Abstract
Research on mortality associated with exposure to the Holocaust is relevant for a better understanding of the effects of genocides on survivors. To our knowledge, previous studies have not investigated the long-term cause-specific mortality of Holocaust survivors. We compared mortality rates among Israelis born in European countries controlled by the Nazis during World War II with those among Israelis of European descent who did not have this exposure. Records of 22,671 people (45% women; 5,042 survivors) from the population-based Jerusalem Perinatal Study (1964–1976) were linked to the Israeli Population Registry, which was updated through 2016. Cox models were used for analysis, with 2-sided tests of statistical significance. Risk of all-cause mortality was higher among exposed women (hazard ratio (HR) = 1.15, 95% confidence interval (CI): 1.05, 1.27) than in unexposed women. No association was found between Holocaust exposure and male all-cause mortality. In both sexes, survivors had higher cancer-specific mortality (HR = 1.17 (95% CI: 1.01, 1.35) in women and HR = 1.14 (95% CI: 1.01, 1.28) in men). Exposed men also had excess mortality due to coronary heart disease (HR = 1.39, 95% CI: 1.09, 1.77) and lower mortality from other known causes combined (HR = 0.86, 95% CI: 0.75, 0.99). In summary, experiencing the Holocaust was associated with excess all-cause and cancer-specific mortality in women and cancer- and coronary heart disease–specific mortality in men.
Keywords: cancer, cohort studies, coronary heart disease, genocide, Holocaust, mortality, survival analysis
Abbreviations
- CHD
coronary heart disease
- CI
confidence interval
- HR
hazard ratio
- ICD-9
International Classification of Diseases, Ninth Revision
- ICD-10
International Classification of Diseases, Tenth Revision
- SEP
socioeconomic position
- SHARE-IL
Israeli component of the Survey of Health, Ageing and Retirement in Europe
The Holocaust undoubtedly stands out in human history as an extraordinary outburst of extreme violence. Tragically, genocides continue to occur and are not diminishing in frequency (1). In order to better understand long-term effects of extreme violence on human biology, research on mortality and morbidity associated with the Holocaust is as relevant in the 21st century as ever.
Epidemiologic literature has demonstrated that social environment at different stages of life can have substantial health consequences over the life course (2–6). Whether as captives in concentration/death camps or as escapees, Jewish survivors of the Holocaust have faced a combination of severe multifaceted stress conditions—for example, extreme physical and emotional abuse, sleep deprivation, strenuous physical activity, and exposure to infectious diseases and toxic waste (7–9). Many victims died quite soon after the Holocaust (10) or were too physically or mentally injured to resume a “normal” life (11). While a considerable number of survivors nevertheless managed to rebuild their lives (12, 13), it is a reasonable hypothesis that the Holocaust may have had a profound long-term impact on survivors’ health.
At the time we conducted this research, extant studies on the health consequences of the Holocaust had yielded conflicting findings, particularly with respect to mortality (14, 15). While some recent research in Israel found that the Holocaust did not affect old-age all-cause mortality (13, 16, 17), other studies showed that it was even lower among the survivors (18, 19).
Several explanations have been proposed to account for the conflicting evidence (15, 16, 20), yet Fund et al. (19) recently noted that more research is warranted to understand Holocaust-related death hazards. Moreover, we are not aware of any study that has investigated long-term cause-specific mortality in Holocaust survivors. Using data from a population-based cohort with mortality records spanning the period from the 1960s to 2016, we aimed to assess whether exposure to the Holocaust affected all-cause and cause-specific mortality among survivors who were able to immigrate to Israel and had offspring.
METHODS
Study design and data source
The Jerusalem Perinatal Study is a population-based cohort study of 92,408 live births and stillbirths that occurred in Jerusalem, Israel, during the period 1964–1976, as well as the parents in those births (21). The current analysis focused on the parents’ generation, baseline data on whom included detailed sociodemographic characteristics (e.g., date and country of birth, education) and health-related and perinatal factors in mothers (21). Using unique Israeli identity numbers, vital status and dates of death (up to December 31, 2016) were obtained by linkage of the cohort members’ baseline records to the Israeli Population Registry. For a very small number of participants (0.06%), these data were verified through April 1, 2005. Underlying causes of death were available through the end of 2015 via record linkage with the Israel Ministry of Health. The cohort was also linked to the Israel National Cancer Registry, which was established in 1960 and updated through December 31, 2014. Deceased subjects for whom the cause of death was unknown were determined to have died from cancer if they had been diagnosed with cancer and passed away within 5 years following the first diagnosis. The remaining cases with unknown causes of death were combined and analyzed as a separate category.
The study was approved by the Institutional Review Board of Hadassah-Hebrew University Medical Center in Jerusalem.
Selection of the study sample and definition of exposure status
This work relied on a definition of a Holocaust survivor used by Israel’s research and governmental institutes, according to which a Holocaust survivor is a person who lived in one of the countries occupied by or under the influence of the Nazi regime for any length of time between 1933 and 1945. According to this definition, the survivor population also includes persons who were forced to leave their place of residence because of the Nazi regime (22).
