Abstract
Predicting long-term outcome in infants with hypoxic-ischemic encephalopathy (HIE) remains an ongoing clinical challenge. We investigated plasma biomarkers and their association with 6-month outcomes in a nonhuman primate model of HIE with or without therapeutic hypothermia (TH) and erythropoietin (Epo). Twenty-nine Macaca nemestrina were randomized to control cesarean section (n = 7) or 20 min of umbilical cord occlusion (UCO, n = 22) with either no treatment (n = 11) or TH/Epo (n = 11). Initial injury severity was scored using 30-min arterial pH, base deficit, and 10-min Apgar score. Twenty-four plasma cytokines, chemokines, and growth factors were measured 3, 6, 24, 72, and 96 h after UCO. Interleukin 17 (IL-17) and macrophage-derived chemokine (MDC) differentiated the normal/mild from moderate/severe injury groups. Treatment with TH/Epo was associated with increased monocyte chemotactic protein-4 (MCP-4) at 3 h–6h, and significantly lower MCP-4 and MDC at 24 h–72h, respectively. IL-12p40 was lower at 24 h–72h in animals with death/cerebral palsy (CP) compared to survivors without CP. Baseline injury severity was the single best predictor of death/CP, and predictions did not improve with the addition of biomarker data. Circulating chemokines associated with the peripheral monocyte cell lineage are associated with severity of injury and response to therapy, but do not improve ability to predict outcomes.
Keywords: Asphyxia, erythropoietin, hypothermia, hypoxic-ischemic encephalopathy, neonatal
Introduction
Perinatal hypoxia-ischemia with subsequent hypoxic-ischemic encephalopathy (HIE) remains a significant problem in the United States, affecting 1.3–4.7/1000 liveborn infants.1,2 Current criteria for treatment of infants with moderate or severe HIE include a combination of clinical (Apgar scores, Sarnat or Thompson scores) and laboratory assessments (lactic acid, pH, base deficit) with or without electrophysiologic function on electroencephalography (EEG). Although therapeutic hypothermia (TH) has been the standard of care for infants with moderate or severe HIE since 2010, the most recent clinical trials suggest that around 1/3 of infants still experience a poor outcome (death or severe disability).3 Though TH does significantly reduce the disease burden of infants with HIE, early prognostication remains relatively poor,4 and we cannot accurately predict which infants will respond to therapy. New biomarkers are needed to more accurately identify patients who will benefit from TH, those who might require additional therapies, and those for whom treatment might be futile. One of the most promising current adjunctive therapies for HIE is erythropoietin (Epo), which has anti-inflammatory, antioxidant, and neuroreparative properties in preclinical studies of term neonatal brain injury.5–7 Epo has been shown to be neuroprotective in multiple small and large animal models of HIE, including a nonhuman primate (NHP) umbilical cord occlusion (UCO) model developed in our laboratory.8
Biomarkers that accurately reflect the severity and evolution of injury as well as response to therapy would have the potential to improve clinical management of newborns with HIE and facilitate new research.9 As HIE is often associated with a coordinated systemic immune response, examining the dynamics of cytokines and chemokines after injury may provide biomarkers with potential for clinical translation.10–12 Nonhuman primates are the ideal preclinical model for human disease because information gained from these animals is most likely to translate to the human condition. We developed an NHP model of HIE in which we can assess sequential longitudinal biomarkers and correlate these with behavioral and structural outcomes.13–15 Using this model, we have examined the time course of plasma chemokines and cytokines in the first 96 h after injury with and without treatment with TH and Epo, particularly with respect to cytokine and chemokine dynamics, in order to assess the association between cytokines and severity of injury, alterations in response to therapy, and ability to predict later death or cerebral palsy (CP).
Methods
Animals
The Institutional Animal Care and Use Committee (IACUC) at the University of Washington approved all experimental protocols, and all experimental procedures were conducted in accordance with these approved protocols as well as the National Institutes of Health Guide for Care and Use of Laboratory Animals. All data are reported in accordance with the ARRIVE (Animal Research: Reporting in Vivo Experiments) guidelines.
Timed matings occured by monitoring adult female menstrual cycles and pairing sexes during the time of peak ovulation for 24–48 h. Pregnant females were monitored by uterine palpation and ultrasound, and pregnancies were grouped such that 2–3 deliveries occurred per month so that animals had play group cohorts. Deliveries were scheduled 5 ± 2 days prior to term, as previously described.8 Hysterotomy was performed through a sterile ventral midline incision of the abdomen under sevoflurane surgical anesthesia. After umbilical cord occlusion (UCO, see below), during which an umbilical arterial catheter was placed, the fetuses were delivered by caesarian (C)-section. Control animals were also delivered by C-section after intrauterine installation of an umbilical arterial catheter (2–3 min procedure). All surgical procedures were followed by post-operative analgesics (ketoprofen 5 mg/kg i.m., butorphanol 0.15 mg/kg i.m. or s.c., and acetaminophen 1–2 children's tablets) for a minimum of 48 h. Twenty-nine Macaca nemestrina (pigtailed macaque) neonates were delivered for the purposes of this study. Animals were randomized prior to C-section and UCO to either control (n = 7) or 18–20 min of UCO (n = 22) followed by either no treatment (n = 11) or 72 h of TH plus Epo (n = 11).
Umbilical cord occlusion and resuscitation
Procedures for UCO were performed as previously described.8 Briefly, after incising the uterus, the umbilical cord was exteriorized while keeping the amniotic fluid and the fetus in the womb. During UCO, the uterus was supported with saline-soaked towels while a sterile 2.5 French Vygon™ umbilical artery catheter was placed. Cord blood was obtained before clamping the cord for 18–20 min, during which time the fetal heart rate was monitored by femoral pulse Doppler. Fetuses were delivered by the surgical team, which included a neonatologist, and stabilized by a team of neonatologists using standardized neonatal resuscitation practice. Resuscitation included endotracheal intubation, positive pressure ventilation, chest compressions, and bolus epinephrine as indicated. Apgar scores were assigned at 1, 5, 10, and 20 min of life. Continuous monitoring included a pulse oximeter and rectal thermometer. For animals not randomized to TH/Epo, a covered heating pad, radiant warmer, and polyethylene sheet were used to provide thermal support during stabilization, then the animals were moved to a thermal-neutral incubator. After delivery, an experienced laboratory staff member remained with the infant for the first 96 h of life, and then as needed based on clinical stability. A neonatologist and veterinarian were on call for the animals at all times. In order to examine the association between severity of initial injury, later cytokine profiles, and long-term outcome, animals were assigned a composite severity score on a scale of 3 (normal/mild) to 6 (most severe). Based on post-resuscitation blood gas assessments, scores were assigned based on standard neonatal clinical parameters including pH (≥7.0 = 1, <7.0 = 2), base deficit (≤15 = 1, >15 = 2), and Apgar score at 10 min (≥5 = 1, <5 = 2).16 Animals with a score of 3 were assigned to the normal/mild group, with animals scoring >3 assigned to the moderate/severe group.
