Abstract
Theories of consciousness and cognition that assume a neural substrate automatically regard phylogenetically basal, nonneural systems as nonconscious and noncognitive. Here, we advance a scale-free characterization of consciousness and cognition that regards basal systems, including synthetic constructs, as not only informative about the structure and function of experience in more complex systems but also as offering distinct advantages for experimental manipulation. Our “minimal physicalist” approach makes no assumptions beyond those of quantum information theory, and hence is applicable from the molecular scale upwards. We show that standard concepts including integrated information, state broadcasting via small-world networks, and hierarchical Bayesian inference emerge naturally in this setting, and that common phenomena including stigmergic memory, perceptual coarse-graining, and attention switching follow directly from the thermodynamic requirements of classical computation. We show that the self-representation that lies at the heart of human autonoetic awareness can be traced as far back as, and serves the same basic functions as, the stress response in bacteria and other basal systems.
Keywords: active inference, aneural systems, basal cognition, classical computation, integrated information, memory, quantum computation, self-representation, state broadcasting
Introduction
The starting point for sciences of consciousness and cognition has traditionally been human consciousness and cognition. The Cartesian presumption against nonhuman consciousness and cognition remained strong enough, even just two decades ago, that prominent researchers found it necessary to argue in print that nonhuman animals and even human infants experienced perception and interoception and engaged in intentional actions (e.g. Panksepp 2005; Trevarthen 2010; Rochat 2012). Those days are thankfully behind us. While researchers in comparative cognition may debate the levels of complexity, compositionality, or stimulus-independence an information-processing system must have to be regarded as “cognitive” (see Bayne et al. 2019 for a recent snapshot), big-brained creatures that learn readily and display flexible behavior in complex environments are now regularly regarded as cognitive systems that are aware of both their environments and their own bodily states, even if they are birds (e.g. Güntürkün and Bugnyar 2016; Nieder et al. 2020) or cephalopods (e.g. Mather 2019; Schnell et al. 2020). To insist that such creatures altogether lack phenomenal consciousness, that they are incapable of experiencing pain and likewise experience nothing whatsoever while hunting, improvising tools, or engaging in mating displays as a doctrinaire Cartesian presumably would, seems by current standards chauvinistic or even perverse.
Characterizing birds and cephalopods, and for that matter other mammals and even other people, as phenomenally conscious begs, of course, the philosophical questions of what phenomenal consciousness is ontologically, and of how it relates to observable structures, functions or behaviors. These questions constitute the “hard problem” (HP) of consciousness (Chalmers 1996; cf. Dietrich and Hardcastle 2005; Dietrich et al. 2021). We agree with Klein and Barron (2020) that the HP is not a problem to be solved, but rather a set of intuitions to be overcome. The questions of interest regarding birds, cephalopods, other mammals, and other people are not, as far as we are concerned, whether or why, in some philosophical sense, they are capable of experience, but rather: (1) what any particular organism or other system of interest is aware of, and (2) how its awareness drives its cognition. Our interest here is not, moreover, in what any particular experience is like for any given system, but in whether it is like something or other; to employ a common example, it is not the particular characters of red or green qualia that are of interest (see e.g. Hacker 2002 for an argument that no such particular characters even exist), but whether some difference between what we, as 3rd party observers, call “red” and “green” is experienced by the system of interest, and whether this difference has some effect on what the system does. Hence we regard consciousness as a (potential) property that a system may have, independently of any details about what it experiences: it is enough, in our view, that it experiences something, that some experience occurs (see Lamme 2018 for a discussion of some of the subtleties that this “binary” view elides). We will use the terms “awareness” and “consciousness” to mean the capacity for or capability of having phenomenal experiences, however basic or minimally structured, and will focus exclusively in what follows on the “what” and “how” questions above.
A well-established literature extends the concepts of consciousness—the capability of having phenomenal experiences, however basic or minimally structured—and cognition to phylogenetically basal systems, including free-living or facultatively communal unicells, whether pro- or eukaryotic (Maturana and Varela 1980; Pattee 1982; Stewart 1996; di Primio et al. 2000; Lyon 2015; Baluška and Levin 2016; Baluška and Reber 2019, 2021; Levin 2019, 2020; Fields and Levin 2020a; Lyon 2020; Baluška et al. 2021), plants (Calvo and Kiejzer 2009; Gagliano and Grimonprez 2015; Baluška et al. 2018; Debono and Souza 2019; Calvo et al. 2020), and aneural or lower (than mammals, birds, or cephalopods) complexity neural metazoa, particularly flatworms (Shomrat and Levin 2013; Inoue et al. 2015; Levin 2020) and insects (Menzel 2012; Perry et al. 2017; Pfeffer and Wolf 2020). Like the extension of these concepts from humans to nonhuman mammals and then to big-brained nonmammals, this extension to more basal organisms was initially motivated by observations of communication, learning, and behavioral flexibility, and by functional similarities between the mechanisms supporting information processing and learning in basal systems and in more complex systems such as mammals. Both molecular and bioelectric mechanisms of cellular information processing, memory, communication, and error correction are, in particular, evolutionarily ancient and conserved across phylogeny (Fields and Levin 2018, 2020b; Fields et al. 2020; Levin 2020). Neither the specialization of some epithelial cells for communication, i.e. as neurons (Nickel 2010; Arendt et al. 2015; Burkhardt and Sprecher 2017) nor the gradual elaboration of brains (Holland et al. 2013; Liebeskind et al. 2017) introduce fundamentally novel molecular or bioelectric mechanisms. Hence while claims of specific kinds of experience, e.g. pain in plants (Draguhn et al. 2020), are controversial, there is no evident biological discontinuity below which organisms can clearly be viewed as completely unaware of their bodily states or their environments, i.e. as utterly lacking phenomenal consciousness. Nor is it obvious that supra-organismal systems utterly lack phenomenal consciousness (Friedman and Søvik 2021). Indeed, the criteria for “having experiences” may be as vague, general, and extensible to non-Terrestrial or even artificial systems as plausible criteria for “life” are (e.g. Bartlett and Wong 2020; but see also Mariscal and Doolittle 2020 for an argument that such “definitionism” is scientifically pointless).
With the development of more sophisticated, biologically motivated theoretical models of consciousness and cognition, the functional similarities between information processing in basal and in more elaborated systems have become even more evident. It is now well-known that signal transduction and gene regulatory networks in single cells share the scale-free, small-world topology (Agrawal 2002; Barabási and Oltvai 2004; Schlitt and Brazma 2007) found ubiquitously in nervous systems (Sporns and Honey 2006; Hagmann et al. 2008; Shanahan 2012; Sporns 2013) and even in social and technological networks (Watts 1999; Barabási 2016; but see Broido and Clauset 2019 for evidence that some biological and social networks are small-world but not strictly scale-free). Networks with high fan-in/high fan-out “bow-tie” topology (Wang et al. 2016; Niss et al. 2020), in particular, implement state-broadcasting functions analogous, at the cellular level, to the broadcasting functions of long-distance connections between network hubs in global neuronal workspace (GNW) models (Dehaene and Naccache 2001; Baars and Franklin 2003; Wallace 2005; Baars et al. 2013; Dehaene et al. 2014; Mashour et al. 2020). Signal transduction and gene regulatory networks are, even when they contain bow-tie nodes, characterized by multiple, typically cross-modulating feedback loops that enable both behavioral plasticity and learning (e.g. Brun-Usan et al. 2020); hence they have positive integrated information Φ and render their minimal containing systems conscious by the criteria of Integrated Information Theory (IIT; Tononi 2008; Oizumi et al. 2014; Tononi and Koch 2015). As the locus of molecular, thermodynamic, and bioelectric exchange with the environment, the cell membrane implements a Markov Blanket (MB) that renders its interior conditionally independent of its exterior (Pearl 1988; Clark 2017); this allows the cell to be described as a Bayesian active inference system (Friston 2010, 2013; see also Cooke 2020 for a variation on this approach). The utility of this Bayesian approach has been demonstrated in simulation models of cell–cell communication driving morphoghenesis (Friston et al. 2015; Kuchling et al. 2020). These cross-scale similarities motivate a hypothesis that consciousness and cognition are scale-free phenomena that characterize all living systems (Levin 2019, 2020; Fields and Levin 2020a).
If consciousness and cognition are scale-free phenomena, we can expect them to be supported by common, scalable mechanisms that can be investigated in whatever systems permit the most straightforward theoretical and experimental approaches. Phylogenetically basal organisms, in vitro preparations, and synthetic constructs (e.g. Kriegman et al. 2020) provide obvious advantages of manipulability and environmental control. Studies of basal systems are, moreover, especially effective in overcoming the intuitions that give rise to the HP, as they allow the mechanisms via which single cells and relatively simple multicellular organisms navigate their environments—mechanisms that they share with most of our cells, and with us as organisms—to be investigated in detail. A theoretical framework suitable for such systems should be scalable, provide a full suite of formal capabilities, and make as few assumptions as possible. It should, in particular, make no scale-dependent architectural assumptions.
Here, we provide a straightforward construction of fundamental, scale-free features of consciousness and cognition within a generic description of system-environment information exchange as bipartite physical interaction (Fields and Glazebrook 2020a; Fields and Marcianò 2020a,b). We term this description “minimal physicalism” (MP) as it makes no assumptions about classical computational architecture, in particular, no assumptions about network architecture (Broido and Clauset 2019), and no physical assumptions beyond those of quantum information theory (Nielsen and Chuang 2000 provide a standard introduction). At the level of molecular interactions at the Angstrom, femtosecond (Å, fs) scale of molecular dynamics calculations (Zwier and Chong 2010; Vlachakis et al. 2014), biological systems are quantum systems, and biological information processing is quantum computation: cellular energy budgets of both prokaryotes and eukaryotes fall orders of magnitude short of the power required to maintain classical states of just protein conformation and localization at this scale (Fields and Levin 2021), despite the massive consumption of ATP by big-brained eukaryotes such as humans (Ueno et al. 2005; Okuno et al. 2011). Hence cellular information processing cannot be entirely, or even primarily classical, the experimental difficulties (Cao et al. 2020) of unambiguously observing quantum coherence in biological systems notwithstanding.
As we show below, the thermodynamic requirements of classical computation by quantum systems have profound consequences for cellular, and hence for organismal, awareness and information processing. In general, basing MP on the physical assumptions of quantum information theory has significant empirical consequences that extend far beyond a mere rejection of dualism. These include:
MP is completely scale-free, applying in the same form to interactions between molecules, cells, tissues, organisms, social groups, or ecosystems and their respective environments. Hence it predicts common mechanisms that can be probed empirically at any scale. It makes no assumptions about the structure or dynamics of the environment, at any scale, beyond its being a physical system.
MP requires every property of either itself or the environment to which an organism or other living system is differentially sensitive to be specified explicitly in terms of the information processing employed by the organism or system to detect and respond to that property. This includes properties often taken for granted, such as the existence of external objects or their embedding in three-dimensional (3d) space, and applies regardless of whether detection and/or response is recorded to memory or “reportable” via any specific assay.
MP treats information and energy as formally equivalent, explicitly enforces conservation of energy, and hence requires the thermodynamics of classical computation (Landauer 1961; Bennett 1982) to be represented explicitly. It requires the thermodynamic cost of memory to be accounted for at every scale, and automatically enforces a metabolism—cognition tradeoff.
