Abstract
Purpose.
Increasing measures of adiposity have been correlated with poor oncologic outcomes and a lack of response to anti-angiogenic therapies. Limited data exists on the impact of subcutaneous fat density (SFD) and visceral fat density (VFD) on oncologic outcomes. This ancillary analysis of GOG-218, evaluates whether imaging markers of adiposity were predictive biomarkers for bevacizumab (bev) use in epithelial ovarian cancer (EOC).
Patients and methods.
There were 1249 patients (67%) from GOG-218 with imaging measurements. SFD and VFD were calculated utilizing Hounsfield units (HU). Proportional hazards models were used to assess the association between SFD and VFD with overall survival (OS).
Results.
Increased SFD and VFD showed an increased HR for death (HR per 1-SD increase 1.12, 95% CI:1.05–1.19 p = 0.0009 and 1.13, 95% CI: 1.05–1.20 p = 0.0006 respectively). In the predictive analysis for response to bev, high VFD showed an increased hazard for death in the placebo group (HR per 1-SD increase 1.22, 95% CI: 1.09–1.37; p = 0.025). However, in the bev group there was no effect seen (HR per 1-SD increase: 1.01, 95% CI: 0.90–1.14) Median OS was 45 vs 47 months in the VFD low groups and 36 vs 42 months in the VFD high groups on placebo versus bev, respectively.
Conclusion.
High VFD and SFD have a negative prognostic impact on patients with EOC. High VFD appears to be a predictive marker of bev response and patients with high VFD may be more likely to benefit from initial treatment with bev.
Keywords: Ovarian cancer, Bevacizumab, Imaging biomarkers, Plasma biomarkers
1. Introduction
Ovarian cancer will have approximately 22,530 new cases and 13,980 deaths expected in 2019 in the US, making it the most deadly gynecologic malignancy [1]. Epithelial ovarian cancer (EOC) is the most common subtype encompassing serous, mucinous, clear cell, and endometrioid histologies. Standard therapy for newly diagnosed EOC consists of a combination of surgery and chemotherapy. The backbone of chemotherapy involves the combination of carboplatin and paclitaxel. While the optimal route and schedule of these drugs is still under debate, there still exists a great need to improve long term, disease free survival.
Utilizing angiogenesis inhibitors has been one such strategy for improvement. It has been established that vascular endothelial growth factor (VEGF) levels are higher in EOC patients and is an independent predictor of worse survival [2]. Bevacizumab (bev) is the most extensively studied anti-angiogenic agent to date in EOC. As a result of several randomized phase 3 trials (RPh3), bev has been FDA approved in both front line and platinum sensitive recurrent disease concurrent with chemotherapy and as maintenance [3–8]. In addition, it is approved concurrent with non-platinum based therapy in platinum resistant disease [9]. With increasing use of bev in front line therapy, retrospective and now prospective data confirm continued benefit in patients previously treated with for bev without added toxicity [10,11].
While an active agent, the benefit from the addition of bev is modest and, to date, there have been no validated predictive biomarkers. There are many different types of biomarkers to help clinicians reliably predict response to a therapy. Clinical features, tumor characteristics, and serum markers are all being explored to help predict response to anti-angiogenic therapy. In addition, measures of adiposity, which include both clinical features such as BMI, as well as imaging characteristics such as visceral and subcutaneous fat area (VFA and SFA) and density (SFD and VSD) are being investigated as biomarkers for response to anti-angiogenic therapy. Increasing measures of adiposity have been shown to correlate with not only the development of various malignancies but also with poor oncologic outcomes and a lack of response to antiangiogenic therapies [12]. In melanoma, a high VFA/SFA ratio was associated with decreased PFS and OS in patients treated with anti-angiogenic therapy [13]. Contradictory reports exist in colon cancer patients with one study showing a high VFA predicting a poor response to anti-angiogenic therapy, and another showing a low VFA predicting worse OS in those treated with bev based therapies [14,15]. In patients with renal cell carcinoma, higher VFA has been shown to show worse response to anti-angiogenic therapy but also improved OS [16,17]. More recently measures of adiposity have also been shown to improve response to patient with platinum resistant EOC treated with apatinib, another angiogenic inhibitor [18].
While these studies have shown conflicting results, they have focused solely on fat area and not the quality of the fat. Fat “quality” including adipocyte size, distribution of macrophages, and vessel density have been shown to correlate with metabolic risk but these characteristics are only assessable by invasive biopsies [19]. Therefore, fat density has been hypothesized to serve as a non-invasive marker of fat quality. Density on CT scan is measured in Hounsfeld Units (HU) where the density of water is 0 HU and the density of air is −1000 HU. Previous studies have shown that lower HU measurements for fat is associated with higher lipid content and has been shown to be associated with adverse metabolic risk factors [19].
It has been hypothesized that obesity-related diseases are related to the inflammatory profile produced by adipose tissue. Increasing macrophages and upregulation of cytokines such as TNF-alpha have been linked to obesity related comorbidities [20,21]. Therefore, we hypothesized that patients with increasing fat density and changes in fat quality may have upregulation of these pro-inflammatory pathways. Previous data presented by Alvarez Secord et al. demonstrated that IL-6, one such pro-inflammatory cytokine, was linked to bev response [22]. This analysis, also an ancillary analysis of a subset of patients on GOG-218, reported that patients with high IL-6 benefitted from the addition of treatment of bev with an improvement in PFS, while those with low IL-6 did not. In another ancillary study of GOG-218, high tumor VEGF-A expression was been correlated with improvement in OS in those treated with bev [23].
Given the need for a cost-effective and accessible biomarker for utilization of anti-angiogenic therapies, one objective of our study was to investigate if visceral and subcutaneous fat density were prognostic of response to bev in EOC. Secondarily, our study examined the utility of these imaging biomarkers to predict a patient’s response to bev in EOC and be applied clinically as predictive markers. We also sought to see if these measurements correlated with other pro-inflammatory serum biomarkers previously studied in the same clinical trial population.