Figure 1 shows how exposed, unexposed, and excluded groups were defined on the basis of the initial sample. As in similar studies (12, 17, 23), to minimize biases related to cultural, environmental, and genetic differences between survivors and unexposed individuals, we included only persons of European descent born before or during 1945. Following the approach used by Lurie et al. (23), survivors were further defined as those who had been born in a Nazi-occupied country and had immigrated to Israel during or after the year in which Nazi persecutions in that particular country had started (see Web Table 1, available online at https://doi.org/10.1093/aje/kwab021). The comparison group was defined as either 1) persons born in the same country who immigrated to Israel before the Nazi persecutions or 2) persons of European descent born in any other country (including Israel) before or during 1945. Since the country-of-birth variable did not specify a particular republic for immigrants from the Union of Soviet Socialist Republics, we could not establish whether they had been born under Nazi occupation and therefore excluded these participants (about 2% of the sample) from all analyses. The resulting exposure variable was coded as a dummy variable (1, exposed; 0, unexposed). Following this exposure definition, 10,210 women and 12,461 men (comprising 25% and 30% of the original samples, respectively) were included in the reported analyses, of whom 2,133 females and 2,909 males were Holocaust survivors (Figure 1).
Figure 1.
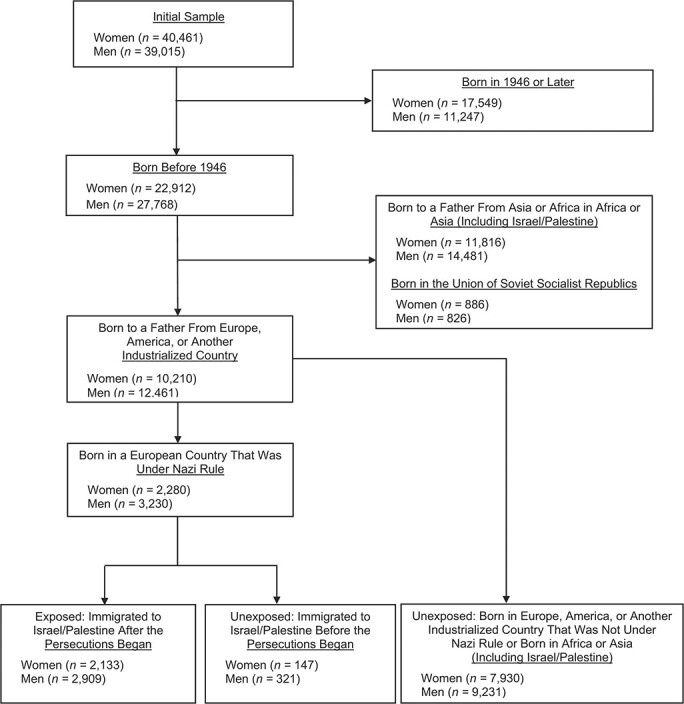
Selection of participants for a study of Holocaust experience and mortality patterns, Jerusalem Perinatal Study, Israel, 1964–1976.
We tested this exposure definition using data from the Israeli component of the Survey of Health, Aging and Retirement in Europe (SHARE-IL) (24–26), which includes self-reported information about subjects’ Holocaust experiences (e.g., see Shrira et al. (27)). Applying our definition of exposure to the SHARE-IL data and comparing it with the SHARE-IL self-reports demonstrated high reliability of our definition: 93% overall agreement (Cohen’s κ = 0.72) including countries within the Union of Soviet Socialist Republics (Belarus, Estonia, Latvia, Lithuania, Moldova, and Ukraine) and 94% agreement (κ = 0.75) excluding these countries.
Key variables
The outcomes studied were all-cause and cause-specific mortality. The underlying causes of deaths that occurred between the 1960s and 1997 were categorized according to International Classification of Diseases, Ninth Revision (ICD-9) codes, and the causes of deaths that occurred from 1998 onwards were categorized according to International Classification of Diseases, Tenth Revision (ICD-10) codes (28). Causes of death were categorized into the following groups: deaths due to all circulatory conditions (ICD-9 codes 390–459; ICD-10 codes I0–I99); deaths due specifically to coronary heart disease (CHD) (ICD-9 codes 410–414, 427.4, and 427.5; ICD-10 codes I20–I25, I46, and I49); deaths due to all neoplasms (ICD-9 codes 140–239; ICD-10 codes C0–C99 and D0–D48); deaths due to unnatural causes (ICD-9 codes 80–99; ICD-10 codes S0–T88 and V0–Y99); and deaths due to all other known causes combined. We created a separate category for persons with an unknown cause of death.