Information processing functions, including writing to or reading from a classical memory, are specified in this framework using the scale- and implementation-independent, category-theoretic language of Barwise-Seligman (“token” – “type”) classifiers and their associated infomorphisms (Barwise and Seligman 1997; see Fields and Glazebrook 2019a for review and example applications). This generic specification language has been previously employed to characterize human object and event categorization using both abstraction and mereological hierarchies (Fields and Glazebrook 2019b) and human problem solving within a GNW context (Fields and Glazebrook 2020b). As it requires the reference frames employed to give internal, e.g. perceptual or motor, representations operational meaning to be explicitly specified, this language requires all semantic hypotheses to be formulated in explicit, experimentally accessible molecular or network-theoretic terms.
We outline the main components of MP in the next section, keeping formalism to the minimum and relying on previous work for details. We show that MBs and a notion of actionable or “meaningful” (Bateson 1972; Roederer 2005) information emerge naturally in an MP framework. We then discuss predictions of MP regarding perception, memory, and attention, providing examples primarily from basal systems for illustration. We show that requirements for coarse-graining and attention switching emerge naturally from the thermodynamics of classical computation. We then consider resource-usage monitoring as a requirement of homeo- or allostasis, and the emergence of a “self” as a representation of resource usage.
Components of MP: Information Exchange, Markov Blankets, and Reference Frames
Physical interaction is information exchange
The re-interpretation of physics as an information theory was initiated by Feynman (1982) and Wheeler (1990), but has its roots in the work of Boltzmann (1995), who first recognized that reducing uncertainty has an energetic cost, and Shannon (1948), who linked uncertainty reduction with communication. It is deeply rooted in Wheeler’s (1983) idea of “observer-participancy”: that information can only be obtained by intervention, i.e. by actively posing a question (see Mermin 2018; Müller 2020 for recent, somewhat philosophical discussions). Physics is intrinsically quantum due to the quantization of obtainable information into bits: answers to yes/no questions (Wheeler 1983). The basic formalism of this quantum information theory is surprisingly simple, and rests on only the two assumptions specified below.
The simplest physical interactions are bipartite: some physical, i.e. quantum system A (conventionally called “Alice”) interacts with some other system B (“Bob”). We can therefore write:
| (1) |
or more explicitly, in terms of Hilbert spaces:
| (2) |
where U is the joint system (“universe”) comprising A and B and ⊗ is the Hilbert-space tensor product. The dynamics of the joint system is completely described by a Hamiltonian operator:
| (3) |
where HA and HB are the internal or “self” interactions of A and B respectively and HAB is their mutual interaction. The operator HU has units of energy and drives state change via the Schrödinger equation:
| (4) |
where i = √-1, ℏ = h/2π, h is Planck’s constant, and |U(t)> is the time-dependent state of U. The mutual interaction HAB describes Alice’s observations of and actions on Bob, and Bob’s observations of and actions on Alice. The internal interactions HA and HB describe Alice’s and Bob’s internal state changes.
The above descriptions are completely generic and characterize any bipartite interaction between quantum systems (Nielsen and Chuang 2000). As a quantum system is simply a collection of physical degrees of freedom, this formalism makes, in particular, no assumptions about implementation: it applies equally to matter (e.g. molecules) or fields (e.g. electromagnetic fields as in McFadden 2020). We now impose two assumptions:
We require the dimension of the joint system U to be finite. This limits both A and B to finite energy resources and HAB to finite resolution.
We require the joint state |U> = |AB> to be separable, i.e. |U> = |AB> = |A>|B>. Equivalently, A and B are not entangled. In this case, HAB specifies the entire dependence of A on B and vice-versa, and we can talk about the state |A> of A and the state |B> of B independently.
With these two assumptions, the interaction HAB can be written, without loss of generality, as:
| (5) |
where k = A or B, i = 1 … N for finite N, kB is Boltzmann’s constant, Tk is k’s temperature, βk ≥ ln 2 is an inverse measure of k’s average thermodynamic efficiency that depends on the internal dynamics Hk, the αki(t) are N real functions such that, for any macroscopic time interval Δt:
| (6) |
and the Mki are N Hermitian operators with binary eigenvalues +1 or -1 that can be regarded as “questions to Nature” with yes/no answers [Wheeler 1983; see Fields and Glazebrook 2020a; Fields and Marcianò 2019, 2020b for details on Equations (5) and (6) and their interpretation]. The requirement that βk ≥ ln 2 in Equation (5) enforces Landauer’s principle of the finite per-bit cost of irreversibly acquiring an observational outcome (Landauer 1961; Bennett 1982), while Equation (6) imposes a normalization condition, equivalent to the requirement that the probability of acquiring some outcome whenever a measurement is made is unity. As we discuss below, Equations (5) and (6) are the only physical requirements needed to describe both free-energy exchange and information transfer in a generic bipartite interaction between finite quantum systems in a separable joint state. We can write Equation (5) in English as:
This is the conceptual heart of quantum information theory. It, like Equations (5) and (6), applies at any scale.
Interactions induce MBs
The complete generality of Equation (5) allows us to view any interaction between two finite quantum systems in a separable joint state as classical communication: the exchange of some finite number of finite bit strings during any finite time interval Δt. The interaction can be depicted as in Fig. 1: A and B can be regarded as alternately preparing, and then measuring N independent, mutually noninteracting quantum bits (qubits), e.g. photon polarizations, electron spins, or any other systems having two clearly distinguishable values of a manipulable state variable. In each cycle of interaction, A prepares the N qubits and then B measures them; the roles then reverse, with B preparing and A measuring. As N becomes large, the interaction may appear “continuous” at macroscopic scales, but remains constrained to finite resolution, and hence a finite bit rate, by the restriction to finite thermodynamic resources built into Equation (5). The time required for A to prepare, and then B to measure (or vice versa), the N systems can be regarded as the smallest possible “macroscopic” and hence effectively classical time interval (see Fields and Glazebrook 2020 for an explicit construction of this interval). The collection of all N qubits encodes, at each such minimal macroscopic time, one N-bit string specifying one of the 2N eigenvalues of HAB; each cycle of interaction is thus an exchange of N-bit strings, each one encoding an (in general different) eigenvalue.
Figure 1.
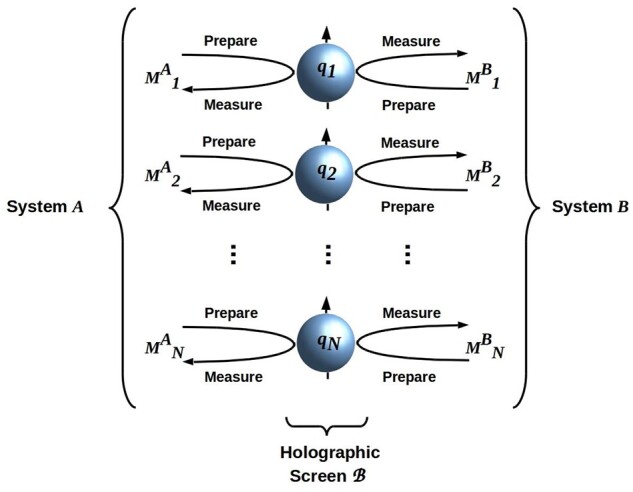
The interaction HAB specified by Equation (5) can, without loss of generality, be implemented by alternating preparation and measurement of N mutually noninteracting qubits. Each cycle of interaction has two phases: first, A prepares the N qubits and then B measures them, then B prepares the N qubits and A measures them. The qubit array defines a holographic screen separating A from B. This screen enforces conditional independence between A and B, and hence functions as a MB. There is no source of classical noise in the interaction; however, there is quantum noise, and hence potential classical communication error, whenever A and B employ different reference frames (e.g. different z-axes) to prepare and measure the qubits. Adapted from Fields and Marcianò (2020b); CC BY license.
The encoding of eigenvalues by qubits shown in Fig. 1 satisfies the requirements for a holographic encoding: the only information about B available to A, or vice versa, is the sequence of eigenvalues of HAB encoded by the qubit array. Hence we can consider the qubit array as a holographic screen separating A from B. Any such holographic screen defines a classical communication channel, i.e. a channel via which A and B exchange finite bit strings (Fields and Marcianò 2020b; Fields et al. 2021). As no other system interferes with this channel, it is free of classical noise. However, it is characterized by quantum noise whenever A and B employ different reference frames (e.g. different z-axis reference frames in the case of qubits implemented by electron spins) to prepare and measure the qubits. The effect of such quantum noise is to impose a classical probability distribution Prob(measured|prepared) on each qubit; i.e. its observable effect is indistinguishable from classical noise.
If we now consider the internal (quantum) states |A> and |> and the states |> of the qubit array forming the holographic screen between them, we see that |A> affects |B> only via its effect on |>, i.e. only via the action of HAB. This is, as noted above, a consequence of separability: separability implies conditional independence. Conditional independence of |A> from |B> and vice-versa is, however, also the MB condition (Pearl 1988; Friston 2013; Clark 2017); hence we can view as an MB. Indeed, the generalization of the holographic principle stated by Equation (5) and illustrated in Fig. 1 implies that any holographic screen defines a classical information channel that functions as an MB, and vice versa (Fields and Marcianò 2020a; Fields et al. 2021).
As discussed in Friston (2013), MBs are ubiquitous features of living systems, and are indeed what distinguish living systems from their environments (see also Friston et al. 2015; Kuchling et al. 2020; Fields and Levin 2020c). Indeed MBs are ubiquitous features of all physical systems with ergodic behavior (Friston 2019). The MB concept is scale-free; Rubin et al. (2020) show, e.g. that it applies to Earth’s biosphere as a whole. The cell membrane, and in eukaryotic cells, organelle membranes, implement the most fundamental biological MBs. The blanket states of these MBs comprise the states of the embedded receptors, channels, and other exchange molecules, together with the permeability, elasticity, and other properties of the membrane itself. The existence of the MB enables the cell to have a well-defined state, conditionally independent of its environment; hence it enables homeostatic/allostatic processes. The information about the environment available to the cell, and vice-versa, is limited to that encoded by the state of the MB. A similarly strong conditional independence result has been obtained via evolutionary game theory by considering generic systems subjected to competition for environmental resources (Hoffman et al. 2015; Prakash et al. 2020a,b).
The central importance to the cell of the MB implemented by its membrane strongly suggests that enclosure by a membrane was the fundamental requirement for the origin of life (Friston 2013; Fields and Levin 2020c). What was enclosed by the MB/membrane of the last universal common ancestor (LUCA) represents the initial condition and oldest memory (Fields and Levin 2018) of all subsequent life. Viewed from an informational perspective, the state space of LUCA’s membrane-enclosed cytoplasm, including its nucleic-acid, protein, and small-molecule components, specifies the initial information about the environment available prior to membrane enclosure and hence physical separation from the environment. While the cytoplasmic conditions of later cells have diverged from these initial conditions, sometimes radically, they remain a fixed constraint with which all paths of divergence must be consistent. Here, the classical meaning of “separation” from the environment as the presence of a physical barrier accords with its quantum-theoretic meaning of state distinguishability. We can, indeed, see the former as implied by the latter.
Actionable information is encoded by quantum reference frames
The information that transits the cell membrane, and is thereby encoded on the MB implemented by the membrane, is actionable or meaningful to the cell: it “makes a difference” to what the cell does (Bateson 1972; Roederer 2005). When the cell’s interaction with its environment is represented as measurement as in Equation (5) or Fig. 1, what renders the information meaningful becomes clear: meaning requires measurement with respect to some reference frame (Fields and Levin 2020a; Fields et al. 2021). Viewed abstractly, a reference frame is a value, or more generally a vector, from which deviation is detectable. Consider, e.g. measuring the states of an array of qubits implemented by electron spins. Determining whether the spin of a qubit is up (+1) or down (–1) requires a reference frame that defines a particular z-axis, a vector in 3d space. Such a vector can be defined by, e.g. a Stern-Gerlach apparatus oriented in some particular way relative to the Earth’s gravitational field. Only the presence of a common reference frame allows multiple measurements to be compared, and hence makes any differences between them meaningful. Sequential measurements of electron spin with respect to a randomly varying z-axis merely yield noise.
