Abstract
Reducing population levels of frailty is an important goal, and preventing its development in midadulthood could be pivotal. There is limited evidence on associations between childhood socioeconomic position (SEP) and frailty. Using data on the 1958 British birth cohort (followed from 1958 to 2016; n = 8,711), we aimed to 1) establish the utility of measuring frailty in midlife, by examining associations between a 34-item frailty index at age 50 years (FI50y) and mortality at ages 50–58 years, and 2) examine associations between early-life SEP and FI50y and investigate whether these associations were explained by adult SEP. Hazard ratios for mortality increased with increasing frailty; for example, the sex-adjusted hazard ratio for the highest quintile of FI50y versus the lowest was 4.07 (95% confidence interval (CI): 2.64, 6.25). Lower early-life SEP was associated with higher FI50y. Compared with participants born in the highest social class, the estimated total effect on FI50y was 42.0% (95% CI: 35.5, 48.4) for participants born in the lowest class, with the proportion mediated by adult SEP being 0.45% (95% CI: 0.35, 0.55). Mediation by adult SEP was negligible for other early-life SEP classes. Findings suggest that early-life SEP is associated with frailty and that adult SEP only partially explains this association. Results highlight the importance of improving socioeconomic circumstances across the life course to reduce inequalities in midlife frailty.
Keywords: birth cohort, childhood circumstances, early-life socioeconomic position, frailty, healthy aging, life course, socioeconomic status
Abbreviations
- CI
confidence interval
- FI
frailty index
- FI50y
frailty index at age 50 years
- NDE
natural direct effect
- NIE
natural indirect effect
- SEP
socioeconomic position
- TE
total effect
Frailty, a state of increased vulnerability resulting from age-related decline in physiological reserves (1), is associated with adverse health outcomes, including falls, hospitalizations, and premature mortality (1, 2). While there is no universal consensus regarding the operationalization of frailty (3), the 2 most common approaches define frailty as a phenotype (4) (based on 5 predefined physical frailty criteria (5)) or a frailty index (FI), based on an accumulation of health-related deficits (6, 7). Despite lack of a standard definition, there is acknowledgement that frailty presents a global challenge because of population aging (8, 9). Although the prevalence of frailty increases with age, it is not limited to older ages (10). However, most epidemiologic studies assessing predictors of frailty have focused exclusively on adults aged 65 years or more (11–13). This omission is important, because frailty reflects biological rather than chronological age (14) and is a dynamic process that may be reversible (15). However, increasing age (from 65 years onwards) is associated with a lower probability of improvement in frailty status (16). Thus, there is emerging recognition that attention to frailty in midadulthood could be pivotal in terms of identifying, managing, and preventing severe frailty at older ages (17, 18).
Reducing frailty at the population level is a desirable goal. To achieve this, a more precise understanding of predictors of frailty from midlife onwards is key to delaying its onset. A life-course approach to frailty has been discussed theoretically (19, 20) and has the potential to identify when and how to intervene at different life stages to maximize the chance of healthy population aging (19). However, to date, only a few empirical life-course studies have examined frailty. For example, a body of literature is emerging on links between early-life socioeconomic position (SEP) and frailty at older ages (13, 21–25). However, these studies have relied on relatively small sample sizes (n < 1,100) (13, 25) and retrospective reports of early-life SEP (21, 22). Importantly, previous studies have examined mainly older adults, and where younger adults have been evaluated (21, 23–25), the age range has been broad, with little consideration for age-related differences in associations. While associations between frailty in adulthood and mortality are well established (1), evidence suggests that frailty levels may have increased in recent generations (26). In addition, some (27) but not all (28) studies suggest that the strength of the frailty-mortality association may have weakened in more recent generations. Thus, there is utility in examining associations of frailty with both mortality and upstream factors, such as early-life SEP, in a single-aged sample from midadulthood to help clarify when in the life course these associations emerge.
