ABSTRACT
Background
Chronic consumption of dairy products with an SFA-reduced, MUFA-enriched content was shown to impact favorably on brachial artery flow-mediated dilatation (FMD). However, their acute effect on postprandial cardiometabolic risk biomarkers requires investigation.
Objective
The effects of sequential high-fat mixed meals rich in fatty acid (FA)–modified or conventional (control) dairy products on postprandial FMD (primary outcome) and systemic cardiometabolic biomarkers in adults with moderate cardiovascular risk (≥50% above the population mean) were compared.
Methods
In a randomized crossover trial, 52 participants [mean ± SEM age: 53 ± 2 y; BMI (kg/m2) 25.9 ± 0.5] consumed a high-dairy-fat breakfast (0 min; ∼50 g total fat: modified: 25 g SFAs, 20 g MUFAs; control: 32 g SFAs, 12 g MUFAs) and lunch (330 min; ∼30 g total fat; modified: 15 g SFAs, 12 g MUFAs; control: 19 g SFAs, 7 g MUFAs). Blood samples were obtained before and until 480 min after breakfast, with FMD assessed at 0, 180, 300, and 420 min. Data were analyzed by linear mixed models.
Results
Postprandial changes in cardiometabolic biomarkers were comparable between the different dairy meals, with the exception of a tendency for a 4% higher AUC for the %FMD response following the modified-dairy-fat meals (P = 0.075). Plasma total lipid FA analysis revealed that incremental AUC responses were 53% lower for total SFAs, 214% and 258% higher for total cis-MUFAs (predominantly cis-9 18:1), and trans-18:1, respectively, following the modified relative to the control dairy meals (all P < 0.0001).
Conclusions
In adults at moderate cardiovascular risk, acute consumption of sequential high-fat meals containing FA-modified dairy products had little impact on postprandial endothelial function or systemic cardiometabolic biomarkers, but a differential effect on the plasma total lipid FA profile, relative to conventional dairy fat meals.
This trial was registered at clinicaltrials.gov as NCT02089035.
Keywords: endothelial function, monounsaturated fatty acids, postprandial lipemia, saturated fatty acids, sequential test meal protocol
Introduction
The replacement of dietary SFAs with unsaturated fatty acids (FAs) is advised as a key public health strategy for cardiovascular disease (CVD) risk reduction (1, 2). Milk and dairy products are a major source of SFAs in the diet (3). However, it is known that the food matrix can influence the nutritional properties, nutrient absorption, and associated health effects of an SFA-rich food (e.g., cheese) (4). Indeed, evidence from prospective studies and meta-analyses indicates that full-fat dairy products are not linked to increased CVD risk, except for butter fat (4). As dairy products play a substantial role in meeting essential nutrient requirements, limiting their consumption to reduce SFA intake may inadvertently affect public health (5–7).
Exaggerated postprandial triacylglycerol (TG) is an independent CVD risk factor, with high-fat meals known to lead to a transient increase in TG-rich lipoproteins during the postprandial phase (8, 9). A meta-analysis of postprandial lipemic responses to single high-fat meals highlighted that, over an 8-h period, there was a benefit or a tendency towards a beneficial effect of replacing SFAs (mainly butter) by either PUFA- or MUFA-rich oils, respectively (10). Additionally, the type of dairy product incorporated into fat- and FA-matched meals has been shown to differently affect the postprandial TG response, with sour cream inducing a larger TG incremental AUC (iAUC) over 6 h compared with whipped cream, butter, and cheese (11). However, little is known about how manipulating the FA composition of high-dairy-fat load affects postprandial lipemia, particularly using a sequential 2-meal protocol that contains a combination of commonly consumed dairy products and, thus, more closely reflects real-life eating patterns.
Postprandial TG may trigger a cascade of proinflammatory events that lead to endothelial dysfunction, an early event in the pathogenesis of atherosclerosis (12, 13). Although single high-fat meals (36–80 g total fat) are known to impair postprandial endothelial function, including flow-mediated dilatation (FMD) (14), evidence regarding the comparative effects of test meals rich in unsaturated FAs with SFAs or different dairy products on postprandial FMD or biomarkers of endothelial function is inconclusive (14, 15).
In a proof-of-concept study, we recently reported that 12-wk intake of SFA-reduced, MUFA-enriched milk, cheese, and butter (∼41 g dairy fat/d) that were FA-modified via a dairy-cow feeding strategy (16), attenuated the rise in fasting LDL-cholesterol concentrations and had a beneficial effect on FMD, relative to conventional dairy product consumption (17). As individuals spend up to 18 h/d in the nonfasting state (9, 18), there is a clear need to gain an understanding of the postprandial cardiometabolic responses to FA-modified dairy products (5). Therefore, we investigated the acute effect of sequential high-fat mixed meals containing FA-modified dairy products on the postprandial %FMD response, systemic cardiometabolic risk biomarkers, and plasma total lipid FA responses in adults with moderate CVD risk. We hypothesized that meals rich in FA-modified milk, cheese, and butter would beneficially impact on the postprandial %FMD response (primary outcome) and other cardiometabolic risk biomarkers compared with matched meals containing dairy foods with an FA profile typical of conventional retail products (control).
Methods
Participants
The REplacement of SaturatEd fat in dairy on Total cholesterol (RESET) Study was performed at the Hugh Sinclair Unit of Human Nutrition, University of Reading (Berkshire, UK). Men and women [aged 25–70 y; BMI (kg/m2)19–32] at moderate CVD risk were recruited from the Berkshire area between February 2014 and September 2015. To assist with participant recruitment, the study upper age limit was extended from 65 y to 70 y in May 2014. Details of the participant recruitment strategy, inclusion and exclusion criteria, and dietary intervention are presented in detail elsewhere (17, 19). In brief, a modified Framingham CVD risk score identified individuals at moderate risk of developing CVD (≥50% greater CVD risk compared with the population mean) (19). Eligible participants were nonsmokers with mild/moderate hypercholesterolemia (fasting total cholesterol ≥5.2–8.0 mmol/L); not diagnosed with CVD and diabetes (and fasting glucose concentration <7 mmol/L); not taking medication for hyperlipidemia, hypertension, hypercoagulation, or inflammation; not pregnant or lactating; not consuming excessive amounts of alcohol (<14 and 21 units/wk for women and men, respectively); not regularly engaging in vigorous-intensity aerobic activities (>3 times × 20 min/wk); and presenting with normal biochemistry for liver and kidney function (19). The study was given a favorable ethical opinion for conduct by the University of Reading's Research Ethics Committee (project 13/43), registered with clinicaltrials.gov (NCT02089035), and carried out in accordance with the Declaration of Helsinki of 1975, as revised in 1983. Participants provided written informed consent before enrollment.
Study design
This trial was an acute randomized, double-blind, sequential-meal, crossover dietary study nested within the larger RESET proof-of-concept chronic intervention study, which examined the impact of long-term consumption of FA-modified dairy products on novel and traditional markers of CVD risk (17). The chronic intervention study consisted of two 12-wk dietary periods where FA-modified or control dairy products were consumed in a randomized crossover manner, separated by an 8-wk washout period (17). As outlined in Supplemental Figure 1, the 2 baseline acute study visits were performed prior to commencing each 12-wk dietary intervention period. With the addition of the 8-wk washout period, the duration between the acute study visits examined in the present trial was 20 wk. None of the results reported here for our subcohort have been previously published. Minimization was used to randomly allocate participants to their first treatment (17, 19). The researchers responsible for conducting and/or analyzing measurements (OM, DV, KEK and RS) and the participants were blinded to treatment allocations.
Postprandial 2-meal protocol
A sequential 2-meal protocol was used to examine the effect of dairy FA manipulation on postprandial endothelial function and cardiometabolic responses. The details of our high-oleic sunflower oil (HOS) dairy-cow feeding strategy, as well as the production, FA profile, and consumer acceptance of the FA-modified milk and dairy products, are published elsewhere (16, 20).
The energy, macronutrient, and FA compositions of the sequential high-fat mixed meals consumed at breakfast and lunch are presented in Table 1. Nutritional analysis (energy and macronutrient content) of modified and control dairy samples was conducted in duplicate by SGS United Kingdom Ltd. (Ealing, London; ISO 17,025 accredited laboratory), as described elsewhere (16, 20). In brief, total fat content was determined by low-resolution proton nuclear magnetic resonance. Protein content was calculated by multiplying the total nitrogen, based on the Dumas method, using a standard nitrogen conversion factor of 6.25 to account for the fraction of nonprotein nitrogen in each sample (21). Carbohydrate content was calculated by difference using the Atwater general system (22). Using a standardized procedure (16), lipids extracted from the modified and control dairy products were analyzed in triplicate for FA composition by GC (Bruker 350; Bruker), equipped with a flame ionization detector (FID) and 100-m fused silica capillary column (CP-SIL 88; Agilent Technologies). The breakfast test meal consisted of a toasted sandwich prepared with white bread (75 g; Kingsmill; Allied Bakeries UK), Cheddar cheese (32.6 g) and butter (modified: 32.6 g; control: 29.4 g), cornflakes (38 g, Kellogg's UK) served with ultra-high temperature (UHT) milk (195 g), and a milkshake prepared with UHT milk (330 g) and strawberry sauce (19 g; Askeys; Silver Spoon Company UK). The lunch test meal consisted of a toasted sandwich prepared with white bread (60 g) with cheddar cheese (15 g) and butter (modified: 19.8 g; control: 18.6 g) and a milkshake prepared with UHT milk (modified: 352 g; control: 350 g) and strawberry sauce (27 g).
TABLE 1.
Nutritional composition of the sequential high-fat mixed test breakfast (0 min) and lunch meals (330 min) that incorporated the fatty acid–modified or conventional (control) dairy products1
| Modified | Control | |||||
|---|---|---|---|---|---|---|
| Breakfast | Lunch | Total | Breakfast | Lunch | Total | |
| Energy,2 MJ | 4.3 | 2.6 | 6.9 | 4.1 | 2.5 | 6.6 |
| Total fat,2 g | 50.6 | 30.6 | 81.2 | 49.9 | 30.3 | 80.2 |
| SFAs,3 g | 24.5 | 14.8 | 39.3 | 31.7 | 19.1 | 50.8 |
| MUFAs,3 g | 20.0 | 12.1 | 32.1 | 12.3 | 7.4 | 19.7 |
| PUFAs,3 g | 2.9 | 1.8 | 4.7 | 2.8 | 1.8 | 4.6 |
| TFAs,3 g | 3.9 | 2.6 | 6.5 | 2.2 | 1.4 | 3.6 |
| Protein,2 g | 36.1 | 20.9 | 57.0 | 39.7 | 19.6 | 59.3 |
| CHO,2 g | 105.9 | 64.6 | 170.5 | 101.4 | 63.3 | 164.7 |
| Free sugars, g | 16.5 | 22.3 | 38.8 | 15.0 | 21.5 | 36.5 |
Values are total energy and macronutrient quantities of each test meal according to modified and control diet. CHO, carbohydrate; FA, fatty acid; TFA, trans fatty acid.