While in classical physics a reference frame is an abstraction that can be completely described to arbitrary precision, this is not true in quantum theory (Bartlett et al. 2007). Quantum theory requires all reference frames to be implemented by physical systems, but forbids the description of any physical system to arbitrary precision. Hence quantum reference frames (QRFs) cannot be completely described to arbitrary precision; they encode, in virtue of their physical implementation, “unspeakable” (Bell 1987) or “nonfungible” (Bartlett et al. 2007) information. This is even true of meter sticks, the lengths of which become uncertain as the energies required to probe them become comparable to the binding energies of nucleii. It is much more obviously true of complex systems such as Stern-Gerlach apparatus or atomic clocks.
The intrinsically nonclassical nature of QRFs has some surprising consequences. Transferring a QRF from one observer to another requires transferring the physical implementation; transferring a finite bit string is provably insufficient (see Bartlett et al. 2007 for proof and examples). Any such transfer requires, moreover, that sender and receiver already share a QRF sufficient to identify the transferred system (Fields and Marcianò 2019). We can, therefore, without loss of generality regard all QRFs available to any observer, including any organism, as physically implemented by the dynamical structure of that system, i.e. by its internal Hamiltonian. All QRFs are, therefore, quantum informational processes, i.e. quantum computations, implemented by physical systems that act as observers. The computations that implement QRFs are, as we will see below, key to understanding both what organisms are aware of and how their awareness drives their cognition. They are, therefore, the central components of any scientific theory of consciousness and cognition that is mathematically consistent with quantum information theory.
The Che-Y system employed by bacterial chemotaxis, e.g. in Escherichia coli, provides a simple example of a biological QRF. If the concentration [Che-Y-P] of phosphorylated Che-Y is high, the flagellar motor spins counter-clockwise and the bacterium swims in a straight line; if [Che-Y-P] is low, the motor spins clockwise and the bacterium tumbles (Wadhams and Armitage 2004; Micali and Endres 2016). “High” and “low” concentrations of phosphorylated Che-Y are defined with respect to some default concentration ratio [Che-Y-P]/[Che-Y] of phosphorylated to unphosphorylated forms, which in turn depends on the balance of relevant kinases and phosphorylases. The default ratio [Che-Y-P]/[Che-Y] is the QRF for the Che-Y system; it fixes a particular value at which behavior switches from straight-line swimming to tumbling. Whether the measured [Che-Y-P]/[Che-Y] at any given time is above or below this fixed value effectively determines whether a stimulus is approached, i.e. is considered “good” by E. coli or avoided, i.e. considered “bad.”
In any electrically excitable cell, the resting membrane potential V0mem is a QRF for polarization: values of Vmem > V0mem are depolarized, while values of Vmem < V0mem are hyperpolarized. The setting of this reference frame is critical to cell behavior in both neurons and nonneural cells. In the planarian Dugesia japonica, altering V0mem of blastomere cells by blocking cell communication via gap junctions during regeneration can effect a homeotic transformation of the posterior from tail morphology to head morphology, including a complete functional brain (Oviedo et al. 2010; Durant et al. 2017). In both neurons and electrically excitable plant cells, blocking gap junctions via anesthetics induces cell quiescence (Baluška et al. 2016; Gremiaux et al. 2014; see also Kelz and Mashour 2019 for a general discussion of anesthetic effects across phylogeny).
The quantum theory of complex, macroscopic systems is generally intractable; hence investigating the general properties of biological QRFs requires an abstract, scale-free specification language. The category-theoretic formalism of Channel Theory, developed by Barwise and Seligman (1997) to describe networks of communicating information processors, provides a suitably general language for specifying the functions of QRFs without any specific assumptions about their implementation. The information processing elements in this representation are logical constraints termed “classifiers” that can be thought of as quantum logic gates; they are connected by “infomorphisms” that preserve the imposed constraints (numerous application examples, mainly in computer science, are reviewed in Fields and Glazebrook 2019a). Combinations of these elements are able to implement “models” in the sense of good-regulator theory (Conant and Ashby 1970). Suitable networks of classifiers and their connecting infomorphisms, e.g. provide a generalized representation of artificial neural networks (ANNs) and support standard learning algorithms such as back-propagation. Networks satisfying the commutativity requirements that define “cones” and “cocones” (“limits” and “colimits,” respectively, when these are defined) in category theory (Goguen 1991) provide a natural representation of both abstraction and mereological hierarchies (Fields and Glazebrook 2019b) and of expectation-driven, hierarchical problem solving, e.g. hierarchical Bayesian inference and active inference (Fields and Glazebrook 2020b,c). Commutativity within a cone—cocone structure, in particular, enforces Bayesian coherence on inferences made by the structure; failures of commutativity indicate “quantum” context switches (Fields and Glazebrook 2020c).
Predictions of MP: Awareness, Memory, and Attention
Awareness of X requires a QRF for X
Theories of consciousness typically assume that the agent of interest is embedded in an environment that has observer-independent features and contains observer-independent objects. The visual system, e.g., is often described as using “inverse optics” to compute an “observer-independent” 3d layout from a 2d image (e.g. Pizlo 2001). Assuming observer-independent, ontological “givens” both restricts the agent of interest to some subset of our (typically assumed a priori) ontology and risks under-predicting the agent’s computational requirements. It makes von Uexküll’s (1957) question of defining the organism’s umwelt, and hence Nagel’s (1974) “what is it like?” question harder, and makes the Cartesian response that most organisms are aware of nothing, and hence have no umwelt easier.
Quantum theory itself forces MP to reject environmental “givens” on purely formal grounds. The Hilbert space B in Equation (2) and internal Hamiltonian HB in Equation (3) can be decomposed in any arbitrary way without affecting the interaction HAB at all (Fields 2012, 2016); hence HAB can communicate no information to A about any such decompositions. This is the case for any bipartite interaction, and hence any interaction described by Equation (5), regardless of the physical makeup of the interacting components. It is the conditional independence between the inside and outside of an MB expressed in more fundamental physical terms.
By rejecting any a priori ontology for the environment, MP requires any perceived ontology to be fully supported by the information-processing capabilities of the perceiver. In particular, any perceived feature of, or perceived object embedded in, the environment B of any agent of interest A must be rendered both detectable and meaningful by a QRF implemented by A. This is illustrated in cartoon form in Fig. 2: detecting an environmental feature (a box) with a property (a color) requires a QRF for “box-ness” and a specific detector for the color. The box-detecting QRF encodes those properties (reference state observables) that boxes must have to qualify as boxes; hence it picks out boxes, and only boxes, against the general background of B. Informally, the QRF can be thought of as an attractor in an “interpretation space” associated with the system implementing the QRF. The property detector determines the value of some “pointer” observable that characterizes all systems that qualify as boxes; here, the box’s color. In quantum-theoretic language, identifying a bounded system (feature or object) having some specific property “decoheres” B relative to A (see Fields and Glazebrook 2020a; Fields and Marcianò 2020b for formal analysis). It is important to emphasize that A’s perceptual and cognitive capabilities have no effect on the physical state |B>, and in particular do not render it separable. The features or objects that A detects in B are strictly relative to A, not “objective” in any observer-independent sense (again see Mermin 2018; Müller 2020 for discussion from a purely quantum-theoretic perspective).
Figure 2.
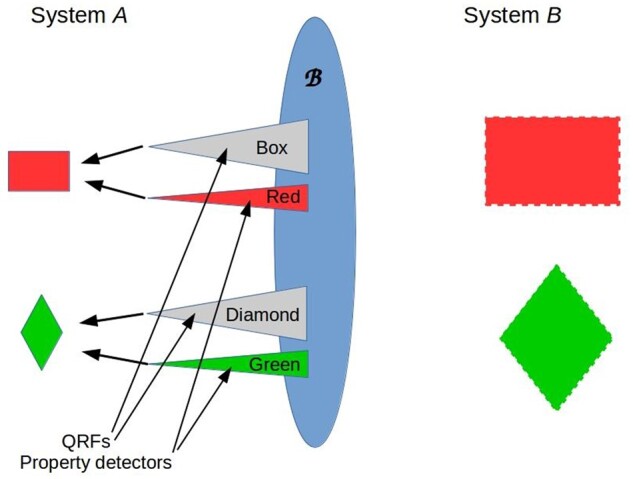
Simplified cartoon of feature or object perception in MP. The depicted relationship between A and B is topological: they are separated by the boundary . There is no implied geometry, and the interaction is bipartite: there is no third system “outside” U = AB with which A or B interact. Features or objects “embedded” in the environment B are perceptible only by systems A equipped with QRFs and property detectors that render the features/objects both detectable and meaningful, and are defined only relative to such systems; this lack of observer-independent ontology is indicated here by dashed boundaries. Triangles within A suggest the form of classifier cocones when drawn as diagrams (Fields and Glazebrook 2019a,b; 2020a,b,c); arrows indicate binding operations. The analogy with mammalian visual feature detection is obvious; see Fields and Glazebrook (2019b) for a detailed formal construction.
Specifying “objects of awareness” entirely in terms of QRFs, with no claims of observer-independent ontology, renders typical formulations of the “combination problem” ill-posed. Combination is well-defined for QRFs: two QRFs implemented by a single system can be combined if, but only if, all of the operators (i.e. the Barwise-Seligman infomorphisms) involved mutually commute (Fields and Glazebrook 2020c). This is the “combination” implemented by standard binding processes, e.g. feature or feature-motion binding during object categorization (Fields and Glazebrook 2019b). “Combination” of QRFs implemented by different systems is, on the hand, strictly undefined: the measurement operators implemented by different systems act on different environments, each of which by definition contains (at least partially) the other system. Hence what Chalmers (2017) calls the “quality” and “structure” combination problems cannot be coherently posed in MP. What Chalmers calls the “subject” combination problem is solved, for living systems, by their environments; systems that cannot maintain homeostasis/allostasis do not survive, and are unlikely to assemble at all. Systems that do survive are described by MP if they satisfy Equations (5) and (6), but they may implement only trivial QRFs. Hence we agree with the deflationary position advocated by Montero (2017), though for more technical reasons.
This universal requirement that awareness be supported by QRFs yields somewhat counter-intuitive predictions of minimality in basal organisms:
Prediction 1: Moving in 3d space does not require a QRF for 3d space, and hence does not require experiencing 3d space. E. coli chemotaxis provides an example. E. coli has a 1d spatial QRF: its body axis, with (mainly) anterior chemoreceptors and (mainly) posterior flagella. Directed “approach” motion is along this axis; undirected “tumbling” re-orients this axis randomly in the 3d “lab” frame of an observer equipped with a 3d QRF. E. coli has no known means of computing the relative angle between its pre- and post-tumbling linear motion, i.e. it has no known 3d QRF; hence tumbling appears to implement a 3d random walk (Wadhams and Armitage 2004). Colonial microbes living in planar mats, on the other hand, can potentially use differential cell–cell or cell–substrate interactions to distinguish left from right (Jauffred et al. 2017) and hence establish a 2d QRF; microbes inhabiting multi-species 3d mats with vertical division of labor may have 3d QRFs (Prieto-Barajas et al. 2018). Differentiated cells of multicellular eukaryotes clearly employ such QRFs (e.g. Bajpai et al. 2021); interestingly, multi-axis morphology correlates with the presence of neurons and appears to be directly instructed by neural signaling (Fields et al. 2020). How the representation of 3d space in migrating cells is coupled to the representations of cell- or extra-cellular surface characteristics, bioelectric and morphogen gradients, and other morphogenetic signals remains a central question in developmental biology (Grossberg 1978; Pezzulo and Levin 2015).
