Abstract
Patients with Parkinson’s disease (PD) often suffer from non-motor symptoms, which may be caused by serotonergic dysfunction. Apart from alleviating the motor symptoms, Deep Brain Stimulation (DBS) in the subthalamic nucleus (STN) may also influence non-motor symptoms. The aim of this study is to investigate how turning DBS off affects the serotonergic system. We here exploit a novel functional PET neuroimaging methodology to evaluate the preservation of serotonergic neurons and capacity to release serotonin. We measured cerebral 5-HT1BR binding in 13 DBS-STN treated PD patients, at baseline and after turning DBS off. Ten age-matched volunteers served as controls. Clinical measures of motor symptoms were assessed under the two conditions and correlated to the PET measures of the static and dynamic integrity of the serotonergic system. PD patients exhibited a significant loss of frontal and parietal 5-HT1BR, and the loss was significantly correlated to motor symptom severity. We saw a corresponding release of serotonin, but only in brain regions with preserved 5-HT1BR, suggesting the presence of a presynaptic serotonergic deficit. Our study demonstrates that DBS-STN dynamically regulates the serotonin system in PD, and that preservation of serotonergic functions may be predictive of DBS-STN effects.
Keywords: Deep brain stimulation, symptoms, Parkinson’s disease, positron emission tomography, serotonin (5-HT)
Introduction
Parkinson’s disease (PD) is one of the most common movement disorders, characterized by neuronal degeneration and a well-described progressive loss of the dopamine producing cells, primarily in the nigrostriatal pathways,1 eventually, this dopaminergic dysfunction results in the characteristic features of bradykinesia and rigidity seen in PD. Medical therapy of PD has primarily focused on pharmacological modulation of the dopamine system, aiming to alleviate the motor symptoms and executive deficits in these patients. However, non-motor symptoms, particularly depression and fatigue,2,3 constitute a huge burden on quality of life in many PD patients, and dopaminergic therapy may either ameliorate or exacerbate non-motor symptoms, depending on their character.4
Although only applicable to a select population of PD patients, deep brain stimulation (DBS) with electrodes placed in the subthalamic nucleus (STN) is a supplementary surgical treatment for alleviation of motor symptoms in PD. Affective side effects are commonly seen within the first three months after DBS surgery, but there are also multiple case series suggesting that DBS improves the burden of non-motor symptoms. A recent review concludes that there is level I evidence for the effect of DBS on mood: Two randomized prospective studies reported no change in depression while improvement of anxiety was seen.5 Although the therapeutic effect of DBS is well established, the physiological mechanisms underlying its effect is still unclear.6 It has been speculated that DBS acts by inducing changes in neurotransmitter levels in targets and connected regions,7 changing firing rates in afferents and efferents, causing distant effects,8 long-term reorganization of neural networks, and neuroprotection.9
Lately, increasing attention has been given to neuronal degeneration outside the nigrostriatal pathways and to other neurotransmitter systems involved in PD. As reviewed by Huot et al.,10 a growing amount of research supports the presence of serotonergic deficits in PD. Post-mortem, biochemical and neuroimaging studies point to a reduction in various serotonin associated markers, with a regional distribution distinct from that of dopamine. Such deficits in the serotonin system are speculated to account for some of the non-motor symptoms commonly found in PD.
Neuroimaging presents a unique opportunity to investigate the serotonin system in PD patients. As reviewed,10 studies of non-depressed PD patients have revealed a substantial decrease in serotonin transporter (SERT) availability, while the postsynaptic serotonin receptors generally seem less affected, or even increased, suggesting that presynaptic serotonin function may be preferentially affected.
To the best of our knowledge, no studies have so far investigated the effects of DBS to probe serotonergic neurotransmission in humans, most likely because the technology for such an investigation has been missing. We recently showed in a combined PET/microdialysis pig study that the 5-HT1B receptor antagonist radioligand [11C]AZ10419369 is sensitive to pharmacologically-induced changes in cerebral synaptic serotonin level,11 and others have demonstrated a similar displacement in the non-human primate brain.12
The aim of the present study was to investigate changes in cerebral serotonin levels, as indexed by regional [11C]AZ10419369 binding, when DBS is turned off in PD patients treated with DBS-STN. To identify group differences in baseline 5-HT1B receptor availability, and to investigate if such a difference was associated with the clinical characteristics of PD, we also included a group of age-matched healthy volunteers.
We hypothesized that the extent to which PD patients had lower 5-HT1B receptor availability than controls would be linearly correlated to PD motor symptom severity. Secondly, we hypothesized that turning off the DBS-STN stimulator would be associated with a change in cerebral serotonin, as indexed by an inverse change in 5-HT1B receptor binding.
Material and methods
Participants
We included 13 (9 M/4F) DBS-STN treated PD patients (60 ± 7 years, mean±SD) from our specialized movement disorder clinic. Eleven age-matched (58 ± 10 years) healthy volunteers served as controls, five of whom also entered in other ongoing PET studies.13,14 One control had to discontinue the study for reasons of discomfort while being placed in the PET scanner, so the final group of age-matched controls included 10 (7 M, 3 F) individuals.