The following variables were included in the analyses as covariates, in addition to the main explanatory variable: sex (unless stated otherwise, all of the analyses were performed separately in subsamples of men and women), a 6-point socioeconomic position (SEP) scale (ranging from 1 (highest) to 6 (lowest)) based on men’s occupations, and educational level in years (categorized as 0, 1–4, 5–8, 9–12, and ≥13). For women, we were also able to control for parity (reported number of children born before first registration in the Jerusalem Perinatal Study cohort, plus those born within the cohort (categorized as 1, 2–4, 5–9, and ≥10)), the presence of obstetrical conditions (toxemia, heart disease, diabetes, or prediabetes) (1, yes; 0, no), and average birth weight of all of a woman’s offspring who were registered in the cohort, in grams (categorized as <2,500, 2,500–2,999, 3,000–3,499, and ≥3,500). The values for SEP and education were copied from the record of a person’s last offspring born in the cohort. Although some people in the cohort could have increased their achievements later, it is reasonable to assume that these variables reflect well the participants’ lifetime social position.
Statistical analysis
To investigate mortality hazards, we employed survival analysis based on the age scale. We faced the statistical issue of left-truncation (also known as delayed entry). In particular, we had no information on Holocaust survivors who died before the Jerusalem Perinatal Study was initiated on January 1, 1964. To address the left-truncation issue, we adopted the risk-set correction method (29). An unbiased comparison of survival distributions between the exposed and the unexposed groups requires that the minimal ages at recruitment in the 2 groups were similar (29). This assumption is verified in Web Table 2.
Cox proportional hazards regression models were used to assess the association between exposure to the Holocaust and all-cause and cause-specific mortality, controlling for educational level and SEP. A separate Cox model with a multiplicative interaction term (exposure × sex) was fitted to the pooled sample of women and men to formally test whether a difference between male and female all-cause mortality was statistically significant. Cox models that also controlled for obstetrical conditions (toxemia, heart disease, diabetes, or prediabetes), parity, and the offspring’s birth weight were fitted to the subsample of women. Tests of statistical significance were 2-sided, and we report hazard ratios and 95% confidence intervals obtained from the Cox regressions. The models’ goodness of fit was evaluated and confirmed graphically using plots of Cox-Snell residuals (29), as well as numerically using the Gronnesby and Borgan test (30). Finally, we examined the proportional hazards assumption using the Grambsch and Therneau test (31), as well as by plotting −log[−log(S(t))] as a function of log(t), and did not find evidence for time dependency in the reported results.
Data on education were missing for 4% of women and 3% of men, and obstetrical data were missing for a slightly higher percentage of women (12%). Persons with missing information were excluded from the analyses. The analyses were repeated using a multiple imputation by chained equations (MICE) algorithm and yielded similar estimates. Analyses were performed in Stata 12 (StataCorp LLC, College Station, Texas).
RESULTS
Descriptive statistics
Table 1 shows that at the (country-specific) time when the Holocaust persecutions began, most of the survivors were young children or teenagers, and male survivors were older than female survivors. SEP was slightly lower among the Holocaust survivors, as was the number of years of education. In women, obstetrical conditions, parity, and offspring’s birth weight were roughly similar among the exposed and unexposed.
Table 1. Characteristics of Participants in a Study of Holocaust Experience and Mortality Patterns, by Sex and Exposure Status, Jerusalem Perinatal Study, Israel, 1964–1976.
| Sex and Exposure Status | ||||||||
|---|---|---|---|---|---|---|---|---|
| Women (n = 10,210) | Men (n = 12,461) | |||||||
| Characteristic |
Unexposed (n = 8,077) |
Holocaust Survivor (n = 2,133) |
Unexposed (n = 9,552) |
Holocaust Survivor (n = 2,909) |
||||
| No. | % | No. | % | No. | % | No. | % | |