Despite the burgeoning literature linking early-life SEP to frailty, only a few studies (13, 24, 25) have examined whether this association is due to life-course continuities in disadvantage. Limited evidence suggests that adult socioeconomic circumstances fully explain associations between early-life SEP and frailty at older ages (13, 24, 25). However, these studies have had several methodological limitations. For example, use of a single indicator of adult SEP, such as occupational class (13, 25), may result in mismeasurement of the mediator, potentially undermining the analysis (29). Moreover, confounders of the adult SEP-frailty relationship, such as smoking (30, 31), that are themselves influenced by early-life SEP (32) were not accounted for. To our knowledge, no study has examined these chains of associations in midlife, and almost all have operationalized frailty as a phenotype (13, 21, 23–25), with few using an FI (22). The validated FI (6) is particularly suitable for examining frailty in midadulthood because, compared with other frailty measures (including the frailty phenotype), it is more sensitive to small changes in health status (33), making it particularly suitable for examining frailty in midadulthood, a life stage in which health deficits are accumulating at a slower rate than at older ages (34).
We aimed to address several outstanding research gaps regarding the utility of measuring frailty in midlife and the links between early-life SEP and frailty. Specifically, using data from the 1958 British birth cohort, we derived an index of frailty at age 50 years (FI50y). To provide construct validity and establish the utility of measuring frailty in midlife, we examined associations between FI50y and mortality over an 8-year follow-up period. We then examined associations between early-life SEP and FI50y and investigated whether these associations were explained by adult SEP.
METHODS
The 1958 British birth cohort includes over 17,000 participants who have been followed since their birth in England, Scotland, or Wales during a single week in March 1958 (35). Ethical approval was given, including approval at age 50 years, by the London Multi-Centre Research Ethics Committee, and participants gave informed consent at various ages. Persons who responded in midadulthood were broadly representative of the surviving cohort (36). At age 50 years, 9,789 cohort members participated; of these, 8,711 had a valid measure of FI50y (see Web Figure 1, available online at https://doi.org/10.1093/aje/kwab038) and were included in the analysis. Compared with cohort members who took part at age 50 years but had insufficient information for creation of an FI (n = 1,078), participants included in this study had a more favorable SEP in early life and in adulthood (Web Table 1).
Measurement of variables
Frailty index.
The FI was derived following guidelines outlined elsewhere (37). An included variable was required to be 1) a health-associated deficit with a prevalence that generally increases with age and 2) a factor that is not universal in the adult population by midlife (e.g., myopia was not included, but age-related sight changes (presbyopia) were included); additionally, when considered together, 3) the included variables had to cover a range of physiological systems and processes. The FI50y included 34 variables (Table 1) covering 8 broad health domains (comorbidity, physical functioning, sensory functioning, mental health, cognitive function, fatigue, sleep, and general health). Most variables were dichotomized and given a score of 1 (deficit present) or 0 (deficit absent). Following the guidelines (37), cohort members (n = 8,711; 89.0%) were included if they had information on at least 30 deficits. For each included individual, an FI50y was generated by summing the total number of deficits reported and dividing by the total number of deficits considered (the number of deficits considered varied from 30 to 34), producing a continuous score between 0 and 1.
Table 1.