2. Patients and methods
This was post hoc, ancillary analysis of GOG-218, which was an RPh3 double-blinded placebo controlled trial whose full methodology has been previous published [6]. Briefly, the study included stage III (incompletely resected) or stage IV EOC patients who were post cytoreductive surgery and then randomized to one of three chemotherapy arms. All arms utilized the same backbone of carboplatin AUC 6 and paclitaxel 175 mg/m2. Arm 1 included chemotherapy with placebo followed by placebo maintenance (CT+ P → P). Arm 2 combined chemotherapy with bev 15 mg/m2 IV every 3 weeks, followed by placebo maintenance (CT + B → P). Arm 3 combined chemotherapy with bev followed by bev maintenance for cycles 2–22 (CT + B → B). The study randomized 1873 patients of whom, 1249 had adiposity measurements available and were utilized in this study. Clinical data from GOG-218 was retrieved on December 9, 2015.
For the imaging portion, The University of Oklahoma imaging core (two radiologists) reviewed all available imaging and were blinded to treatment information. VFA/VFD and SFA/SFD were measured on computed tomography (CT) scans performed after primary debulking surgery but before chemotherapy administration. A computer-aided detection (CAD) scheme was applied to process the CT images of each patient as shown in Fig. 1. CAD scheme first applies a deep learning algorithm to automatically detect abdominal region depicting on CT images volume of interest and then segment images of human body in each CT slide into 3 categories of SFA, VFA and other non-fat related human organs or tissues, which use densities −140 HU to −40 HU as the threshold to define the subcutaneous and visceral fat areas. After segmentation, the cross-sectional subcutaneous and visceral fat area was calculated in cm2 in each CT slide. Density was calculated based on the sum of pixel values multiplied to pixel size. By combining the computation results of all involved CT slides in the abdominal region, the volume of SFA and VFA of each patient are computed. The details of the CAD scheme has been reported in our previous studies [24,25].
Fig. 1.
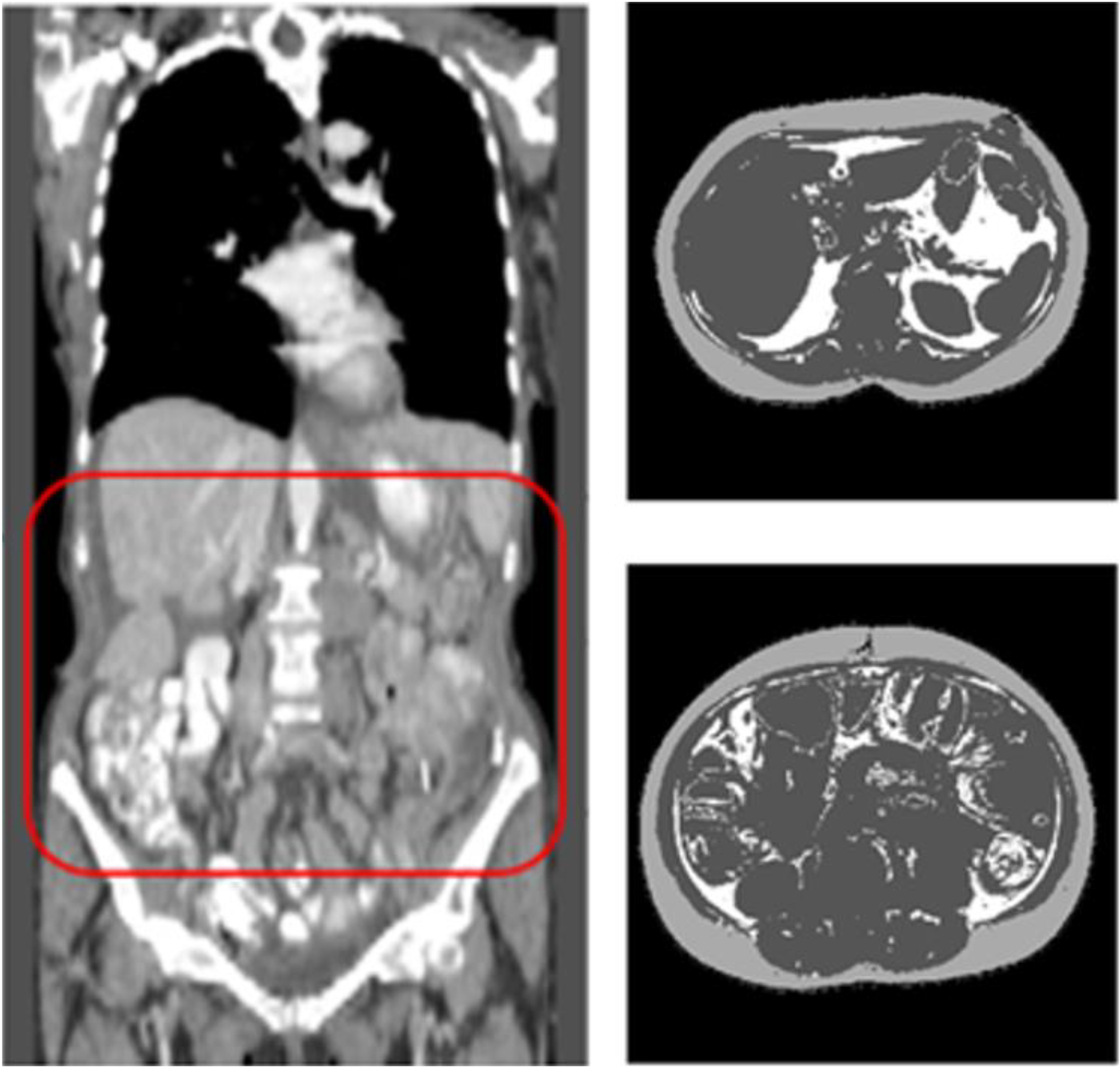
Illustration of measuring VFA and SFA from the CT images by applying a computer-aided detection scheme to automatically (a) detect abdominal section of CT images (inside a box with Red frame) and (b) segment each CT image slide into 3 categories (in which SFA is shown in light gray color, VFA in white color and other non-fat human organs in dark gray color).