| Age at exposure, yearsa | ||||||||
| Unexposed | 8,077 | 100.0 | 9,552 | 100.0 | ||||
| <1 (newborn) | 873 | 40.9 | 774 | 26.6 | ||||
| 1–12 (child) | 1,172 | 54.9 | 1,652 | 56.8 | ||||
| 13–19 (adolescent) | 88 | 4.1 | 421 | 14.5 | ||||
| ≥20 (adult) | 0 | 0 | 62 | 2.1 | ||||
| Socioeconomic positionb | ||||||||
| Low | 661 | 8.2 | 212 | 9.9 | 821 | 8.6 | 304 | 10.5 |
| Middle | 2,932 | 36.3 | 770 | 36.1 | 3,548 | 37.1 | 1,029 | 35.4 |
| High | 4,484 | 55.5 | 1,151 | 54.0 | 5,183 | 54.3 | 1,576 | 54.2 |
| Participant’s duration of education, years | ||||||||
| 0 | 29 | 0.4 | 10 | 0.5 | 18 | 0.2 | 5 | 0.2 |
| 1–4 | 33 | 0.4 | 22 | 1.0 | 15 | 0.2 | 16 | 0.6 |
| 5–8 | 1,060 | 13.1 | 277 | 13.0 | 728 | 7.6 | 274 | 9.4 |
| 9–12 | 2,553 | 31.6 | 725 | 34.0 | 3,038 | 31.8 | 903 | 31.0 |
| ≥13 | 4,136 | 51.2 | 997 | 46.7 | 5,469 | 57.3 | 1,600 | 55.0 |
| Unknown | 266 | 3.3 | 102 | 4.8 | 284 | 3.0 | 111 | 3.8 |
| Ever being diagnosed with an obstetrical conditionc | ||||||||
| No | 6,690 | 82.8 | 1,802 | 84.5 | ||||
| Yes | 416 | 5.2 | 104 | 4.9 | ||||
| Unknown | 971 | 12.0 | 227 | 10.6 | ||||
| No. of live births | ||||||||
| 1 | 954 | 11.8 | 254 | 11.9 | ||||
| 2–4 | 5,586 | 69.2 | 1,454 | 68.2 | ||||
| 5–9 | 1,334 | 16.5 | 346 | 16.2 | ||||
| ≥10 | 190 | 2.4 | 75 | 3.5 | ||||
| Unknown | 13 | 0.2 | 4 | 0.2 | ||||
| Average offspring birth weight, g | ||||||||
| <2,500 | 406 | 5.0 | 119 | 5.6 | ||||
| 2,500–2,999 | 1,518 | 18.8 | 392 | 18.4 | ||||
| 3,000–3,499 | 3,599 | 44.6 | 907 | 42.5 | ||||
| 3,500–3,999 | 2,075 | 25.7 | 551 | 25.8 | ||||
| ≥4,000 | 449 | 5.6 | 149 | 7.0 | ||||
| Unknown | 30 | 0.4 | 15 | 0.7 | ||||
a At the beginning of Nazi persecutions (country-specific year).
b By husband’s occupation for women and by occupational status for men.
c Toxemia, heart disease, diabetes, or prediabetes.
Table 2 shows that during the period of follow-up (i.e., from the first offspring registered in the Jerusalem Perinatal Study to December 31, 2016), women contributed 471,316 person-years of observation, and 2,270 (22%) of them died. Men contributed 539,876 person-years, and 4,665 (37%) of them died. For participants who died, the mean age at death was 69 years for women and 71 years for men.
Table 2. Age and Follow-up Time in a Study of Holocaust Experience and Mortality Patterns, by Sex, Jerusalem Perinatal Study, Israel, 1964–1976.
| Variable | Women (n = 10,210) | Men (n = 12,461) | ||
|---|---|---|---|---|
|
No. of Persons or PY |
Mean (SD) |
No. of Persons or PY |
Mean (SD) | |
| Age at death or end of follow-up, years | 10,210 | 75.4 (8.0) | 12,461 | 75.4 (9.3) |
| Age at death among persons who died, years | 2,270 | 68.7 (12.3) | 4,665 | 70.7 (12.3) |
| PY of observationa | 471,316 | 539,876 | ||
Abbreviations: PY, person-years; SD, standard deviation.
a PY from birth of the first offspring in the cohort to death or the end of follow-up.
The all-cause mortality rate (number of deaths per 1,000 person-years) was 4.8 in women and 8.6 in men. With the exception of mortality due to unnatural causes, all-cause and cause-specific mortality rates were higher among Holocaust survivors than among the unexposed for both sexes (Table 3).
Table 3. Mortality by Cause of Death, Exposure to the Holocaust, and Sex in a Study of Holocaust Experience and Mortality Patterns, Jerusalem Perinatal Study, Israel, 1964–1976.
| Sex and Exposure Status | ||||||||
|---|---|---|---|---|---|---|---|---|
| Women (n = 2,270 Deaths) | Men (n = 4,665 Deaths) | |||||||
| Cause of Death a | Unexposed | Holocaust Survivor | Unexposed | Holocaust Survivor | ||||
|
No. of Deaths |
Mortality Rateb |
No. of Deaths |
Mortality Rate |
No. of Deaths |
Mortality Rate |
No. of Deaths |
Mortality Rate |
|
| CHD | 58 | 0.2 | 27 | 0.3 | 220 | 0.5 | 105 | 0.9 |
| All circulatory disordersc | 223 | 0.6 | 95 | 1.0 | 703 | 1.7 | 309 | 2.5 |
| Cancer | 729 | 1.9 | 244 | 2.5 | 950 | 2.3 | 397 | 3.2 |
| Unnatural causes | 31 | 0.1 | 11 | 0.1 | 117 | 0.3 | 32 | 0.3 |
| Any other known cause | 341 | 0.9 | 123 | 1.3 | 815 | 2.0 | 313 | 2.6 |
| Unknown cause | 338 | 0.9 | 135 | 1.4 | 743 | 1.8 | 286 | 2.3 |
| All-cause mortality | 1,662 | 4.4 | 608 | 6.3 | 3,328 | 8.0 | 1,337 | 10.9 |
Abbreviation: CHD, coronary heart disease.
a Causes of death were updated through the end of 2015.
b Number of deaths per 1,000 person-years.
c Including CHD.
