral Center in Iran. Tohoku J Exp Med. 2020;252(1):73–84. doi: 10.1620/tjem.252.73. [DOI] [PubMed] [Google Scholar]
- 19.Bruce E, Barlow-Pay F, Short R, Vilches-Moraga A, Price A, McGovern A, et al. Prior routine use of non-steroidal anti-inflammatory drugs (NSAIDs) and important outcomes in hospitalised patients with COVID-19. J Clin Med. 2020;9(8):2586. doi: 10.3390/jcm9082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, et al. Factors associated with death in critically ill patients with Coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1436–1447. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang JM, Kim JH, Park JS, Chang MC, Park D. Neurological diseases as mortality predictive factors for patients with COVID-19: a retrospective cohort study. Neurol Sci. 2020;41(9):2317–2324. doi: 10.1007/s10072-020-04541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imam Z, Odish F, Gill I, O'Connor D, Armstrong J, Vanood A, et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J Intern Med. 2020;288(4):469–476. doi: 10.1111/joim.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong AY, MacKenna B, Morton CE, Schultze A, Walker AJ, Bhaskaran K, et al. Use of non-steroidal anti-inflammatory drugs and risk of death from COVID-19: an OpenSAFELY cohort analysis based on two cohorts. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2020-219517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drake TM, Fairfield CJ, Pius R, Knight SR, Norman L, Girvan M, et al. Non-steroidal anti-inflammatory drug use and outcomes of COVID-19 in the ISARIC clinical characterisation protocol UK cohort: a matched, prospective cohort study. Lancet Rheumatol. 2021 doi: 10.1016/S2665-9913(21)00104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41–51. doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rinott E, Kozer E, Shapira Y, Bar-Haim A, Youngster I. Ibuprofen use and clinical outcomes in COVID-19 patients. Clin Microbiol Infect. 2020;26(9):1259.e5–1259.e7. doi: 10.1016/j.cmi.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong HE, Lee H, Shin HJ, Choe YJ, Filion KB, Shin JY. Association between NSAIDs use and adverse clinical outcomes among adults hospitalized with COVID-19 in South Korea: a nationwide study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kragholm K, Gerds TA, Fosbol E, Porsborg Andersen M, Phelps M, Butt JH, et al. Association between prescribed ibuprofen and severe COVID-19 infection: a nationwide register-based cohort study. Clin Transl Sci. 2020;13(6):1103–1107. doi: 10.1111/cts.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perkins SJ, Holmes AR, Nelson JR, Hirschl JR, Chopra Z, Medlin R, et al. Clinical outcomes among COVID-19 patients taking non-steroidal anti-inflammatory drugs. Ann Emerg Med. 2020;76(Suppl 4):S17. doi: 10.1016/j.annemergmed.2020.09.051. [DOI] [Google Scholar]
- 30.Sahai A, Bhandari R, Godwin M, McIntyre T, Chung MK, Iskandar JP, et al. Effect of aspirin on short-term outcomes in hospitalized patients with COVID-19. Vasc Med. 2021 doi: 10.1177/1358863X211012754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiao W, Wang C, Chen B, Zhang F, Liu Y, Lu Q, et al. Ibuprofen attenuates cardiac fibrosis in streptozotocin-induced diabetic rats. Cardiology. 2015;131(2):97–106. doi: 10.1159/000375362. [DOI] [PubMed] [Google Scholar]
- 32.Micallef J, Soeiro T, Jonville-Bera AP. COVID-19 and NSAIDs: primum non nocere. Therapie. 2020;75(5):514–515. doi: 10.1016/j.therap.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P, et al. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur Heart J. 2020;41(19):1801–1803. doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trifiro G, Crisafulli S, Ando G, Racagni G, Drago F, Italian Society of P Should patients receiving ace inhibitors or angiotensin receptor blockers be switched to other antihypertensive drugs to prevent or improve prognosis of novel Coronavirus disease 2019 (COVID-19)? Drug Saf. 2020;43(6):507–509. doi: 10.1007/s40264-020-00935-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin–aldosterone system blockers and the risk of COVID-19. N Engl J Med. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, et al. Renin–angiotensin–aldosterone system inhibitors and risk of COVID-19. N Engl J Med. 2020;382(25):2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ip A, Parikh K, Parrillo JE, Mathura S, Hansen E, Sawczuk IS, et al. Hypertension and renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. medRxiv. 2020. 10.1101/2020.04.24.20077388.