For the biomarker analysis, IL-6 and VEGF-A were assessed by multiplex ELISA as previously described [22].
Statistical analysis was performed by the NRG Oncology statistical core. Descriptive statistics were calculated for age, BMI, SFA, SFD, VFA, and VFD. Kruskal-Wallis nonparametric ANOVA test used to assess differences between treatment groups. Proportional hazard models were used to assess the association between OS and BMI and adiposity variables. Models were adjusted for treatment, stage, and performance status. Analysis of the predictive value on bevacizumab response was only assessed on the 851 patients randomized to Arm 1 or 3 (CT+ P → P) or (CT + B → B), respectively. Correlations were assessed using the Spearman correlation method. Statistical significance was met for p-values less than 0.05. All statistical analysis was completed with SAS version 9.4 (Cary, NC).
3. Results
Demographics from this cohort did not differ statistically from the entire group included in GOG-218 where baseline demographics and tumor characteristics were evenly distributed between treatment groups. Table 1 summarizes the demographic variables of this cohort.
Table 1.
Characteristics of patients on GOG-218 with complete adiposity measurements.
| Treatment group | |||
|---|---|---|---|
| CT + P → P | CT + B → P | CT + B → B | |
| Age (Median (Q1,Q3)) | 58 (51, 66) | 60 (53, 67) | 59 (52, 65) |
| Race (N, (%)) | |||
| Non-Hispanic white | 350 (83) | 329 (83) | 355 (83) |
| Asian | 28 (7) | 29 (7) | 25 (6) |
| Non-Hispanic black | 21 (5) | 18 (5) | 20 (5) |
| Hispanic | 17 (4) | 16 (4) | 16 (4) |
| Other | 4 (1) | 3 (1) | 4 (1) |
| Not specified | 4 (1) | 3 (1) | 7 (2) |
| Performance status (N, (%)) | |||
| 0 | 215 (51) | 218 (55) | 213 (50) |
| 1 | 182 (43) | 157 (39) | 179 (42) |
| 2 | 27 (6) | 23 (6) | 35 (8) |
| Histology (N, (%)) | |||
| Papillary serous | 361 (85) | 330 (83) | 357 (84) |
| Endometrioid | 14 (3) | 7 (2) | 19 (4) |
| Clear cell | 10 (2) | 17 (4) | 15 (4) |
| Mucinous | 5 (1) | 5 (1) | 3 (1) |
| Other | 34 (8) | 39 (10) | 33 (8) |
| Stage (N, (%)) | |||
| III-optimal | 152 (36) | 134 (34) | 149 (35) |
| III-suboptimal | 167 (39) | 159 (40) | 160 (38) |
| IV | 105 (25) | 105 (26) | 118 (28) |
Table 2 shows the descriptive statistics of the adiposity measures. There were no statistically significant differences in distribution of these characteristics across treatment groups. Fat density is reported as a negative number. Larger numbers, or less negative numbers, reflect more dense fat. There was no effect of BMI, SFA, or VFA on oncologic outcomes. However, both density measures did show a statistically significant impact on OS (Table 3). An increase in SFD had a significant increase in the hazards of death with a HR of 1.12 (95% confidence interval (CI): 1.05–1.19) for each 1-standard deviation (SD) increase. VFD is also associated with an increased hazard ratio of death with a HR of 1.13 (95% CI: 1.05–1.20) for each 1-SD increase.
Table 2.
Medians (Q1, Q3) of adiposity measures by treatment group.
| Treatment Group | |||
|---|---|---|---|
| CT + P → P | CT + B → P | CT + B → B | |
| BMI, kg/m2 | 25.8 (22.2, 30.1) | 25.7 (22.5, 30.7) | 25.4 (22.1, 30.2) |
| SFA, cm2 | 185.4 (105.7, 253.6) | 174.2 (118.1, 264.5) | 174.5 (111.8, 260.0) |
| SFD, HU | −93.6 (−99.2, −84.9) | −94.4 (−99.4, −85.4) | −94.4 (−99.9, −85.3) |
| VFA, cm2 | 71.2 (31.9, 119.5) | 70.8 (33.6, 122.9) | 67.8 (36.8, 115.7) |
| VFD, HU | −76.7 (−81.7, −72.1) | −77.2 (−81.8, −72.2) | −76.7 (−81.2, −72.3) |
Table 3.
Hazard ratios of overall survival for biomarkers adjusted for treatment, stage and performance status.
| HR per 1-SD Increase | 95% CI | p-Value | |
|---|---|---|---|
| BMI | 1.02 | (0.95–1.08) | 0.65 |
| SFA | 0.98 | (0.92–1.05) | 0.57 |
| SFD | 1.12 | (1.05–1.19) | 0.0009 |
| VFA | 1 | (0.94–1.07) | 0.93 |
| VFD | 1.13 | (1.05–1.20) | 0.0004 |
Each of these variables were categorized into quartiles and Kaplan-Meier OS curves were created (Fig. 2). Fig. 2A shows no difference in OS as BMI increases. Fig. 2B–C also show a lack of association between OS and either SFA or VFA. However, Fig. 1D–E demonstrate the differences in OS by quartile for both SFD and VFD. A stepwise progression is demonstrated with worse survival by quartile. There is an approximate 10-month difference between the 2nd and 4th quartiles, with both SFD and VFD. Overall, this data is suggestive that SFD and VFD are both prognostic variables in EOC.
Fig. 2.