The leading cause of death for both women and men was cancer (Table 4). However, while 44% of the deceased women died from cancer, in men this percentage was lower (29%). Except for the 2 composite categories (any other known cause of death and unknown cause of death), the second-largest cause of death in both sexes was circulatory disorders. However, while among women circulatory disorders caused only 14% of deaths, among men they accounted for 22% of deaths.
Table 4. All-Cause and Cause-Specific Mortality in the Study Sample and Mortality Risk as a Function of Exposure to the Holocaust (Cox Models), Jerusalem Perinatal Study, Israel, 1964–1976.
| Cause of Death a | Women (n = 9,842 Observations) | Men (n = 12,066 Observations) | ||||||
|---|---|---|---|---|---|---|---|---|
|
No. of Deaths |
% of Deaths |
HR b | 95% CI |
No. of Deaths |
% of Deaths |
HR b | 95% CI | |
| All-cause mortality | 2,137 | 100.0 | 1.15 | 1.05, 1.27 | 4,448 | 100.0 | 1.02 | 0.95, 1.09 |
| Specific causes | ||||||||
| CHD | 77 | 3.6 | 1.28 | 0.78, 2.12 | 306 | 6.9 | 1.39 | 1.09, 1.77 |
| All circulatory disordersc | 298 | 13.9 | 1.22 | 0.94, 1.57 | 960 | 21.6 | 1.12 | 0.97, 1.29 |
| Cancer | 930 | 43.5 | 1.17 | 1.01, 1.35 | 1,282 | 28.8 | 1.14 | 1.01, 1.28 |
| Unnatural causes | 40 | 1.9 | 1.34 | 0.67, 2.70 | 141 | 3.2 | 0.77 | 0.51, 1.16 |
| Any other known cause | 425 | 19.9 | 1.06 | 0.85, 1.32 | 1,087 | 24.4 | 0.86 | 0.75, 0.99 |
| Unknown cause | 444 | 20.8 | 1.17 | 0.95, 1.45 | 978 | 22.0 | 1.00 | 0.87, 1.15 |
Abbreviations: CHD, coronary heart disease, CI, confidence interval; HR, hazard ratio.
a Causes of death were updated through the end of 2015.
b The models adjusted for participant’s education and socioeconomic position.
c Including CHD.
Cox proportional hazards models
Table 4 shows that after controlling for the sociodemographic variables, exposure to the Holocaust was associated with a significant increase in all-cause mortality in women yet had no relationship to male all-cause mortality. Specifically, compared with unexposed women, the hazard ratio for the female Holocaust survivors was 1.15 (95% confidence interval (CI): 1.05, 1.27). The differential association between Holocaust exposure and survival in men and women was formally examined using a multiplicative interaction term (i.e., Holocaust exposure × sex); results were significant (P for interaction = 0.028).
In the female subsample, we also fitted a model (Web Table 3) that controlled for perinatal and obstetrical characteristics at baseline as well as for socioeconomic variables. This further adjustment only slightly attenuated the effect of exposure on all-cause mortality produced by the model in Table 4 (hazard ratio (HR) = 1.12, 95% CI: 1.01, 1.24).
Next, we examined relationships between the Holocaust and specific causes of death (Table 4). In women, excess risk was found only for mortality due to cancer (HR = 1.17, 95% CI: 1.01, 1.35). This risk decreased somewhat after the model further adjusted for perinatal and obstetrical conditions (HR = 1.12, 95% CI: 0.95, 1.31) (Web Table 3). In men, among Holocaust survivors we observed statistically significant excess mortality due to CHD (HR = 1.39, 95% CI: 1.09, 1.77) and cancer (HR = 1.14, 95% CI: 1.01, 1.28) as compared with nonexposed men. Mortality due to “any other known cause” was significantly lower in male Holocaust survivors than in the unexposed (HR = 0.86, 95% CI: 0.75, 0.99). To further investigate this observed relationship, we broke down this composite category into 7 subcomponents of diseases based on ICD-10 codes and analyzed each of them separately. This analysis did not yield hazard ratios significantly different from 1 in any of the 7 categories (Web Tables 4 and 5), probably because of the small number of observations in each category.
We conducted several sensitivity analyses that all produced similar results. First, models were fitted using 1939 as the year in which the Holocaust began instead of the country-specific year of Nazi invasion. Additional analyses were carried out in a sample that also included immigrants from the Union of Soviet Socialist Republics. In a further analysis, we used a sample that excluded the Israeli-born group and fitted Cox models with and without control for participant’s age at immigration. Finally, we conducted an analysis where instead of counting time from the participant’s own birth and correcting for left-truncation, we counted time from the moment a participant entered the study while controlling for her or his age at that moment.