Measurement of energy, total fat, protein, and carbohydrate content of the dairy product samples was conducted in duplicate by SGS UK Ltd. (Ealing, London; ISO 17,025 accredited laboratory).
Lipids extracted from the dairy product samples were analyzed in triplicate for FA composition by GC–flame ionization detection using a standardized procedure, as described previously (16).
Study visits
Participants completed a 4-d weighed food diary to assess habitual dietary intake in the 1-wk run-in period to each study visit, which was analyzed using Dietplan 7 Professional (Forestfield Software Ltd.) (19). Habitual physical activity was assessed using the last 7-d version of the International Physical Activity Questionnaire (IPAQ) long form. For 24 h before each study visit, participants were instructed to avoid alcohol and aerobic exercise and were provided with a low-fat evening ready meal (<1.46 MJ; <7 g total fat) to standardize short-term fat intake. Fasted (12 h) participants arrived at the Clinical Unit after only drinking low-nitrate water overnight (Buxton Mineral Water; Nestlé Waters UK).
Postprandial study visits (480 min) took place in a temperature-controlled clinical room maintained between 22° ± 1°C. Following body-composition assessment, a cannula was inserted into an antecubital vein of the forearm to allow for frequent blood sampling. After a baseline blood sample and a 30-min period of supine rest, endothelial function was assessed by FMD. Subsequently, a second baseline sample was drawn (0 min) before a standardized breakfast test meal was provided and consumed within a period of 20 min. Blood samples were collected at regular intervals (every 30 min until 180 min, and then every 60 min until 300 min). A standardized lunch test meal was provided immediately after the 330-min blood draw and was consumed within 15 min. Blood samples were then collected every 30 min up to 420 min, with the final sample obtained at 480 min after the breakfast meal. FMD was assessed immediately after obtaining blood samples at 180, 300, and 420 min postprandially. Participants remained in the Clinical Unit for the duration of the study visit and were continuously monitored. Low-nitrate water was permitted ad libitum during the study visits.
Assessment of endothelial function
Three-lead electrocardiogram-gated brachial artery FMD measurements were conducted in a standardized manner using a CX50 CompactXtreme Ultrasound System (Philips HealthCare, UK), as previously reported (17, 23). For each participant, the initial baseline scan image (visit 1) was used as a visual aid to help position the probe on a similar section of the artery for subsequent FMD measurements. Image sequences were assessed in a blinded manner using automated wall-tracking software (Vascular Research Tools 5; Medical Imaging Applications LLC). The %FMD response was calculated at each time point as the relative increase in brachial artery diameter from baseline to maximum dilation, expressed as a percentage.
Biochemical analyses
Plasma/serum separation was carried out, as previously detailed (17), and samples were subsequently stored at −80°C until analysis. For determination of serum TG, nonesterified fatty acids (NEFAs), insulin, and glucose, blood was drawn at all time points. Samples for serum apoB were collected at 0, 60, 120, 180, 240, 300, 360, 390, 420, and 480 min. At 180, 300, and 420 min (i.e., the designated FMD time points), blood samples were also collected for the assessment of plasma nitrite, nitrate, markers of endothelial activation, total lipid FA responses, as well as whole blood culture for determination of LPS-stimulated cytokine production.
Serum TG, apoB, NEFA, and glucose concentrations were quantified on an ILAB 600 autoanalyzer (Instrumentation Laboratory Ltd.) with the use of colorimetric assay kits (TG and glucose reagents: Instrumentation Laboratory Ltd.; NEFA reagent: Alpha Laboratories Ltd.) and the use of an immunoturbidimetric assay (apoB reagent: Randox Laboratories Ltd.). Serum insulin concentrations were determined by ELISA (Dako UK Ltd.). Plasma nitrite and nitrate concentrations were measured by HPLC (ENO-30; EiCom Corporation) with online reduction of nitrate to nitrite and subsequent post-column derivatization with the Griess reagent (ENO-30 Analyzer; Eicom Corporation) (17). Plasma soluble vascular cell adhesion molecule 1 (sVCAM-1), soluble intercellular adhesion molecule 1 (sICAM-1), E-selectin, and P-selectin concentrations were quantified by the Human Adhesion Molecule Magnetic Luminex Performance Assay 4-Plex kit (R&D Systems Europe Ltd.) with the use of Luminex 200 (Invitrogen) and xPONENT software 3.1 (Luminex). All samples for each participant were analyzed within a single run or kit to reduce interbatch variations. Mean inter-assay CVs were <5% for the ILAB automated assays and <10% for all other analyses.
LPS-stimulated cytokine analyses
For the determination of whole blood culture LPS-stimulated cytokines, blood samples collected into K2-EDTA tubes were not centrifuged and stored at 4°C until processing as previously described (24). Briefly, whole-blood samples were diluted 1:9 with Roswell Park Memorial Institute 1640 medium (Sigma) supplemented with 1% antibiotics, 1% l-glutamine, and 1% nonessential amino acids (Bioscience). Diluted blood samples were cultured in 12-well plates (Greiner Bio-one) with 0.5 μg bacterial LPS/mL (Escherichia coli 026:B6; Sigma), at a final concentration of 0.05 μg/mL. Whole blood cultures were subsequently incubated at 37°C for 24 h before centrifugation at 700 × g (1000 rpm) for 5 min at room temperature to isolate the supernatant, which was stored at −20°C until analysis. Measurement of the monocyte count of each sample was performed by the Pathology Department at the Royal Berkshire Hospital (Reading, UK). A human cytokine premixed 5-Plex Panel (TNF-α, IL-6, IL-8, IL-1β, IL-10; R&D Systems Europe Ltd.) and a Luminex 200 with xPONENT software 3.1 was used to measure the concentrations of cytokines in the whole blood culture supernatant in a 1:2 dilution. Cytokine production was corrected for the number of monocytes in the whole-blood sample (micrograms × 103 monocytes).
Total lipid FA analyses
To assess changes in plasma FA status in response to our sequential 2-meal fat challenge, we measured the plasma total lipid FA pool; this pool is representative of immediate FA intake and gives an indication of all FA-containing plasma lipid fractions, including cholesteryl esters, NEFAs, phospholipids, and TG (25). Total lipid in 0.5 mL K3-EDTA plasma was extracted using 5.0 mL chloroform-methanol [2:1, vol:vol; Burdge et al. (24)]. After shaking for 15 min, the aqueous and solvent layers were separated using centrifugation (1125 × g for 10 min at 4°C), and the solvent layer dried under nitrogen at 40°C. Dried lipid was redissolved in 0.5 mL toluene and methylated by the addition of 1.0 mL 2% H2SO4 in methanol, and incubated for 2 h at 50°C. After neutralization, FAMEs were dissolved in hexane and centrifuged (1125 × g for 10 min at 4°C). Resulting FAMEs were separated on a 100-m fused silica capillary column (CP-SIL 88; Agilent Technologies) using GC (Bruker 350; Bruker) with FID (26). Plasma FAMEs were identified based on retention time comparisons with an authentic standard (GLC #463; Nu-Chek-Prep, Inc.) and cross-referenced with previously published chromatograms (27). Carbon deficiency in the FID response for FAMEs containing 4- to 10-carbon atoms was accounted for using a combined correction factor, which also converted FAMEs to FAs (28).
Power calculation and statistical analyses
An a priori power calculation was performed for the nested study's primary outcome: change in the postprandial %FMD response. At 80% power and 5% significance, the minimum number of participants required to complete both arms of the study in order to detect a 1.4% difference in the postprandial %FMD response between dairy fat treatments with an SD of 2.3% was calculated as n = 45 (29). To allow for a 15% drop-out rate, 52 participants were recruited into the study and randomly assigned. A P value <0.05 was considered significant for the primary outcome measure (postprandial %FMD response; n = 45). No formal sample-size calculations were performed for secondary outcomes variables, which included lipid metabolism [TG, apoB, NEFA (n = 43–49)], glucose and insulin responses (n = 46), circulating biomarkers of endothelial activation and inflammation [nitrite and nitrate (n = 49); sVCAM-1, sICAM-1, E-selectin, and P-selectin (n = 50); whole blood culture LPS-stimulated cytokines (TNF-α, IL-6, IL-1β, IL-8, IL-10) ( n = 43–48)], and plasma total lipid FA responses (n = 47–48). The FAs deemed relevant to our dietary intervention (from 65 identified FAs) were selected for statistical analysis a priori (19). P ≤ 0.01 was chosen a priori when assessing significance for secondary outcome variables to acknowledge the issue of multiplicity (17). Data presented in the text, tables, and figures represent the unadjusted means ± SEMs, unless otherwise stated. We chose to follow a per-protocol analysis approach a priori, as this was a proof-of-concept study rather than a confirmatory trial (17). This approach was taken, rather than intention-to-treat, as the trial was designed to test efficacy (biological effects) rather than effectiveness (30).
Summary measures of postprandial responses included the following: AUC (calculated using the trapezoidal rule) and iAUC (calculated by subtracting the fasting value from the AUC). Inclusion of the iAUC summary measure provided an indication of the change in the postprandial response to the sequential meal ingestion (31). The time interval for AUC and iAUC was 420 min for %FMD, plasma nitrite, nitrate, cell adhesion molecules, whole blood culture LPS-stimulated cytokines, and total lipid FA responses and 480 min for serum TG, apoB, glucose, and insulin responses. Due to the shape of the postprandial NEFA time-course profile, AUC and iAUC for this outcome were calculated from 120 min (approximate time of NEFA minimum concentration) to 480 min postprandially (i.e., 360-min interval) (32).