Prediction 2: Successful interaction with an object does not require a QRF that identifies that object, and hence does not require experiencing that object. E. coli mating provides an example: the mating pilus extends randomly in 3d space, and is tipped by an adhesin of unknown specificity (Cabezón et al. 2015). Commonplace lateral gene transfer (LGT) between members of distant microbial lineages (Robbins et al. 2016) suggests that mating without mate detection is routine in the microbial world; viral-mediated gene transfer and direct uptake of nucleic acids from the environment provide even more extreme examples. Communal microbes such as Myxococcus xanthus that differentiate kin from nonkin even below the species level, however, appear to have sophisticated, though yet uncharacterized, QRFs for other organisms (Muñoz-Dorado et al. 2016; Thiery and Kaimer 2020). Fungi that engage in differential anastomosis appear similarly equipped (de la Providencia et al. 2005). Cell-type identification QRFs appear to be implemented in part bioelectrically in multi-species microbial mats (Humphries et al. 2017; Yang et al. 2020); the ubiquitous use of bioelectric signaling in fungi suggests that this may be the case for fungal cell-type QRFs as well.
Prediction 3: Successful causation does not require a mechanism for detecting causation, and hence does not require experiencing causation. Hunting swarms of M. xanthus kill and eat other microbes, but appear to have separate, noncommunicating detection systems for prey species and edible prey components (Thiery and Kaimer 2020). Hence they have no way of causally associating the killing of prey with the subsequent availability of edible prey components.
These can be summarized by the following, which recognizes the key role of memory in enabling the experiences of space, objects, and causation:
Prediction 4: Having a memory does not require a QRF for linear time, and hence does not require experiencing time or retrievable memory. All organisms have genomes that record their phylogenetic history, but they have no mechanism for reading their previous genomic states. The genome does not, therefore, function as an internal QRF for linear time. This applies, in particular, to us, although we can employ the genomes of other organisms as external linear time QRFs (Kumar 2005).
If this last prediction is correct, microbes and perhaps other organisms lacking linear time QRFs may live in a “continuous present” characterized mainly by chemoreception (“taste”) and stress (see below). Their sensory capabilities may change radically via LGT or other mechanisms, but they cannot “notice” such changes.
Biological memories exist at multiple scales (Fields and Levin 2018). Characterizing the scales at which memories are encoded, and the QRFs that enable them, emerges as a central task for any theory of consciousness that assumes MP. Bioenergetic studies may provide a content-independent means of approaching these questions, as discussed below.
Experienced memories are encoded on boundaries
Actions by an organism on its environment are, in the language of Fig. 1, preparations of an MB state |> that its environment observes. A swimming bacterium, e.g. prepares a new environmental state by expending energy to change its location. In Friston’s (2013) terms, this is active inference: acting on the environment to change its state alters, in consequence, the subsequent environmental states observed by the active system.
Actions are, by definition, thermodynamically irreversible: they transfer classical information, via , from the organism to its environment. Transfers of classical information across boundaries such as are, moreover, the only thermodynamically irreversible events in MP. As with the requirement for QRFs to enable awareness, this is a consequence of MP’s quantum theoretic foundation. The state |A> is not, in general, assumed to be separable; hence its Schrödinger evolution is time-reversible and incurs no energetic cost (Bennett 1982). Information can only be retrievable at some later time if it is irreversibly and hence classically encoded. Hence retrievable memories can, in MP, only be encoded on boundaries such as . As experiencing a memory as such requires an ability to compare two experiences—either two memories or a memory and a current state—it requires retrieval. Any experienced memory must, therefore, be both encoded on, and retrievable via an appropriate QRF from .
This fundamental thermodynamic constraint has immediate consequences for biological architecture and for the structure of QRFs for linear time:
Prediction 5: All retrievable biological memories are stigmergic. Beginning with bacteria (Gloag et al. 2015), biological systems ubiquitously employ stigmergic memories (Heylighen 2016). This is not a surprising observation to be explained, but rather an empirical confirmation in MP. The idea that the experiencing mind “extends” (Clark 1998) into the environment via stigmergic memory is a direct consequence of quantum theory.
The stigmergic nature of memory can be reconciled with the experience of memory as an internal, private phenomenon only if the agent A is compartmentalized by internalizing part of to provide an internal boundary on which classical information can be encoded. This internal boundary imposes a separability condition |A> = |A1>|A2> as shown in Fig. 3; A1 becomes part of the “environment” of A2 and vice-versa. The interaction between A1 and A2 can be written in the form of Equation (5); hence information flow across is bidirectional classical communication between the components A1 and A2. If we view A2 as implementing perception and A1 as implementing action, this communication, together with the environment’s response, forms a closed loop. Hence we have:
Figure 3.
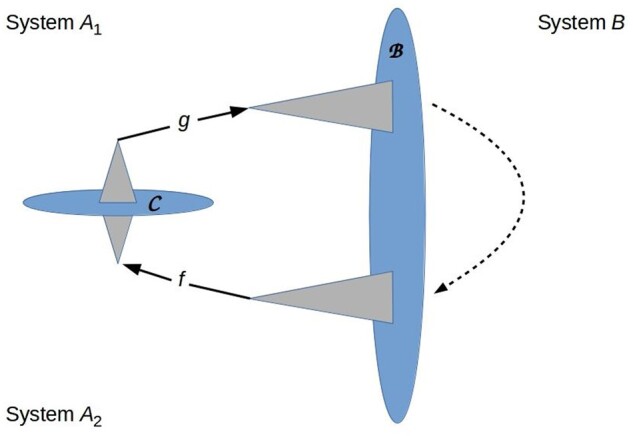
Cartoon representation of a system A with an internal boundary and hence a separable state |A> = |A1>|A2>. Again the relationships depicted are topological, not geometric. Triangles represent QRFs; f and g are internal informational states. Information flow across is bidirectional by Equation (5); information also flows through the environment (dashed arrow). The communication loop is closed, generating positive Φ (Oizumi et al. 2014).
Prediction 6: Internal awareness requires internal boundaries. Any system A capable of experiencing internal memories, in particular, has a separable internal state and positive integrated information Φ. Internal memories are built into all systems that qualify as conscious in IIT (Oizumi et al. 2014, see especially Fig. 19) and are the basis for such systems having Φ > 0. Here, we see this as a consequence of quantum information theory.
As discussed above in connection with MBs, this prediction links separability in its quantum-theoretic sense of state distinguishability with separability in its classical sense of separation by a boundary: the internal boundary functions as an MB that encodes classical information and imposes conditional independence. It provides for a general expectation that living systems will be compartmentalized by internal boundaries on which classical information can be encoded. Because MBs limit, as well as enabling, information transfer, it also predicts a systematic inability to determine the source of a memory. In particular:
Prediction 7: Compartmentalized systems cannot determine the sources of their encoded memories. Systems can be expected to behave as if memories they encode reflect their own past experience, whether they do or not. Studies of memory transplantation (Pietsch and Schneider 1969) and false-memory induction (Ramirez et al. 2013) in nonhuman animals provide mechanistic support for this prediction (see Levin 2020 for additional examples and discussion), as does the psychology (Henkel and Carbuto 2008) and neuroscience (Straube 2012) of false-memory induction in humans.
Organisms record memories as messages to their future selves. Neither the mechanism that recorded a memory, the internal or environmental events that induced recording, nor the context in which the recording occurred are discoverable, however, when the memory is later retrieved. This fundamental uncertainty about the sources of memories can be seen as a consequence of a more general uncertainty about whether QRFs are shared, either by distinct observers at a single time, or by a single observer across time. This question of QRF sharing is provably finite Turing undecidable (Fields et al. 2021).
The compartmentalization in Fig. 3 can be arbitrarily generalized:
Prediction 8: Experiential complexity scales with internal compartmentalization. Evolution “discovered” the benefits of internal compartmentalization ca. 3.5 billion years ago with the development of microbial biofilms exhibiting differential exposure to the open environment and division of metabolic labor (Stal 2012). The organelles of eukaryotic cells, including internal membrane complexes such as the Golgi apparatus, are canonical intracellular compartments. Multicellularity is the most common form of macroscopic compartmentalization, but is not required; examples such as Acetabularia (Schweiger 1969), glass sponges (Leys 2016), and syncitial fungi (Roper et al. 2015) all illustrate complex functional compartmentalization within single giant cells.
This prediction is clearly in line with the expectations of IIT, and again provides a physical basis for these expectations.
Cellular compartments and intercellular boundaries are, from a biological perspective, dynamic structures that must be preserved through metabolic activity. This requirement can be stated abstractly:
Prediction 9: Memory stability scales with the frequency of read/write cycles. The stabilization of classical bit values by repeated cycles of preparation followed by observation is called the “quantum Zeno effect” (Misra and Sudarshan 1977); the probability that the state remains stable is proportional to the frequency of observations. Hence memory decay—“forgetting”—is predicted whenever memories are not routinely accessed. This is broadly observed across phylogeny. Repair systems for nucleic acids and degradation systems for proteins provide molecular-scale examples.
Rewriting classical information costs free energy, i.e. requires metabolism as discussed below. This requirement for read/write cycles suggests that compartmentalization plays a key role in the implementation of linear time QRFs:
Prediction 10: Any ordered sequence of separate memories together with a comparison function can serve as a linear time QRF. Trajectories, including looming, are the simplest linear time QRFs; in the limit, they may support only sequential comparisons and hence only distinguish “then” from “now.” Insects are capable of at least short-sequence linear time perception; spiders are capable of longer sequence perception (Japyassú and Laland 2017). Merely executing a fixed action pattern does not require experiencing linear time, although it clearly requires an internal clock. Molecular cell-cycle clocks are as old as life, and circadian clocks are as old as cyanobacteria (Johnson 2004); both are highly conserved across phylogeny (Doherty and Kay 2010). Possessing a molecular clock does not, by itself, enable time perception.
Prediction 11: Time and object/feature identity are duals. Perceiving a trajectory requires perceiving an object or feature executing that trajectory; conversely, motion perception is the basis of object identity (Fields 2011). Whether insects or relatively low-complexity vertebrates are aware of objects as such or only features of their environments is unclear; bees at least have robust spatial memory and feature perception, and may recognize objects as such (Chittka 2017), while fish appear to recognize conspecifics as distinct objects (Sovrano et al. 2018). Spiders are capable of robust object perception and object-directed planning (Japyassú and Laland 2017), as are cephalopods (Mather 2019), birds and mammals.
Environmental objects and features that are experienced as having stable identities are, to close the circle, the canonical bearers of stigmergic memories. Without perceptible object identity, time is not perceptible and hence memories cannot be recognized as such. Only an organism capable of experiencing linear time and objects or features with stable identities can experience memories. Such an organism inhabits a rich and meaningful umwelt, whether its ontology easily relates to our own or not.
The free-energy costs of irreversibility induce coarse-graining and attention-switching
With the understanding of QRFs developed above, we can return to the question of bioenergetics, see why living systems can have only limited awareness, cognitive capacity, and memory, and understand the tradeoffs between these.
The dynamics described by Equation (5) explicitly conserves energy: βATA = βBTB. Hence some fraction of the bits transferred from B to A must be “burned” by A to supply the free energy required to irreversibly encode classical information (Fields and Glazebrook 2020a). These free-energy supplying bits are not available as input to any QRF (Fields et al. 2021). As no QRF can read the values encoded by these bits, their values are irrelevant and indeed “invisible” to A. They can, therefore, be considered random for A, i.e. to constitute environmental “noise” or in thermodynamic terms, a heat bath. As noted above, however, the source of this noise is quantum, not classical; there is no third system injecting noise into the bipartite A-B interaction (see Fields et al. 2021, for further discussion).