DISCUSSION
Strikingly, our findings show that among persons who sired or gave birth to children in midlife, exposure to the Nazi regime early in life was associated with excess all-cause and cause-specific mortality later in life. Differences in mortality rates were also found by survivor’s sex. Overall, compared with women who were not exposed to the Holocaust, female survivors exhibited higher all-cause mortality rates. Furthermore, we detected statistically significant excess mortality due to cancer in survivors of both sexes and, in exposed men, excess mortality due to CHD as well. It is noteworthy that in men, death due to other known causes combined was the second-largest category, accounting for almost one-quarter (24%) of deaths. The fact that the mortality rate among male survivors was significantly lower in this category than among the unexposed probably explains why there was no significant excess all-cause mortality in male survivors, even though risks of mortality due to cancer and CHD were significantly increased.
These results have several implications. First, they differ from those of other studies on mortality among Holocaust survivors in Israel. As we noted above, previous research did not find excess all-cause mortality among Holocaust survivors (13, 15–19). Our analyses point out the importance of considering specific causes of death. Indeed, although in the male subsample there was no excess all-cause mortality among Holocaust survivors, when mortality was broken down into specific causes of death, it appeared that survivors were in fact affected by higher rates of mortality due to some causes but not others. In addition, some studies that did not find excess all-cause mortality among survivors studied only those who lived a relatively long time (e.g., conducting interviews in the late 1990s or the 2000s) or used relatively late mortality data (i.e., only records from the late 1980s onwards) (16, 17, 19). However, many frail survivors might have died much earlier. Instead, our mortality data spanned several decades.
The excess mortality due to cancer and CHD in this study is in line with recent research showing higher prevalence of cancer (7, 9, 32, 33) and cardiovascular morbidity and associated risk factors (8, 12, 14, 33, 34) among Holocaust survivors. Several mechanisms have been proposed to explain the survivors’ vulnerability to these ailments. Apart from studies that found detrimental health effects of undernutrition around the time of birth (3–6, 9), others have suggested that the abrupt increase in caloric intake upon arrival in Israel might have been responsible for late-life morbidity (34). Furthermore, acutely stressful events have also been shown to affect cancer and cardiovascular morbidity by distressing various physiological systems, such as the hypothalamic-pituitary-adrenal axis or the immune system (7, 12, 14, 28). Additionally, researchers have pointed to the exposure of survivors to such carcinogens as infectious diseases (e.g., hepatitis) (7) and toxic waste (33).
Second, it is important to note that in female survivors, mortality due to other known causes of death combined was not higher than among the unexposed, and in exposed men it was even lower. Breaking the category “other known causes of death” down into its separate components yielded nonsignificant results in both sexes. Although this lack of findings might be attributed to the small number of subjects who died from these causes, previous research has suggested that the lack of excess all-cause mortality might hint at survivors’ resilience (18). Indeed, a theoretical model has suggested that Holocaust survivors can be characterized by general health resilience combined with specific vulnerabilities (15, 20). Thus, although the vulnerabilities to cancer and CHD had persisted throughout the survivors’ lives, they had not necessarily precluded the possibility of a normal life after the trauma.
Third, we note the aforementioned differences in mortality patterns by sex. It should be kept in mind, however, that on average our female survivors were younger than the males. This dissimilarity in age structure could explain some of the observed sex differences in mortality patterns, both by differential timing of exposure to the Holocaust and by differential risks of CHD mortality according to age and sex (35). It is therefore possible that future follow-up in this population might also show increased CHD mortality in women.
Several limitations of this study should be mentioned. One limitation was the inability to adjust for risky health behaviors (e.g., diet or a sedentary lifestyle) or psychological variables (e.g., personality characteristics or mental health). In addition, some scholars have claimed that the ecological definition of a Holocaust survivor used in this paper might be inferior to respondents’ self-reports or the Israeli state codes that designate survivors, because it does not provide adequate details on individual exposure (7). However, authors who have relied mainly on the Israeli codes have admitted that their exposure definition, too, might have been subject to some misclassification (7, 8). Furthermore, researchers who have used self-reports have suggested that some of their “unexposed” participants in fact have been persecuted due to residing as Jews in Nazi-occupied countries (13). Finally, we found a substantial degree of agreement between our definition of exposure and the self-reports in the SHARE-IL data set.
A further limitation was the lack of information on Holocaust survivors who were not included in the cohort. To account for the lack of records on survivors who died before the study was initiated, we corrected the risk set for left-truncation (29). Nevertheless, the absence of information about survivors who immigrated elsewhere or did not have children might affect the generalizability of our results. While country of immigration might affect a person’s ability to adapt to a new environment and thus mortality, a meta-analysis by Barel et al. (36) found no difference between elderly survivors living in Israel and those living elsewhere with respect to physical health. In addition, people without offspring might, on average, have worse health and a shorter life span due to both causal effects (e.g., social support from one’s children in old age) and/or selection (e.g., not having children for health reasons). On the basis of these considerations, we believe that while settling in Israel (and specifically in Jerusalem) possibly did not affect mortality, the absence of data on immigrants without offspring might have led to underestimation of the survivors’ mortality rates.