For postprandial variables with 13 (TG, NEFA, glucose, and insulin) or 10 (apoB) time points, maximum (peak) concentration reached after the test meal (Cmax) and time to reach maximum concentration (Tmax) were also calculated. Additional outcome measures for NEFAs included minimum concentration reached after the first test meal (Cmin), time to reach minimum concentration (Tmin), and percentage of NEFA suppression from fasting (32). A participant's study visit data were excluded from biochemical analyses if ≥40% of data or 3 consecutive blood time points were missing. Since ingestion of sequential meals leads to biphasic lipid and glycemic control responses (33, 34), AUC and iAUC were also calculated for the time period between breakfast and lunch (0–330 min) and post-lunch (330–480 min) for serum TG, apoB, glucose, and insulin responses (i.e., variables with ≥10 time points). As NEFA AUC and iAUC responses were calculated from 120 to 480 min post-breakfast, it was not considered physiologically relevant to calculate before and after the lunch for this outcome.
Before analysis, suitable checks for normality were conducted on all variables and the ln transformation was used for skewed outcomes. Statistical analyses were performed with the use of SAS 9.4 University edition statistical software (SAS Institute, Inc.). The baseline characteristics prior to the modified and control study visits were assessed by paired t tests and chi-square test for continuous and categorical variables, respectively. For all primary and secondary outcomes, linear mixed-model analyses (PROC MIXED) were used to evaluate fasting and treatment effects of meal dairy FA composition on postprandial summary data. Postprandial time-course responses to the meal dairy FA composition were also assessed using linear mixed models. Treatment × time interaction effects were initially included in the model and retained where found to be significant. In the absence of a significant interaction, the interaction term was removed from the model so that the overall treatment effect could be assessed. Fixed (period, time, treatment, gender, age, and BMI) and random (participant) effects were retained in all linear mixed models, regardless of their degrees of significance.
Results
Study participation
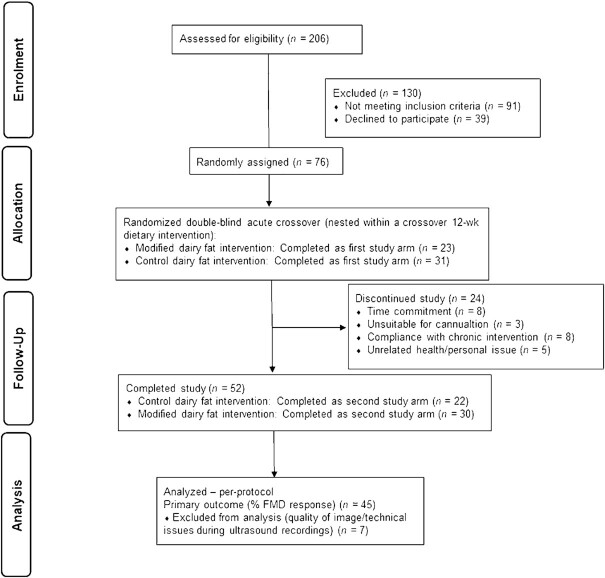
A total of 52 participants successfully completed the study (see Figure 1 for flowchart). There were no large biases in the sex and age of individuals who were randomly assigned to the intervention (n = 76) and those who completed the intervention (n = 52; data not shown). Baseline characteristics of participants at the beginning of each acute study visit are presented in Table 2. The mean CVD risk score of participants at screening, as assessed with the use of a modified Framingham risk score, was 3.0 ± 0.2. As outlined in Table 2, there were no differences in the baseline characteristics or the habitual dietary intake of participants prior to the modified and control study visits (P > 0.01). Similarly, physical activity scores (as assessed by IPAQ) did not significantly differ prior to the study visits (data not shown). The sequential test meals were fully consumed and well tolerated by all participants, without side effects.
FIGURE 1.
Flow of participants through the study. %FMD, percentage of flow-mediated dilatation response.
TABLE 2.
Baseline characteristics of participants at the beginning of each acute study visit1
| Characteristics | Modified | Control |
|---|---|---|
| Sex, n (%) | ||
| Men | 31 (59.6) | |
| Women | 21 (40.4) | |
| Age, y | 53 ± 2 | |
| Ethnicity, n (%) | ||
| White | 46 (88) | |
| Black | 2 (4) | |
| Chinese/Far Eastern | 2 (4) | |
| Asian | 2 (4) | |
| Body weight, kg | 77.6 ± 1.9 | 77.8 ± 1.9 |
| BMI, kg/m² | 25.9 ± 0.5 | 25.9 ± 0.5 |
| Waist circumference, cm | 90.1 ± 1.5 | 89.2 ± 1.4 |
| Blood pressure, mm Hg | ||
| Systolic | 120 ± 2 | 120 ± 2 |
| Diastolic | 69 ± 1 | 70 ± 1 |
| Fasting serum lipid profile, mmol/L | ||
| TC | 5.55 ± 0.14 | 5.48 ± 0.12 |
| LDL-C | 3.46 ± 0.11 | 3.44 ± 0.10 |
| HDL-C | 1.52 ± 0.05 | 1.52 ± 0.04 |
| Habitual dietary intake2 | ||
| Energy, MJ/d | 8.3 ± 0.3 | 8.5 ± 0.4 |
| Total fat, %TE | 35.9 ± 0.8 | 36.5 ± 0.9 |
| SFAs, %TE | 14.1 ± 0.5 | 13.9 ± 0.5 |
| MUFAs, %TE | 11.8 ± 0.4 | 11.9 ± 0.4 |
| n–6 PUFAs, %TE | 4.0 ± 0.2 | 4.6 ± 0.2 |
| n–3 PUFAs, %TE | 0.7 ± 0.0 | 0.8 ± 0.1 |
| Total PUFAs, %TE | 4.6 ± 0.2 | 5.3 ± 0.3 |
| TFAs, %TE | 0.9 ± 0.1 | 1.0 ± 0.1 |
| Protein, %TE | 17.0 ± 0.5 | 16.3 ± 0.5 |
| Carbohydrates, %TE | 46.7 ± 1.1 | 46.4 ± 1.0 |
| Alcohol, %TE | 2.9 ± 0.5 | 3.0 ± 0.5 |
| Dietary fiber (AOAC), g/d | 20.5 ± 1.0 | 20.2 ± 1.2 |
| Sodium, g/d | 2.6 ± 0.2 | 2.7 ± 0.2 |
Values are given as untransformed and unadjusted means ± SEMs or n (%); n = 52 (overall group). Ethnicity was determined by self-report. No significant differences were observed between the baseline characteristics of participants prior to modified and control study visits, as assessed by paired t-tests (continuous variables) and chi-square test (categorical variable). AOAC, Association of Analytical Chemists; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; TC, total cholesterol; TFA, trans fatty acid; %TE, percentage of total energy intake.
Assessed by 4-d weighed food diet diaries in the 1-wk run-in period to each study visit.
Postprandial endothelial function response
The fasting %FMD response was comparable between study days. There was no difference in the postprandial %FMD iAUC or time-course profile (overall treatment) response following consumption of the FA-modified and control sequential test meals (Table 3; Supplemental Figure 2). However, there was a tendency for an effect of meal dairy FA composition on the AUC for the %FMD response, with a 4% higher response observed after consumption of the FA-modified relative to the control meals (P = 0.075).
TABLE 3.
Fasting and postprandial summary endothelial function and circulating biomarkers of endothelial activation and inflammatory responses following sequential high-fat mixed-meal challenges rich in fatty acid–modified or conventional (control) dairy products in adults with moderate cardiovascular disease risk1
| Modified | Control | P | |
|---|---|---|---|
| %FMD response | |||
| Fasting,2 % | 4.50 ± 0.27 | 4.66 ± 0.28 | 0.112 |
| AUC,2 % × min | 2040 ± 108 | 1960 ± 111 | 0.075 |
| iAUC, % × min | 146 ± 78 | 5 ± 81 | 0.315 |
| Plasma nitrite | |||
| Fasting, μmol/L | 0.14 ± 0.02 | 0.16 ± 0.02 | 0.570 |
| AUC, μmol/L × min | 64.6 ± 7.9 | 65.5 ± 8.5 | 0.980 |
| iAUC, μmol/L × min | 4.3 ± 4.9 | -0.8 ± 4.7 | 0.480 |
| Plasma nitrate | |||
| Fasting,2 μmol/L | 16.1 ± 0.9 | 16.6 ± 1.0 | 0.970 |
| AUC,2 μmol/L × min | 5280 ± 285 | 5290 ± 271 | 0.990 |
| iAUC, μmol/L × min | −1480 ± 249 | −1700 ± 248 | 0.730 |
| Adhesion molecules | |||
| Plasma sVCAM-1 | |||
| Fasting,2 ng/mL | 540 ± 32 | 554 ± 31 | 0.623 |
| AUC,2 ng/mL × min | 226 ± 12 | 237 ± 13 | 0.180 |
| iAUC, ng/mL × min | -0.8 ± 3.4 | 3.7 ± 4.5 | 0.489 |
| Plasma sICAM-1 | |||
| Fasting, ng/mL | 88.2 ± 6.9 | 84.3 ± 7.3 | 0.534 |
| AUC, ng/mL × min | 37.8 ± 3.0 | 36.4 ± 3.1 | 0.461 |
| iAUC, ng/mL × min | 0.7 ± 1.2 | 1.0 ± 1.5 | 0.959 |
| Plasma E-selectin | |||
| Fasting, ng/mL | 25.2 ± 2.0 | 25.2 ± 1.7 | 0.953 |
| AUC, ng/mL × min | 10.3 ± 0.8 | 10.3 ± 0.7 | 0.992 |
| iAUC, ng/mL × min | −0.28 ± 0.2 | −0.27 ± 0.1 | 0.934 |
| Plasma P-selectin | |||
| Fasting,2 ng/mL | 25.7 ± 1.4 | 26.7 ± 1.5 | 0.257 |
| AUC,2 ng/mL × min | 10.7 ± 0.6 | 10.7 ± 0.6 | 0.912 |
| iAUC, ng/mL × min | −0.05 ± 0.2 | −0.51 ± 0.2 | 0.090 |
| Whole blood culture LPS-stimulated cytokines (per 103 monocytes) | |||
| TNF-α | |||
| Fasting,2 μg | 12.3 ± 0.9 | 11.5 ± 0.6 | 0.729 |
| AUC,2 mg × min | 5.00 ± 0.3 | 4.99 ± 0.3 | 0.250 |
| iAUC, mg × min | −0.18 ± 0.2 | 0.16 ± 0.2 | 0.319 |
| IL-6 | |||
| Fasting,2 μg | 82.6 ± 4.3 | 79.1 ± 4.2 | 0.869 |
| AUC,2 mg × min | 32.8 ± 1.7 | 33.2 ± 1.7 | 0.453 |
| iAUC, mg × min | −1.88 ± 1.0 | 0.03 ± 1.2 | 0.343 |
| IL-1β | |||
| Fasting,2 μg | 27.4 ± 1.5 | 26.2 ± 1.3 | 0.516 |
| AUC,2 mg × min | 11.9 ± 0.6 | 12.5 ± 0.6 | 0.181 |
| iAUC, mg × min | 0.36 ± 0.4 | 1.50 ± 0.4 | 0.024 |
| IL-8 | |||
| Fasting, μg | 124 ± 10 | 130 ± 10 | 0.653 |
| AUC, mg × min | 48.6 ± 4.1 | 51.3 ± 6.0 | 0.502 |
| iAUC, mg × min | −3.35 ± 2.4 | −3.13 ± 4.4 | 0.921 |
| IL-10 | |||
| Fasting, μg | 0.77 ± 0.08 | 0.91 ± 0.09 | 0.060 |
| AUC,2 mg × min | 0.28 ± 0.02 | 0.35 ± 0.04 | 0.083 |
| iAUC, mg × min | −0.04 ± 0.02 | −0.03 ± 0.02 | 0.645 |
Values are untransformed and unadjusted means ± SEMs. For all variables, n = 50, except for nitrite and nitrate, n = 49; TNF-α, IL-1β, IL-6, IL-10, n = 48; %FMD response, n = 45; and IL-8, n = 43. The time interval for AUC and iAUC: 420 min for all variables. Linear mixed-model analyses were used to calculate overall treatment effect in postprandial summary measures, with adjustments made for fixed effects of period, treatment, gender, age, and BMI. Participant was included as a random effPostprandial endothelial function response intercellular adhesion molecule 1; sVCAM-1, soluble vascular adhesion molecule 1, %FMD, percentage of flow-mediated dilatation.