- 38.Dambha-Miller H, Albasri A, Hodgson S, Wilcox CR, Khan S, Islam N, et al. Currently prescribed drugs in the UK that could upregulate or downregulate ACE2 in COVID-19 disease: a systematic review. BMJ Open. 2020;10(9):e040644. doi: 10.1136/bmjopen-2020-040644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sriram K, Insel PA. Risks of ACE inhibitor and ARB usage in COVID-19: evaluating the evidence. Clin Pharmacol Ther. 2020 doi: 10.1002/cpt.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with COVID-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen JS, Alfajaro MM, Wei J, Chow RD, Filler RB, Eisenbarth SC, et al. Cyclooxgenase-2 is induced by SARS-CoV-2 infection but does not affect viral entry or replication. bioRxiv. 2020. 10.1101/2020.09.24.312769.
- 42.Kelleni MT. ACEIs, ARBs, ibuprofen originally linked to COVID-19: the other side of the mirror. Inflammopharmacology. 2020;28(6):1477–1480. doi: 10.1007/s10787-020-00755-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelleni MT. Early use of non-steroidal anti-inflammatory drugs in COVID-19 might reverse pathogenesis, prevent complications and improve clinical outcomes. Biomed Pharmacother. 2021;133:110982. doi: 10.1016/j.biopha.2020.110982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailey E, Worthington H, Coulthard P. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review. Br Dent J. 2014;216(8):451–455. doi: 10.1038/sj.bdj.2014.330. [DOI] [PubMed] [Google Scholar]
- 45.Moore RA, Derry S, Aldington D, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2015;2015(9):CD008659. doi: 10.1002/14651858.CD008659.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore RA, Wiffen PJ, Derry S, Maguire T, Roy YM, Tyrrell L. Non-prescription (OTC) oral analgesics for acute pain—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2015;2015(11):CD010794. doi: 10.1002/14651858.CD010794.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furtado RHM, Berwanger O, Fonseca HA, Correa TD, Ferraz LR, Lapa MG, et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020;396(10256):959–967. doi: 10.1016/S0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.RECOVERY Collaborative Group Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10274):605–612. doi: 10.1016/S0140-6736(21)00149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lesko SM, O'Brien KL, Schwartz B, Vezina R, Mitchell AA. Invasive group A streptococcal infection and nonsteroidal antiinflammatory drug use among children with primary varicella. Pediatrics. 2001;107(5):1108–1115. doi: 10.1542/peds.107.5.1108. [DOI] [PubMed] [Google Scholar]
- 50.De Flora S, Balansky R, La Maestra S. Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19. FASEB J. 2020;34(10):13185–13193. doi: 10.1096/fj.202001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sestili P, Fimognari C. Paracetamol-induced glutathione consumption: is there a link with severe COVID-19 illness? Front Pharmacol. 2020;11:579944. doi: 10.3389/fphar.2020.579944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silvagno F, Vernone A, Pescarmona GP. The role of glutathione in protecting against the severe inflammatory response triggered by COVID-19. Antioxidants (Basel) 2020;9(7):624. doi: 10.3390/antiox9070624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verd S, Verd M. Commentary: paracetamol-induced glutathione consumption: is there a link with severe COVID-19 illness? Front Pharmacol. 2020;11:625295. doi: 10.3389/fphar.2020.625295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torjesen I. Covid-19: ibuprofen can be used for symptoms, says UK agency, but reasons for change in advice are unclear. BMJ. 2020;369:m1555. doi: 10.1136/bmj.m1555. [DOI] [PubMed] [Google Scholar]
- 55.WHO. Updated: WHO Now Doesn't Recommend Avoiding Ibuprofen For COVID-19 Symptoms 2020. https://www.sciencealert.com/who-recommends-to-avoid-taking-ibuprofen-for-covid-19-symptoms. Accessed 1 Mar 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.