All participants were assessed by a structured interview for a variety of medical disorders, major depression, and severe cognitive deficits, and selected according to the in- and exclusion criteria. Specific inclusion criteria for the PD patients were current DBS-STN treatment, and exclusion criteria were DBS surgery within 3 months from PET scan. General exclusion criteria for all participants were: Severe or symptomatic somatic, psychiatric or neurological illness not related to PD; use of medicine which may influence the research results; severe cognitive deficits or Mini Mental State Examination (MMSE) scores <27; severe hearing or visual impairment; being non-fluent in Danish; or current substance or alcohol abuse.
None of the participants had any significant somatic, psychiatric, or neurological history or abnormalities on clinical examination other than those related to PD. None of the participants’ cerebral MRI scans were abnormal. Apart from slightly elevated blood cholesterol in a few participants, blood chemistry was unremarkable. All participants were screened and negative for drugs of abuse on a urine test (Rapid Response™ Multi-Drug Test Panel; BTNX Inc., Markham, Ontario, Canada), except for one patient who reported having taken one tablet of 5 mg morphine for acute back pain 5 days prior to the scanning.
The day before the PET scan, patients were admitted to the Department of Neurology. Throughout the PET scan, the patient was attended by their designated nurse and a neurosurgeon. After the PET scan, upon return to the ward, all patients reported themselves in normal condition and were discharged either immediately or the day after, according to their own wish.
Ethical statement
The study was approved by the Capital Region’s ethics committee (H-1-2014-002, H-3-2013-100, H-6-2014-057, and H-KF-2006-20) and the Danish Data Protection Agency (30-1450). All participants provided written informed consent according to the Declaration of Helsinki. The age-matched controls received monetary compensation for their participation.
Rating scales
The PD patients were clinically staged, and presence and severity of non-motor and motor symptoms was assessed by a MDS-UPDRS certified neurosurgeon using two rating scales for PD: the Hoehn and Yahr Rating Scale (HYR) and the revised Unified Parkinson’s Disease Rating Scale (MDS-UPDRS).15 All participants were scored with the self-reported rating scale of The Major Depression Inventory (MDI)16 and patients were scored with Mini-Mental Status Examination (MMSE).17 The patients were assessed while on their usual anti-parkinson medication.
Study design
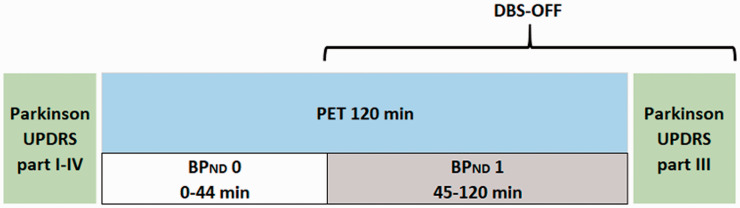
The study design is illustrated in Figure 1. In all PD patients, the DBS-STN was turned off 45 minutes after the radiotracer was injected, and the PET scanning was continued for another 75 min. That is, the PET scan generated two non-displaceable binding potentials (BPND): BPND0 (0–44 min, DBS-ON) and BPND1 (45 min-end of scan, DBS-OFF). All patients were assessed at baseline in the DBS-ON condition by means of MDS-UPDRS (part I–IV) with repeated measures of MDS-UPDR (part III of motor scores) post-scan while still in the DBS-OFF condition.
Figure 1.
The experimental design of the study. DBS was turned off in a within-scan design to generate a BPND0 (DBS-ON) and BPND1 (DBS-OFF). Patients were assessed with MDS-UPDRS (part I–IV) at baseline (DBS-ON) with repeated measures (part III, motor scores) post-scan while still in the DBS-OFF condition.
Neuroimaging
An MRI brain scan was conducted in all age-matched controls with the purpose of co-registration and alignment to the PET scan. For reasons of relative MRI contraindications and likely MRI artefacts by the DBS implants, we used the pre-operative MRI scan of the PD patients. The time interval between the structural MRI and the PET scan was 2.6 ± 1.8 years.
The radiochemical [11C]AZ10419369 was produced as previously reported.18 PET scanning was conducted with a high-resolution research tomography (HRRT) PET scanner (CTI/Siemens, Knoxville, TN, USA). BPND’s were computed by the Extended Simplified Reference Tissue Model,19 with cerebellum as a reference. The MRI and PET scanning protocols and quantification of [11C]AZ10419369 binding is further detailed in the Supplementary Material, Figure A and Figure B.
Statistical analyses
Based on a previous test-retest study in pigs with the PET radioligand [11C]AZ10419369,11 eleven PD patients is the estimated sample size required to demonstrate a 4% change in cortical binding. The estimate is given a significance level of 0.05, power of 0.8 and an effect size determined by the BPND0 (mean ± SD) at baseline (0.71 ± 0.10), BPND1 after intervention with escitalopram (0.68 ± 0.12) in a therapeutically relevant dose (0.28 mg/kg), and the Interclass Correlation Coefficient of 0.98 between groups. The power analysis was performed using G*Power 3.1 software, and the following statistical analysis were performed using IBM SPSS Statistics 24.
Group differences in demographics and injected mass per kilogram bodyweight were evaluated with unpaired two-tailed t-test. The difference in state scores (DBS OFF versus ON) of PD patients was evaluated with a paired Wilcoxon test.