Our study had several strengths. This is one of the rare works to have evaluated cause-specific mortality in Holocaust survivors. Our high-quality data spanned the period from the 1960s to 2016, thus allowing estimation of mortality over the course of 4–5 decades. The rich data set also enabled us to control for several important covariates on which data were collected between 1964 and 1976, such as education and SEP, as well as perinatal and obstetrical characteristics in women.
In summary, this study contributes to understanding of the consequences of extreme adversity early in life with regard to long-term mortality among parous Holocaust survivors. Our results suggest that sex-specific intervention strategies may be warranted to treat and prevent cancer and cardiovascular ailments in Holocaust survivors. In addition, our findings are relevant for understanding and predicting the life-course mortality of survivors of more recent genocides, for whom fewer long-term data are currently available (9).
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Braun School of Public Health, Hebrew University of Jerusalem-Hadassah Medical School, Jerusalem, Israel (Iaroslav Youssim, Ronit Calderon-Margalit, Orly Manor, Ora Paltiel, Yechiel Friedlander, Hagit Hochner); Department of Statistics and Operations Research, School of Mathematical Sciences, Tel Aviv University, Tel Aviv, Israel (Malka Gorfine); and New York Academy of Medicine, New York, New York, United States (David S. Siscovick).
This work was supported by US National Institutes of Health grant R01-CA80197 and Israel Science Foundation grant 1065/16.
We thank the investigators of the Survey of Health, Ageing and Retirement in Europe, who collected the data used to test the exposure definition. (For the full text of the acknowledgment see: http://www.share-project.org/data-access/citation-requirements.html.)
Conflict of interest: none declared.
REFERENCES
- 1. Stone L. Quantifying the Holocaust: hyperintense kill rates during the Nazi genocide. Sci Adv. 2019;5(1):eaau7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rutter M. Achievements and challenges in the biology of environmental effects. Proc Natl Acad Sci U S A. 2012;109(suppl 2):17149–17153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Almond D, Currie J. Killing me softly: the fetal origins hypothesis. J Econ Perspect. 2011;25(3):153–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ravelli AC, van der Meulen JH, Michels RP, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351(9097):173–177. [DOI] [PubMed] [Google Scholar]
- 5. Lumey LH, Stein AD, Susser E. Prenatal famine and adult health. Annu Rev Public Health. 2011;32:237–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82(8):485–491. [DOI] [PubMed] [Google Scholar]
- 7. Sadetzki S, Chetrit A, Freedman LS, et al. Cancer risk among Holocaust survivors in Israel—a nationwide study. Cancer. 2017;123(17):3335–3345. [DOI] [PubMed] [Google Scholar]
- 8. Zamstein O, Biderman A, Sherf M, et al. Cardiovascular morbidity and risk factors in Holocaust survivors in Israel. J Am Geriatr Soc. 2018;66(9):1684–1691. [DOI] [PubMed] [Google Scholar]
- 9. Keinan-Boker L. Increased cancer incidence in Holocaust survivors and the implications for survivors of other extreme events. Expert Rev Anticancer Ther. 2018;18(11):1059–1062. [DOI] [PubMed] [Google Scholar]
- 10. Knobler HY, Abramowitz MZ, Lindert J. Survival and resilience versus psychopathology: a seven-decade perspective post-Holocaust. In: Lindert J, Marsoobian A, eds. Multidisciplinary Perspectives on Genocide and Memory. New York, NY: Springer Publishing Company; 2018:103–113. [Google Scholar]
- 11. Hursting SD, Forman MR. Cancer risk from extreme stressors: lessons from European Jewish survivors of World War II. J Natl Cancer Inst. 2009;101(21):1436–1437. [DOI] [PubMed] [Google Scholar]
- 12. Greenblatt Kimron L, Marai I, Lorber A, et al. The long-term effects of early-life trauma on psychological, physical and physiological health among the elderly: the study of Holocaust survivors. Aging Ment Health. 2019;23(10):1340–1349. [DOI] [PubMed] [Google Scholar]
- 13. Collins C, Burazeri G, Gofin J, et al. Health status and mortality in Holocaust survivors living in Jerusalem 40–50 years later. J Trauma Stress. 2004;17(5):403–411. [DOI] [PubMed] [Google Scholar]
- 14. Kagansky N, Knobler H, Stein-Babich M, et al. Holocaust survival and the long-term risk of cardiovascular disease in the elderly. Isr Med Assoc J. 2019;21(4):241–245. [PubMed] [Google Scholar]
- 15. Shrira A, Palgi Y, Ben-Ezra M, et al. Functioning and mortality of Holocaust survivors: physical resilience and psychosocial vulnerabilities. J Loss Trauma. 2011;16(1):67–83. [Google Scholar]