Treatment groups
UCO animals were treated with ether saline and normothermia (maintenance of target rectal temperature at 36–37°C) or TH (72 h at 33.5°C rectal temperature) plus Epo (1000 U/kg on day 1, 2, 3, 5, and 7) in the form of Epogen® (Epoetin Alfa Recombinant, Amgen, Thousand Oaks, CA).7 To produce initial TH, animals were not actively warmed at delivery. Active cooling was begun after resuscitation of the infants and always occurred by the third hour of life. To maintain TH, a water blanket was applied to the animal’s head and body and thermal support from the incubator was adjusted to achieve a rectal temperature of 33.5 °C for 72 h. Rectal temperature was maintained by a servo-controlled cooling machine (CritiCool, MTRE Advanced Technologies, Israel) that altered the temperature of the cooling blanket in order to maintain target temperature. Rewarming was done slowly, raising the target rectal temperature by 0.5 °C per hour until regular body temperature (36–37°C) was reached.
Animal care
Post-resuscitation care was conducted as previously reported.8 Briefly, arterial blood gas, lactate, and electrolytes (iSTAT®, HESKA Corp., Loveland, Colorado) were measured at multiple scheduled intervals. Study animals were maintained for a minimum of 3 days on parenteral fluids including 2% amino acids (Aminosyn II) adjusted to maintain euglycemia, electrolyte balance, and hydration. Enteral feedings were started on postnatal day 4. Weight was followed daily.
Blood sampling and circulating cytokines
The umbilical artery catheter was maintained for the first 96 h of life to allow for blood access, fluid and nutrient administration, and continuous blood pressure monitoring. At scheduled intervals (3, 6, 24, 72, and 96 h), blood was drawn from the catheter in an aseptic manner. Total blood volume taken was tracked. After collection, blood was promptly spun at 4000 RPM for 10 min, the plasma collected, and immediately frozen at −70°C until further use. Meso Scale Discovery (MSD) technology was used to measure the following inflammatory markers and growth factors: Interferon-gamma (IFN-γ),17 Interleukin (IL)-1β,18 IL-2, IL-6, IL-5, IL-7, IL-8, IL-10, IL-15, IL-16, IL-17, IL-12p40, tumor necrosis factor-α (TNF-α), transforming growth factor (TGF)-β, macrophage inflammatory protein-1α (MIP-1α), MIP-1β,19 monocyte chemotactic protein-1 (MCP-1),19 MCP-4, eotaxin-3, Interferon gamma-induced protein 10 (IP-10), macrophage-derived chemokine (MDC), thymus and activation regulated chemokine (TARC), granulocyte-macrophage colony-stimulating factor (GM-CSF), and vascular endothelial growth factor (VEGF). MSD assays were performed according to the manufacturer’s recommended protocols.
Developmental evaluations
Developmental assessment was performed by the Infant Primate Research Laboratory (IPRL) at the University of Washington, as previously described.21,22 To assess for the presence or absence of CP, animals were assessed by a single senior staff member mentored by a physical therapist with a special interest in neonates, as peviously described.8 CP was graded on a scale of 0 (normal), 1 (mild), 2 (moderate), or 3 (severe). Sequential exams were done at 1 week, 1 month, and 6 months to document any evidence of motor abnormalities and contractures. Assessment included evaluation of their ability to control active movement. Muscle tone at each joint was graded on the Ashford scale of 0 (normal) to 4 (affected parts rigid in flexion or extension).20
Statistical analysis
Prior to all analyses, calculated concentrations of biomarkers were log-transformed (base 2) to alleviate the skewness observed in the raw data. Samples outside of the fitted curve’s range (n = 2 extreme outliers above the normal calibration curve and n = 8 missing values) were also removed. The primary outcomes of interest were death or any CP (both individually and combined) among animals that were randomized to treatment. Descriptive statistics were provided for all study animals.
Associations between initial injury severity and biomarker values at different time points in all study animals were first assessed by fitting linear regressions with robust standard errors. The mean biomarker concentrations were compared between the normal/mild group (injury score = 3) and the moderate/severe group (injury score >3).
The non-UCO animals were then excluded from treatment analysis. The associations between treatment (TH/Epo vs. not treated) and biomarker values at different time points were also evaluated using linear regressions with robust standard errors. Due to sex imbalance later observed across treatment groups, the models were adjusted for initial injury score and sex. Mean biomarker concentrations were compared between the TH/Epo and not treated groups.
Non-UCO animals were also excluded from analyses of outcomes. The mean log-transformed biomarker concentrations between groups defined by the primary outcome of interest (death or CP) were first examined, before evaluating how well the primary outcome (death or any CP) was predicted by logistic regressions using (1) initial injury score only, (2) a single biomarker at one specific time point only, or (3) a combination of both initial injury score and the biomarker. In-sample receiver-operator characteristic (ROC) curves were constructed, in which the area under the curve (AUC) indicated how well each model performed at distinguishing UCO animals that died or had any CP from those that survived or had no CP. For each assay, the AUC of the model using combination of both initial injury score and the biomarker was compared with the AUC of the model using only initial injury score using DeLong’s Method.21 Similar analyses were repeated for CP outcome (any CP vs. no CP) among UCO animals that survived.
Due to data availability and group sizes, the predictiveness of combinations of biomarkers at the same time points for outcomes were not explored. Statistical signficance was evaluated at level 0.05 and a Bonferroni-corrected level for multiple testing22 relevant to each analysis. Graphical methods were used to assess model assumption for linear regressions. All statistical analyses were performed using the R statistical analysis package version 3.6.3.23
Results
Animal characteristics
The characteristics of 29 study animals are shown in Table 1. Out of 22 UCO animals, 11 (50%) were not treated and 11 (50%) were treated with TH/Epo. In the UCO groups, median UCO times were 20 min. One animal in the untreated group and two in the TH/Epo group had UCO times of 18 min. In the untreated group, one animal’s UCO was stopped after 8 min due to technical difficulties (Table 1). One control animal inadvertently had a 9 min period of UCO due to complications while placing the umbilical line that delayed delivery but did not require resuscitation. Median (IQR) time to intubation was 6 min (2.8–9 min) in the untreated group, and 2.3 min (1.5–5.8 min) in the TH/Epo group. Neither UCO time nor time to intubation was statistically different between the two treatment groups. None of the control animals had moderate/severe injury, while there were 8 (72.7%) and 10 (90.1%) moderate/severe animals in the untreated and TH/Epo groups, respectively. Sex was imbalanced between the two treatment groups, with n = 3 (27.3%) versus n = 8 (72.7%) female animals in the untreated and TH/Epo groups, respectively, and therefore sex was adjusted in later statistical models.