Group differences in regional BPND0 were tested by general linear model analyses with covariates of group (controls, PD) and age. Age was included in the regression model as previous PET studies have demonstrated a negative correlation between [11C]AZ10419369 BPND and age.20,21 Although the molar radioactivity of [11C]AZ10419369 was high in all cases, we initially included injected mass per kilogram of bodyweight as a covariate, because of theoretical effect of unlabeled AZ10419369 on BPND. However, given that the injected doses were low and that we did not identify injected mass as a significant covariate, it was excluded from the final analysis. To test for differences between BPND0 and BPND1, we tested each volume of interest (VOI) with a paired sample t-test, independently for both groups. Our primary analysis included the following VOI’s: frontal, parietal, temporal, and occipital cortices and limbic cortex, and we subsequently conducted a post-hoc analyses of additional VOI’s: neocortex, superior frontal gyrus, primary motor cortex, dorsolateral prefrontal cortex, ventrolateral prefrontal cortex, medial inferior frontal gyrus, orbitofrontal gyrus, superior temporal gyrus, medial inferior temporal gyrus, somatosensory cortex, anterior cingulate cortex, posterior cingulate cortex, insular cortex, caudate, putamen and thalamus. BPND estimates with a coefficient of variation (COV) larger than 15%, e.g. raphe nucleus, were excluded from the analyses and only regions where more than 50% of the subjects’ data fit the kinetic model were included.
Significance level was set at p-value of 0.05. One regression analysis was done for each brain region of interest, and the p-value of each primary brain VOI (n = 5) that survived correction for multiple comparisons by the Bonferroni-Holm method was marked with an Asterix (*). In the post hoc analyses, p-values were not corrected for multiple comparisons.
For primary VOI’s with significant group difference in BPND, we analyzed if there was an association between BPND and UPDRS motor scores both at baseline and with DBS turned off. The association between regional BPND and the UPDRS motor scores was evaluated by general linear model, with BPND as the dependent and UPDRS-motor score (DBS-ON), age, and L-DOPA equivalents as covariates.
We performed a post hoc analysis of the relative change in binding potential when turning DBS-STN off. The percent decrease in BPND in PD patients when turning the DBS off was associated to the ratio between patients and healthy controls BPND across region using linear regression preceded by a normal distribution test. The standard coefficient of variance (COV) was below 15% for all BPNDs. Data that did not fulfill this criterion were not included in the analysis. The raphe did not fit well in more than half the cases and the region was excluded. For reasons given below, putamen was not included in the statistical regression analysis. Also, regions comprising sub-regions (neocortex, frontal, parietal, limbic, and temporal cortex) were not included in the statistical regression analysis.
Results
Clinical measures
Demographic and clinical variables of the patients are displayed in Table 1. There was no significant difference between PD patients and healthy controls with regards to age or sex. All patients had MMSE scores > 27 and the healthy volunteers were cognitively well-functioning, as assessed by neuropsychological testing. None of the participants met the criteria for major depression. There was no significant difference in injected mass per kg bodyweight between patients and controls (22 ± 13 vs. 24 ± 19 ng/kg). The PD patients only displayed slightly more head movements than controls during the PET scan, judged insufficient to create any consistent bias in the observed BPND (Figure A, Supplementary Material).
Table 1.
Patient characteristics.
| PD | Sex | Age | Years since surgery | L-DOPA equivalents | UPDRS p. I-IV | UPDRS p. III |
HYR | |
|---|---|---|---|---|---|---|---|---|
| DBS-ON | DBS-ON | DBS-OFF | DBS-OFF | |||||
| 1 | M | 55 | 7 | 531 | n.a | n.a | n.a | 2 |
| 2 | M | 66 | 1.7 | 469 | 48 | 20 | 47 | 2 |
| 3 | M | 53 | 3.4 | 967 | 15 | 7 | 34 | 2 |
| 4 | M | 59 | 2.7 | 391 | 34 | 25 | 66 | 2 |
| 5 | M | 72 | 5.5 | 815 | 44 | 11 | 37 | 3 |
| 6 | M | 67 | 0.5 | 430 | 9 | 0 | 26 | 2 |
| 7 | F | 56 | 0.8 | 532 | 21 | 4 | 15 | 1 |
| 8 | F | 63 | 2.5 | 479 | 22 | 7 | 41 | 2 |
| 9 | F | 50 | 1.2 | 500 | 36 | 7 | 55 | 2 |
| 10 | F | 65 | 1.8 | 305 | 36 | 7 | 29 | 2 |
| 11 | M | 50 | 2.6 | 385 | 36 | 7 | 28 | 2 |
| 12 | M | 62 | 2.6 | 1896 | 73 | 27 | 41 | 2 |
| 13 | M | 56 | 0.7 | 835 | 22 | 0 | 23 | 2 |
| Mean ± SD | 60 ± 7 | 2.5 ± 1.8 | 656 ± 405 | 33 ± 16 | 10 ± 9 | 37 ± 14 | 2.0 ± 0.4 | |
Male (M), Female (F), levodopa (L-DOPA) or equipotent to L-DOPA, Deep Brain Stimulation (DBS), Unified Parkinson Disease Rating Scale (UPDRS) and motor scores part III (UPDRS-motor), Hoehn and Yahr Rating scale (HYR).