- 16. Ayalon L, Covinsky KE. Late-life mortality in older Jews exposed to the Nazi regime. J Am Geriatr Soc. 2007;55(9):1380–1386. [DOI] [PubMed] [Google Scholar]
- 17. Stesssman J, Cohen A, Hammerman-Rozenberg R, et al. Holocaust survivors in old age: the Jerusalem Longitudinal Study. J Am Geriatr Soc. 2008;56(3):470–477. [DOI] [PubMed] [Google Scholar]
- 18. Sagi-Schwartz A, Bakermans-Kranenburg MJ, Linn S, et al. Against all odds: genocidal trauma is associated with longer life-expectancy of the survivors. PLoS One. 2013;8(7):e69179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fund N, Ash N, Porath A, et al. Comparison of mortality and comorbidity rates between Holocaust survivors and individuals in the general population in Israel. JAMA Netw Open. 2019;2(1):e186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shmotkin D. Vulnerability and resilience intertwined: a review of research on Holocaust survivors. In: Jacoby R, Keinan G, eds. Between Stress and Hope: From a Disease-Centered to a Health-Centered Perspective. Westport, CT: Praeger Publishers; 2003:213–233. [Google Scholar]
- 21. Harlap S, Davies AM, Deutsch L, et al. The Jerusalem Perinatal Study cohort, 1964–2005: methods and a review of the main results. Paediatr Perinat Epidemiol. 2007;21(3):256–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Myers-JDC-Brookdale Institute . Holocaust Survivors in Israel: Population Estimates, Demographic, Health and Social Characteristics, and Needs . Jerusalem, Israel: Myers-JDC-Brookdale Institute; 2010. (Publication no. RR-553-09). http://claimscon.org/forms/Holocaust_Survivors_Israel-jdc.pdf. Accessed January 23, 2021. [Google Scholar]
- 23. Lurie I, Gur A, Haklai Z, et al. Suicide risk among Holocaust survivors following psychiatric hospitalizations: a historic cohort study. Arch Suicide Res. 2018;22(3):496–509. [DOI] [PubMed] [Google Scholar]
- 24. Börsch-Supan A. Survey of Health, Ageing and Retirement in Europe (SHARE) Wave 1. Release version: 7.0.0. SHARE-ERIC [data set]. Munich, Germany: Munich Center for the Economics of Aging, Max Planck Institute for Social Law and Social Policy; 2019. http://www.share-project.org/data-documentation/waves-overview/wave-1.html. Accessed October 10, 2019. [Google Scholar]
- 25. Bӧrsch-Supan A, Brandt M, Hunkler C, et al. Data resource profile: the Survey of Health, Ageing and Retirement in Europe (SHARE). Int J Epidemiol. 2013;42(4):992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mannheim Research Institute for the Economics of Aging . The Survey of Health, Ageing and Retirement in Europe—Methodology. Mannheim, Germany: Mannheim Research Institute for the Economics of Aging; 2005. http://www.share-project.org/fileadmin/pdf_documentation/Methodology/Methodology_2005.pdf. Accessed January 23, 2021. [Google Scholar]
- 27. Shrira A, Palgi Y, Ben-Ezra M, et al. Do Holocaust survivors show increased vulnerability or resilience to post-Holocaust cumulative adversity? J Trauma Stress. 2010;23(3):367–375. [DOI] [PubMed] [Google Scholar]
- 28. Schorr L, Burger A, Hochner H, et al. Mortality, cancer incidence, and survival in parents after bereavement. Ann Epidemiol. 2016;26(2):115–121. [DOI] [PubMed] [Google Scholar]
- 29. Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. 2nd ed. New York, NY: Springer Science + Business Media; 2006. [Google Scholar]
- 30. Hosmer DW Jr, Lemeshow S, May S. Applied Survival Analysis: Regression Modeling of Time-to-Event Data. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2008. [Google Scholar]
- 31. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 32. Keinan-Boker L, Vin-Raviv N, Liphshitz I, et al. Cancer incidence in Israeli Jewish survivors of World War II. J Natl Cancer Inst. 2009;101(21):1489–1500. [DOI] [PubMed] [Google Scholar]
- 33. Ben David R, Biderman A, Sherf M, et al. Elevated cancer risk in Holocaust survivors residing in Israel: a retrospective cohort study. Eur J Cancer. 2018;95:85–92. [DOI] [PubMed] [Google Scholar]
- 34. Keinan-Boker L, Shasha-Lavsky H, Eilat-Zanani S, et al. Chronic health conditions in Jewish Holocaust survivors born during World War II. Isr Med Assoc J. 2015;17(4):206–212. [PubMed] [Google Scholar]
- 35. Bots SH, Peters SA, Woodward M. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Glob Health. 2017;2(2):e000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barel E, Van IJzendoorn MH, Sagi-Schwartz A, et al. Surviving the Holocaust: a meta-analysis of the long-term sequelae of a genocide. Psychol Bull. 2010;136(5):677–698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