A central question of biology is: how can organisms maintain their phenomenal complexity with such small free energy budgets? The answer is alluded to above: quantum computational complexity, implemented by unitary Schrödinger evolution, is energetically free (Bennett 1982). Classical, i.e. observable or experienceable computational complexity is expensive, ln2 kBT per bit. Hence we have:
Prediction 12: Organisms only require the energy needed to maintain their classical states. As seen above, these are encoded on either exterior or intercompartmental boundaries. An organism’s energy budget must, therefore, supply the free energy needed to maintain, via Zeno-effect read/write cycles, the classical states of their compartment boundaries. Nonboundary states can remain quantum, evolve unitarily, and consume no free energy. Only the inputs and outputs of these quantum computations are classically encoded, all on boundaries.
Clearly not all boundary-localized processes are classical; electron-transport processes operating in the THz range could consume a cell’s entire energy budget if fully classical. The technical difficulty of observing nonclassical behavior in such systems (e.g. Cao et al. 2020) is not surprising. All of our observational outcomes are classical by definition; observing quantum coherence requires observing expectation violations in probability distributions over recorded classical events (e.g. Mermin 1993). This suggests that an indirect approach to quantitating nonclassicality in biological systems is required. From the above considerations, stable memories provide a quantitative lower limit on classicality, while the cellular energy budget provides an upper limit. Determining what states a cell or organism commits free energy to maintain, i.e. what the set points for homeostasis/allostasis are, may be the best approach to estimating the net classicality of biologically encoded information.
Biological encodings of classical information have lower limits of 1–2 nm in radius, e.g. the size of a typical protein active site (Liang et al. 1998) or a gap-junction channel (Sosinsky and Nicholson 2005), and about 200 fs in time, e.g. the response time of rhodopsin to photons (Wang et al. 1994). Cellular response times, even for bioelectric responses, are orders of magnitude larger and involve much larger areas. This loss in resolution is a consequence of bioenergetics:
Prediction 13: Actionable classical encodings are coarse-grained. Actionability requires irreversible encoding as in Fig. 3. The free energy to support this encoding must come from , and hence must consume some of the bits encoded on as fuel. The information encoded by these bits is irreversibly lost to A; hence all of A’s irreversible encodings are coarse-grained.
Any system A that encodes information irreversibly is, therefore, faced with a choice that its computational architecture must resolve: the tradeoff between preserving information via memory and losing information due to coarse-graining. A flexible solution to this tradeoff is to devote memory resources to only some input information, i.e. to the results computed by only some QRFs, allowing these selected results to be recorded at higher resolution while recording others either at low resolution or not at all. Hence we have:
Prediction 14: Living systems in complex, dynamic environments will evolve attention-switching systems. Attention has long been associated with consciousness (Engel and Singer 2001) and attention allocation is one of the main functions of competition for access to the workspace in GNW models (Dehaene and Naccache 2001), whether formulated in terms of the “rich club” (Sporns 2013), “connective core” (Shanahan 2012) or “giant component” (Wallace 2005) of the larger network. Indeed the “self” has been described as a working model of attention allocation (Graziano and Webb 2014; see also below). In humans, attention can drive entirely illusory object perception (Ongchoco and Scholl 2019), consistent with active-inference models of Bayesian-precision allocation (Kanai et al. 2015). Here, we see a requirement for active attention as a consequence of the thermodynamic requirements of classically encoding information.
The approach/avoid switching in simple chemotactic systems such as E. coli provides a basal model of the switch between active exploration and expectation revision at the heart of active inference theory (Friston 2010, 2013). Such models suggest that every such switch is governed by a reference value set by some QRF. Dorsal/ventral attention system switching in humans (Vossel et al. 2014) is sensitive to a vast array of expectations, the reference values of which are unknown and in at least some cases subject to considerable individual variation. Salience assignments driving both exploratory and reactive behavior are, in particular, highly dependent on individual experience, strongly coupled to the core self-representation and the reward system, and subject to variation in both prosocial and pathological directions (Uddin 2015). Much of ethology can be viewed as the comparative study of salience. Understanding how QRFs that regulate salience vary across phylogeny will be a major step toward answering the “what is it like?” question in a systematic way.
Resource Usage, Interoception, and the Self
The considerations above reinforce the obvious point that acquiring and monitoring the usage of free-energy resources is one of the core functions of any living system. A second core function, equally necessary for the maintenance of homeostasis/allostasis, is damage control. Within the MP framework, these core functions are supported by a QRF that encodes the set points that serve as the overall homeostatic attractor. This QRF defines the “self” in MP.
Prediction 15: The “self” comprises three core monitoring functions, for free-energy availability, physiological status, and organismal integrity, and three core response functions, free-energy acquisition, physiological damage control, and defense against parasites and other invaders. These will be found in every organism. Indeed they are found even in E. coli, which has inducible metabolite acquisition and digestion systems (Jacob and Monod 1961), the generalized “heat shock” stress response system (Burdon 1986), and restriction enzymes that detect and destroy foreign, e.g. viral DNA (Horiuchi and Zinder 1972). All of these responses act to restore an overall homeostatic setpoint, i.e. an expected nonequilibrium state; hence they can all be viewed as acting to minimize environmental variational free energy or Bayesian expectation violation (Friston 2010; 2013).
Specialized molecular pathways for the core functions of the self are supplemented by specialized intercellular communication pathways in multicellular organisms and by inter-individual communication pathways in social organisms, e.g. eusocial insects (Robinson 1992) or humans. Networks of neurons support feeding, locomotion (a primary stress response) and defense already in Cnidarians (Bosch et al. 2017) and at least feeding and locomotion in Ctenophores (Moroz 2015); these functions become far more complex in bilaterian animals, particularly in active animals including arthropods, cephalopods, and vertebrates (Liebeskind et al. 2017), and form the basis of “core consciousness” in Damasio’s (2000) framework.
Considerable evidence now indicates that interoception in humans, and so presumably in mammals generally, employs a predictive coding mechanism (Hohwy 2013; Seth 2013) and is strongly coupled to the core self-representation and the salience network via the insula—cingulate—orbitofrontal loop (Craig 2010; Uddin 2015; Seth and Tsakiris 2018). This predictive coding system manages homeostasis/allostasis across the scale hierarchy from cells to organ systems (Corcoran and Hohwy 2019) and couples interoception to exteroception and proprioception (Barrett and Simmons 2015; Seth 2013; Barrett 2017; Seth and Tsakiris 2018). Intriguingly, emotional and stress responses, core components of the self, are highly sensitive to gut microbiome activity in humans and other animals (Vuong et al. 2017; Sarkar et al. 2018). As all multicellular eukaryotes have obligate symbiotic microbiomes (hence are “holobionts”; Gilbert 2014), one can expect that microbial contributions to stress detection and response are universal.
The thermodynamic costs of memory impose a particular restriction on the self, which predatory eukaryotic unicells such as Paramecium, animals, and quite possibly plants solve by weighting the less-certain future lower in resource priority than the (by default assumed to be) more-certain past:
Prediction 16: Organisms bias their “cognitive light cones” (Levin 2019), their representational and computational boundaries of concern or goal-directedness, toward the past. Memory, in other words, takes precedence over planning. The extent to which food caching, cooperative hunt organization, and other future-directed activities by nonhuman animals provides evidence of deliberative, explicit planning remains controversial (Bayne et al. 2019), with some arguing that all forms of imaginative “mental time travel” are human-specific (Suddendorf and Corballis 2007). It is, however, clear that explicit planning requires classically encoded representations of future events and so competes for resources with memory. As planning also requires explicit memory, it cannot win this competition.
The free-energy costs of mental time travel in either direction constrain it to an “off-line” activity when organisms are faced with rapid change that requires high-resolution perception and action. Such constraints also apply to the real-time representational costs of the self. Hence we can predict:
Prediction 17: Increasing real-time response requirements will disrupt encoding of the self-representation. This is observed in humans in “flow” states (Csikszentmihályi 1990), in highly automated, expertise-dependent activities including most social interaction (Bargh and Ferguson 2000; Bargh et al. 2012), and in experimental manipulations in which fatigue in various modalities affects cognitive performance (Massar et al. 2018). The extent to which nonhuman animals are able to apply “theory of mind” reasoning to themselves, and hence maintain a metacognitive self-representation, remains controversial (Martin and Santos 2016).
The picture that emerges from these energetic considerations is of a self-representation with critical functions and deep evolutionary roots (Levin 2020), but with only limited and transient exposure to awareness via explicit encoding. This is broadly consistent with the constructive views of the self outlined by Blackmore (2002), Chater (2018), Graziano and Webb (2014) among others.
Context Change Drives Evolution, Development, and Learning
Psychology in the 20th century was consumed with controversies pitting evolution (“Nature”) against learning (“Nurture”), with developmental processes straddling the conceptual gap in between. The development of robust machine learning algorithms, the advent of evo–devo (Müller 2007; Carroll 2008), the recognition that evolution itself can be viewed as a learning process (Power et al. 2015; Watson and Szathmáry 2016; Kouvaris et al. 2017), and in particular, the scale-free applicability of active inference models (Friston 2013; Friston et al. 2015; Fields and Levin 2020b,c; Kuchling et al. 2020) have done much to deconstruct these distinctions. As a scale-free approach in which any lineage can also be considered an individual (Fields and Levin 2020c), MP treats evolution, development, and learning as instances of a common mechanism. It distinguishes two nonoverlapping cases: gradual alterations in the relative contributions of QRFs or components of QRFs to outcomes, and saltatory changes in QRFs themselves.
Prediction 18: Context changes drive QRF changes. Here, we define context changes strictly: For a fixed set {oi} of observables, a context change x → y has occurred if the probability distributions Prob(oi|x) and Prob (oi|y) are well defined but the joint distribution Prob(oi|x∨y) is not (Kochen and Specker 1967; Dzhafarov et al. 2017). Here, “observables” are by definition classifiers, each of whose components consist of event/(condition, context)/valuation triples (Fields and Glazebrook 2020c; Fields et al. 2021). Under these conditions, QRF change in response to context change is required to maintain Bayesian coherence (Fields and Glazebrook 2020c).
Context switches are hard to measure, as doing so requires predicting what “background” variables are relevant in fact to how an event is processed, a prediction problem that is in general intractable (Dietrich and Fields 2020). Saltatory changes in event representations, e.g. canonical “Aha!” moments (Kounios and Beeman 2015), may reflect context-driven QRF changes. Basal systems may provide uniquely manipulable windows into such processes: e.g. Pezzulo et al. (2020) have recently suggested that bioelectrically driven saltatory morphological changes in planaria may employ the same underlying mechanism as saltatory changes in episodic memories in mammals.
Conclusions
Quantum theory has had a long and somewhat disreputable association, dating back at least to von Neumann (1932), with the science of consciousness. A dominant concern, beginning at least with Wigner (1961) and extending through Penrose (1989), Schwartz et al. (2005), and Hameroff and Penrose (2014) to Georgiev (2020) has been to employ quantum theory to establish an ontological foundation for a science of consciousness. Our interest here has not been ontological, but rather empirical: to derive as much as possible from the simple assumption that consciousness involves information exchange subject to the constraints of quantum information theory. We have shown that the MP framework that follows from this assumption allows many of the key features of consciousness to be understood as simple, scale-independent consequences of thermodynamics.