Table 1.
Animal characteristics of the three experimental groups.
| Control(n = 7) |
UCO Animals |
|||
|---|---|---|---|---|
| UCO + Not Treated(n = 11) | UCO + TH/Epo(n = 11) | P-valued | ||
| Demographics: | ||||
| Female – n (%) | 4 (57.1%) | 3 (27.3%) | 8 (72.3%) | 0.03 |
| Birthweight (g) – median (IQR) | 558 (5,09,599) | 625 (5,39,631) | 526 (4,95,586) | 0.25 |
| Days delivered early – median (IQR) | 3.0 (3.0, 4.5) | 4.0 (3.5, 5.0) | 4.0 (3.0, 4.0) | 0.37 |
| UCO (min) – median (range) | 0 (0–9) | 20 (8–20) | 20 (18–20) | 0.46 |
| Clinical parameters: | ||||
| Time to intubation (min) – median (range) | – | 6 (0–13) | 2.3 (0–10) | 0.77 |
| Apgar at 10 min – median (IQR) | 9 (8.0, 9.0) | 3 (2.5, 6.0) | 3 (2.5, 3.0) | 0.38 |
| pH – median (IQR) | 7.32 (7.26, 7.34) | 7.08 (7.01, 7.13) | 7.10 (7.06, 7.18) | 0.61 |
| Base deficit (mEq/L) – median (IQR) | –2.6 (–6.0, 1.0) | –15.1 (–18.2, –12.4) | –16.5 (–19.5, –14.5) | 0.32 |
| Lactate (mmol/L) – median (IQR)a | 1.4 (1.1, 2.0) | 10.5 (9.3, 13.3) | 11.1 (9.7, 12.3) | 0.30 |
| pCO2 (mmHg) – median (IQR)b | 43.4 (39.0, 50.4) | 44.5 (39.6, 45.7) | 39.9 (33.9, 50.8) | 0.44 |
| Moderate or severe injury – n (%)c | 0 (0%) | 8 (72.3%) | 10 (90.1%) | 0.29 |
aThere was 1 animal with missing data on lactate, and 1 animal with measure “>20” which was conservatively assumed to be 20.1.
bThere was 1 animal with missing data on pCO2.
cDerivation and classification of injury score was based on pH, base deficit, and apgar score at 10 min. Animals with a score of 3 were assigned to the normal/mild group, with animals scoring >3 assigned to the moderate/severe group.
dP-values were included from two-sample t-tests to assess the differences in baseline characteristics between between UCO + TH/Epo animals and UCO + Not Treated aninmals.
Biomarkers and intial injury severity
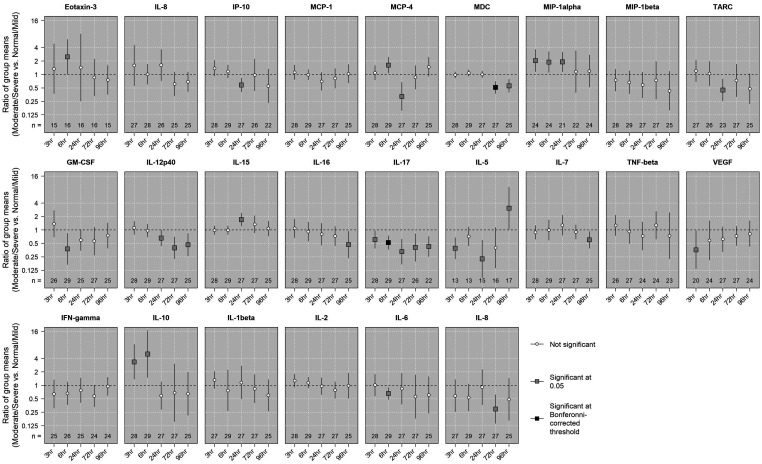
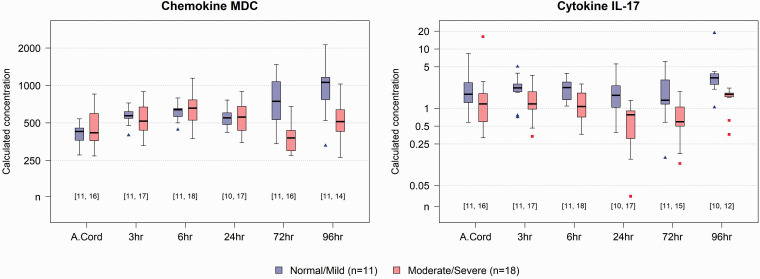
Based on pH, base deficit, and Apgar score at 10 min, 11 out of 29 animals were in the normal/mild group, and 18 animals were in the moderate/severe group. Results of statistical tests and comparisons of mean biomarker concentrations between moderate/severe and normal/mild groups over time are shown in Figure 1. Noteably, the ratio of mean concentrations between the moderate/severe group and the normal/mild group was 0.52 (95% confidence interval (CI) of [0.37, 0.74]) for IL-17 at 6 h, and 0.51 (95% CI of [0.37, 0.70]) for MDC at 72 h. These results were significant even after being adjusted with a highly-conservative Bonferroni-corrected threshold to account for 120 multiple tests. Figure 2 shows the distribution of MDC and IL-17 over time by intial injury severity for all study animals.
Figure 1.
Comparing mean concentrations by initial injury severity groups among study animals. The associations between the mean log-transformed concentration of each biomarker at each time point and initial injury group (moderate/severe vs. normal/mild) were assessed using linear regressions with robust standard errors. An estimated ratio >1 indicates that the mean concentration in the moderate/severe group was higher than the mean concentration in the normal/mild group, and vice versa. Statistical signficance was evaluated using both an undajusted level of 0.05 and a conservative Bonferroni-corrected level (0.05 divided by 120 tests).
Figure 2.
MDC and IL-17 by initial injury severity over time. Distributions of MDC (left) and IL-17 (right) over time, including initial cord blood, by initial injury group. Numbers in square brackets indicate number of animals from which data was available at each time point in the Mild/Normal (first number) and Moderate/Severe (Second number) groups.