DBS-STN treated patients with PD versus controls
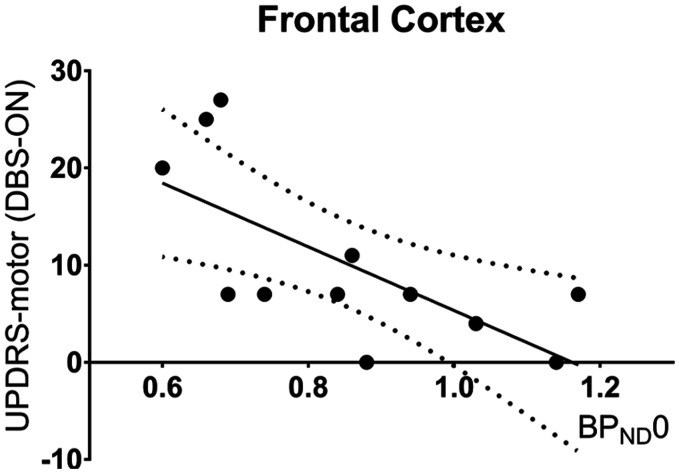
Table 2 shows BPND0 in patients with DBS-ON and in healthy controls. The standard coefficient of variance (COV) was below 15% for all regional BPND; data that did not fulfill this criterion were not included in the analysis. We found that the regional BPND0 values were numerically consistently lower in PD patients, with significantly lower BPND0 in the frontal and parietal cortices. The UPDRS motor score was inversely correlated with frontal cortex BPND0 (Figure 2), meaning that PD patients who suffered the most pronounced clinical symptoms also had the lowest frontal cortex BPND0. The correlation was also present in the DBS-OFF condition (p = .01, R2 = 0.61). This inverse relationship was not significant for the parietal cortex (p = .23, r2 = 0.17).
Table 2.
BPND0 in PD patients and age-matched controls and the relative change in BPND (%).
| VOI | BPND0 |
ΔBPND(%) |
|||||
|---|---|---|---|---|---|---|---|
| PD (n = 13) | HC (n = 10) | p-value | PD (n = 13) | p-value | HC (n = 10) | P-value | |
| FC | 0.85 ± 0.17 | 1.12 ± 0.27 | .01 * | −5 ± 11 | .08 | 6 ± 12 | .12 |
| TC | 0.79 ± 0.15 | 0.95 ± 0.25 | .09 | −11 ± 9 | .002** | 3 ± 11 | .21 |
| PC | 0.79 ± 0.13 | 1.00 ± 0.21 | .01 * | −2 ± 11 | .33 | 12 ± 12 | .02 |
| LC | 1.02 ± 0.21 | 1.28 ± 0.34 | .05 | −9 ± 12 | .01* | 2 ± 7 | .17 |
| OC | 1.06 ± 0.16 | 1.16 ± 0.22 | .26 | −8 ± 9 | .02* | 0 ± 8 | .74 |
| Post-hoc analyses | |||||||
| Neo | 0.84 ± 0.14 | 1.05 ± 0.24 | .02 | −7 ± 10 | .03 | 6 ± 10 | ns |
| SFG | 0.80 ± 0.20 | 1.07 ± 0.25 | .01 | −6 ± 12 | ns | 5 ± 13 | ns |
| PMC | 0.86 ± 0.19 | 1.11 ± 0.27 | .02 | −6 ± 16 | ns | 7 ± 14 | ns |
| dlPFC | 0.75 ± 0.22 | 1.12 ± 0.31 | .005 | −2 ± 20 | ns | 8 ± 14 | ns |
| vlPFC | 1.02 ± 0.19 | 1.26 ± 0.32 | .05 | −2 ± 11 | ns | 9 ± 12 | .04 |
| MIFG | 0.90 ± 0.18 | 1.19 ± 0.29 | .01 | −2 ± 12 | ns | 8 ± 12 | ns |
| OFG | 0.86 ± 0.19 | 1.00 ± 0.27 | ns | −11 ± 14 | .03 | 4 ± 19 | ns |
| STG | 0.79 ± 0.18 | 0.97 ± 0.31 | ns | −15 ± 10 | .002 | 4 ± 12 | ns |
| MITG | 0.80 ± 0.14 | 0.94 ± 0.22 | ns | −6 ± 8 | .02 | 2 ± 10 | ns |
| SSC | 0.73 ± 0.19 | 1.00 ± 0.24 | .01 | −5 ± 15 | ns | 8 ± 12 | ns |
| ACC | 1.09 ± 0.23 | 1.38 ± 0.33 | .03 | −7 ± 10 | .04 | 2 ± 11 | ns |
| PCC | 0.88 ± 0.19 | 0.89 ± 0.21 | ns | −17 ± 15 | ns | −5 ± 11 | ns |
| Ins | 0.99 ± 0.21 | 1.23 ± 0.36 | ns | −11 ± 13 | .02 | 1 ± 6 | ns |
| Cau | 0.66 ± 0.28 | 1.07 ± 0.37 | .006 | 2 ± 29 | ns | 8 ± 21 | ns |
| Put | 1.25 ± 0.25 | 1.42 ± 0.46 | ns | 4 ± 12 | ns | 20 ± 13 | .004 |
| Tha | 0.49 ± 0.12 | 0.55 ± 0.14 | ns | −16 ± 21 | ns | −3 ± 14 | ns |
Abbreviations: frontal cortex (FC), parietal cortex (PC), temporal cortex (TC), occipital cortex (OC), limbic cortex (LC), neocortex (Neo), superior frontal gyrus (SFG), primary motor cortex (PMC), dorsolateral prefrontal cortex (dlPFC), ventrolateral prefrontal cortex (vlPFC), medial inferior frontal gyrus (MIFG), orbitofrontal gyrus (OFG), superior temporal gyrus (STG), medial inferior temporal gyrus (MITG), somatosensory cortex (SSC), anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), insular cortex (Ins), caudate (Cau), putamen (Put) and thalamus (Tha). For primary VOI’s, the given p-values are uncorrected for multiple corrections; those p-values that survive the Bonferoni-Holm correction, are marked with an Asterix (*).