In direct contrast with strict Cartesianism, MP holds that we can better understand our own awareness by understanding the awareness of our more basal cousins. Our homeostatic/allostatic drives and the mechanisms that satisfy them are phylogenetically continuous with those of prokaryotic unicells including E. coli. Our concepts and categories are implemented by QRFs playing the same roles, and satisfying the same basic requirements, as the Che-Y system regulating bacterial chemotaxis. The tradeoffs that we implement, and adjust in real time, between perception, memory, and planning are tradeoffs that have been explored and adjusted in niche-specific ways by all organisms throughout evolutionary history. We can take advantage of these fundamental mechanistic similarities to design theoretical and experimental paradigms that reveal and assess scale-free properties of consciousness in both natural and engineered systems.
Acknowledgments
The authors thank E. Dietrich, D. Hoffman, A Marcianò, C. Prakash, and R. Prentner for relevant discussions, noting that they may nonetheless reject some or all of our conclusions. They also thank the two reviewers for their careful reading and helpful comments.
Funding
M.L. gratefully acknowledges support of the Barton Family Foundation, the Elisabeth Giauque Trust, and the Templeton World Charity Foundation.
Conflict of interest statement. None declared.
References
- Agrawal H. Extreme self-organization in networks constructed from gene expression data. Phys Rev Lett 2002;89:268702. [DOI] [PubMed] [Google Scholar]
- Arendt D, Benito-Gutierrez E, Brunet T, et al. Gastric pouches and the mucociliary sole: setting the stage for nervous system evolution. Philos Trans R Soc Lond B Biol Sci 2015;370:20150286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars BJ, Franklin S.. How conscious experience and working memory interact. Trends Cogn Sci 2003;7:166–72. [DOI] [PubMed] [Google Scholar]
- Baars BJ, Franklin S, Ramsoy TZ.. Global workspace dynamics: cortical “binding and propagation” enables conscious contents. Front Psychol 2013;4:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai S, Prabhakar B, Chelakkot R, Chelakkot R, Inamdar M.. Role of cell polarity dynamics and motility in pattern formation due to contact dependent signalling. bioRxiv 10.1101/2020.10.09.331009, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F, Gagliano M, Wizany G (eds). Memory and Learning in Plants. Cham: Springer, 2018. [Google Scholar]
- Baluška F, Levin M.. On having no head: cognition throughout biological systems. Front Psychol 2016;7:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F, Reber A.. Sentience and consciousness in single cells: how the first minds emerged in unicellular species. BioEssays 2019;41:1800229. [DOI] [PubMed] [Google Scholar]
- Baluška F, Reber A.. Senomic and ephaptic principles of cellular consciousness: the biomolecular basis for plant and animal sentience. J Cons Stud 2021;28:31–49. [Google Scholar]
- Baluška F, Miller WB, Reber A.. Biomolecular basis of cellular consciousness via subcellular nanobrains. Int J Mol Sci 2021; 22:25452021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F, Yokawa K, Mancuso S, et al. Understanding of anesthesia - Why consciousness is essential for life and not based on genes. Commun Integr Biol 2016;9:e1238118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabási A-L. Network Science. Cambridge, UK: Cambridge University Press, 2016. [Google Scholar]
- Barabási A-L, Oltvai ZN.. Network biology: understanding the cell’s functional organization. Nat Rev Genet 2004;5:101–14. [DOI] [PubMed] [Google Scholar]
- Bargh JA, Ferguson MJ.. Beyond behaviorism: on the automaticity of higher mental processes. Psychol Bull 2000;126:925–45. [DOI] [PubMed] [Google Scholar]
- Bargh JA, Schwader KL, Hailey SE, et al. Automaticity in social-cognitive processes. Trends Cogn Sci 2012;16:593–605. [DOI] [PubMed] [Google Scholar]
- Barrett LF. The theory of constructed emotion: an active inference account of interoception and categorization. Soc Cogn Affect Neurosci 2017;12:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Simmons WK.. Interoceptive predictions in the brain. Nat Rev Neurosci 2015;16:419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett SD, Rudolph T, Spekkens RW.. Reference frames, superselection rules, and quantum information. Rev Mod Phys 2007;79:555–609. [Google Scholar]
- Bartlett S, Wong ML.. Defining Lyfe in the Universe: from three privileged functions to four pillars. Life 2020;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barwise J, Seligman J, Information Flow: The Logic of Distributed Systems (Cambridge Tracts in Theoretical Computer Science, 44). Cambridge, UK: Cambridge University Press, 1997. [Google Scholar]
- Bateson G. Steps to an Ecology of Mind: Collected Essays in Anthropology, Psychiatry, Evolution, and Epistemology. Northvale, NJ, USA: Jason Aronson, 1972. [Google Scholar]
- Bayne T, Brainard D, Bryne RW, et al. What is cognition? Curr Biol 2019;29:R608–15. [DOI] [PubMed] [Google Scholar]
- Bell JS. Speakable and unspeakable in quantum mechanics. In: Bell, JS (ed.), Speakable and Unspeakable in Quantum Mechanics. Cambridge, UK: Cambridge University Press, 1987, 169–72. [Google Scholar]
- Bennett CH. The thermodynamics of computation. Int J Theor Phys 1982;21:905–40. [Google Scholar]
- Blackmore S. There is no stream of consciousness. J Cons Stud 2002;9:17–28. [Google Scholar]
- Boltzmann L. Lectures on Gas Theory. New York, NY: Dover, 1995. [Google Scholar]
- Bosch TCG, Klimovich A, Domazet-Lošo T, et al. Back to the basics: Cnidarians start to fire. Trends Neurosci 2017;40:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broido AD, Clauset A.. Scale-free networks are rare. Nat Comms 2019;10:1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun-Usan M, Thies C, Watson RA.. How to fit in: the learning principles of cell differentiation. PLoS Comput Biol 2020;16:e1006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon RH. Heat shock and the heat shock proteins. Biochem J 1986;240:313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt P, Sprecher SG.. Evolutionary origin of synapses and neurons – Bridging the gap. BioEssays 2017;39:1700024. [DOI] [PubMed] [Google Scholar]
- Cabezón E, Ripoll-Rozada J, Peña A, et al. Towards an integrated model of bacterial conjugation. FEMS Microbiol Rev 2015;39:81–95. [DOI] [PubMed] [Google Scholar]
- Calvo P, Baluška F, Trewavas A, Integrated information as a possible basis for plant consciousness. Biochem Biophys Res Comm 2020. 10.1016/j.bbrc.2020.10.022 [DOI] [PubMed] [Google Scholar]
- Calvo P, Kiejzer F.. Plant cognition. In: Baluška F (ed.), Plant-Environment Interactions, Signaling and Communication in Plants. Berlin: Springer, 2009, 247–66. [Google Scholar]
- Cao J, Cogdell RJ, Coker DF, et al. Quantum biology revisited. Sci Adv 2020;6:eaaz4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SB. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 2008;134:25–36. [DOI] [PubMed] [Google Scholar]
- Chalmers D. The Conscious Mind: In Search of a Fundamental Theory. Oxford: Oxford University Press, 1996. [Google Scholar]
- Chalmers D. The combination problem for panpsychism. In: Brüntrup G, Jaskolla L (eds.), Panpsychism: Contemporary Perspectives. Oxford: Oxford University Press, 2017, 179–214. [Google Scholar]
- Chater N. The Mind is Flat. London: Allen Lane, 2018. [Google Scholar]
- Chittka L. Bee cognition. Curr Biol 2017;27:R1037–59. [DOI] [PubMed] [Google Scholar]
- Clark A. Where brain, body, and world collide. Daedalus 1998;127:257–80. [Google Scholar]
- Clark A. How to knit your own Markov blanket: Resisting the Second Law with metamorphic minds. In: Metzinger T, Wiese W (eds), Philosophy and Predictive Processing: 3. Frankfurt am Main, MIND Group, 2017. [Google Scholar]
- Conant RC, Ashby SW.. Every good regulator of a system must be a model of that system. Int J Syst Sci 1970;1:89–97. [Google Scholar]
- Cooke JE. The living mirror theory of consciousness. J Cons Stud 2020;27:127–47. [Google Scholar]
- Corcoran AW, Hohwy J.. Allostasis, interoception, and the free energy principle: feeling our way forward. In: Tsakiris M, De Preester H (eds.), The Interoceptive Mind: From Homeostasis to Awareness. Oxford: Oxford University Press, 2019, 272–92. [Google Scholar]
- Craig AD. The sentient self. Brain Struct Funct 2010;214:563–77. [DOI] [PubMed] [Google Scholar]
- Csikszentmihályi M. Flow: The Psychology of Optimal Experience. New York, NY: Harper and Row, 1990. [Google Scholar]
- Damasio A. The Feeling of What Happens: Body, Emotion and the Making of Consciousness. London: Heinemann, 2000. [Google Scholar]
- de la Providencia IE, de Souza FA, Fernández F, et al. Arbuscular mycorrhizal fungi reveal distinct patterns of anastomosis formation and hyphal healing mechanisms between different phylogenic groups. New Phytol 2005;165:261–71. [DOI] [PubMed] [Google Scholar]
- Debono M-W, Souza GM.. Plants as electromic plastic interfaces: a mesological approach. Prog. Biophys Mol Biol 2019;146:123–33. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Charles L, King J-R, et al. Toward a computational theory of conscious processing. Curr Opin Neurobiol 2014;25:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Naccache L.. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition 2001;79:1–37. [DOI] [PubMed] [Google Scholar]
- Dietrich E, Fields C.. Equivalence of the Frame and Halting problems. Algorithms 2020;13:175. [Google Scholar]
- Dietrich E, Fields C, Sullins JP, et al. Great Philosophical Objections to Artificial Intelligence: The History and Legacy of the AI Wars. London: Bloomsbury, 2021. [Google Scholar]
- Dietrich E, Hardcastle V.. Sisyphus’s Boulder: Consciousness and the Limits of the Knowable. Amsterdam: John Benjamins, 2005. [Google Scholar]
- di Primio F, Müller BF, Lengeler JW.. Minimal cognition in unicellular organisms. In: Meyer JA, Berthoz A, Floreano D, Roitblat HL, Wilson SW (eds), From Animals to Animats. Honolulu, HI: International Society for Adaptive Behavior, 2000, 3–12. [Google Scholar]
- Doherty CJ, Kay SA.. Circadian control of global gene expression patterns. Annu Rev Genet 2010; 44:419–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draguhn A, Mallatt JM, Robinson DG.. Anesthetics and plants: no pain, no brain, and therefore no consciousness. Protoplasma 2021; 258: 239-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant F, Morokuma J, Fields C, et al. Long-term, stochastic editing of regenerative anatomy via targeting endogenous bioelectric gradients. Biophys J 2017;112:2231–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhafarov EN, Cervantes VH, Kujala JV.. Contextuality in canonical systems of random variables. Philos Trans R Soc A 2017;375:20160389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Singer W.. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci 2001;5:16–25. [DOI] [PubMed] [Google Scholar]
- Feynman RP. Simulating physics with computers. Int J Theor Phys 1982;21:467–88. [Google Scholar]
- Fields C. Trajectory recognition as the basis for object individuation: a functional model of object file instantiation and object token encoding. Front Psychol Percept Sci 2011;2:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields C. If physics is an information science, what is an observer? Information 2012;3:92–123. [Google Scholar]
- Fields C. Building the observer into the system: toward a realistic description of human interaction with the world. Systems 2016; 4:32. [Google Scholar]
- Fields C, Bischof J, Levin M.. Morphological coordination: a common ancestral function unifying neural and non-neural signaling. Physiology 2020; 35:16–30. [DOI] [PubMed] [Google Scholar]
- Fields C, Glazebrook JF.. A mosaic of Chu spaces and Channel Theory I: category-theoretic concepts and tools. J Expt Theor Artif Intell 2019a;31:177–213. [Google Scholar]
- Fields C, Glazebrook JF.. A mosaic of Chu spaces and Channel Theory II: applications to object identification and mereological complexity. J Expt Theor Artif Intell 2019b;31:237–65. [Google Scholar]
- Fields C, Glazebrook JF.. Representing measurement as a thermodynamic symmetry breaking. Symmetry 2020a;12:810. [Google Scholar]
- Fields C, Glazebrook JF.. Do Process-1 simulations generate the epistemic feelings that drive Process-2 decision making? Cogn Proc 2020b;21:533.53. [DOI] [PubMed] [Google Scholar]
- Fields C, Glazebrook JF.. Information flow in context-dependent hierarchical Bayesian inference. J Expt Theor Artif Intell 2020c. 10.1080/0952813X.2020.1836034 [DOI] [Google Scholar]
- Fields C, Glazebrook JF, Marcianò A.. Reference frame induced symmetry breaking on holographic screens. Symmetry 2021; 13:408. [Google Scholar]
- Fields C, Levin M.. Multiscale memory and bioelectric error correction in the cytoplasm-cytoskeleton-membrane system. WIRES Syst Biol Med 2018;10:e1410. [DOI] [PubMed] [Google Scholar]
- Fields C, Levin M.. How do living systems create meaning? Philosophies 2020a;5:36. [Google Scholar]
- Fields C, Levin M.. Integrating evolutionary and developmental thinking into a scale-free biology. BioEssays 2020b;42:1900228. [DOI] [PubMed] [Google Scholar]
- Fields C, Levin M.. Does evolution have a target morphology? Organisms 2020c;4:57–76. [Google Scholar]
- Fields C, Levin M.. Metabolic limits on classical information processing by biological cells. Preprint Arxiv 2103;17061v1 [quant.ph], 2021. [DOI] [PubMed]
- Fields C, Marcianò A.. Sharing nonfungible information requires shared nonfungible information. Quant Rep 2019; 1:252–9. [Google Scholar]
- Fields C, Marcianò A.. Markov blankets are general physical interaction surfaces. Phys Life Rev 2020a;33:109–11. [DOI] [PubMed] [Google Scholar]
- Fields C, Marcianò A.. Holographic screens are classical information channels. Quant Rep 2020b;2:326.36. [Google Scholar]
- Friedman DA, Søvik E.. The ant colony as a test for scientific theories of consciousness. Synthese 2021;198:1457–80. [Google Scholar]
- Friston KJ. The free-energy principle: a unified brain theory? Nat Rev Neurosci 2010;11:127–38. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Life as we know it. J R Soc Interface 2013;10:20130475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. A free energy principle for a particular physics. Preprint arxiv:1906.10184v1 [q-bio.NC], 2019.