Treatment and cytokine dynamics
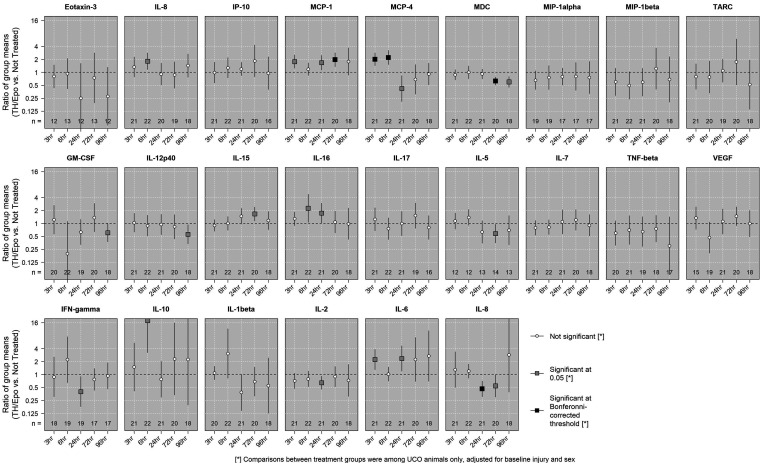
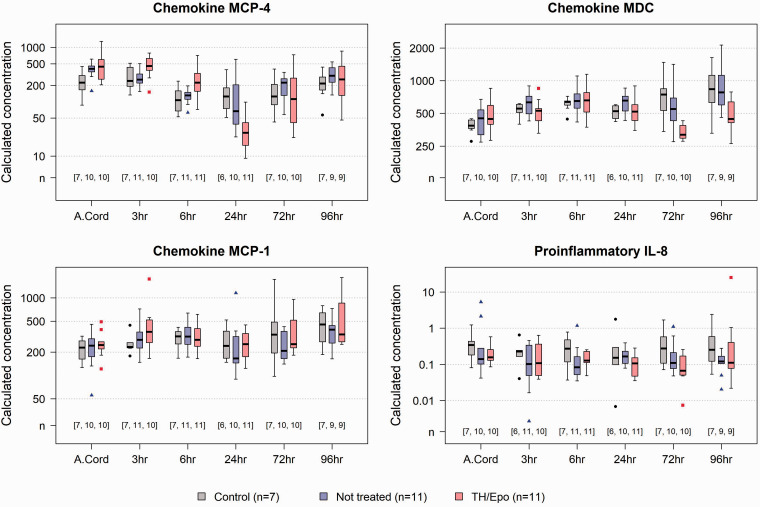
The mean concentrations of biomarkers at different time points were compared between the UCO+TH/Epo group and the UCO untreated group and adjusted for the potential confounding effects of initial injury and sex. Results of statistical tests and comparisons of mean concentratrions between the UCO+TH/Epo and untreated UCO groups over time are shown in Figure 3. Results were statistically significant at the Bonferroni-corrected level for MCP-4 at 3 h, 6 h, and 24 h, and for MDC at 72 h. On average, animals in the TH/Epo group had higher levels of MCP-4 at 3 h and 6 h, and lower levels at 24 h, compared to those in the untreated group. The ratio (95% CI) of mean concentrations of MCP-4 between the TH/Epo group and the untreated group was estimated to be 2.00 (1.42, 2.82) at 3 h, 2.20 (1.50, 3.23) at 6 h, and 0.42 (0.21, 0.84) at 24 h. For MDC at 72 h, the ratio (95%) of mean concentrations was 0.63 (0.50, 0.78). Corresponding results were 1.96 (1.36, 2.84) for MCP-1 at 72 h, and 0.47 (0.31, 0.71) for IL-8 at 24 h. Figure 4 shows the distributions of MCP-4, MDC, MCP-1, and IL-8 over time by treatment group for all study animals.
Figure 3.
Comparing mean concentrations in treatment groups among UCO animals. The associations between the mean log-transformed concentration of each biomarker at each time point and treatment group (TH/Epo vs untreated) among UCO animals were assessed using linear regressions with robust standard errors. An estimated ratio >1 indicates that the mean concentration in the TH/Epo group was higher than the mean concentration in the untreated group after adjustment for initial injury and sex. Statistical signficance was evaluated using both an undajusted level of 0.05 and a conservative Bonferroni-corrected level (0.05 divided by 120 tests).
Figure 4.
MCP-4, MDC, MCP-1, and IL-8 by treatment group over time. Distributions of MCP-4, MDC, MCP-1, and IL-8 over time, including initial cord blood, by treatment group, including non-UCO animals. Numbers in square brackets indicate number of animals from which data was available at each time point in the Control (first number), untreated UCO (middle number), and TH/Epo (right number) groups.
Cytokine dynamics and neurodevelopmental outcome in UCO animals
Six month outcomes among the 22 UCO animals with respect to death or death/any CP varied by initial injury score. None of the 4 animals with an injury score of 3 (lowest score) died or had CP, while all 5 animals with an injury score of 6 (maximum possible score) died or had CP. None of the 4 animals with a score of 4 died, but 2 (50%) had CP. Out of 9 animals with injury score of 5, 3 (33%) died and 4 (44%) had CP. The primary outcome was similar between treatment groups (UCO with and without TH/Epo treatment), with 7 out of 11 (64%) animals in each group dying or surviving with CP.
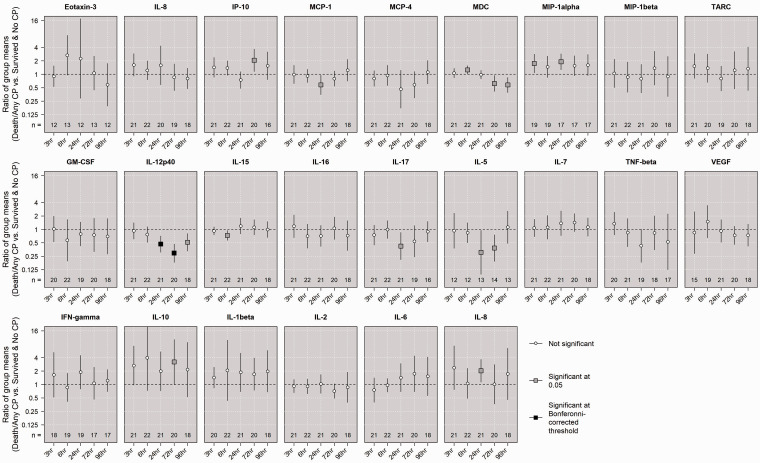
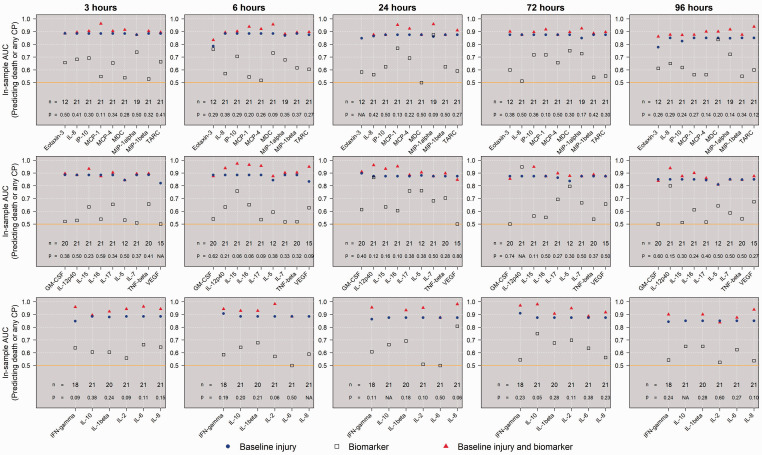
We first compared the mean log-transformed concentrations between groups defined by the primary outcome of interest in unadjusted linear regressions, and the results are shown in Figure 5. In UCO animals who died or survived with CP, IL-12p40 levels were lower at 24 h and 72 h, and these results were statistically significant at the Bonferroni-corrected threshold. For each biomarker at each timepoint, in-sample ROC curves were generated to assess the performance of initial injury severity score alone, biomarker alone, or combined, at predicting the primary outcome of death or any CP. Figure 6 shows these AUC comparisons. When analyzing all 22 UCO animals, the AUC resulting from a logistic model using only initial injury score was 0.88, which suggested that the severity of the initial injury alone was a good predictor of the primary outcome. In general, using only a single biomarker resulted in poor classification of primary outcome. IL-12p40 at 24 h and 72 h alone was relatively good at predicting the primary outcome; however, IL-12p40 did not add to the predictive performance of the initial injury severity. Though some biomarkers added apparent improvement to the overall predictiveness, none of these results were significant at level 0.05.