Figure 2.
5-HT1BR availability vs motor symptoms severity. Association between frontal cortex BPND0 and the UPDRS motor scores at baseline (p = .02, r2 = 0.53).
The BPND (%) after switching DBS-STN off was calculated as (BPND1-BPND0)/BPND0)*100%. Values are given as mean ± SD. Primary VOIs (n = 5) that survived multiple comparisons are labelled for significance level p < .05 (*) and p < .01 (**).
Turning the DBS-STN off
When the DBS stimulator was turned off in the patients, we observed a significant decrease in BPND in the temporal, limbic, and occipital cortex (Table 2 and Supplementary Material Figure C). The controls did not show any significant changes in BPND0 vs. BPND1, except in the putamen where BPND1 was found to be larger than BPND0.
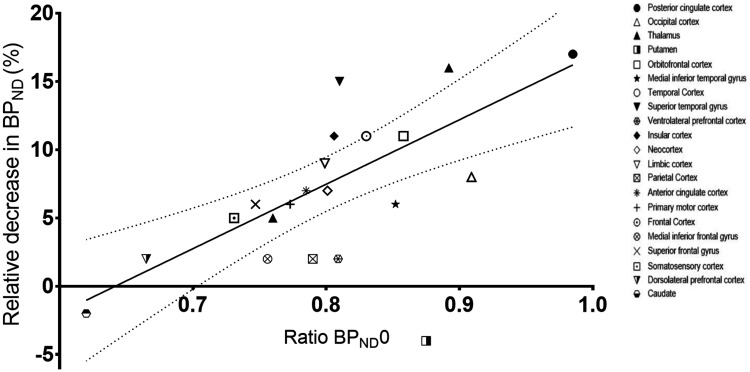
Next, in a post-hoc analysis across all brain regions, we investigated whether the extent to which PD patients had regionally preserved BPND0 as compared to their age-matched controls was related to the regional brain response to switching the DBS-STN off. We excluded the putamen from the analysis since the healthy controls showed an increase in BPND1 in this region, despite no intervention taking place (Table 2), consistent with what we have seen in previous analyses in healthy volunteers.22 Generally, putamen 5-HT1B receptor PET binding is for some reason not easy to fit well kinetically.23 Interestingly, we found a correlation between the relative change in BPND and preservation of 5-HT1B receptor binding in PD patients (Figure 3). That is, in the least affected brain regions, serotonin was released when DBS was turned off, whereas in brain regions with the largest reductions in 5-HT1B receptor binding, no significant serotonin release was detected.
Figure 3.
5-HT1BR preservation vs serotonin release. Percent decrease in BPND in PD patients when turning the DBS off versus the BPND0 ratio between patients and healthy controls across region, calculated as the mean BPND0[single PD]/BPND0[average all HC]. A significant correlation between the two measures was found, (p = .0005, r2 = 0.62).
Test-retest data from the healthy controls, including intercorrelation coefficients as well as the effect size of the DBS-STN intervention, are given in Table A in the Supplementary Material. VOI volumes are given in Table B (Supplementary Material).
Data availability
Data is available upon request to the corresponding author.
Discussion
Firstly, we find that compared to controls, PD patients treated with DBS-STN have lower 5-HT1B receptor availability in frontal and parietal cortex, and that the frontal cortex 5-HT1B receptor availability is negatively correlated with motor symptom severity in PD. Secondly, when DBS-STN is turned off, we see a decrease in 5-HT1B receptor binding, interpreted as an increase in serotonin levels, in temporal, limbic, and occipital cortex. Importantly, the degree to which serotonin levels increase after turning the DBS-STN off is linearly correlated to the regional preservation of 5-HT1B receptors in PD.