Health Deficits Used to Construct a 34-Item Index of Frailty at Age 50 Years for Participants in the 1958 British Birth Cohort (n = 8,711), 2008
| Health Deficit Value a | Missing Data | |||||
|---|---|---|---|---|---|---|
| Domain and Health Variable | 0 | 1 | No. | % | ||
| No. | % | No. | % | |||
| Comorbidity | ||||||
| Asthma/wheezy bronchitisb | 7,878 | 90.44 | 833 | 9.56 | 0 | 0 |
| Type 2 diabetesb | 8,340 | 95.74 | 371 | 4.26 | 0 | 0 |
| Recurrent backache, prolapsed disc, or sciaticab | 7,221 | 82.90 | 1,490 | 17.10 | 0 | 0 |
| Body painc,d | 7,984 | 91.94 | 700 | 8.06 | 27 | 0.31 |
| High blood pressureb | 7,395 | 84.89 | 1,316 | 15.11 | 0 | 0 |
| Stomach issuesb,e | 7,987 | 96.86 | 259 | 3.14 | 465 | 5.34 |
| Kidney/bladder problemsb | 8,499 | 97.57 | 212 | 2.43 | 0 | 0 |
| Persistent cough/bringing up phlegmb | 8,349 | 95.84 | 362 | 4.16 | 0 | 0 |
| Sensory factorsb | ||||||
| Hearing problemsf | 7,868 | 90.32 | 843 | 9.68 | 0 | 0 |
| Eyesight problemsg | 8,441 | 97.11 | 251 | 2.89 | 19 | 0.22 |
| Cognitive functionh | ||||||
| Delayed recall of 10 words | 6,176 | 71.80 | 2,426 | 28.2 | 109 | 1.25 |
| Immediate recall of 10 words | 6,853 | 79.14 | 1,806 | 20.9 | 52 | 0.60 |
| No. of animals named in 1 minute | 6,402 | 73.93 | 2,257 | 26.1 | 52 | 0.60 |
| No. of accurately crossed out P’s and W’s in a letter grid | 6,442 | 75.57 | 2,082 | 24.4 | 187 | 2.15 |
| General health | ||||||
| Self-rated general healthi | 1,731 | 19.88 | 455 | 5.23 | 3 | 0.03 |
| Self-rated health compared with 1 year agoj | 7,420 | 85.18 | 193 | 2.22 | 0 | 0 |
| Health limiting social activities (e.g., visiting friends, relatives)k | 8,307 | 95.71 | 372 | 4.29 | 32 | 0.37 |
| Expecting one’s health to get worsel | 6,715 | 94.10 | 510 | 5.90 | 61 | 0.70 |
| Mental health | ||||||
| Physical/emotional problems interfering with normal social activities with family, friends, groups, neighborsd,m | 7,972 | 91.80 | 712 | 8.20 | 27 | 0.31 |
| Feeling so down in the dumps that nothing could cheer you upn | 8,469 | 97.37 | 229 | 2.63 | 13 | 0.15 |
| Having seen a doctor or specialist or having been to a hospital because one was feeling low, depressed, or sad (and was still symptomatic)b | 8,404 | 96.50 | 305 | 3.50 | 2 | 0.02 |
| Having seen a doctor or specialist or having been to a hospital because one was feeling generally anxious or jittery (and was still symptomatic)b | 8,564 | 98.32 | 146 | 1.68 | 1 | 0.01 |
| Physical functiono | ||||||
| Moderate physical activities (e.g., pushing vacuum, moving table, bowling, playing golf) | 8,241 | 94.70 | 461 | 5.30 | 9 | 0.10 |
| Lifting groceries | 8,257 | 94.95 | 439 | 5.05 | 15 | 0.17 |
| Climbing 1 flight of stairs | 8,353 | 96.09 | 340 | 3.91 | 18 | 0.21 |
| Bending, kneeling, and stooping | 8,025 | 92.22 | 677 | 7.78 | 9 | 0.10 |
| Walking 100 yards (91.4 m) | 8,365 | 96.14 | 336 | 3.86 | 10 | 0.11 |
| Bathing | 8,444 | 96.99 | 262 | 3.01 | 5 | 0.06 |
| Fatigued | ||||||
| Having a lot of energyp | 8,179 | 94.05 | 518 | 5.96 | 14 | 0.16 |
| Feeling worn outq | 7,992 | 91.90 | 704 | 8.10 | 15 | 0.17 |
| Feeling full of lifer | 8,326 | 95.73 | 371 | 4.27 | 14 | 0.16 |
| Feeling tireds | 8,323 | 95.71 | 373 | 4.29 | 15 | 0.17 |
| Sleep | ||||||
| Amount of time usually needed to fall asleept | 8,152 | 93.76 | 543 | 6.24 | 16 | 0.18 |
| Getting enough sleep to feel rested upon waking in the morningd,u | 8,097 | 92.99 | 610 | 7.01 | 4 | 0.05 |
a All variables were self-reported and were given a score of 1 (deficit present) or 0 (deficit absent). Deficits that included an intermediary response (e.g., “sometimes” or “a little”) were assigned a score between 0 and 1 as appropriate.