Indicates data were log-transformed prior to analysis.
Postprandial lipid, glucose, and insulin responses
Fasting serum concentrations of TGs, apoB, NEFAs, glucose, and insulin were comparable between study days. There was no differential impact of meal dairy FA composition on the postprandial lipid (TGs and NEFAs), apoB, glucose, and insulin summary measures (Table 4) or time-course responses (0–480 min) (data not shown).
TABLE 4.
Fasting and postprandial serum lipids, glucose, and insulin responses following sequential high-fat mixed-meal challenges rich in fatty acid–modified or conventional (control) dairy products in adults with moderate cardiovascular disease risk1
| Modified | Control | P | |
|---|---|---|---|
| TGs2 | |||
| Fasting, mmol/L | 1.25 ± 0.08 | 1.15 ± 0.06 | 0.163 |
| Cmax, mmol/L | 2.83 ± 0.15 | 2.58 ± 0.12 | 0.080 |
| Tmax, min | 355 ± 9 | 334 ± 9 | 0.045 |
| AUC, mmol/L × min | 950 ± 53 | 879 ± 41 | 0.163 |
| iAUC, mmol/L × min | 351 ± 26 | 325 ± 23 | 0.651 |
| apoB | |||
| Fasting,2 mg/mL | 1.01 ± 0.03 | 1.00 ± 0.03 | 0.324 |
| Cmax,2 mg/mL | 1.03 ± 0.03 | 1.04 ± 0.03 | 0.725 |
| Tmax, min | 212 ± 24 | 207 ± 22 | 0.790 |
| AUC,2 mg/mL × min | 472 ± 14 | 470 ± 14 | 0.477 |
| iAUC, mg/mL × min | -14 ± 3 | -11 ± 2 | 0.284 |
| NEFAs | |||
| Fasting,2 μmol/L | 557 ± 25 | 554 ± 31 | 0.412 |
| Cmin30–330,2 μmol/L | 113 ± 5 | 111 ± 6 | 0.212 |
| Tmin30–330,2 min | 142 ± 6 | 135 ± 7 | 0.394 |
| Suppression30–330,2 % | 79 ± 1 | 77 ± 2 | 0.318 |
| Cmax120–480,2 μmol/L | 436 ± 19 | 481 ± 21 | 0.083 |
| Tmax120–480,2 min | 361 ± 5 | 360 ± 6 | 0.806 |
| AUC120–480,2 mmol/L × min | 91 ± 3 | 97 ± 4 | 0.192 |
| iAUC120–480, mmol/L × min | 45 ± 3 | 51 ± 4 | 0.223 |
| Glucose | |||
| Fasting,2 mmol/L | 5.41 ± 0.11 | 5.39 ± 0.10 | 0.870 |
| Cmax,2 mmol/L | 8.27 ± 0.22 | 7.99 ± 0.21 | 0.086 |
| Tmax, min | 230 ± 25 | 243 ± 25 | 0.620 |
| AUC,2 mmol/L × min | 2940 ± 64 | 2870 ± 64 | 0.055 |
| iAUC,2 mmol/L × min | 347 ± 41 | 286 ± 40 | 0.145 |
| Insulin | |||
| Fasting, pmol/L | 41.2 ± 3.6 | 41.3 ± 4.0 | 0.948 |
| Cmax, pmol/L | 551 ± 51 | 509 ± 42 | 0.108 |
| Tmax, min | 170 ± 25 | 222 ± 25 | 0.062 |
| AUC, μmol/L × min | 121 ± 11 | 114 ± 9 | 0.204 |
| iAUC, μmol/L × min | 102 ± 9 | 95 ± 8 | 0.151 |
Values are untransformed and unadjusted means ± SEMs. For all variables, n = 46, except n = 49 for apoB and n = 43 for NEFAs. Time interval for AUC and iAUC: 480 min for all variables, except for 360 min NEFA. Linear mixed-model analyses were used to calculate overall treatment effect in postprandial summary measures, with adjustments made for fixed effects of period, treatment, gender, age, and BMI. Participant was included as a random effect. For all outcome measures, P ≤ 0.01 was deemed significant to acknowledge multiplicity. Cmax, maximum concentration; Cmin, minimum concentration; iAUC, incremental AUC; NEFA, nonesterified fatty acid; TG; triacylglycerol; Tmax, time to reach maximum concentration; Tmin, time to reach minimum concentration.
Indicates data were log-transformed prior to analysis.
As outlined in Supplemental Table 1, subanalyses of AUC and iAUC responses for the time period between the breakfast and lunch meals (0–330 min) and after the lunch meal (330–480 min) revealed differences in the TG and glucose responses between the modified and control dairy fat meals. For the TG response, there was a 2.8% higher iAUC after the modified dairy compared with the control breakfast meal (0–330 min) (P = 0.009). No other pre- or post-lunch differences in AUC or iAUC were evident between the modified and control dairy meals (i.e., P > 0.01).
Postprandial response for circulating markers of endothelial activation and inflammation
Baseline (fasting) concentrations of all plasma markers of endothelial activation and inflammation were comparable between study days. Meal dairy FA composition had no effect on postprandial endothelial activation or whole blood culture LPS-stimulated cytokine summary measures (Table 3) or time-course profile responses (data not shown), with the exception of a tendency towards a 76% lower iAUC for the LPS-stimulated whole-blood IL-1β response after consumption of the modified, relative to the control, dairy fat meals (P = 0.024).
Postprandial plasma total lipid FA responses
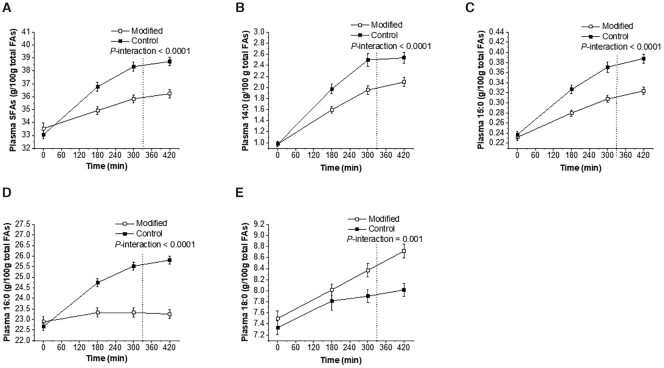
As outlined in Table 5, baseline (fasting) abundance of all plasma total lipid FAs was comparable between treatments. The abundance of total SFAs, 14:0 (myristic acid), 15:0 (pentadecanoic acid), and 16:0 (palmitic acid) was significantly lower following the FA-modified dairy compared with the control dairy fat meals (all P < 0.0001 for the AUC, iAUC, and the time-course interaction effect) (Table 5; Figure 2). The abundance of 18:0 (stearic acid) over the postprandial period was higher following the FA-modified compared with the control dairy meals (P = 0.0002 and P = 0.001 for the AUC response and the time-course interaction effect, respectively).
TABLE 5.