5-Ht1b receptor availability in PD patients
When conducting a rigorous statistical analysis with conservative corrections for multiple comparisons, we observed significantly lower 5-HT1B receptor binding in frontal and parietal cortex only. As can be seen from Table 2, however, PD patients had numerically lower 5-HT1B receptor binding in all brain regions. This observation is consistent with a prior non-DBS study of PD patients PET-scanned with [11C]AZ10419369, where a voxel-based analysis showed lower 5-HT1B receptors binding in the orbitofrontal cortex, but PD patients overall had lower cerebral 5-HT1B receptor binding.21
Theoretically, the lower baseline 5-HT1B receptor binding in PD patients could be caused by 1) a decrease in receptor density (Bmax), 2) lower tissue free fraction (fND), or 3) lower radioligand affinity (higher KD), for example caused by higher serotonin levels. These options are discussed below, in reverse order:
There is ample evidence from cerebrospinal fluid and brain autopsies studies10,24,25 that PD patients have lower levels of serotonin and its metabolite, 5-HIAA, but if anything, because of competition with radioligand binding, this would be expected to lead to higher 5-HT1B receptor binding. Yet, we cannot ignore that synaptic serotonin levels may vary regionally and thereby potentially (partially) explain the regional variation in brain binding in PD patients compared to controls. Further, although it cannot be ruled out, we fail to find any good explanation for why PD patients should have lower free fraction of radioligand in their brain tissue. That is, our observation is most likely due to PD patients having lower 5-HT1B receptor density, possibly due to loss of brain cells that normally express 5-HT1B receptors. Since the 5-HT1B receptor functions both as an autoreceptor modulating serotonin release and as an heteroceptor, modulating other neurotransmitter systems,26 the lower 5-HT1B receptor density could be caused by a reduction in either autoreceptor or heteroreceptor density, or both. We find it most likely that the observed lower 5-HT1B receptor density is due to presynaptic dysfunction or axonal loss. As recently reviewed,10 several in vivo and in vitro studies have found widespread reductions in cerebral SERT in PD. It was also recently discovered that a decline in serotonin transporter binding in premotor A53T SNCA carriers preceded development of dopaminergic pathology and motor symptoms and was associated with disease burden,27 in a regional pattern fairly consistent with what we observe here (Figure 3), whereas postsynaptic serotonin receptors are better preserved or even upregulated.10 It is also well-known that more advanced stages of PD are associated with loss of serotonergic fibers,28,29 possibly due to Lewy body deposits preferentially forming at the axonal terminals, leading to cellular dysfunction and eventually neuronal death. The observation supports the idea that in PD, proximate cellular structures to the Lewy body deposits in the axonal terminal are affected more than somatodendritic cellular components.
The role of 5-HT1B receptors for motor disability
In the PD patients, we observed an inverse correlation between motor symptom severity and frontal cortex 5-HT1B receptor availability. This suggests that either the 5-HT1B receptor is important for motor function or that the decline in 5-HT1B receptor availability is a proxy of the disease progression, or a combination. There is some preclinical evidence for 5-HT1B receptors being involved in motor function: pharmacological stimulation of the 5-HT1B receptor is associated with strong locomotor response in mice,26 and 5-HT1B receptor stimulation also facilitates dopamine release.30 We examined if frontal subregions involved in motor function seemed to drive the association, but that was not obvious. In a previous PET study of 5-HT1B receptor binding they did not identify a statistically significant correlation between 5-HT1B receptor binding and stage or severity of the disease;21 the authors ascribe this failure to lack of power or medication effects.
Turning off STN-DBS in PD patients
When we turned off the DBS-STN in patients with PD, BPND decreased between zero and 15% across a number of brain regions (Figure 3). Based on data from the pig brain,11 one can estimate that a 7% decline in [11C]AZ10419369 BPND corresponds to a 3-fold increase in cerebral serotonin level, achievable with a single intravenous dose of the potent serotonin releasing agent fenfluramine (0.5 mg/kg). But how does turning off the DBS stimulator lead to increased cerebral serotonin release? In rat studies,31–33 high frequency stimulation of the STN caused a decrease in DRN firing and a decrease in serotonin in prefrontal cortex. Here, we observe an increase when DBS is turned off. Besides changes in dopamine levels, known to regulate the dorsal raphe nucleus firing rate, we propose that the serotonergic response to cessation of DBS-STN stimulation extends beyond the basal ganglia and thalamocortical circuits and stimulates the raphe nuclei serotonergic neurotransmission, possibly in response to arousal.
Intriguingly, unrelated to the baseline BPND, we find that the degree to which regional cerebral 5-HT1B receptors are preserved in PD predicts the capacity to elicit serotonin release when the DBS stimulator is turned off. From Figure 3 it can be seen that when the regional 5-HT1B receptor binding is normal, switching DBS off leads to a 15% decrease in BPND, corresponding to an 8-fold increase in cerebral serotonin levels. Or conversely, when 5-HT1B receptor binding is reduced in PD patients to ⅔ of control levels, no serotonin is released in response to turning off the stimulator. This observation further supports the idea that the abnormalities in cerebral 5-HT1B receptors found in PD patients are primarily caused by presynaptic degeneration. Since our study, for logistical reasons, only involved turning off DBS-STN, we cannot know if the reverse actions occur when turning DBS-STN on. Also, the temporal evolvement of the response cannot be assessed beyond 60 min since our experimental setup does now allow for extended measurements of cerebral 5-HT1B receptor binding in the off condition.
Limitations
Patients displayed slightly more head motion than controls, in average 1 mm more. It is unlikely that head motion could explain the differences observed between controls and PD patients because firstly, head motion was corrected for and secondly, even with the HRRT PET scanner, the spatial resolution is 3 mm.
Our study design was a single PET-scan where DBS was switched off after 45 min. We cannot conclude anything about what would happen in the reverse situation, i.e., when DBS is turned on during the experiment. We abstained from the latter design because it would require considerations about how long time it would take to reach a new steady state in the DBS OFF condition. Furthermore, our results are based on the assumption that our PET data reflect serotonin release instantaneously, which may not be the case. If the serotonin release is picked up more slowly by PET, if anything, the total release may have been underestimated.