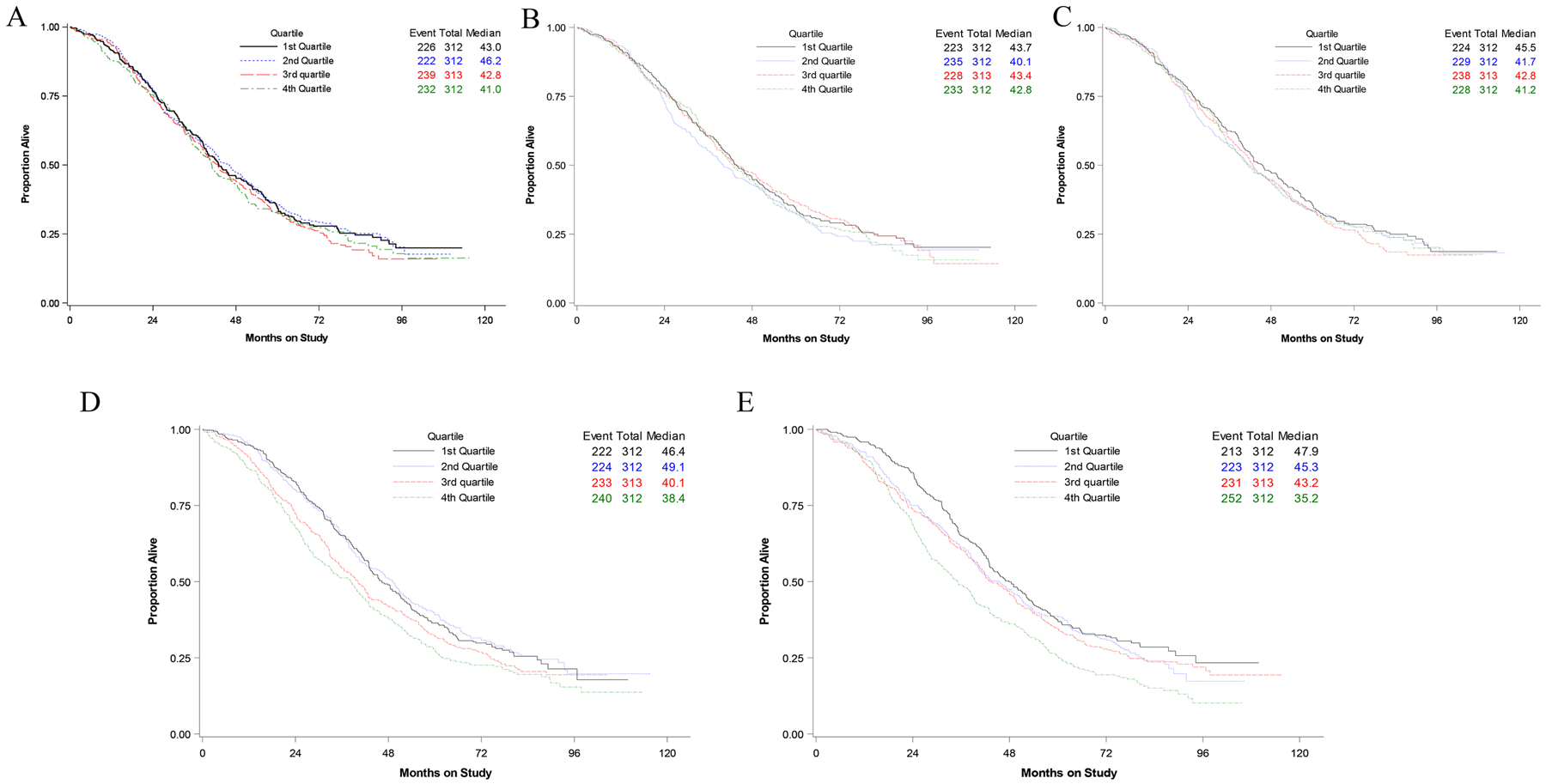
BMI (A), SFA (B), VFA (C), SFD (D), VFD (E) divided into quartiles and the median overall survival by months.
In the predictive analysis, each measurement was divided into a dichotomous variable and categorized as either high or low (ex. high or low BMI based on the median). This analysis was performed only on the CT + P → P versus CT + B → B. There was no predictive value of BMI, SFA, VFA or SFD on benefit for bev (Data not shown). The only variable that met statistical significance was the VFD group shown in Fig. 3 (p = 0.025). The high VFD patients (black lines) that were treated with bev had a median OS of 42 months (solid line) versus those on placebo (dotted line) with a median OS of 36 months. Those with low VFD (blue lines) treated with or without bev had similar OS (45 and 47 months, respectively). Table 5 shows the demographic distribution of these two groups. The only category that met statistical significance was in stage. There was a higher percentage of patients in the VFD high group with stage IV disease (29 vs. 24%) but a lower percentage of patients with optimally resected stage III disease (30 vs. 39%). Overall, this suggests that VFD is not only prognostic for oncologic outcomes in EOC but also predictive for benefit of bev.
Fig. 3.