- Friston K, Levin M, Sengupta B, et al. Knowing one’s place: a free-energy approach to pattern regulation. J R Soc Interface 2015;12:20141383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliano M, Grimonprez M.. Breaking the silence–Language and the making of meaning in plants. Ecopsychol 2015;7:145. 52. [Google Scholar]
- Georgiev DD. Quantum information theoretic approach to the mind–brain problem. Prog Biophys Mol Biol 2020;158:16–32. [DOI] [PubMed] [Google Scholar]
- Gilbert SF. Symbiosis as the way of eukaryotic life: the dependent co-origination of the body. J Biosci 2014;39:201–9. [DOI] [PubMed] [Google Scholar]
- Gloag ES, Turnbull L, Whitchurch CB.. Bacterial stigmergy: an organising principle of multicellular collective behaviours of bacteria. Scientifica 2015;2015:387342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goguen JA. A categorical manifesto. Math Struct Comp Sci 1991;1:49–67. [Google Scholar]
- Graziano MSA, Webb TW.. A mechanistic model of consciousness. Int J Mach Cons 2014;6:1–14. [Google Scholar]
- Gremiaux A, Yokawa K, Mancuso S, et al. Plant anesthesia supports similarities between animals and plants: Claude Bernard’s forgotten studies. Plant Signal Behav 2014;9:e27886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg S. Communication, memory and development. Prog Theor Biol 1978;5:183–232. [Google Scholar]
- Güntürkün O, Bugnyar T.. Cognition without cortex. Trends Cogn Sci 2016;20:291–303. [DOI] [PubMed] [Google Scholar]
- Hacker PMS. Is there anything it is like to be a bat? Philosophy 2002;77:157–74. [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, et al. Mapping the structural core of the human cerebral cortex. PLoS Biol 2008;6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameroff S, Penrose R.. Consciousness in the universe: a review of the ‘OrchOR’ theory. Phys Life Rev 2014;11:39–78. [DOI] [PubMed] [Google Scholar]
- Henkel LA, Carbuto M.. Remembering what we did: how source misattributions arise from verbalization, mental imagery, and pictures. In: Kelley MR (ed.), Applied Memory. Hauppauge, NY: Nova, 2008;213–34. [Google Scholar]
- Heylighen F. Stigmergy as a universal coordination mechanism II: varieties and evolution. Cogn Syst Res 2016;38:50–9. [Google Scholar]
- Hoffman DD, Singh M, Prakash C.. The interface theory of perception. Psych Bull Rev 2015;22:1480–506. [DOI] [PubMed] [Google Scholar]
- Hohwy J, The Predictive Mind. Oxford, UK: Oxford University Press, 2013. [Google Scholar]
- Holland LZ, Carvalho JE, Escriva H, et al. Evolution of bilaterian central nervous systems: a single origin? EvoDevo 2013;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K, Zinder ND.. Cleavage of bacteriophage fl DNA by the restriction enzyme of Escherichia coli B. Proc Natl Acad Sci USA 1972;69:3220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries J, Xiong L, Liu J, et al. Species-independent attraction to biofilms through electrical signaling. Cell 2017;168:200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Hoshino H, Yamashita T, et al. Planarian shows decision-making behavior in response to multiple stimuli by integrative brain function. Zool Lett 2015;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F, Monod J.. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol 1961;3:318–56. [DOI] [PubMed] [Google Scholar]
- Japyassú HF, Laland KN.. Extended spider cognition. Anim Cogn 2017;20:375–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauffred L, Vejborg RM, Korolev KS, et al. Chirality in microbial biofilms is mediated by close interactions between the cell surface and the substratum. ISME J 2017;11:1688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH. Precise circadian clocks in prokaryotic cyanobacteria. Curr Issues Mol Biol 2004;6:103–10. [PubMed] [Google Scholar]
- Kanai R, Komura Y, Shipp S, et al. Cerebral hierarchies: predictive processing, precision and the pulvinar. Philos Trans R Soc Lond B Biol Sci 2015;370:20140169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelz MB, Mashour GA.. The biology of general anesthesia from paramecium to primate. Curr Biol 2019;29:R1199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Barron AB.. How experimental neuroscientists can fix the hard problem of consciousness. Neurosci Cons 2020;6:niaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochen S, Specker EP.. The problem of hidden variables in quantum mechanics. J Math Mech 1967;17:59–87. [Google Scholar]
- Kounios J, Beeman M.. The Eureka Factor: Creative Insights and the Brain. London: Windmill, 2015. [Google Scholar]
- Kouvaris K, Clune J, Kounious L, et al. How evolution learns to generalise: using the principles of learning theory to understand the evolution of developmental organisation. PLoS Comp Biol 2017;13:e1005358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegman S, Blackiston D, Levin M, et al. A scalable pipeline for designing reconfigurable organisms. Proc Natl Acad Sci USA 2020;117:1853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchling F, Friston K, Georgiev G, et al. Morphogenesis as Bayesian inference: a variational approach to pattern formation and control in complex biological systems. Phys Life Rev 2020;33:88–108. [DOI] [PubMed] [Google Scholar]
- Kumar S. Molecular clocks: four decades of evolution. Nat Rev Genet 2005;6:654–62. [DOI] [PubMed] [Google Scholar]
- Lamme VAF. Challenges for theories of consciousness: seeing or knowing, the missing ingredient and how to deal with panpsychism. Philos Trans R Lond Soc B Biol Sci 2018;373:20170344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landauer R. Irreversibility and heat generation in the computing process. IBM J Res Devel 1961;5:183–95. [Database]. [Google Scholar]
- Levin M. The computational boundary of a “self”: developmental bioelectricity drives multicellularity and scale-free cognition. Front Psychol 2019;10:2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. Life, death, and self: fundamental questions of primitive cognition viewed through the lens of body plasticity and synthetic organisms. Biochem Biophys Res Comm 2020. doi.org/10.1016/j.bbrc.2020.10.077 [DOI] [PubMed] [Google Scholar]
- Leys SP. Elements of a ‘nervous system’ in sponges. J Expt Biol 2016;218:581–91. [DOI] [PubMed] [Google Scholar]
- Liang J, Edelsbrunner H, Woodward C.. Anatomy of protein pockets and cavities: measurement of binding site geometry and implications for ligand design. Protein Sci 1998;7:1884–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebeskind BJ, Hofmann HA, Hillis DM, et al. Evolution of animals neural systems. Annu Rev Ecol Evol Syst 2017;48:377–98. [Google Scholar]
- Lyon P. The cognitive cell: bacterial behavior reconsidered. Front Microbiol 2015;6:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon P. Of what is “minimal cognition” the half-baked version? Adapt Behav 2020;28:407–28. [Google Scholar]
- Mariscal C, Doolittle WF.. Life and life only: a radical alternative to life definitionism. Synthese 2020;197:2975–89. [Google Scholar]
- Martin A, Santos LR.. What cognitive representations support primate Theory of Mind? Trends Cogn Sci 2016;20:375–82. [DOI] [PubMed] [Google Scholar]
- Mashour GA, Roelfsema P, Changeux J-P, et al. Conscious processing and the global neuronal workspace hypothesis. Neuron 2020;105:776–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massar SAA, Csathó A, Van der Linden D.. Quantifying the motivational effects of cognitive fatigue through effort-based decision making. Front Psychol 2018;9:843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather J. What is in an octopus’s mind? Animal Sent 2019;4:26.1. [Google Scholar]
- Maturana HR, Varela FJ.. Autopoesis and Cognition: The Realization of the Living. D. Reidel: Dordrecht, 1980.