Conclusions
By applying a novel functional PET methodology, we investigated the static and dynamic integrity of the serotonin system in PD patients. We find that DBS-STN treated PD patients exhibit a loss of frontal 5-HT1B receptors, a deficit that correlates to the degree of motor dysfunction. When the DBS-STN is turned off, the brain regions with the best preserved presynaptic serotonin function respond by releasing serotonin. This suggests that the presynaptic terminals are still relatively preserved in those brain areas, whereas the more affected brain regions have lost their serotonin releasing capacity. These deficits in the regulation of the serotonin may contribute to PD patients’ non-motor or motor symptom severity. Our study is a novel demonstration that DBS-STN dynamically regulates the serotonin system and that preservation of the presynaptic serotonin function may be predictive of the effects of DBS-STN.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20982389 for Parkinson patients have a presynaptic serotonergic deficit: A dynamic deep brain stimulation PET study by Louise M Jørgensen, Tove Henriksen, Skirmante Mardosiene, Sune H Keller, Dea S Stenbæk, Hanne D Hansen, Bo Jespersen, Carsten Thomsen, Pia Weikop, Claus Svarer and Gitte M Knudsen in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
The authors gratefully thank Anders Lundetoft Clausen, Bente Dall, Szabolcs Lehel, Vibeke Naja Høyrup Dam and Brice Ozenne for excellent assistance and statistical support. We thank Marie Deen Christensen and Sofi da Cunha-Bang for sharing data from six controls. We thank the Movement Disorder Society for granting permission to use the MDS-UPDRS score.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Lundbeck Foundation [R170 2014 994 and R183 2014 3836]; Aase and Ejnar Danielsens Fond [10-001296] and Fonden til Lægevidenskabens Fremme [13-216]. The study design, collection, analysis and interpretation of data, writing of the paper and in the decision to submit the paper for publication were not influenced by any of the funds supporting the study. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit the data for publication.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: All authors revised and gave final approval of the manuscript. Following authors contributed to the conception (LMJ and GMK), drafting of the article (LMJ, SHK and GMK), design (LMJ, TH, SM and GMK), acquisition of data (LMJ, TH, SM, DSS and SHK), and analysis and interpretation of data (LMJ, DSS, CS, PW, BJ, CT, SHK, HDH, and GMK).
Full financial disclosures (past 3 years): SM has received honorary compensation for given lectures to Abbvie. TH has received honorary compensation for lectures given to Abbvie, Nordic Infucare, EVER Pharma and Britannia. LMJ and CT have filed for patency (EPS 17195244.3 – 1666) for an fMRI compatible electrical stimulator. GMK has received honoraria as a speaker for Janssen Pharmaceuticals and as advisor for Sage Therapeutics and Sanos. SHK, DSS, HDH, BJ, PW and CS report no conflict of interest these activities had no involvement or restrictions regarding publication of this work.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
Louise M Jørgensen https://orcid.org/0000-0002-1009-7139
Hanne D Hansen https://orcid.org/0000-0001-5564-7627
Claus Svarer https://orcid.org/0000-0001-7811-1825
References
- 1.Goetz CG, Pal G.Initial management of Parkinson’s disease. BMJ 2014; 349: g6258. [DOI] [PubMed] [Google Scholar]
- 2.Müller B, Assmus J, Herlofson K, et al. Importance of motor vs. non-motor symptoms for health-related quality of life in early Parkinson’s disease. Parkinsonism Relat Disord 2013; 19: 1027–1032. [DOI] [PubMed] [Google Scholar]
- 3.Politis M, Wu K, Molloy S, et al. Disease symptoms: the patient’s perspective. Mov Disord 2010; 25: 1646–1651. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri KR, Schapira AHV.Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 2009; 8: 464–474. [DOI] [PubMed] [Google Scholar]
- 5.Kurtis MM, Rajah T, Delgado LF, et al. The effect of deep brain stimulation on the non-motor symptoms of Parkinson’s disease: a critical review of the current evidence. NPJ Park Dis 2017; 3: 16024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voon V, Kubu C, Krack P, et al. Deep brain stimulation: neuropsychological and neuropsychiatric issues. Mov Disord 2006; 21: S305–S327. [DOI] [PubMed] [Google Scholar]
- 7.van Dijk A, Mason O, Klompmakers AA, et al. Unilateral deep brain stimulation in the nucleus accumbens core does not affect local monoamine release. J Neurosci Methods 2011; 202: 113–118. [DOI] [PubMed] [Google Scholar]
- 8.Luigjes J, van den Brink W, Feenstra M, et al. Deep brain stimulation in addiction: a review of potential brain targets. Mol Psychiatry 2012; 17: 572–583. [DOI] [PubMed] [Google Scholar]
- 9.Winn HR.Chapter 83: deep brain stimulation: mechanisms of action. In: Youmans neurological neurosurgery. Philadelphia: Elsevier, 2011, pp. 975–986. [Google Scholar]
- 10.Huot P, Fox SH, Brotchie JM.The serotonergic system in Parkinson’s disease. Prog Neurobiol 2011; 95: 163–212. [DOI] [PubMed] [Google Scholar]