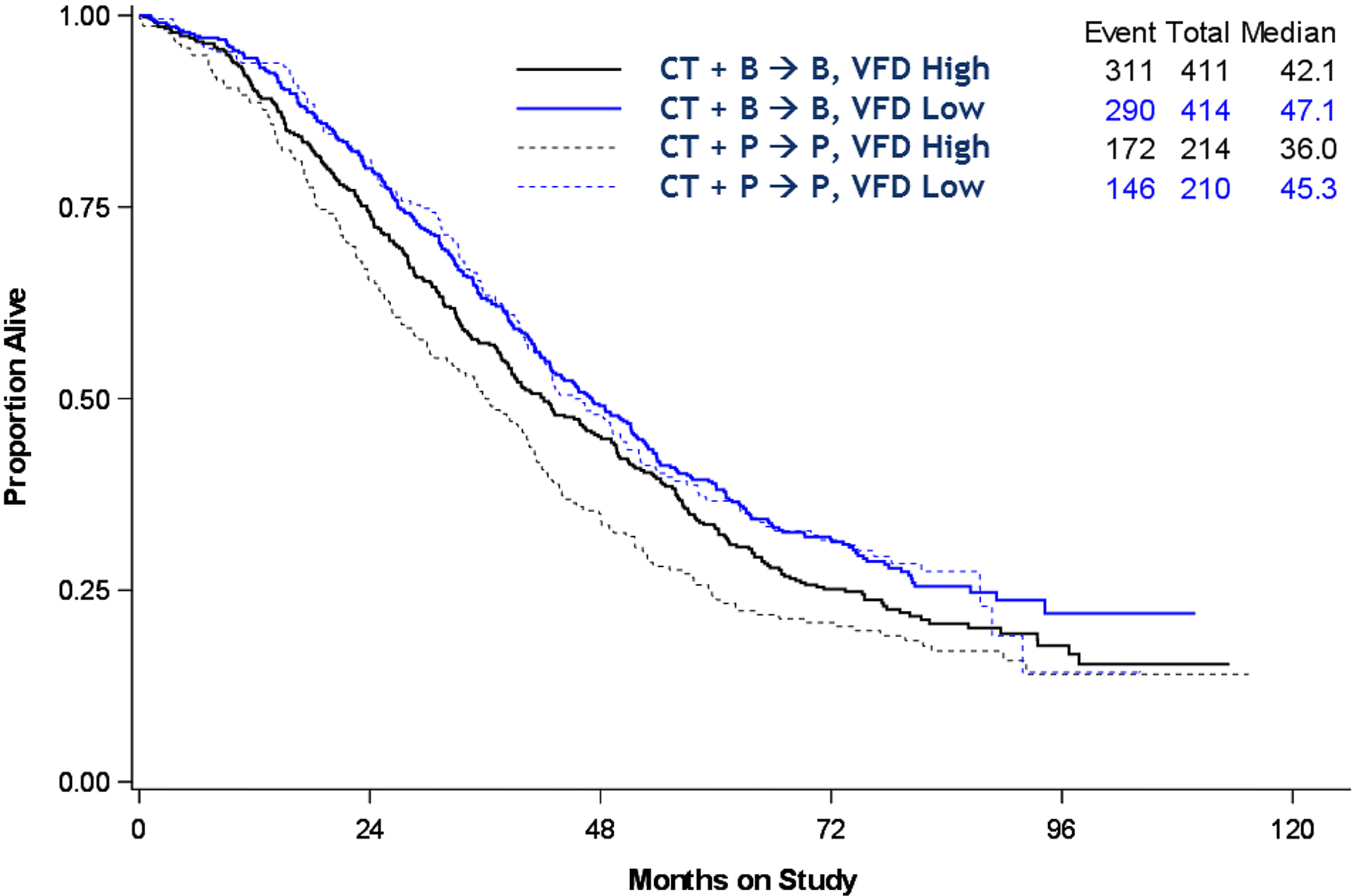
Effect of high and low VFD on overall survival by treatment group. P = 0.025.
Table 5.
Characteristics of patients on GOG-218 with complete adiposity measurements by VFD Category.
| VFD low | VFD high | P value | |
|---|---|---|---|
| Age (Median (Q1,Q3)) | 59.2 (51.9, 65.8) | 58.9 (51.2, 66.3) | 0.71 |
| Race (N, (%)) | 0.28 | ||
| Non-Hispanic white | 511 (82) | 523 (84) | |
| Asian | 43 (7) | 39 (6) | |
| Non-Hispanic black | 26 (4) | 33 (5) | |
| Hispanic | 31 (5) | 18 (3) | |
| Other | 4 (1) | 7 (1) | |
| Not specified | 9 (1) | 5 (1) | |
| Performance status (N, (%)) | 0.06 | ||
| 0 | 337 (54) | 309 (50) | |
| 1 | 254 (41) | 264 (42) | |
| 2 | 33 (5) | 52 (8) | |
| Histology (N, (%)) | 0.10 | ||
| Papillary serous | 512 (82) | 536 (86) | |
| Endometrioid | 30 (5) | 10 (2) | |
| Clear cell | 22 (4) | 20 (3) | |
| Mucinous | 7 (1) | 6 (1) | |
| Other | 53 (8) | 53 (8) | |
| Stage (N, (%)) | 0.002 | ||
| III-optimal | 246 (39) | 189 (30) | |
| III-suboptimal | 231 (37) | 255 (41) | |
| IV | 147 (24) | 181 (29) |
Similar to the predictive effect seen with VFD, previous post hoc analyses of patients on GOG-218 looked at the prognostic and predictive effect of VEGF-A and IL-6 levels as previously discussed [22,23]. It was therefore hypothesized that patients with higher VFD may be the same patient population with high IL-6 or high VEGF-A levels. Results of this analysis are presented in Table 4. Of the 1249 patients included in the imaging analysis, 522 had IL-6 measurements and were in the CT + P → P and CT + B → B groups. A Spearman correlation was estimated between the VFD and IL-6. The median IL-6 level was 10 pg/ml in patients with low VFD and 12.1 pg/ml in those with high VFD. We were unable to detect a correlation between VFD and IL-6 (r = 0.02, p = 0.57). Of the 1249 patients, 616 had VEGF-A measurements in the three treatment arms. The median VEGF-A was 115.5 pg/ml in patients with low VFD and 159.3 pg/ml in patients with high VFD. A Spearman correlation between VFD and VEGF-A was estimated. There was no statistically significant relationship between VFD and VEGF-A (r = 0.07, p = 0.071).
Table 4.
Medians (Q1, Q3) of IL-6 and VEGF-A and Spearman correlations with VFD.
| Treatment group | VFD | ||||||
|---|---|---|---|---|---|---|---|
| CT + P → P | CT + B → P | CT + B → B | VFD low groupa | VFD high groupa | r Value | p-Value | |
| IL6, n = 522 | 10.8 (4.3, 24.7) | - | 11.3 6.0, 23.0) | 10.0 (5.1, 21.0) | 12.1 (4.6, 26.8) | 0.02 | 0.57 |
| VEGF-A, n = 616 | 148.0 (77.0, 371.6) | 124 (77.0, 254.9) | 134.4 (77.0, 275.6) | 115.5 (77.0, 240.3) | 159.3 (77.0, 341.6) | 0.07 | 0.071 |
VFD Low and VFD High groups represent patients with VFD values below and above the median respectively.
4. Discussion
While markers of adiposity have been shown to negatively affect the prognosis of many cancers, this is the first report of SFD and VFD as prognostic markers in EOC. While data in other tumor types of have focused on SFA and VFA, we found no association of these markers with bev response [26]. VFD does appear to be a predictive marker of bev response and patients with high VFD may be more likely to benefit from initial treatment with bev. There was no statistically significant correlation between VFD and IL-6 but a trend was seen with VEGF-A that did not meet statistical significance.