- McFadden J. Integrating information in the brain's EM field: the cemi field theory of consciousness. Neurosci Cons 2020;2020:niaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R. The honeybee as a model for understanding the basis of cognition. Nat Rev Neurosci 2012;13:758–68. [DOI] [PubMed] [Google Scholar]
- Mermin ND. Hidden variables and the two theorems of John Bell. Rev Mod Phys 1993;65:803–15. [Google Scholar]
- Mermin ND. Making better sense of quantum mechanics. Rep Prog Phys 2018;82:012002. [DOI] [PubMed] [Google Scholar]
- Micali G, Endres RG.. Bacterial chemotaxis: information processing, thermodynamics, and behavior. Curr Opin Microbiol 2016;30:8–15. [DOI] [PubMed] [Google Scholar]
- Misra B, Sudarshan ECG.. The Zeno’s paradox in quantum theory. J Math Phys 1977;18:756–63. [Google Scholar]
- Montero BG. What combination problem? In: Brüntrup G, Jaskolla L (eds.), Panpsychism: Contemporary Perspectives. Oxford: Oxford University Press, 2017, 215–28. [Google Scholar]
- Moroz LL. Convergent evolution of neural systems in ctenophores. J Expt Biol 2015;218:598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller GB. Evo-devo: extending the evolutionary synthesis. Nat Rev Genet 2007;8:943–9. [DOI] [PubMed] [Google Scholar]
- Müller MP. Law without law: from observer states to physics via algorithmic information theory. Quantum 2020;4:301. [Google Scholar]
- Muñoz-Dorado JM, Torres FJ, García-Bravo E, et al. Myxobacteria: moving, killing, feeding, and surviving together. Front Microbiol 2016;7:781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel T. What is it like to be a bat? Philos Rev 1974;83:435–50. [Google Scholar]
- Nickel M. Evolutionary emergence of synaptic nervous systems: what can we learn from the non-synaptic, nerveless Porifera? Invert Biol 2010;129:1–16. [Google Scholar]
- Nieder A, Wagener L, Rinnert P.. A neural correlate of sensory consciousness in a corvid bird. Science 2020; 369:1626–9. [DOI] [PubMed] [Google Scholar]
- Nielsen MA, Chuang IL, Quantum Computation and Quantum Information. Cambridge, UK: Cambridge University Press, 2000. [Google Scholar]
- Niss K, Gomez-Casado C, Hjaltelin JX, et al. Complete topological mapping of a cellular protein interactome reveals bow-tie motifs as ubiquitous connectors of protein complexes. Cell Rep 2020;31:107763. [DOI] [PubMed] [Google Scholar]
- Oizumi M, Albantakis L, Tononi G.. From the phenomenology to the mechanisms of consciousness: Integrated Information Theory 3.0. PLoS Comp Biol 2014;10:e1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno D, Iino R, Noji H.. Rotation and structure of FoF1-ATP synthase. J Biochem 2011;149:655–64. [DOI] [PubMed] [Google Scholar]
- Ongchoco JDK, Scholl BJ.. How to create objects with your mind: from object-based attention to attention-based objects. Psychol Sci 2019;30:1648–55. [DOI] [PubMed] [Google Scholar]
- Oviedo NJ, Morokuma J, Walentek P, et al. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev Biol 2010;339:188–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. Affective consciousness: core emotional feelings in animals and humans. Cons Cognit 2005;14:30–80. [DOI] [PubMed] [Google Scholar]
- Pattee HH. Cell psychology. Cognit Brain Theory 1982;5:325–41. [Google Scholar]
- Pearl J. Probabilistic Reasoning in Intelligent Systems: Networks of Plausible Inference. San Mateo, CA: Morgan Kaufmann, 1988. [Google Scholar]
- Penrose R. The Emperor’s New Mind: Concerning Computers, Minds, and the Laws of Physics. Oxford: Oxford University Press, 1989. [Google Scholar]
- Perry CJ, Barron AB, Chittka L.. The frontiers of insect cognition. Curr Opin Behav Sci 2017;16:111–8. [Google Scholar]
- Pezzulo G, LaPalme J, Durant F, et al. Bistability of somatic pattern memories: stochastic outcomes in bioelectric circuits underlying regeneration. Philos Trans R Soc Lond B Biol Sci 2021; 376: 20190765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzulo G, Levin M.. Re-membering the body: applications of computational neuroscience to the top-down control of regeneration of limbs and other complex organs. Integr Biol 2015;7:1487–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S, Wolf H.. Arthropod spatial cognition. Anim Cogn 2020;23:1041–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietsch P, Schneider CW.. Brain transplantation in salamanders – an approach to memory transfer. Brain Res 1969;14:707–15. [DOI] [PubMed] [Google Scholar]
- Pizlo Z. Perception viewed as an inverse problem. Vis Res 2001;41:3145–61. [DOI] [PubMed] [Google Scholar]
- Power DA, Watson RA, Szathmáry E, et al. What can ecosystems learn? Expanding evolutionary ecology with learning theory. Biol Direct 2015;10:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash C, Fields C, Hoffman DD, et al. Fact, fiction, and fitness. Entropy 2020;22:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash C, Stephens KD, Hoffman DD, et al. Fitness beats truth in the evolution of perception. Acta Biotheor 2020. doi:10.1007/s10441-020-09400-0 [DOI] [PubMed] [Google Scholar]
- Prieto-Barajas CM, Valencia-Cantero E, Santoyo G.. Microbial mat ecosystems: structure types, functional diversity, and biotechnological application. Electr J Biotech 2018; 31:48–56. [Google Scholar]
- Ramirez S, Liu X, Lin PA, et al. Creating a false memory in the hippocampus. Science 2013;341:387–e91. [DOI] [PubMed] [Google Scholar]
- Robbins RJ, Krishtalka L, Wooley JC.. Advances in biodiversity: metagenomics and the unveiling of biological dark matter. Stand Genomic Sci 2016;11:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GE. Regulation of division of labor in insect societies. Annu Rev Entomol 1992;37:637.65. [DOI] [PubMed] [Google Scholar]
- Rochat P. Primordial sense of embodied self-unity. In: Slaughter V, Brownell CA (eds.), Early Development of Body Representations. Cambridge, UK: Cambridge University Press, 2012, 3–18. [Google Scholar]
- Roederer J. Information and Its Role in Nature. Berlin: Springer, 2005. [Google Scholar]
- Roper M, Lee C-H, Hickey PC, et al. Life as a moving fluid: fate of cytoplasmic macromolecules in dynamic fungal syncytia. Curr Opin Microbiol 2015;26:116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin S, Parr T, Da Costa L, et al. Future climates: markov blankets and active inference in the biosphere. J R Soc Interface 2020;17:20200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Harty S, Lehto SM, et al. The microbiome in psychology and cognitive neuroscience. Trends Cogn. Sci 2018; 22:611–36. [DOI] [PubMed] [Google Scholar]
- Schlitt T, Brazma A.. Current approaches to gene regulatory network modelling. BMC Bioinformatics 2007;8:(Suppl S9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell AK, Amodio P, Boeckle M, et al. How intelligent is a cephalopod? Lessons from comparative cognition. Biol Rev 2021; 96: 162-78. [DOI] [PubMed] [Google Scholar]
- Schwartz JM, Stapp HP, Beauregard M.. Quantum physics in neuroscience and psychology: a neurophysical model of mind-brain interaction. Philos Trans R Soc Lond B Biol Sci 2005;360:1309–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger HG. Cell biology of Acetabularia. Current Topics in Microbiology and Immunology 1969; 50: 1–36 [DOI] [PubMed] [Google Scholar]
- Seth AK. Interoceptive inference, emotion, and the embodied self. Trends Cogn Sci 2013;17:565–73. [DOI] [PubMed] [Google Scholar]
- Seth AK, Tsakiris M.. Being a beast machine: the somatic basis of selfhood. Trends Cogn Sci 2018;22:969–81. [DOI] [PubMed] [Google Scholar]
- Shanahan M. The brain's connective core and its role in animal cognition. Philos Trans R Soc Lond B Biol Sci 2012;367:2704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon CE. A mathematical theory of communication. Bell Syst Tech J 1948;27:379–423. [Google Scholar]
- Shomrat T, Levin M.. An automated training paradigm reveals long-term memory in planarians and its persistence through head regeneration. J Expt Biol 2013;216:3799–810. [DOI] [PubMed] [Google Scholar]
- Sosinsky GE, Nicholson BJ.. Structural organization of gap junction channels. Biochem Biophys Acta 2005;1711:99–125. [DOI] [PubMed] [Google Scholar]
- Sovrano VA, Baratti G, Potrich D.. A detour task in four species of fishes. Front Psychol 2018;9:2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Network attributes for segregation and integration in the human brain. Curr Opin Neurobiol 2013;23:162–71. [DOI] [PubMed] [Google Scholar]
- Sporns O, Honey CJ.. Small worlds inside big brains. Proc Natl Acad Sci USA 2006;111:15220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stal LJ, Cyanobacterial mats and stromatolites. In: Whitton BA (ed.), Ecology of Cyanobacteria II: Their Diversity in Space and Time. Berlin: Springer, 2012, 65–125. [Google Scholar]
- Stewart J. Cognition = Life: implications for higher-level cognition. Behav Process 1996;35:311–26. [DOI] [PubMed] [Google Scholar]
- Straube B. An overview of the neuro-cognitive processes involved in the encoding, consolidation, and retrieval of true and false memories. Behav Brain Funct 2012;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC.. The evolution of foresight: what is mental time travel and is it unique to humans? Behav Brain Sci 2007;30:299–313. [DOI] [PubMed] [Google Scholar]
- Thiery S, Kaimer C.. The predation strategy of Myxococcus xanthus. Front Microbiol 2020;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G. Consciousness as integrated information: a provisional manifesto. Biol Bull 2008;215:216–42. [DOI] [PubMed] [Google Scholar]
- Tononi G, Koch C.. Consciousness: here, there and everywhere? Philos Trans R Soc Lond B Biol Sci 2015;370:20140167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevarthen C. What is it like to be a person who knows nothing? Defining the active intersubjective mind of a newborn human being. Inf Child Dev 2010;20:119–35. [Google Scholar]
- Ueno H, Suzuki T, Kinosita K Jr, et al. ATP-driven stepwise rotation of FoF1-ATP synthase. Proc Natl Acad Sci USA 2005;102:1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 2015;16:55–61. [DOI] [PubMed] [Google Scholar]
- Vlachakis D, Bencurova E, Papangelopoulos N, et al. Current state-of-the-art molecular dynamics methods and applications. Adv Prot Chem Struct Biol 2014;94:269–313. [DOI] [PubMed] [Google Scholar]
- von Neumann J. Mathematical Foundations of Quantum Mechanics. Princeton: Princeton University Press, 1955. [Google Scholar]
- von Uexküll J, A Stroll through the worlds of animals and men. In: Schiller C (ed.), Instinctive Behavior. New York, NY: International Universities Press, 1957, 5–80. [Google Scholar]
- Vossel S, Geng JJ, Fink GR.. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist 2014;20:150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong HE, Yano JM, Fung TC, Hsiao EY.. The microbiome and host behavior. Annu Rev Neurosci 2017;40:21–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhams GH, Armitage JP.. Making sense of it all: bacterial chemotaxis. Nat Rev Mol Cell Biol 2004;5:1024–37. [DOI] [PubMed] [Google Scholar]
- Wallace R, Consciousness: A Mathematical Treatment of the Global Neuronal Workspace Model. New York, NY: Springer, 2005. [Google Scholar]
- Wang D, Jin S, Zou X.. Crosstalk between pathways enhances the controllability of signalling networks. [Database] IET Syst Biol 2016;10:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Schoenlein RW, Peteanu LA, et al. Vibrationally coherent photochemisty in the femtosecond primary event of vision. Science 1994;266:422–5. [DOI] [PubMed] [Google Scholar]
- Watson RA, Szathmáry E.. Can evolution learn? Trends Ecol Evol 2016;31:147–57. [DOI] [PubMed] [Google Scholar]
- Watts D. Small Worlds: The Dynamics of Networks Between Order and Randomness. Princeton NJ, Princeton University Press, 1999. [Google Scholar]
- Wheeler JA. Law without law. In: Wheeler JA, Zurek WH (eds.), Quantum Theory and Measurement. Princeton: Princeton University Press, 1983, 182–213. [Google Scholar]
- Wheeler JA. Information, physics, quantum: the search for links. In: Zurek WH (ed.), Complexity, Entropy, and the Physics of Information. Boulder, CO: Westview, 1990, 3–28. [Google Scholar]
- Wigner EP. Remarks on the mind-body question. In: Good IJ (ed.), The Scientist Speculates. London: Heinemann, 1961, 284–302. [Google Scholar]
- Yang C-Y, Bialecka-Fornal M, Weatherwax C, et al. Encoding membrane-potential-based memory within a microbial community. Cell Syst 2020;10:417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwier MC, Chong LT.. Reaching biological timescales with all-atom molecular dynamics simulations. Curr Opin Pharmacol 2010;10:745–52. [DOI] [PubMed] [Google Scholar]