Figure 5.
Comparing mean concentrations in primary outcome groups among UCO animals. The ratios of the mean log-transformed concentration of each biomarker at each time point between UCO animals that died or had any CP and UCO animals that survived and had no CP, were assessed using linear regressions with robust standard errors. An estimated ratio >1 indicates that the mean concentration in the death/any CP group was higher than the mean concentration in those who survived without CP. Statistical signficance was evaluated using both an undajusted level of 0.05 and a conservative Bonferroni-corrected level (0.05 divided by 120 tests).
Figure 6.
In-sample ROC for predicting outcome of death or any CP among UCO animals. The AUCs were calculated for in-sample ROC curves generated from logistic regression models using initial injury alone, biomarker alone, or their combination, to predict the primary outcome of death or any CP among UCO animals. For each assay, the p-value, if possible to be calculated, evaluated whether the model using a combination of biomarker and initial injury score can lead to a statistcally significant improvement in AUC compared to the model using only initial injury score to predict death or any CP.
Supplemental Figure 1 and Figure 2 show comparisons of the unadjusted mean ratios as well as the AUCs when either initial injury severity alone, biomarker alone, or their combination, was used to predict CP among the 15 UCO animals who survived. A few biomarkers substantially improved the AUC, e.g. MIP-1α, IFN-γ, IL-10, and IL-8 at 24 h. However, these results were not statisticially significant at level 0.05.
Discussion
Perinatal hypoxia-ischemia is a common cause of neonatal encephalopathy in term infants, often called HIE, a condition associated with significant risk of death or long-term disability. Sequelae of moderate to severe HIE include intellectual disability, CP, hydrocephalus, seizures, and death. Loss of productivity, dependency, recurrent use of medical and rehabilitation services, and reduced life expectancy all exacerbate the burden.24 Research directed at neuroprotection for children with these conditions is hampered by a lack of sensitive and specific biomarkers with which to predict outcomes and response to potential therapies. Using an NHP model of term HIE, we show for the first time that certain circulating cytokines/chemokines, particularly the IL-12p40 subunit and chemokines associated with the monocyte cell lineage, are associated with severity of injury and treatment with TH/Epo. However, no cytokine or chemokine at any timepoint was able to significantly improve prognostic accuracy above the use of basic clinical parameters, and further work is required to determine whether these potential biomarkers have clinical promise.
The goal of this study was to track multiple putative immune markers over time after UCO in NHPs in a hypothesis-free manner in order to determine whether it was possible to identify biomarkers of injury, treatment response, and long-term neurodevelopmental outcome. Of particular note was the fact that early clinical markers (pH, base deficit, and 10-min Apgar score) were associated with a number of differences in biomarker levels, as well as highly predictive of long-term outcome. The latter finding is in slight opposition to the clinical HIE literature, where these parameters are used as inclusion criteria for TH but tend to have poor long-term predictive value compared to other indicators such as clinical magnetic resonance imaging or EEG.4 This is likely partly due to the controlled nature of the model, which is a pure acute hypoxic-ischemic insult with known timing relative to the clinical measurements. From animal models, it is known that a defined injury produced by a discrete hypoxic-ischemic insult will evolve through discrete stages including primary and secondary energy failure, cell death, inflammation, and late repair.25 By comparison, many factors can influence the outcome of clinical HIE including the duration and severity of injury, brain maturity at time of injury, as well as the general condition of the maternal-infant pair prior to the injury (nutrition, hypoxic preconditioning, infection, stress, etc.).26–31 When a baby is born with evidence of HIE, most of these factors are unknown, resulting in greater variability in the available clinical parameters that can be measured before the onset of cooling. Therefore, circulating peripheral immune markers associated with the severity of the insult may have greater utility in the clinical setting compared to biochemical parameters that can change rapidly as the infant is stabilized.
Two notable differences in biomarker levels based on severity of initial injury were an approximately 50% decrease in IL-17 at 6 h and MDC at 72 h in the moderate/severe group compared to the normal/mild group. IL-17 is a cytokine produced by T helper (Th) 17 cells, as well as other T cell lineage including γδ T cells and natural killer (NK) cells.32 Mean levels of IL-17 were consistently, though not always significantly, lower in the moderate/severe injury group at every time point (Figure 2). To our knowledge, this is the first time that IL-17 has been measured in a preclinical model in HIE, with data also lacking in the clinical literature. MDC, also known as C-C motif chemokine 22 (CCL22), is a chemokine secreted by macrophages and dendritic cells that is both constitutively and actively expressed. While MDC has been associated with increased cellular recruitment and inflammatory responses in preclinical models of peripheral injury such as lung injury after hemorrhage and resuscitation,33 its association with acute brain injury, particularly in the neonate, is less clear. We found that MDC was significantly decreased at 72 h in animals with moderate/severe initial injury. Similar to IL-17, no published data on MDC in models of HIE appears to exist. However, in one small clinical study of adults with acute ischemic stroke, levels of MDC 24 h and 72 h after the injury were inversely correlated with stroke severity, similar to what was seen with initial injury severity in our model.34 As the combination of TH/Epo has previously been shown to be neuroprotective in this model,8 the fact that TH/Epo suppressed a cytokine negatively associated with severity of injury is therefore unexpected. Finally, TH/Epo treatment was associated with almost immediate changes in MCP-4, also known as chemokine ligand 13 (CCL13), which is a chemoattractant for monocytes and eosinophils and can stimulate degranulation of basophils.35 Here, MCP-4 was initially increased by TH/Epo treatment at 3 h and 6 h, suggesting an early effect of the treatment on the immediate peripheral immune response to injury. However, due to the design of the study we were not able to determine whether one aspect of TH/Epo treatment, or the combination of TH/Epo, resulted in these changes, and greater exploration of whether IL-17, MDC, and MCP-4 are clinically-meaningful markers of injury in neonatal HIE is required.