It has been clearly demonstrated that bev impacts PFS in front line EOC but this impact is modest and better patient selection via imaging or serum biomarkers may help select those patients most likely to gain benefit and limit exposure and toxicity to those patients unlikely to benefit. However, we are still in need of a way to accurately identify the population that will benefit the most from the addition of bev despite much investigation into this topic. Table 4 summarizes the range of biomarkers that have been investigated in front line EOC. Each of the biomarkers in Table 4 were from post hoc analyses of either ICON7 or GOG-218. Clinical biomarkers such as the presence of ascites and stage IV disease have shown both prognostic and predictive associations with OS in GOG-218 [27,28]. As previously mentioned, the addition of bev improved PFS in GOG-218 [6]; however, it failed to improve OS. Therefore, these clinical biomarkers may help delineate a subset of patients with improved OS. In addition, in ICON 7, a high-risk subgroup defined as those with stage IV disease, inoperable stage III disease, or suboptimally debulked stage III disease showed improvement in OS with the addition of bev [29].
Tumor associated biomarkers such as tumor microvessel density as measured by expression of anti-CD31 antibody appeared to have predictive value for both PFS and OS [23]. Molecular characterization of patients on ICON 7 identified a 63 gene expression signature. They showed that those with the immune signature (which had down-regulation of angiogenesis markers) had worse OS and no benefit from the treatment of bev [30]. Serum markers are also of great desire to incorporate into clinical practice. IL-6 as well as a combined signature of mesothelin, fms-like tyrosine kinase-4 (FLT4), α1-acid glycoprotein (AGP), and CA-125 were predictive biomarkers of PFS in patients treated with bev [22,31]. Now we propose that imaging biomarkers also be included in the discussion moving forward.
It is important to note that many of these biomarkers, including imaging, have been identified via exploratory analyses and none have been validated. Exploratory analyses have inherent bias. Complete data is often not available and distribution of biomarkers across treatment arms may not be balanced as evidenced in Table 5 with a statistically significant difference noted in stage at presentation between the two groups. With these limitations, this data suggests that prospective validation of VFD as a biomarker is needed. In addition, the clinical significance of changes in VFD is not fully explained. An underlying explanation for why VFD plays a role as both a prognostic and predictive biomarker is only speculative at this point in time. Studies on cardiovascular risk suggest that this fat may have different biochemical characteristics, however, much more work is needed to understand differences in VFD.
We sought to see if any tumor or serum biomarkers correlated with VFD to help understand an underlying mechanism but did not find any statistically significant correlations with previously explored potential biomarkers. As we continue to incorporate new therapies in the treatment of EOC it is important to identify patients that benefit from such additions. Additional therapies often expose patients to additional toxicity and identification of those who will ultimately receive clinical benefit is imperative. This analysis suggests that imaging biomarkers will likely play a role in this discussion for EOC and are important to incorporate into future study designs.
HIGHLIGHTS.
Subcutaneous fat density (SFD) and visceral fat density (VFD) appear to be prognostic biomarkers for EOC.
High VFD may also be a predictive biomarker for use of bevacizumab in this patient population.
There was no correlation between IL-6 and VFD as predictive biomarkers for use of bevacizumab.
Acknowledgements
The authors would like to acknowledge Dr. Howard D. Homesley for his significant contribution to this study.
The following NRG Oncology/Gynecologic Oncology Group member institutions participated in this study: Cancer Trials Support Unit, University of Oklahoma Health Sciences Center, Gynecologic Oncology Network/Brody School of Medicine, Ohio State University Comprehensive Cancer Center, University of California Medical Center at Irvine-Orange Campus, Metro-Minnesota CCOP, Saitama Medical University International Medical Center, University of Alabama at Birmingham, Abington Memorial Hospital, Rush University Medical Center, Mayo Clinic, University of Kentucky, Roswell Park Comprehensive Cancer Center, Abramson Cancer Center of The University of Pennsylvania, University of North Carolina at Chapel Hill, Walter Reed National Military Medical Center, University of Iowa Hospitals and Clinics, Memorial Sloan Kettering Cancer Center, Mount Sinai School of Medicine, Washington University School of Medicine, Northwestern University, Indiana University Hospital/Melvin and Bren Simon Cancer Center, University of Colorado Cancer Center – Anschutz Cancer Pavilion, Fox Chase Cancer Center, Women’s Cancer Center of Nevada, University of Chicago, Women and Infants Hospital, Seoul National University Hospital, University of New Mexico, University of Virginia, University of California at Los Angeles Health System, Yale University, Duke University Medical Center, University of Pittsburgh Cancer Institute, University of Hawaii, University of Minnesota Medical Center-Fairview, Cleveland Clinic Foundation, The Hospital of Central Connecticut, Cooper Hospital University Medical Center, Cancer Research for the Ozarks NCORP, University of Mississippi Medical Center, Moffitt Cancer Center and Research Institute, University of Wisconsin Hospital and Clinics, Northern Indiana Cancer Research Consortium, University of Massachusetts Memorial Health Care, Wake Forest University Health Sciences, Case Western Reserve University, Michigan Cancer Research Consortium Community Clinical Oncology Program, Wayne State University/Karmanos Cancer Institute, Gynecologic Oncology of West Michigan PLLC, Cancer Research Consortium of West Michigan PLLC, Cancer Research Consortium of West Michigan NCORP, Saint Vincent Hospital, University of Cincinnati, State University of New York Downstate Medical Center, MD Anderson Cancer Center, Georgia Center for Oncology Research and Education (CORE), Delaware/Christiana Care COP, Virginia Commonwealth University, Northern New Jersey CCOP, Penn State Milton S. Hershey Medical Center, Tufts-New England Medical Center, Stony Brook University Medical Center, Wisconsin NCI Community Oncology Research Program, Tacoma General Hospital, New York University Medical Center, Scott and White Memorial Hospital, Virginia Mason CCOP, Aurora Women’s Pavilion of Aurora West Allis Medical Center, University of Illinois, Evanston CCOP-North Shore University Health System, Kalamazoo CCOP, Carle Cancer Center, William Beaumont Hospital, Colorado Cancer Research Program NCORP, Saint Louis-Cape Girardeau CCOP, University of Texas Southwestern Medical Center, Fletcher Allen Health Care, University of Texas – Galveston, Kansas City CCOP, Missouri Valley Cancer Consortium CCOP, Central Illinois CCOP, Dayton Clinical Oncology Program, Mainline Health CCOP, Meharry Medical College Minority Based CCOP and Heartland Cancer Research CCOP.