Fasting and postprandial summary responses of selected plasma total lipid fatty acids following sequential high-fat mixed-meal challenges rich in fatty acid–modified or conventional (control) dairy products in adults with moderate cardiovascular disease risk1
| Modified | Control | P | |
|---|---|---|---|
| SFAs | |||
| 12:0 | |||
| Fasting, g/100 g total FAs | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.489 |
| AUC,2 g/100 g total FAs × min | 47 ± 7 | 68 ± 13 | 0.446 |
| iAUC,2 g/100 g total FAs × min | 7 ± 7 | 29 ± 14 | 0.446 |
| 14:02 | |||
| Fasting, g/100 g total FAs | 0.98 ± 0.05 | 0.98 ± 0.05 | 0.861 |
| AUC, g/100 g total FAs × min | 689 ± 24 | 835 ± 28 | <0.0001 |
| iAUC, g/100 g total FAs × min | 279 ± 15 | 424 ± 25 | <0.0001 |
| 15:0 | |||
| Fasting, g/100 g total FAs | 0.23 ± 0.01 | 0.24 ± 0.01 | 0.468 |
| AUC, g/100 g total FAs × min | 119 ± 2 | 138 ± 3 | <0.0001 |
| iAUC,2 g/100 g total FAs × min | 22 ± 1 | 39 ± 2 | <0.0001 |
| 16:0 | |||
| Fasting,2 g/100 g total FAs | 22.89 ± 0.25 | 22.67 ± 0.19 | 0.451 |
| AUC,2 g/100 g total FAs × min | 9760 ± 91 | 10,400 ± 73 | <0.0001 |
| iAUC, g/100 g total FAs × min | 142 ± 33 | 837 ± 48 | <0.0001 |
| 17:0 | |||
| Fasting, g/100g total FAs | 0.28 ± 0.01 | 0.28 ± 0.01 | 0.537 |
| AUC,2 g/100 g total FAs x min | 117 ± 3 | 128 ± 3 | 0.603 |
| iAUC,2 g/100 g total FAs × min | 1 ± 3 | 12 ± 4 | 0.458 |
| 18:0 | |||
| Fasting,2 g/100 g total FAs | 7.50 ± 0.13 | 7.33 ± 0.12 | 0.103 |
| AUC,2 g/100 g total FAs × min | 3410 ± 42 | 3260 ± 45 | 0.0002 |
| iAUC, g/100 g total FAs × min | 255 ± 38 | 181 ± 43 | 0.132 |
| Total SFAs3 | |||
| Fasting,2 g/100 g total FAs | 33.30 ± 0.36 | 32.91 ± 0.28 | 0.277 |
| AUC,2 g/100 g total FAs × min | 14,700 ± 126 | 15,400 ± 111 | <0.0001 |
| iAUC, g/100g total FAs × min | 741 ± 69 | 1580 ± 109 | <0.0001 |
| MUFAs | |||
| cis-9 18:1 | |||
| Fasting,2 g/100 g total FAs | 21.75 ± 0.38 | 21.56 ± 0.35 | 0.923 |
| AUC,2 g/100 g total FAs × min | 9350 ± 161 | 8730 ± 146 | <0.0001 |
| iAUC, g/100 g total FAs × min | 219 ± 76 | -323 ± 67 | <0.0001 |
| Total cis-18:14 | |||
| Fasting,2 g/100 g total FAs | 23.53 ± 0.32 | 23.36 ± 0.30 | 0.975 |
| AUC,2 g/100 g total FAs × min | 10,100 ± 133 | 9460 ± 124 | <0.0001 |
| iAUC, g/100 g total FAs × min | 204 ± 63 | −353 ± 56 | <0.0001 |
| Total cis-MUFAs5 | |||
| Fasting,2 g/100 g total FAs | 25.19 ± 0.36 | 24.99 ± 0.32 | 0.996 |
| AUC,2 g/100 g total FAs × min | 10,800 ± 150 | 10,300 ± 139 | <0.0001 |
| iAUC,2 g/100 g total FAs × min | 272 ± 67 | −238 ± 59 | <0.0001 |
| trans-9 18:1 | |||
| Fasting, g/100 g total FAs | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.309 |
| AUC, g/100 g total FAs × min | 85 ± 3 | 53 ± 2 | <0.0001 |
| iAUC,2 g/100 g total FAs × min | 33 ± 5 | 4 ± 4 | <0.0001 |
| trans-10 18:1 | |||
| Fasting,2 g/100 g total FAs | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.239 |
| AUC, g/100 g total FAs × min | 222 ± 9 | 57 ± 2 | <0.0001 |
| iAUC, g/100 g total FAs × min | 184 ± 9 | 16 ± 3 | <0.0001 |
| trans-11 18:1 | |||
| Fasting,2 g/100 g total FAs | 0.14 ± 0.01 | 0.15 ± 0.01 | 0.421 |
| AUC,2 g/100 g total FAs × min | 100 ± 3 | 102 ± 2 | 0.198 |
| iAUC, g/100 g total FAs × min | 41 ± 3 | 41 ± 3 | 0.811 |
| Total trans-18:16 | |||
| Fasting,2 g/100 g total FAs | 0.56 ± 0.03 | 0.57 ± 0.03 | 0.679 |
| AUC,2 g/100 g total FAs × min | 657 ± 19 | 358 ± 7 | <0.0001 |
| iAUC, g/100 g total FAs × min | 422 ± 22 | 118 ± 12 | <0.0001 |
| Total trans-MUFAs7 | |||
| Fasting,2 g/100 g total FAs | 1.03 ± 0.03 | 1.02 ± 0.03 | 0.934 |
| AUC,2 g/100 g total FAs × min | 855 ± 21 | 552 ± 9 | <0.0001 |
| iAUC, g/100 g total FAs × min | 424 ± 22 | 122 ± 13 | <0.0001 |
| Total TFAs8 | |||
| Fasting, g/100 g total FAs | 1.36 ± 0.04 | 1.36 ± 0.04 | 0.699 |
| AUC, g/100 g total FAs × min | 1000 ± 24 | 695 ± 11 | <0.0001 |
| iAUC, g/100 g total FAs × min | 434 ± 25 | 124 ± 16 | <0.0001 |
| PUFAs | |||
| Total CLAs9 | |||
| Fasting,2 g/100 g total FAs | 0.19 ± 0.01 | 0.20 ± 0.01 | 0.561 |
| AUC,2 g/100 g total FAs × min | 118 ± 4 | 108 ± 4 | 0.019 |
| iAUC,2 g/100g total FAs × min | 39 ± 3 | 25 ± 2 | 0.001 |
| Total n–3 PUFAs10 | |||
| Fasting,2 g/100 g total FAs | 3.72 ± 0.12 | 3.86 ± 0.15 | 0.426 |
| AUC,2 g/100 g total FAs × min | 1460 ± 50 | 1480 ± 52 | 0.786 |
| iAUC,2 g/100 g total FAs × min | −106 ± 24 | −143 ± 29 | 0.211 |
| Total n–6 PUFAs11 | |||
| Fasting, g/100 g total FAs | 34.94 ± 0.66 | 35.43 ± 0.59 | 0.544 |
| AUC,2 g/100 g total FAs × min | 13,400 ± 241 | 13,600 ± 217 | 0.440 |
| iAUC,2 g/100 g total FAs × min | -1250 ± 138 | -1250 ± 139 | 0.896 |
Values are untransformed and unadjusted means ± SEMs. For all variables, n = 47–48. The time interval for AUC and iAUC: 420 min for all variables. Linear mixed-model analyses were used to calculate overall treatment effect in postprandial summary measures, with adjustments made for fixed effects of period, treatment, gender, age, and BMI. Participant was included as a random effect. For all outcome measures, P ≤ 0.01 was deemed significant to acknowledge multiplicity. CLA, conjugated linoleic acid; FA, fatty acid; iAUC, incremental AUC; TFA, trans fatty acid.
Indicates data were log-transformed prior to analysis.
Total SFAs include: 6:0, 7:0, 8:0, 9:0, 10:0, 11:0, 12:0, 13:0 iso, 13:0 anteiso, 13:0, 14:0 iso, 14:0, 15:0 anteiso, 15:0, 16:0 iso, 16:0, 17:0 iso, 17:0 anteiso, 17:0, 18:0 iso, 18:0, 19:0, 20:0, 22:0, and 24:0.
Total cis-18:1 include: cis-9 18:1, cis-11 18:1, cis-12 18:1, cis-13 18:1, cis-14 18:1, cis-15 18:1, and cis-16 18:1.
Total cis-MUFAs include: cis-9 10:1, cis-10 11:1, cis-9 12:1, 13:1 (unknown bond position), cis-9 14:1, cis-10 15:1, cis-9 16:1, cis-13 16:1, cis-10 17:1, cis-9 17:1, cis-9 18:1, cis-11 18:1, cis-12 18:1, cis-13 18:1, cis-14 18:1 cis-15 18:1, cis-16 18:1, 19:1 (unknown bond position), cis-5 20:1, cis-8 20:1, cis-11 20:1, cis-13 22:1, and cis-15 24:1.
Total trans 18:1 include: trans-4 18:1, trans-6 18:1, trans-7 18:1, trans-8 18:1, trans-9 18:1, trans-10 18:1, trans-11 18:1, trans-12 18:1, trans-15 18:1, and trans-16 18:1.
Total trans-MUFAs include: trans-9 14:1, trans-9 16:1, trans-11 16:1, trans-13 16:1, trans-10 17:1, trans-4 18:1, trans-6 18:1, trans-7 18:1, trans-8 18:1, trans-9 18:1, trans-10 18:1, trans-11 18:1, trans-12 18:1, trans-15 18:1, and trans-16 18:1.
Total TFAs include: trans-18:1, trans-11, 15 18:2, trans-9, 12 18:2, cis-9, trans-13 18:2, cis-10, trans-14 18:2, cis-9, trans-12 18:2, trans-9, 12 18:2, trans-11, cis-15 18:2, and trans-12, cis-15 18:2.
Total CLAs include a peak, which contains mainly cis-9, trans-11 CLA, but also trans-7, cis-9 CLA, trans-8, cis-10 CLA, and trans-6, cis-8 CLA.
Total n–3 PUFAs include: trans-11, 15 18:2, trans-11, cis-15 18:2, trans-12, cis-15 18:2, cis-9, 12, 15, 18:3, cis-11, 14, 17 20:3, cis-5, 8, 11, 14, 17 20:5, cis-7, 10, 13, 16, 19 22:5, and cis-4, 7, 10, 13, 16, 19 22:6.
Total n–6 PUFAs include: trans-9, 12 18:2, cis-9, trans-12 18:2, trans-9, cis-12 18:2, cis-9, 12 18:2, cis-6, 9, 12 18:3, cis-11, 14 20:2, cis-8, 11, 14 20:3, cis-5, 8, 11, 14 20:4, cis-13, 16 20:2, and cis-7, 10, 13, 16 22:4.
FIGURE 2.
Time-course profiles of postprandial plasma total lipid FAs in response to sequential high-fat mixed-meal challenges (breakfast at 0 min and lunch at 330 min) rich in FA-modified or conventional dairy products (control) in adults at moderate cardiovascular risk for total SFAs (A), 14:0 (B), 15:0 (C), 16:0 (D), and 18:0 (E). Values are untransformed and unadjusted means ± SEMs, n = 47–48. The dotted lines represent the timing of the second meal (330 min). Linear mixed-model analysis was used to explore the effects of treatment and time, with an adjustment made in all cases for fixed effects of period, time, treatment, gender, age, and BMI. Participant was included as a random effect. P ≤ 0.01 was deemed significant to acknowledge multiplicity. FA, fatty acid.