One of the most pressing requirements for biomarker development in HIE is to assist in the prediction of long-term outcomes, both to help provide information for clinician and family members, as well as to better determine pathways of care including transition to palliative care. As mentioned above, the degree of initial injury as assessed by clinical parameters was highly predictive of long-term outcome. This was particularly the case when using death/CP as a dichotomous outcome. However, one cytokine appeared to have strong predictive value for long-term outcome – the IL-12 subunit p40. At 24 h and 72 h, IL-12p40 was significantly lower in animals who died or had CP compared to those without CP. When only looking at survivors, IL-12p40 was also significantly predictive of CP, particularly at 72 h. IL-12p40 is the β chain of the IL-12 heterodimer, and also forms part of the IL-23, IL-27, and IL-35 heterodimers.36 Though p40 can have pro-inflammatory signaling properties, it can also antagonize IL-12.36,37 As such, increased p40 in animals with a good outcome may be due to its counterregulatory activity, preventing an excessive inflammatory response. However, while IL-12p40 levels remain a promising biomarker of injury, adding IL-12p40 levels to clinical markers did not improve prediction of long-term outcome. Further work is required to determine whether IL-12p40 levels, as well as other cytokines such as IFN-γ, IL-8, and IL-10, in infants with HIE can assist in the prediction of long-term outcomes above current imaging, physiological, and clinical assessments.
This study has a number of limitations. Due to the relatively small group sizes, we were not able to detect a significant effect of TH/Epo on long-term outcome; however, the study was powered to determine biomarker responses to therapy rather than changes in long-term outcomes after treatment. Though we have previously described a neuroprotective effect of TH/Epo in this model,8 not all of the preclinical data regarding combined TH/Epo treatment in models of HIE is positive, with some studies suggesting either non-additive or minimal increases in neuroprotection when adding Epo to TH.38–41 Importantly, however, this question is currently being explored in multiple clinical trials such as the HEAL (High-dose Erythropoietin for Asphyxia and Encephalopathy) and EDEN (Erythropoietin and Darbepoetin in Neonatal Encephalopathy) trials. The nature of the study design and small group sizes also resulted in limitations regarding the analysis procedures. For instance, in order to create a single control group for baseline injury, animals with minimal clinical evidence of injury were combined with unexposed controls, which may have masked any effect of mild asphyxia on later chemokine/cytokine levels. However, given the ability of clinical parameters to predict long-term outcomes in this study, we do not believe that this will have had a large effect on the results of those analyses. The lack of separate TH and Epo groups is also a limitation with respect to determining the treatment-specific effects on injury or biomarker responses. Additionally, while no animals showed signs of clinical infection, we cannot rule out the effect of occult infections on cytokine and chemokine dynamics.
In some linear regression models, graphical assessments of residuals also showed mild to moderate violation of normality assumption, which signified that these results should be taken with caution due to the small sample size and potential confounding variables that we did not include. Though non-parametric approaches might be more appropriate considering the nature of some of the data, these were not chosen, largely due to the small sample size and covariate adjustment. Although the study design included randomization of animals, pre-randomization of animals to groups before C-section unfortunately resulted in a sex imbalance among the UCO animals, which was therefore adjusted for in all UCO analyses. Due to the large number of biomarkers tested and the number of time points, as well as comparisons by initial injury, treatment, and outcome, it was highly likely that differences might be found by chance. As such, we employed strict Bonferroni corrections to adjust for multiple comparisons, which conversely may have increased the risk of type II error. This is particularly pertinent to the use of cytokines to improve prediction of long-term outcome when used in addition to clinical markers. When combined with clinical parameters, some AUCs including individual cytokines were close to 1, and unadjusted p-values compared to the model using only initial injury score were 0.05 for IFN-γ, IL-8, and IL-10 at 24 h (Figure 6). The use of in-sample ROC might also be overly optimistic in terms of prediction; however, the small sample size did not allow for more robust strategies such as cross-validation. These data are in line with previous publications in infants with HIE in which levels of IL-8 and IL-10 6 to 24 h after birth were associated with outcomes at 15–18 months of age and severity of injury on early imaging, respectively.42,43
Though group sizes and lack of a priori hypotheses to guide the building of predictive models prevented us from being able to combine multiple biomarkers to predict long-term outcome, there remains the possibility that measuring multiple cytokines around 24 h after birth could provide a model for predicting both short-term and long-term outcomes in infants undergoing active treatment for HIE as well as identifying those with milder HIE not undergoing treatment with TH but who are at greater risk of poor outcomes. Though none have yet translated to clinical practice, a number of promising biomarkers of HIE remain in development. The most well-described is perhaps plasma Tau,44 with metabolomic analyses of the tryptophan/kynurenine pathway,45 plasma-type gelsolin,46 serum neurofilament light,47 serum 24S-hydroxycholesterol,48 and plasma osteopontin49 also receiving recent attention in various models or types of neonatal and pediatric brain injury and holding significant promise for investigation in both large animal models and human infants with HIE. Though the search for circulating biomarkers in HIE still continues, physiological measurements such as continuous EEG or amplitude-integrated EEG (aEEG) are already being implemented clinically and have shown a robust ability to assist in long-term outcome prediction in the era of TH,50–52 as well as having significant promise for automated analysis and combination with other physiological measurements such as near-infra-red spectroscopy (NIRS) to examine neurovascular coupling.53–55 We and others have also shown that monitoring of physiological temperature responses may provide an additional prognostic biomarker in both low and high resource settings.56,57 While the absence of EEG or continuous physiological monitoring is another limitation of our study, we have previously found that EEG monitoring did not improve outcome prediction above the use of clinical parameters in this model,8 which is why those were the focus of baseline injury assessment.