This work was supported by National Cancer Institute grants to the NRG Oncology SDMC (U10 CA180822), NRG Oncology Operations (U10CA 180868) and UG1CA189867 (NCORP). Research reported in this publication was supported in part by the Stephenson Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Stephenson Cancer Center.
Declaration of Competing Interest
Megan Buechel: No conflicts of interest.
Danielle Enserro: No conflicts of interest.
Robert A. Burger: Served as a consultant for Amgen, Astra Zeneca, Tesaro, Clovis Oncology, Genentech, Immunogen, Agenus, Gradalis, Janssen Research & Development, Merck, and VBL Therapeutics.
Mark F. Brady: No conflicts of interest.
Katrina Wade: No conflicts of interest.
Angeles Alvarez Secord: Recieved clinical trial grant funding from AbbVie, Amgen, Astellas Pharma Inc., Astex Pharmaceuticals Inc., Astra Zeneca, Boehringer Ingelheim, Bristol Myers Squibb, Clovis, Eisai, Endocyte, Exelixis, Immutep Ltd., Incyte, Merck, PharmaMar, Roche/Genentech, Seattle Genetics, Inc., Tesaro/GSK, and VBL Therapeutics; honoraria for Advisory Boards from Aravive, AstraZeneca, Clovis, Cordgenics, Eisai, Janssen/Johnson & Johnson, Merck, Mersana, Oncoquest, Roche/Genentech, and Tesaro/GSK; participated on Steering Committees for OVAL and AtTend clinical trials (uncompensated); and is a member of GOG Foundation Board of Directors within the past 36 months.
Andrew B. Nixon: Receives industry funding from Genentech, MedImmune/AstraZeneca, Medpacto, Seattle Genetics, HTG Molecular Diagnostics, Tracon Pharmaceuticals, Eureka Therapeutics, Leadiant Biosciences, Acceleron Pharma, OncoMed Pharmaceuticals.Does consulting for GSK, Promega, Kanghong Pharma, Eli Lilly. Travel with Powering Precision Health.
Seyedehnafiseh Mirniaharikandehei: No conflicts of interest.
H. Liu: No conflicts of interest.
Bin Zheng: No conflicts of interest.
Heidi Gray: No conflicts of interest.
Krishnansu S. Tewari: Consultant and Speaker’s Bureau with Genentech/Roche, Abbvie, Essai, GSK/Tesaro, Merck, Clovis, Astra Zeneca.
David M. O’Malley: Reports personal fees and institutional support for clinical research from AstraZeneca, personal fees and institutional support for clinical research from Clovis, personal fees and institutional support for clinical research from Tesaro, personal fees and institutional support for clinical research from Immunogen, personal fees from Ambry, personal fees and institutional support for clinical research from Janssen/J&J, personal fees and institutional support for clinical research from Abbvie, personal fees and institutional support for clinical research from Regeneron, personal fees and institutional support for clinical research from Amgen, personal fees and institutional support for clinical research from Novocure, personal fees and institutional support for clinical research from Genentech/Roche, institutional support for clinical research from VentiRx, institutional support for clinical research from Array Biopharma, institutional support for clinical research from EMD Serono, institutional support for clinical research from Ergomed, institutional support for clinical research from Ajinomoto Inc., institutional support for clinical research from Ludwig Cancer Research, institutional support or clinical research from Stemcentrx, Inc., institutional support for clinical research from CERULEAN PHARMA, personal fees and institutional support for clinical research from GOG Foundation, institutional support for clinical research from Bristol-Myers Squibb Co, institutional support for clinical research from Serono Inc., institutional support for clinical research from TRACON Pharmaceuticals, institutional support for clinical research from Yale University, institutional support for clinical researchfrom New Mexico Cancer Care Alliance, institutional support for clinical researchfrom INC Research, Inc., institutional support for clinical researchfrom inVentiv Health Clinical, institutional support for clinical researchfrom Iovance Biotherapeutics, Inc., institutional support for clinical research from PRA Intl, personal fees from Myriad Genetics, personal fees and institutional support for clinical research from Eisai, personal fees and institiional support from Agenus, personal fees and institutional support for clinical research from GSK, personal fees from Tarveda, personal fees and institutional support for clinical research from Merck.
Robert S. Mannel: Has received honorarium from Tesaro for an Advisory Board.
Michael J. Birrer: No conflicts of interest.
Kathleen N. Moore: Advisory board participation for Abbvie, Aravive, Astra Zeneca, Clovis, Eisai, Genentech/Roche, GSK/Tesaro, Immunogen, Merck, Mersana, Tarveda, VBL Therapeutics, Vavotar. Her institution receives research funding from PTC Therapeutics, Merck, Genentech/Roche.
References
- [1].American Cancer Society, Cancer Facts and Figures 2019, Atlanta, GA: 2019. [Google Scholar]
- [2].Cooper BC, Ritchie JM, Broghammer CL, et al. , Preoperative serum vascular endothelial growth factor levels: significance in ovarian cancer, Clin. Cancer Res 8 (2002) 3193–3197. [PubMed] [Google Scholar]
- [3].FDA Approves Bevacizumab in Combination With Chemotherapy for Ovarian Cancer, https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm610664.htm 2018.