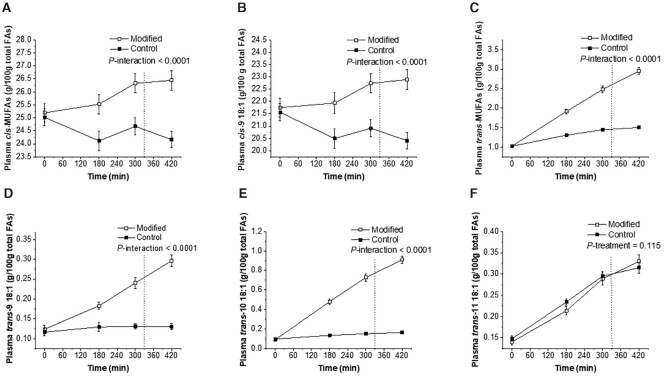
The abundance of total cis-MUFAs (predominantly cis-9 18:1 (oleic acid)), total trans-18:1 (including trans-9 18:1 (elaidic acid) and trans-10 18:1 (octadecenoic acid)), and total trans-MUFAs increased in the plasma total lipid FA pool following the consumption of the FA-modified dairy fat meals compared with control (P < 0.0001 for the AUC, iAUC, and the time-course interaction effect) (Table 5; Figure 3). The increase in the proportion of trans-11 18:1 (vaccenic acid) was similar between control and FA-modified dairy meals (AUC, iAUC, and time-course overall treatment effect: P > 0.05). The abundance of conjugated linoleic acids also increased following consumption of the modified, relative to the control, dairy products (iAUC: P = 0.001; and the time-course overall treatment effect: P = 0.006).
FIGURE 3.
Time-course profiles of postprandial plasma total lipid FAs in response to sequential high-fat mixed-meal challenges (breakfast at 0 min and lunch at 330 min) rich in FA-modified or conventional dairy products (control) in adults at moderate cardiovascular risk for cis-MUFAs (A), cis-9 18:1 (B), trans-MUFAs (C), trans-9 18:1 (D), trans-10 18:1 (E), and trans-11 18:1 (F). Values are untransformed and unadjusted means ± SEMs, n = 49. The dotted lines represent the timing of the second meal (330 min). Linear mixed-model analysis was used to explore the effects of treatment and time, with an adjustment made in all cases for fixed effects of period, time, treatment, gender, age, and BMI. Participant was included as a random effect. P ≤ 0.01 was deemed significant to acknowledge multiplicity. FA, fatty acid.
Discussion
This study highlights that, in adults at moderate CVD risk, acute consumption of high-fat mixed meals rich in modified (SFA-reduced, MUFA-rich) dairy products did not differentially affect postprandial endothelial function or systemic biomarkers of cardiometabolic health, relative to meals that contained control (conventional) dairy products. Postprandial abundance of total lipid FAs in the plasma following sequential FA-modified meal consumption largely reflected the partial replacement of SFAs with MUFAs in our dairy-rich test meals.
Endothelial dysfunction in the postprandial state has been attributed to a progressive detrimental effect on CVD risk (12, 13). Here we demonstrate that acute consumption of dairy meals varying in FA composition did not lead to different postprandial endothelial function responses, although there was a tendency for a beneficial increase in the AUC (but not iAUC) for the %FMD response following consumption of the modified dairy meals. This observation largely agrees with previous acute investigations, which found that consumption of high-fat mixed meals containing specific dietary fats (SFAs, MUFAs, and n–6 PUFAs) did not differentially influence impairments observed in postprandial endothelial function, as assessed by FMD (35–37). In contrast, the parallel LIPGENE postprandial study by Perez-Martinez et al. (38) found that patients with the metabolic syndrome assigned to an MUFA-rich test breakfast (65% total fat: 12% SFAs, 43% MUFAs) had a significantly higher postprandial FMD response compared with those assigned to other test meal conditions, including a fat-matched SFA-rich breakfast (38% SFAs, 21% MUFAs) (38). This study also highlighted that the availability of postprandial NO synthase was greater and plasma sICAM-1 responses were lower following the ingestion of a high-MUFA, relative to the SFA-rich, meal, which contained predominantly butter (38). Rundblad and colleagues (39) demonstrated that a high-fat meal containing butter, but not fermented dairy products (such as cheese), increased postprandial sICAM-1 and sVCAM-1 concentrations. As our modified and control test meals contained a combination of dairy products varying in food matrix and fermentation, this may partly explain why we did not have differential postprandial endothelial activation in response to our high-fat dairy products. However, it is difficult to draw meaningful comparisons between our study and the findings of Perez-Martinez et al. (38), as the postprandial test meal in the latter study was administered at the end of a 12-wk period of dietary FA manipulation. However, another plausible explanation for contrasting endothelial function outcomes could be that our FA-modified and control sequential test meals only had relatively modest difference (∼12 g) in both the content of total SFAs and cis-MUFAs, compared with the LIPGENE postprandial study where test-meal FA modification was not limited specifically to dairy products (38).
Elevated systemic inflammation in the postprandial period is linked to increased risk for atherosclerosis and insulin resistance, with a single high-fat meal known to increase proinflammatory cytokine concentrations, particularly IL-6 (40). Our study findings suggested that the FA composition of the dairy products affected postprandial systemic inflammatory responses to a similar extent, apart from a tendency for a potentially favorable reduction in the whole blood culture LPS-stimulated IL-1β iAUC response following modified dairy meals. This finding may be related to higher MUFA content of these dairy products since a study conducted in insulin-resistant patients found the fasting plasma FA concentration of oleic acid (cis-9 18:1) to be negatively associated with systemic IL-1β concentrations (41). Although not directly comparable, our study agrees with a previous observation highlighting that the dairy content of high-fat meals did not differently affect postprandial IL-6 and TNF-α responses in healthy men (42). Similarly, the FA quality of high-fat meals did not elicit differences in postprandial IL-6 concentrations in metabolic syndrome patients (43) or obese and lean women (44).
We observed no differential effect of our sequential dairy test meal challenge on postprandial lipid, glucose, or insulin concentrations. This finding is largely in agreement with previous acute studies that compared the metabolic effects of sequential high-fat meals rich in SFAs, MUFAs, and n–6 PUFAs among postmenopausal women (35) and single high-fat meals (40 g total fat) containing different proportions of MUFAs (12%, 17%, and 24% of energy) in healthy men (45). In subanalyses, we found a higher TG iAUC response following the modified breakfast meal (0–330 min), relative to the control meal. In line with this, Tholstrup et al. (46) observed higher postprandial plasma and chylomicron TG responses to a high-fat meal containing FA-modified butter at 4 h postprandially in healthy young men, when compared with a conventional Danish butter. However, postprandial responses were assessed after a 4-wk isoenergetic dietary intervention (with 16% of 12:0–16:0 replaced mainly with cis-9 18:1 and 18:0 in the FA-modified butter diet) (46).
Supplementation of the dairy-cow diet with unprotected HOS oil by our group led to a proportion of milk SFAs being replaced by cis-MUFAs, as well as trans-MUFAs through the process of biohydrogenation of unsaturated FAs by rumen bacteria (16). Consequently, our FA-modified, 2-meal challenge each contained a 3-g (2-fold) higher trans-MUFA content compared with the conventional dairy meals. Our bovine supplementation strategy also led to alterations in the trans-18:1 isomer profile, with a shift from the formation of predominantly trans-11 in conventional milk fat toward a greater proportion of trans-10 (and to a lesser extent, trans-9) intermediates in modified milk fat, which was primarily as a result of isomerization of cis-9 18:1 within the rumen (16). The postprandial composition of the plasma total lipid FA pool largely resembled that of the ingested dairy fats (16). We observed a reduction in total SFAs (including 14:0, 15:0, and 16:0) and an increase in the abundance of 18:0, total cis-MUFAs (primarily cis-9 18:1), and total trans-MUFAs (particularly trans-9 and trans-10 18:1 isomers) in the plasma total lipid pool following consumption of the sequential modified dairy, relative to the control, meals. It may be considered that the higher total trans-18:1 content of our FA-modified dairy meals, and abundance of trans-18:1 isomers in the plasma lipid pool, might have counteracted the impairment of endothelial function and inflammation in response to the sequential high-fat meals linked to favorable postprandial changes in the abundance of plasma total SFAs and cis-MUFAs following consumption of the FA-modified dairy meals. Indeed, increased intake of trans-9 18:1 (elaidic acid), one of the major trans isomers produced industrially by the partial hydrogenation of vegetable oils, is adversely linked to cardiovascular health outcomes (47, 48). Cross-sectional data generated from the Nurses’ Health Study illustrated that intake of trans-9 18:1 was positively associated with E-selectin, sVCAM-1, sICAM-1, and soluble TNF-α receptor 2 in women (49). In support of differences in biological mechanisms between trans-18:1 isomers, Iwata et al. (50) reported that a 180-min treatment of human endothelial cells with increasing concentrations (≤0.1 mM) of trans-9 18:1 was associated with increased NF-κB activation and impaired NO production, whereas trans-vaccenic acid (trans-11 18:1) was not related to either of these responses. Taking into consideration that the concentrations of total plasma trans FAs in humans have been shown to be as high as 0.6 mM in the fasted state (51), the FA concentrations used in the aforementioned in vitro study could be deemed physiological (52).
Strengths of the current study include the double-blinded, randomized design and use of a sequential high-fat mixed-meal challenge, which provided a more accurate reflection of Western dietary patterns (32). We studied adults at moderate CVD risk but acknowledge that our findings may not be generalizable across different groups, including young, healthy populations. Our modified foods largely reflected the natural FA profile of milk produced by an HOS-oil bovine feeding strategy (16) but our strategy increased the concentration of trans-18:1 isomers in milk, which were incorporated into the plasma total lipid pool following acute consumption. Further research is warranted to examine the cardiometabolic health effects of FA-reformulated dairy foods that are produced following rumen-protected bovine supplementation strategies, and thus contain a reduced formation of trans-18:1 intermediates.