b 0 = no; 1 = yes.
c 0 = none, very mild, mild, or moderate; 1 = severe or very severe.
d In the past month.
e Stomach issues included gallstones, gastric ulcer, acid reflux, diverticulitis, hernia, tumor, and cancer.
f Hearing problems included hearing loss in 1 or both ears, tinnitus, Ménière disease, age-related degeneration, and inability to hear certain noises.
g Eyesight problems included diabetes-associated eye disease, glaucoma, cataract, macular degeneration, low vision, and blindness.
h 0 = upper 3 quartiles; 1 = lowest quartile.
i 0 = excellent (n = 1,731 (19.9%)); 0.25 = very good (n = 2,906 (33.4%)); 0.5 = good (n = 2,544 (29.2%)); 0.75 = fair (n = 1,072 (12.3%)); 1 = poor (n = 455 (5.2%)).
j 0 = much better/the same (n = 7,420 (85.2%)); 0.5 = worse (n = 1,098 (12.6%)); 1 = much worse (n = 193 (2.2%)).
k 0 = a good bit/some/a little/none of the time (n = 8,307 (95.7%)); 1 = all/most of the time (n = 372 (4.3%)).
l 0 = don’t know/mostly false/definitely false (n = 6,715 (77.6%)); 0.5 = mostly true (n = 1,425 (16.5%)); 1 = definitely true (n = 510 (5.9%)).
m 0 = not at all/slightly/moderately (n = 7,972 (91.8%)); 1 = quite a bit/extremely (n = 712 (8.2%)).
n 0 = a good bit/some/a little/none of the time (n = 8,469 (97.4%)); all/most of the time (n = 229 (2.6%)).
o 0 = limited a little/not limited; 1 = limited a lot.
p 0 = all/most/some of the time (n = 7,159 (82.3%)); 0.5 = a little of the time (n = 1,021 (11.7%)); 1 = none of the time (n = 518 (6.0%)).
q 0 = a good bit of the time/some of the time/a little of the time/none of the time (n = 7,992 (91.9%)); 1 = all/most of the time (n = 704 (8.1%)).
r 0 = all/most of the time/a good bit of the time/some of the time/a little of the time (n = 8,326 (95.7%)); 1 = none of the time (n = 371 (4.3%)).
s 0 = a good bit of the time/some of the time/a little of the time/none of the time (n = 8,323 (95.7%)); 1 = all/most of the time (n = 373 (4.3%)).
t 0 = 1 hour or less (n = 8,152 (93.8%)); 1 = over 1 hour (n = 543 (6.2%)).
u 0 = all/most/a good bit/some/a little bit of the time (n = 8,097 (93.0%)); 1 = none of the time (n = 610 (7.0%)).
Mortality.
Information on deaths occurring from 2008 (when cohort members were aged 50 years) to the end of 2016 (when cohort members were aged 58 years) was ascertained from a variety of sources, the majority (n = 198; 94.7%) through linkage to death certificates from the National Health Service Central Register (38). Information obtained from relatives or close friends during survey activities/cohort maintenance allowed identification of 11 further deaths (see Web Table 2 footnotes).
Early-life SEP.
Early-life SEP was identified from prospectively recorded information on father’s occupation at birth in 1958 or, if missing, at age 7 years in 1965 (n = 631; 7.24%). Using the Registrar General’s Social Classification groupings, 4 SEP categories were identified: professional/managerial (classes I/II), skilled nonmanual (class III nonmanual), skilled manual (class III manual), and partly skilled/unskilled manual (classes IV/V and cases where there was no male head of the household).