- 11.Jørgensen LM, Weikop P, Svarer C, et al. Cerebral serotonin release correlates with [11C]AZ10419369 PET measures of 5-HT1B receptor binding in the pig brain. J Cereb Blood Flow Metab 2018; 38: 1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finnema S. j, Varrone A, Hwang T. j, et al. Fenfluramine-induced serotonin release decreases [11C]AZ10419369 binding to 5-HT1B-receptors in the primate brain. Synapse 2010; 64: 573–577. [DOI] [PubMed] [Google Scholar]
- 13.Deen M, Hansen HD, Hougaard A, et al. Low 5-HT1B receptor binding in the migraine brain: a PET study. Cephalalgia 2018; 38: 519–527. [DOI] [PubMed] [Google Scholar]
- 14.da Cunha-Bang S, Hjordt LV, Dam VH, et al. Anterior cingulate serotonin 1B receptor binding is associated with emotional response inhibition. J Psychiatr Res 2017; 92: 199–204. [DOI] [PubMed] [Google Scholar]
- 15.Goetz CG, Tilley BC, Shaftman SR, et al.; Movement Disorder Society UPDRS Revision Task Force. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008; 23: 2129–2170. [DOI] [PubMed] [Google Scholar]
- 16.Bech P, Rasmussen N-A, Olsen LR, et al. The sensitivity and specificity of the major depression inventory, using the present state examination as the index of diagnostic validity. J Affect Disord 2001; 66: 159–164. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR.Mini-mental state. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 18.da Cunha-Bang S, Hjordt LV, Perfalk E, et al. Serotonin 1B receptor binding is associated with trait anger and level of psychopathy in violent offenders. Biol Psychiatry 2017; 82: 267–274. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Chen M-K, Endres CJ, et al. An extended simplified reference tissue model for the quantification of dynamic PET with amphetamine challenge. NeuroImage 2006; 33: 550–563. [DOI] [PubMed] [Google Scholar]
- 20.Nord M, Cselenyi Z, Forsberg A, et al. Distinct regional age effects on [11C]AZ10419369 binding to 5-HT1B receptors in the human brain. NeuroImage 2014; 103: 303–308. [DOI] [PubMed] [Google Scholar]
- 21.Varrone A, Svenningsson P, Forsberg A, et al. Positron emission tomography imaging of 5-hydroxytryptamine1B receptors in Parkinson’s disease. Neurobiol Aging 2014; 35: 867–875. [DOI] [PubMed] [Google Scholar]
- 22.Hansen HD, da Cunha-Bang S, Svarer C, et al. Validation of the extended simplified reference tissue model for pharmacological within-scan challenges in dynamic PET. Eur Neuropsychopharmacol 2014; 24: S262–S263. [Google Scholar]
- 23.Gallezot J-D, Nabulsi N, Neumeister A, et al. Kinetic modeling of the serotonin 5-HT1B receptor radioligand [11C]P943 in humans. J Cereb Blood Flow Metab J Tab 2010; 30: 196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tohgi H, Abe T, Takahashi S, et al. Concentrations of serotonin and its related substances in the cerebrospinal fluid of Parkinsonian patients and their relations to the severity of symptoms. Neurosci Lett 1993; 150: 71–74. [DOI] [PubMed] [Google Scholar]
- 25.Kish SJ, Tong J, Hornykiewicz O, et al. Preferential loss of serotonin markers in caudate versus putamen in Parkinson’s disease. Brain 2008; 131: 120–131. [DOI] [PubMed] [Google Scholar]
- 26.Barnes NM, Sharp T.A review of Central 5-HT receptors and their function. Neuropharmacology 1999; 38: 1083–1152. [DOI] [PubMed] [Google Scholar]
- 27.Wilson H, Dervenoulas G, Pagano G, et al. Serotonergic pathology and disease burden in the premotor and motor phase of A53T α-synuclein parkinsonism: a cross-sectional study. Lancet Neurol 2019; 18: 748–759. [DOI] [PubMed] [Google Scholar]
- 28.Halliday GM, Li YW, Blumbergs PC, et al. Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease. Ann Neurol 1990; 27: 373–385. [DOI] [PubMed] [Google Scholar]
- 29.D’Amato RJ, Zweig RM, Whitehouse PJ, et al. Aminergic systems in Alzheimer’s disease and Parkinson’s disease. Ann Neurol 1987; 22: 229–236. [DOI] [PubMed] [Google Scholar]
- 30.Filip M, Bader M.Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacol Rep 2009; 61: 761–777. [DOI] [PubMed] [Google Scholar]
- 31.Tan SKH, Hartung H, Visser-Vandewalle V, et al. A combined in vivo neurochemical and electrophysiological analysis of the effect of high-frequency stimulation of the subthalamic nucleus on 5-HT transmission. Exp Neurol 2012; 233: 145–153. [DOI] [PubMed] [Google Scholar]
- 32.Navailles S, Benazzouz A, Bioulac B, et al. High-Frequency stimulation of the subthalamic nucleus and l-3,4-Dihydroxyphenylalanine inhibit in vivo serotonin release in the prefrontal cortex and hippocampus in a rat model of Parkinson’s disease. J Neurosci 2010; 30: 2356–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Temel Y, Boothman LJ, Blokland A, et al. Inhibition of 5-HT neuron activity and induction of depressive-like behavior by high-frequency stimulation of the subthalamic nucleus. Proc Natl Acad Sci U S A 2007; 104: 17087–17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20982389 for Parkinson patients have a presynaptic serotonergic deficit: A dynamic deep brain stimulation PET study by Louise M Jørgensen, Tove Henriksen, Skirmante Mardosiene, Sune H Keller, Dea S Stenbæk, Hanne D Hansen, Bo Jespersen, Carsten Thomsen, Pia Weikop, Claus Svarer and Gitte M Knudsen in Journal of Cerebral Blood Flow & Metabolism
Data Availability Statement
Data is available upon request to the corresponding author.