In conclusion, consumption of sequential high-fat meals rich in FA-modified dairy products led to a lower abundance of total SFAs (including 14:0, 15:0, and 16:0) and higher abundance of 18:0 and total cis- and trans-MUFAs (predominantly trans-18:1 isomers) in the postprandial plasma total lipid pool, when compared with control dairy meals. Meal dairy FA consumption did not elicit significant differences in postprandial endothelial function or systemic cardiometabolic risk markers in our cohort of adults at moderate CVD risk. Previous work has shown that chronic consumption of FA-modified dairy products may have a beneficial impact on the fasting cholesterol profile (17, 53) and endothelial function (17). As the FA composition of the background diet may be of greater health importance than isolated acute differences in FA intake (54), the postprandial effects of long-term FA-modified dairy product consumption need further investigation.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge S Hargreaves for assistance with participant recruitment, the research nurses (R Mihaylova and K Jenkins) for their assistance with cannulation, the technical staff (V Bines, C Humphrey), and research assistants/students (C Brilley, L Bula, Y Chatzidiakou, V Clifton, H Dong, A Garcimartin, C Holland, J Guo, A Kanneganti, P Loughman, E Mertens, J Ryan, L Sellem, T Staff, and H Zhang) for their assistance with data collection, preparation of test meals, data entry, or food diary analysis. We are also grateful to G Kuhnle and V Sagi-Kiss for technical assistance during the NO analysis. The authors’ responsibilities were as follows—KGJ, DIG, and JAL: designed the human study; OM, KGJ, and JAL: designed the test meal protocol; OM, DV, KEK, CCF, ASG, DJH, and DIG: designed and produced the modified dairy products; OM and DV: conducted the research; OM, DV, KEK, and RS: analyzed the data; OM: performed the statistical analysis; ST: provided statistical advice; OM: drafted the manuscript, which was modified by all co-authors; JAL: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
Supported by the UK Medical Research Council (MR/K020218/1). Arla Foods UK and AAKUK supplied in kind the commercially available dairy products and high-oleic sunflower oil, respectively, according to our specification.
Author disclosures: JAL is Deputy Chair of the UK Scientific Advisory Committee for Nutrition (SACN) and was an expert on SACN's Saturated Fats Working Group. All other authors report no conflicts of interest. JAL is a member of the Journal's Editorial Board and played no role in the Journal's evaluation of the manuscript. The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of the manuscript.
Supplemental Table 1 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
OM and DV contributed equally to this study.
Abbreviations used: CVD, cardiovascular disease; FA, fatty acid; FID, flame ionization detector; FMD, flow-mediated dilatation; HOS, high-oleic sunflower oil; iAUC, incremental AUC; IPAQ, International Physical Activity Questionnaire; NEFA, nonesterified fatty acid; RESET, REplacement of SaturatEd fat in dairy on Total cholesterol; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1; TG, triacylglycerol; UHT, ultra-high temperature; %FMD, percentage flow-mediated dilatation.
Contributor Information
Oonagh Markey, Hugh Sinclair Unit of Human Nutrition and Institute for Cardiovascular and Metabolic Research, University of Reading, Reading, United Kingdom.
Dafni Vasilopoulou, Hugh Sinclair Unit of Human Nutrition and Institute for Cardiovascular and Metabolic Research, University of Reading, Reading, United Kingdom.
Kirsty E Kliem, Animal, Dairy, and Food Chain Sciences, University of Reading, Reading, United Kingdom; Institute for Food, Nutrition, and Health, University of Reading, Reading, United Kingdom.
Colette C Fagan, Hugh Sinclair Unit of Human Nutrition and Institute for Cardiovascular and Metabolic Research, University of Reading, Reading, United Kingdom; Institute for Food, Nutrition, and Health, University of Reading, Reading, United Kingdom.
Alistair S Grandison, Hugh Sinclair Unit of Human Nutrition and Institute for Cardiovascular and Metabolic Research, University of Reading, Reading, United Kingdom.
Rachel Sutton, Hugh Sinclair Unit of Human Nutrition and Institute for Cardiovascular and Metabolic Research, University of Reading, Reading, United Kingdom.
David J Humphries, Animal, Dairy, and Food Chain Sciences, University of Reading, Reading, United Kingdom; Institute for Food, Nutrition, and Health, University of Reading, Reading, United Kingdom.
Susan Todd, Department of Mathematics and Statistics, University of Reading, Reading, United Kingdom.
Kim G Jackson, Hugh Sinclair Unit of Human Nutrition and Institute for Cardiovascular and Metabolic Research, University of Reading, Reading, United Kingdom; Institute for Food, Nutrition, and Health, University of Reading, Reading, United Kingdom.
David I Givens, Institute for Food, Nutrition, and Health, University of Reading, Reading, United Kingdom.
Julie A Lovegrove, Hugh Sinclair Unit of Human Nutrition and Institute for Cardiovascular and Metabolic Research, University of Reading, Reading, United Kingdom; Institute for Food, Nutrition, and Health, University of Reading, Reading, United Kingdom.
References
- 1. Scientific Advisory Committee on Nutrition . Saturated fats and health. [Internet]. 2019. [cited 2020 Apr 29]. Available from: https://www.gov.uk/government/publications/saturated-fats-and-health-sacn-report. [Google Scholar]
- 2. World Health Organization . Draft guidelines on saturated fatty acid and trans-fatty acid intake for adults and children. [Internet]. 2018; [cited 2019 Apr 29]. Available from: https://extranet.who.int/dataform/upload/surveys/666752/files/Draft WHO SFA-TFA guidelines_04052018 Public Consultation(1).pdf. [PubMed] [Google Scholar]
- 3. Roberts C, Steer T, Maplethorpe N, Cox L, Meadows S, Nicholson S, Page P, Swan G. National Diet and Nutrition Survey. Results from years 7–8 (combined) of the Rolling Programme (2014/15-2015/16). London: Public Health England; 2018. [Google Scholar]
- 4. Thorning TK, Bertram HC, Bonjour J-P, de Groot L, Dupont D, Feeney E, Ipsen R, Lecerf JM, Mackie A, McKinley MC et al. Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps. Am J Clin Nutr. 2017;105:1033–45. [DOI] [PubMed] [Google Scholar]
- 5. Markey O, Vasilopoulou D, Givens DI, Lovegrove JA. Dairy and cardiovascular health: Friend or foe?. Nutr Bull. 2014;39(2):161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Astrup A, Magkos F, Bier DM, Brenna JT, de Oliveira Otto MC, Hill JO, King JC, Mente A, Ordovas JM, Volek JS et al. Saturated fats and health: a reassessment and proposal for food-based recommendations. J Am Coll Cardiol. 2020;76:844–57. [DOI] [PubMed] [Google Scholar]
- 7. Krauss RM, Kris-Etherton PM. Public health guidelines should recommend reducing saturated fat consumption as much as possible: debate consensus. Am J Clin Nutr. 2020;112:25–6. [DOI] [PubMed] [Google Scholar]
- 8. Nordestgaard BG, Benn M, Schnohr P, Tybjærg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298(3):299–308. [DOI] [PubMed] [Google Scholar]
- 9. Lopez-Miranda J, Williams C, Lairon D. Dietary, physiological, genetic and pathological influences on postprandial lipid metabolism. Br J Nutr. 2007;98(3):458–73. [DOI] [PubMed] [Google Scholar]
- 10. Monfort-Pires M, Delgado-Lista J, Gomez-Delgado F, Lopez-Miranda J, Perez-Martinez P, Ferreira SRG. Impact of the content of fatty acids of oral fat tolerance tests on postprandial triglyceridemia: systematic review and meta-analysis. Nutrients. 2016;8(9):580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hansson P, Holven KB, Øyri LKL, Brekke HK, Biong AS, Gjevestad GO, Raza GS, Herzig K-H, Thoresen M, Ulven SM. Meals with similar fat content from different dairy products induce different postprandial triglyceride responses in healthy adults: a randomized controlled cross-over trial. J Nutr. 2019;149(3):422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Norata GD, Grigore L, Raselli S, Redaelli L, Hamsten A, Maggi F, Eriksson P, Catapano AL. Post-prandial endothelial dysfunction in hypertriglyceridemic subjects: molecular mechanisms and gene expression studies. Atherosclerosis. 2007;193(2):321–7. [DOI] [PubMed] [Google Scholar]
- 13. van Oostrom AJ, Sijmonsma TP, Verseyden C, Jansen EH, de Koning EJ, Rabelink TJ, Castro Cabezas M. Postprandial recruitment of neutrophils may contribute to endothelial dysfunction. J Lipid Res. 2003;44(3):576–83. [DOI] [PubMed] [Google Scholar]
- 14. Vafeiadou K, Weech M, Sharma V, Yaqoob P, Todd S, Williams CM, Jackson KG, Lovegrove JA. A review of the evidence for the effects of total dietary fat, saturated, monounsaturated and n-6 polyunsaturated fatty acids on vascular function, endothelial progenitor cells and microparticles. Br J Nutr. 2012;107(3):303–24. [DOI] [PubMed] [Google Scholar]
- 15. Drouin-Chartier J-P, Côté JA, Labonté M-È, Brassard D, Tessier-Grenier M, Desroches S, Couture P, Lamarche B. Comprehensive review of the impact of dairy foods and dairy fat on cardiometabolic risk. Adv Nutr. 2016;7(6):1041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kliem KE, Humphries DJ, Markey O, Vasilopoulou D, Fagan CC, Grandison AS, Todd S, Givens DI, Lovegrove JA. Food chain approach to lowering saturated fat in milk and dairy products: the RESET study. Int J Dairy Technol. 2019;72:100–9. [Google Scholar]
- 17. Vasilopoulou D, Markey O, Kliem KE, Fagan CC, Grandison AS, Humphries DJ, Todd S, Jackson KG, Givens DI, Lovegrove JA. Reformulation initiative for partial replacement of saturated with unsaturated fats in dairy foods attenuates the increase in LDL cholesterol and improves flow-mediated dilatation compared with conventional dairy: the randomized, controlled REplacement of SaturatEd fat in dairy on Total cholesterol (RESET) study. Am J Clin Nutr. 2020;111(4):739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jackson KG, Poppitt SD, Minihane AM. Postprandial lipemia and cardiovascular disease risk: Interrelationships between dietary, physiological and genetic determinants. Atherosclerosis. 2012;220(1):22–33. [DOI] [PubMed] [Google Scholar]