In summary, using an NHP model of term neonatal HIE, we show a number of alterations in circulating chemokines based on severity of injury and response to therapy with TH/Epo. This was particularly the case with chemokines associated with the peripheral monocyte cell lineage, which to date have received little attention in the field of neonatal HIE and warrant further investigation. As no cytokine or chemokine was able to significantly improve prognostic accuracy above the use of basic clinical parameters, further work is required to determine whether these proteins have promise as clinical biomarkers.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X21991439 for Cytokine and chemokine responses to injury and treatment in a nonhuman primate model of hypoxic-ischemic encephalopathy treated with hypothermia and erythropoietin by Thomas R Wood, Phuong T Vu, Bryan A Comstock, Janessa B Law, Dennis E Mayock, Patrick J Heagerty, Thomas Burbacher, Theo K Bammler and Sandra E Juul in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the US National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development R01-HD-073128-02.
Acknowledgements: The authors would like to thank Noelle McKain, Ron McPherson, Daniel Moralejo, Kylie Corry, and all the members of the Infant Primate Research Laboratory nursery staff for their animal care work and technical assistance.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: TRW, PTV, BAC, JBL, TKB, TB, DEM, PJH, and SEJ conceived the manuscript. TKB oversaw the biomarker measurements. TB oversaw long-term animal care and behavioral assessments. SEJ oversaw the methodology of the experimental protocols. PTV, BAC, and PJH performed the statistical analysis. PTV made the figures. TRW drafted and revised the manuscript. All authors contributed to editing the manuscript and approved the final draft.
Supplementary material: Supplemental material for this article is available online.
References
- 1.Kurinczuk JJ, White-Koning M, Badawi N.Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev 2010; 86: 329–338. [DOI] [PubMed] [Google Scholar]
- 2.Wu YW, Backstrand KH, Zhao S, et al. Declining diagnosis of birth asphyxia in California: 1991-2000. Pediatrics 2004; 114: 1584–1590. [DOI] [PubMed] [Google Scholar]
- 3.Shankaran S, Laptook AR, Pappas A, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network et al. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA 2017; 318: 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabir H, Cowan FM.Prediction of outcome methods assessing short- and long-term outcome after therapeutic hypothermia. Semin Fetal Neonatal Med 2015; 20: 115–121. [DOI] [PubMed] [Google Scholar]
- 5.Parikh P, Juul SE.Neuroprotective strategies in neonatal brain injury. J Pediatr 2018; 192: 22–32. [DOI] [PubMed] [Google Scholar]
- 6.Rangarajan V, Juul SE.Erythropoietin: emerging role of erythropoietin in neonatal neuroprotection. Pediatr Neurol 2014; 51: 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juul SE, Comstock BA, Heagerty PJ, et al. High-dose erythropoietin for asphyxia and encephalopathy (HEAL): a randomized controlled trial - background, aims, and study protocol. Neonatology 2018; 113: 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Traudt CM, McPherson RJ, Bauer LA, et al. Concurrent erythropoietin and hypothermia treatment improve outcomes in a term nonhuman primate model of perinatal asphyxia. Dev Neurosci 2013; 35: 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins RD, Shankaran S.Hypothermia for hypoxic ischemic encephalopathy in infants > or =36 weeks. Early Hum Dev 2009; 85: S49–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai JCY, Rocha-Ferreira E, Ek CJ, et al. Immune responses in perinatal brain injury. Brain Behav Immun 2017; 63: 210–223. [DOI] [PubMed] [Google Scholar]
- 11.Liu F, McCullough LD.Inflammatory responses in hypoxic ischemic encephalopathy. Acta Pharmacol Sin 2013; 34: 1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li B, Concepcion K, Meng X, et al. Brain-immune interactions in perinatal hypoxic-ischemic brain injury. Prog Neurobiol 2017; 159: 50–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juul SE, Aylward E, Richards T, et al. Prenatal cord clamping in newborn Macaca nemestrina: a model of perinatal asphyxia. Dev Neurosci 2007; 29: 311–320. [DOI] [PubMed] [Google Scholar]
- 14.Beckstrom AC, Humston EM, Snyder LR, et al. Application of comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry method to identify potential biomarkers of perinatal asphyxia in a non-human primate model. J Chromatogr A 2011; 1218: 1899–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson Misbe EN, Richards TL, McPherson RJ, et al. Perinatal asphyxia in a nonhuman primate model. Dev Neurosci 2011; 33: 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankaran S, Natarajan G, Chalak L, et al. Hypothermia for neonatal hypoxic-ischemic encephalopathy: NICHD neonatal research network contribution to the field. Semin Perinatol 2016; 40: 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savino C, Pedotti R, Baggi F, et al. Delayed administration of erythropoietin and its non-erythropoietic derivatives ameliorates chronic murine autoimmune encephalomyelitis. J Neuroimmunol 2006; 172: 27–37. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, Shi JX, Hang CH, et al. Inhibitory effect on cerebral inflammatory agents that accompany traumatic brain injury in a rat model: a potential neuroprotective mechanism of recombinant human erythropoietin (rhEPO). Neurosci Lett 2007; 425: 177–182. [DOI] [PubMed] [Google Scholar]
- 19.O’Shea TM, Allred EN, Kuban KCK, et al. Elevated concentrations of inflammation-related proteins in postnatal blood predict severe developmental delay at 2 years of age in extremely preterm infants. J Pediatr 2012; 160: 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohannon RW, Smith MB.Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987; 67: 206–207. [DOI] [PubMed] [Google Scholar]
- 21.DeLong ER, DeLong DM, Clarke-Pearson DL.Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG.Multiple significance tests: the Bonferroni method. BMJ 1995; 310: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical computing, 2019. [Google Scholar]
- 24.Wang B, Chen Y, Zhang J, et al. A preliminary study into the economic burden of cerebral palsy in China. Health Policy 2008; 87: 223–234. [DOI] [PubMed] [Google Scholar]
- 25.Fan X, Kavelaars A, Heijnen CJ, et al. Pharmacological neuroprotection after perinatal hypoxic-ischemic brain injury. Curr Neuropharmacol 2010; 8: 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowan F, Rutherford M, Groenendaal F, et al. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet 2003; 361: 736–742. [DOI] [PubMed] [Google Scholar]