- [4].Aghajanian C, Blank SV, Goff BA, et al. , OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer, J. Clin. Oncol 30 (2012) 2039–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aghajanian C, Goff B, Nycum LR, et al. , Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer, Gynecol. Oncol 139 (2015) 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Burger RA, Brady MF, Bookman MA, et al. , Incorporation of bevacizumab in the primary treatment of ovarian cancer, N. Engl. J. Med 365 (2011) 2473–2483. [DOI] [PubMed] [Google Scholar]
- [7].Coleman RL, Brady MF, Herzog TJ, et al. , Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial, Lancet Oncol. 18 (2017) 779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Perren TJ, Swart AM, Pfisterer J, et al. , A phase 3 trial of bevacizumab in ovarian cancer, N. Engl. J. Med 365 (2011) 2484–2496. [DOI] [PubMed] [Google Scholar]
- [9].Pujade-Lauraine E, Hilpert F, Weber B, et al. , Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial, J. Clin. Oncol 32 (2014) 1302–1308. [DOI] [PubMed] [Google Scholar]
- [10].Coleman RL, Brady MF, Herzog TJ, et al. , Bevacizumab after bevacizumab in platinum-sensitive recurrent ovarian cancer: a subgroup analysis of GOG0213, J. Clin. Oncol 34 (2016) 5523. [Google Scholar]
- [11].Pignata S, Lorusso D, Joley F, et al. , Chemotherapy plus or minus bevacizumab for platinum-sensitive ovarian cancer patients recurring after a bevacizumab containing first line treatment: the randomized phase 3 trial MITO16B-MaNGO OV2BENGOT OV17, J. Clin. Oncol 36 (15_suppl) (2018) (5506–5506). [Google Scholar]
- [12].Renehan AG, Zwahlen M, Egger M, Adiposity and cancer risk: new mechanistic insights from epidemiology, Nat. Rev. Cancer 15 (2015) 484–498. [DOI] [PubMed] [Google Scholar]
- [13].Grignol VP, Smith AD, Shlapak D, Zhang X, Del Campo SM, Carson WE, Increased visceral to subcutaneous fat ratio is associated with decreased overall survival in patients with metastatic melanoma receiving anti-angiogenic therapy, Surg. Oncol 24 (2015) 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Miyamoto Y, Oki E, Emi Y, et al. , Low visceral fat content is a negative predictive marker for bevacizumab in metastatic colorectal cancer, Anticancer Res. 38 (2018) 491–499. [DOI] [PubMed] [Google Scholar]
- [15].Guiu B, Petit JM, Bonnetain F, et al. , Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer, Gut 59 (2010) 341–347. [DOI] [PubMed] [Google Scholar]
- [16].Ladoire S, Bonnetain F, Gauthier M, et al. , Visceral fat area as a new independent predictive factor of survival in patients with metastatic renal cell carcinoma treated with anti-angiogenic agents, Oncologist 16 (2011) 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Steffens S, Grunwald V, Ringe KI, et al. , Does obesity influence the prognosis of metastatic renal cell carcinoma in patients treated with vascular endothelial growth factor-targeted therapy? Oncologist 16 (2011) 1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Huang X, Xie C, Tang J, et al. , Adipose tissue area as a predictor for the efficacy of apatinib in platinum-resistant ovarian cancer: an exploratory imaging biomarker analysis of the AEROC trial, BMC Med. 18 (1) (2020. October 5) 267, 10.1186/s12916-020-01733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rosenquist KJ, Pedley A, Massaro JM, et al. , Visceral and subcutaneous fat quality and cardiometabolic risk, JACC Cardiovasc. Imaging 6 (2013) 762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xu H, Barnes GT, Yang Q, et al. , Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance, J. Clin. Invest 112 (2003) 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Apovian CM, Bigornia S, Mott M, et al. , Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects, Arterioscler. Thromb. Vasc. Biol 28 (2008) 1654–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Secord AA, Tritchler D, Liu Y, et al. , Prognostic and predictive blood-based biomarkers (BMs) in patients (pts) with advanced epithelial ovarian cancer (EOC) treated with carboplatin–paclitaxel (CP) ± bevacizumab (BEV): results from GOG-0218, J. Clin. Oncol 34 (15_suppl) (2016) (5521–5521). [Google Scholar]
- [23].Bais C, Mueller B, Brady MF, et al. , Tumor microvessel density as a potential predictive marker for bevacizumab benefit: GOG-0218 biomarker analyses, J. Natl. Cancer Inst 109 (11) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang Y, Thai T, Moore K, et al. , Quantitative measurement of adiposity using CT images to predict the benefit of bevacizumab-based chemotherapy in epithelial ovarian cancer patients, Oncol. Lett 12 (2016) 680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang Y, Qiu Y, Thai T, et al. , A two-step convolutional neural network based computer-aided detection scheme for automatically segmenting adipose tissue volume depicting on CT images, Comput. Methods Prog. Biomed 144 (2017) 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Slaughter KN, Thai T, Penaroza S, et al. , Measurements of adiposity as clinical biomarkers for first-line bevacizumab-based chemotherapy in epithelial ovarian cancer, Gynecol. Oncol 133 (2014) 11–15. [DOI] [PubMed] [Google Scholar]
- [27].Ferriss JS, Java JJ, Bookman MA, et al. , Ascites predicts treatment benefit of bevacizumab in front-line therapy of advanced epithelial ovarian, fallopian tube and peritoneal cancers: an NRG Oncology/GOG study, Gynecol. Oncol 139 (2015) 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Randall L, Burger R, Nguyen H, et al. , Outcome differences in patients with advanced epithelial ovarian, primary peritoneal and fallopian tube cancers treated with and without bevacizumab, Gynecol. Oncol 130 (2013) e33–e34. [Google Scholar]
- [29].Oza AM, Cook AD, Pfisterer J, et al. , Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial, Lancet Oncol. 16 (2015) 928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gourley C, McCavigan A, Perren T, et al. , Molecular subgroup of high-grade serous ovarian cancer (HGSOC) as a predictor of outcome following bevacizumab, J. Clin. Oncol 32 (2014) 5502. [Google Scholar]
- [31].Collinson F, Hutchinson M, Craven RA, et al. , Predicting response to bevacizumab in ovarian cancer: a panel of potential biomarkers informing treatment selection, Clin. Cancer Res 19 (2013) 5227–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]