- 19. Markey O, Vasilopoulou D, Kliem KE, Koulman A, Fagan CC, Summerhill K, Wang LY, Grandison AS, Humphries DJ, Todd S et al. Plasma phospholipid fatty acid profile confirms compliance to a novel saturated fat-reduced, monounsaturated fat-enriched dairy product intervention in adults at moderate cardiovascular risk: a randomized controlled trial. Nutr J. 2017;16:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Markey O, Souroullas K, Fagan CC, Kliem KE, Vasilopoulou D, Jackson KG, Humphries DJ, Grandison AS, Givens DI, Lovegrove JA. Consumer acceptance of dairy products with a saturated fatty acid–reduced, monounsaturated fatty acid–enriched content. J Dairy Sci. 2017;100(10):7953–66. [DOI] [PubMed] [Google Scholar]
- 21. Sriperm N, Pesti GM, Tillman PB. Evaluation of the fixed nitrogen-to-protein (N:P) conversion factor (6.25) versus ingredient specific N:P conversion factors in feedstuffs. J Sci Food Agric. 2011;91(7):1182–6. [DOI] [PubMed] [Google Scholar]
- 22. Merrill AL, Watt BK. Energy value of foods: basis and derivation. In: Agriculture handbook 74. Washington (DC): US Department of Agriculture, Agricultural Research Service; 1973. [Google Scholar]
- 23. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–65. [DOI] [PubMed] [Google Scholar]
- 24. Burdge GC, Tricon S, Morgan R, Kliem KE, Childs C, Jones E, Russell JJ, Grimble RF, Williams CM, Yaqoob P. Incorporation of cis-9, trans-11 conjugated linoleic acid and vaccenic acid (trans-11 18: 1) into plasma and leucocyte lipids in healthy men consuming dairy products naturally enriched in these fatty acids. British Journal of Nutrition. 2005;94(2):237–43. [DOI] [PubMed] [Google Scholar]
- 25. Glaser C, Demmelmair H, Koletzko B. High-throughput analysis of total plasma fatty acid composition with direct in situ transesterification. PLoS One. 2010;5(8):e12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kliem KE, Reynolds CK, Humphries DJ, Kirkland RM, Barratt CES, Livingstone KM, Givens DI. Incremental effect of a calcium salt of cis-monounsaturated fatty acids supplement on milk fatty acid composition in cows fed maize silage-based diets. J Dairy Sci. 2013;96(5):3211–21. [DOI] [PubMed] [Google Scholar]
- 27. Kliem KE, Shingfield KJ, Livingstone KM, Givens DI. Seasonal variation in the fatty acid composition of milk available at retail in the United Kingdom and implications for dietary intake. Food Chem. 2013;141(1):274–81. [DOI] [PubMed] [Google Scholar]
- 28. Ulberth F, Gabernig RG, Schrammel F. Flame-ionization detector response to methyl, ethyl, propyl, and butyl esters of fatty acids. J Am Oil Chem Soc. 1999;76(2):263–6. [Google Scholar]
- 29. De Roos NM, Bots ML, Schouten EG, Katan MB. Within-subject variability of flow-mediated vasodilation of the brachial artery in healthy men and women: implications for experimental studies. Ultrasound Med Biol. 2003;29(3):401–6. [DOI] [PubMed] [Google Scholar]
- 30. Welch RW, Antoine JM, Berta JL, Bub A, de Vries J, Guarner F, Hasselwander O, Hendriks H, Jäkel M, Koletzko BV et al. Guidelines for the design, conduct and reporting of human intervention studies to evaluate the health benefits of foods. Br J Nutr. 2011;106(Suppl 2):S3–15. [DOI] [PubMed] [Google Scholar]
- 31. Carstensen M, Thomsen C, Hermansen K. Incremental area under response curve more accurately describes the triglyceride response to an oral fat load in both healthy and type 2 diabetic subjects. Metabolism. 2003;52(8):1034–7. [DOI] [PubMed] [Google Scholar]
- 32. Jackson KG, Clarke DT, Murray P, Lovegrove JA, O'Malley B, Minihane AM, Williams CM. Introduction to the DISRUPT postprandial database: subjects, studies and methodologies. Genes Nutr. 2010;5(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burdge GC, Jones AE, Frye SM, Goodson L, Wootton SA. Effect of meal sequence on postprandial lipid, glucose and insulin responses in young men. Eur J Clin Nutr. 2003;57(12):1536–44. [DOI] [PubMed] [Google Scholar]
- 34. Jackson KG, Walden CM, Murray P, Smith AM, Lovegrove JA, Minihane AM, Williams CM. A sequential two meal challenge reveals abnormalities in postprandial TAG but not glucose in men with increasing numbers of metabolic syndrome components. Atherosclerosis. 2012;220(1):237–43. [DOI] [PubMed] [Google Scholar]
- 35. Rathnayake K, Weech M, Jackson KG, Lovegrove JA. Meal fatty acids have differential effects on postprandial blood pressure and biomarkers of endothelial function but not vascular reactivity in postmenopausal women in the randomized, controlled DIVAS-2 study. J Nutr. 2018;148:348–57. [DOI] [PubMed] [Google Scholar]
- 36. Raitakari OT, Lai N, Griffiths K, McCredie R, Sullivan D, Celermajer DS. Enhanced peripheral vasodilation in humans after a fatty meal. J Am Coll Cardiol. 2000;36(2):417–22. [DOI] [PubMed] [Google Scholar]
- 37. Nicholls SJ, Lundman P, Harmer JA, Cutri B, Griffiths KA, Rye K-A, Barter PJ, Celermajer DS. Consumption of saturated fat impairs the anti-inflammatory properties of high-density lipoproteins and endothelial function. J Am Coll Cardiol. 2006;48(4):715–20. [DOI] [PubMed] [Google Scholar]
- 38. Perez-Martinez P, Moreno-Conde M, Cruz-Teno C, Ruano J, Fuentes F, Delgado-Lista J, Garcia-Rios A, Marin C, Gomez-Luna MJ, Perez-Jimenez F. Dietary fat differentially influences regulatory endothelial function during the postprandial state in patients with metabolic syndrome: from the LIPGENE study. Atherosclerosis. 2010;209(2):533–8. [DOI] [PubMed] [Google Scholar]
- 39. Rundblad A, Holven KB, Øyri LKL, Hansson P, Ivan IH, Gjevestad GO, Thoresen M, Ulven SM. Intake of fermented dairy products induces a less pro-inflammatory postprandial peripheral blood mononuclear cell gene expression response than non-fermented dairy products: a randomized controlled cross-over trial. Mol Nutr Food Res. 2020;64:e2000319. [DOI] [PubMed] [Google Scholar]
- 40. Emerson SR, Kurti SP, Harms CA, Haub MD, Melgarejo T, Logan C, Rosenkranz SK. Magnitude and timing of the postprandial inflammatory response to a high-fat meal in healthy adults: a systematic review. Adv Nutr. 2017;8(2):213–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bersch-Ferreira ÂC, Sampaio GR, Gehringer MO, Torres EAFDS, Ross-Fernandes MB, da Silva JT, Torreglosa CR, Kovacs C, Alves R, Magnoni CD et al. Association between plasma fatty acids and inflammatory markers in patients with and without insulin resistance and in secondary prevention of cardiovascular disease, a cross-sectional study. Nutr J. 2018;17(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schmid A, Petry N, Walther B, Bütikofer U, Luginbühl W, Gille D, Chollet M, McTernan PG, Gijs MAM, Vionnet N et al. Inflammatory and metabolic responses to high-fat meals with and without dairy products in men. Br J Nutr. 2015;113(12):1853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meneses ME, Camargo A, Perez-Martinez P, Delgado-Lista J, Cruz-Teno C, Jimenez-Gomez Y, Paniagua JA, Gutierrez-Mariscal FM, Tinahones FJ, Vidal-Puig A et al. Postprandial inflammatory response in adipose tissue of patients with metabolic syndrome after the intake of different dietary models. Mol Nutr Food Res. 2011;55(12):1759–70. [DOI] [PubMed] [Google Scholar]
- 44. Manning PJ, Sutherland WHF, McGrath MM, De Jong SA, Walker RJ, Williams MJA. Postprandial cytokine concentrations and meal composition in obese and lean women. Obesity. 2008;16(9):2046–52. [DOI] [PubMed] [Google Scholar]
- 45. Roche HM, Zampelas A, Jackson KG, Williams CM, Gibney MJ. The effect of test meal monounsaturated fatty acid: saturated fatty acid ratio on postprandial lipid metabolism. Br J Nutr. 1998;79(5):419–24. [DOI] [PubMed] [Google Scholar]
- 46. Tholstrup T, Sandström B, Hermansen JE, Hølmer G. Effect of modified dairy fat on postprandial and fasting plasma lipids and lipoproteins in healthy young men. Lipids. 1998;33(1):11–21. [DOI] [PubMed] [Google Scholar]
- 47. Kuhnt K, Baehr M, Rohrer C, Jahreis G. Trans fatty acid isomers and the trans-9/trans-11 index in fat containing foods. Eur J Lipid Sci Technol. 2011;113(10):1281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schünemann H, Beyene J et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. Br Med J. 2015;351::h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lopez-Garcia E, Schulze MB, Meigs JB, Manson JE, Rifai N, Stampfer MJ, Willett WC, Hu FB. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr. 2005;135(3):562–6. [DOI] [PubMed] [Google Scholar]
- 50. Iwata NG, Pham M, Rizzo NO, Cheng AM, Maloney E, Kim F. Trans fatty acids induce vascular inflammation and reduce vascular nitric oxide production in endothelial cells. PLoS One. 2011;6(12):e29600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang Q, Zhang Z, Loustalot F, Vesper H, Caudill SP, Ritchey M, Gillespie C, Merritt R, Hong Y, Bowman BA. Plasma trans-fatty acid concentrations continue to be associated with serum lipid and lipoprotein concentrations among US adults after reductions in trans-fatty acid intake. J Nutr. 2017;147(5):896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oteng A-B, Kersten S. Mechanisms of action of trans fatty acids. Adv Nutr. 2020;11(3):697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Markey O, Kliem KE. Does modifying dairy fat composition by changing the diet of the dairy cow provide health benefits? In: Givens DI, editor. Milk and dairy foods: Academic Press, London; 2020. p. 51–86. [Google Scholar]
- 54. Burdge GC, Powell J, Calder PC. Lack of effect of meal fatty acid composition on postprandial lipid, glucose and insulin responses in men and women aged 50–65 years consuming their habitual diets. Br J Nutr. 2006;96(3):489–500. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.