- 27.Lee AC, Kozuki N, Blencowe H, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res 2013; 74: 50–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferriero DM.Neonatal brain injury. N Engl J Med 2004; 351: 1985–1995. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Biarge M, Diez-Sebastian J, Wusthoff CJ, et al. Antepartum and intrapartum factors preceding neonatal hypoxic-ischemic encephalopathy. Pediatrics 2013; 132: e952–e959. [DOI] [PubMed] [Google Scholar]
- 30.Badawi N, Kurinczuk JJ, Keogh JM, et al. Antepartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ 1998; 317: 1549–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badawi N, Kurinczuk JJ, Keogh JM, et al. Intrapartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ 1998; 317: 1554–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waisman A, Hauptmann J, Regen T.The role of IL-17 in CNS diseases. Acta Neuropathol 2015; 129: 625–637. [DOI] [PubMed] [Google Scholar]
- 33.Richter JR, Sutton JM, Belizaire RM, et al. Macrophage-derived chemokine (CCL22) is a novel mediator of lung inflammation following hemorrhage and resuscitation. Shock 2014; 42: 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.García-Berrocoso T, Giralt D, Llombart V, et al. Chemokines after human ischemic stroke: from neurovascular unit to blood using protein arrays. Transl Proteomics 2014; 3: 1–9. [Google Scholar]
- 35.Garcia-Zepeda EA, Combadiere C, Rothenberg ME, et al. Human monocyte chemoattractant protein (MCP)-4 is a novel CC chemokine with activities on monocytes, eosinophils, and basophils induced in allergic and nonallergic inflammation that signals through the CC chemokine receptors (CCR)-2 and -3. J Immunol 1996; 157: 5613. [PubMed] [Google Scholar]
- 36.Vignali DA, Kuchroo VK.IL-12 family cytokines: immunological playmakers. Nat Immunol 2012; 13: 722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper AM, Khader SA.IL-12p40: an inherently agonistic cytokine. Trends Immunol 2007; 28: 33–38. [DOI] [PubMed] [Google Scholar]
- 38.Fan X, van Bel F, van der Kooij MA, et al. Hypothermia and erythropoietin for neuroprotection after neonatal brain damage. Pediatr Res 2013; 73: 18–23. [DOI] [PubMed] [Google Scholar]
- 39.Fang AY, Gonzalez FF, Sheldon RA, et al. Effects of combination therapy using hypothermia and erythropoietin in a rat model of neonatal hypoxia–ischemia. Pediatr Res 2013; 73: 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wassink G, Davidson JO, Fraser M, et al. Non-additive effects of adjunct erythropoietin therapy with therapeutic hypothermia after global cerebral ischaemia in near-term fetal sheep. J Physiol 2020; 598: 999–1015. [DOI] [PubMed] [Google Scholar]
- 41.Juul SE.Hypothermia plus erythropoietin for neonatal neuroprotection? Commentary on Fan et al. and Fang et al. Pediatr Res 2013; 73: 10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chalak LF, Sánchez PJ, Adams-Huet B, et al. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J Pediatr 2014; 164: 468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orrock JE, Panchapakesan K, Vezina G, et al. Association of brain injury and neonatal cytokine response during therapeutic hypothermia in newborns with hypoxic-ischemic encephalopathy. Pediatr Res 2016; 79: 742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Massaro AN, Wu YW, Bammler TK, et al. Plasma biomarkers of brain injury in neonatal hypoxic-ischemic encephalopathy. J Pediatr 2018; 194: 67–75. [DOI] [PubMed] [Google Scholar]
- 45.Denihan NM, Kirwan JA, Walsh BH, et al. Untargeted metabolomic analysis and pathway discovery in perinatal asphyxia and hypoxic-ischaemic encephalopathy. J Cereb Blood Flow Metab 2019; 39: 147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benavente-Fernandez I, Ramos-Rodriguez JJ, Infante-Garcia C, et al. Altered plasma-type gelsolin and amyloid-β in neonates with hypoxic-ischaemic encephalopathy under therapeutic hypothermia. J Cereb Blood Flow Metab 2019; 39: 1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinter D, Gattringer T, Enzinger C, et al. Longitudinal MRI dynamics of recent small subcortical infarcts and possible predictors. J Cereb Blood Flow Metab 2019; 39: 1669–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu F, Fan S, Romo AR, et al. Serum 24S-hydroxycholesterol predicts long-term brain structural and functional outcomes after hypoxia-ischemia in neonatal mice. J Cereb Blood Flow Metab 2021; 41: 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao N, Zhang-Brotzge X, Wali B, et al. Plasma osteopontin may predict neuroinflammation and the severity of pediatric traumatic brain injury. J Cereb Blood Flow Metab 2020; 40: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skranes JH, Løhaugen G, Schumacher EM, et al. Amplitude-integrated electroencephalography improves the identification of infants with encephalopathy for therapeutic hypothermia and predicts neurodevelopmental outcomes at 2 years of age. J Pediatr 2017; 187: 34–42. [DOI] [PubMed] [Google Scholar]
- 51.Chandrasekaran M, Chaban B, Montaldo P, et al. Predictive value of amplitude-integrated EEG (aEEG) after rescue hypothermic neuroprotection for hypoxic ischemic encephalopathy: a meta-analysis. J Perinatol 2017; 37: 684–689. [DOI] [PubMed] [Google Scholar]
- 52.Weeke LC, Boylan GB, Pressler RM, NEonatal seizure treatment with Medication Off-patent (NEMO)consortium et al. Role of EEG background activity, seizure burden and MRI in predicting neurodevelopmental outcome in full-term infants with hypoxic-ischaemic encephalopathy in the era of therapeutic hypothermia. Eur J Paediatr Neurol 2016; 20: 855–864. [DOI] [PubMed] [Google Scholar]
- 53.Dereymaeker A, Matic V, Vervisch J, et al. Automated EEG background analysis to identify neonates with hypoxic-ischemic encephalopathy treated with hypothermia at risk for adverse outcome: a pilot study. Pediatr Neonatol 2019; 60: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chalak LF, Tian F, Adams-Huet B, et al. Novel wavelet real time analysis of neurovascular coupling in neonatal encephalopathy. Sci Rep 2017; 7: 45958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitra S, Bale G, Meek J, et al. Cerebral near infrared spectroscopy monitoring in term infants with hypoxic ischemic encephalopathy—a systematic review. Front Neurol 2020; 11: 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mietzsch U, Radhakrishnan R, Boyle FA, et al. Active cooling temperature required to achieve therapeutic hypothermia correlates with short-term outcome in neonatal hypoxic-ischaemic encephalopathy. J Physiol 2020; 598: 415–424. [DOI] [PubMed] [Google Scholar]
- 57.Enweronu-Laryea C, Martinello KA, Rose M, et al. Core temperature after birth in babies with neonatal encephalopathy in a Sub-Saharan African hospital setting. J Physiol 2019; 597: 4013–4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X21991439 for Cytokine and chemokine responses to injury and treatment in a nonhuman primate model of hypoxic-ischemic encephalopathy treated with hypothermia and erythropoietin by Thomas R Wood, Phuong T Vu, Bryan A Comstock, Janessa B Law, Dennis E Mayock, Patrick J Heagerty, Thomas Burbacher, Theo K Bammler and Sandra E Juul in Journal of Cerebral Blood Flow & Metabolism