Adult SEP.
Adult SEP was considered a potential intermediary factor based on established associations with both early-life SEP (39, 40) and frailty (26, 41). It was represented by occupational class at age 42 years (or, if missing, at age 33 years (n = 829; 9.52%)), educational attainment by age 33 years, and household tenure at age 45 years (see Table 2 footnotes).
Table 2.
Early-Life and Adult Socioeconomic Characteristics and Frailty Index Score at Age 50 Years in the 1958 British Birth Cohort (n = 8,711), 1958–2008a
| SEP Variable | Total Population | Women | Men | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Early-life SEPb (father’s occupational class) | ||||||
| I/II | 1,671 | 19.7 | 856 | 19.5 | 815 | 20.0 |
| III nonmanual | 877 | 10.4 | 439 | 9.98 | 438 | 10.8 |
| III manual | 4,054 | 47.9 | 2,110 | 48.0 | 1,944 | 47.8 |
| IV/V | 1,865 | 22.0 | 994 | 22.6 | 871 | 21.4 |
| Adult occupational classb | ||||||
| I/II | 3,368 | 42.1 | 1,530 | 36.9 | 1838 | 47.8 |
| III nonmanual | 1,836 | 23.0 | 1,449 | 35.0 | 387 | 10.1 |
| III manual | 1,475 | 18.5 | 288 | 6.95 | 1,187 | 30.8 |
| IV/V | 1,313 | 16.4 | 876 | 21.1 | 437 | 11.4 |
| Adult educational levelc | ||||||
| <O-levels | 1,596 | 21.4 | 944 | 23.8 | 652 | 18.6 |
| O-levels | 2,548 | 34.1 | 1,503 | 37.9 | 1,045 | 29.8 |
| A-levels | 2,245 | 30.1 | 1,013 | 25.6 | 1,232 | 35.1 |
| Degree or higher | 1,083 | 14.5 | 505 | 12.7 | 578 | 16.5 |
| Adult housing tenured | ||||||
| Owner | 7,346 | 84.4 | 3,816 | 84.4 | 3,530 | 84.5 |
| Renter | 1,145 | 13.2 | 618 | 13.7 | 527 | 12.6 |
| Other | 221 | 2.4 | 88 | 2.0 | 123 | 2.9 |
| Frailty index scoree | 0.07 (0.04–0.13) | 0.07 (0.03–0.13) | 0.07 (0.04–0.13) | |||
Abbreviation: SEP, socioeconomic position.
a Based on observed (i.e., unimputed) data.
b Early-life SEP based on father’s occupation at birth (or, if missing, at age 7 years) and adult occupational class at age 42 years (or, if missing, at age 33 years). Both were classified using the United Kingdom Register General’s classification of occupations and grouped into the following categories: professional/managerial (classes I/II), skilled nonmanual (class III nonmanual), skilled manual (class III manual), and partly/unskilled manual (classes IV/V; in early life, also included cases where there was no male head of the household).
c Educational attainment based on the participant’s highest educational qualification by age 33 years. O-levels: high school qualifications, typically ascertained at age 16 years. A-levels: national qualifications, typically ascertained at age 18 years.
d Housing tenure based on financial circumstances of the participant’s housing arrangements at age 45 years (or, if missing, at age 42/50 years). Tenure was grouped into 3 categories: owning a property (outright or with a mortgage), renting (or having a partial mortgage), and other (e.g., living rent-free with a relative).
e Values are expressed as median (interquartile range).
Confounders.
Confounders were identified a priori on the basis of factors associated with early-life SEP, adult SEP, and frailty. These included sex as a baseline confounder and physical activity (42, 43), smoking (30, 31), and problem alcohol drinking (30, 44) as confounders of the adult SEP-frailty relationship that are influenced by early-life SEP (32, 43, 45) (see Figure 1 and Web Appendix 1).
Figure 1.
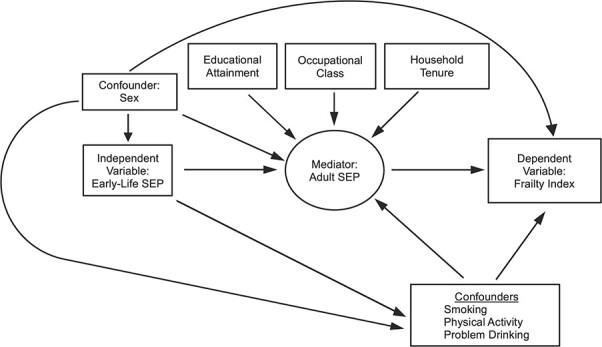
Proposed relationships between early-life socioeconomic position (SEP), adult SEP, and frailty. Boxes represent observed variables; the circle represents a latent variable. (See Methods section of the text for details.)
Statistical analysis
Proportional hazards for mortality were visually assessed using Kaplan-Meier plots. Cox proportional hazards models were used to estimate (sex-adjusted) hazard ratios and 95% confidence intervals for associations between FI50y and all-cause mortality at ages 50–58 years. Associations between FI and mortality are commonly examined using a continuous measure (46) or prespecified FI categories (0–0.1, 0.1–0.2, etc.) (2, 47, 48). In midlife, the FI is highly skewed (e.g., approximately 60% of the sample has an FI < 0.1); thus, these categorizations were not appropriate. Therefore, similar to other investigators, we divided FI50y into quintiles (49). Survival time included time from completion of the age-50 survey to the date of death, censoring (last date of contact), or the end of the study period (December 2016), whichever came first. Schoenfeld residuals were checked to test the assumption of proportional hazards for FI50y and sex; neither violated the assumption.
Using linear regression, we examined whether associations between early-life SEP and FI50y varied by sex. There was no evidence of effect modification (P = 0.35); hence, results from sex-adjusted analyses are presented. For ease of interpretation, FI50y was log-transformed and multiplied by 100, whereby regression coefficients can be interpreted as the symmetrical percent difference in mean values (50). Before log-transforming FI50y, we added 0.01 to the index, as in previous work (51), to circumvent logarithmic values of 0.
Creation of latent classes to represent adult SEP
To account for potential measurement error introduced by using a single indicator of adult SEP, we created a latent class variable using adult occupational class, educational attainment, and household tenure, which capture different aspects of SEP (52). The best-fitting class solution was based on model fit statistics: the Akaike (53) and Bayesian (54) information criteria, with lower values indicating better fit. Class discrimination was assessed using entropy, with values of 0.6 or higher indicating good class separation (55). After identification of the optimal number of classes, participants were assigned to their most likely class, which created a categorical variable representing adult SEP that was used in subsequent analyses.
Mediation analysis
Details on the adopted counterfactual approach are given elsewhere (56). In brief, we compared scenarios whereby the entire population was assigned a specific category for early-life SEP (i.e., classes I/II (baseline), class III nonmanual, class III manual, or classes IV/V) and adult SEP (represented by a latent variable). Our targets of estimation, the total effect (TE), natural direct effect (NDE), and natural indirect effect (NIE), were estimated for each of the 3 levels of early-life SEP as compared with the baseline (classes I/II). For each level of early-life SEP, the TE on frailty was partitioned into the effect mediated via adult SEP (NIE) and a nonmediated effect (NDE). For each level of early-life SEP as compared with baseline, the TE is the percent difference in frailty comparing 2 situations: the percent difference in mean FI50y if all individuals were assigned to 1) the level of early-life SEP under consideration and 2) the baseline category of early-life SEP. For each level of early-life SEP compared with the baseline, the NDE is the percent difference in frailty comparing the situations where all individuals are assigned to early-life SEP at either 1) the level under consideration or 2) the baseline level, with adult SEP set to the value that would be observed if early-life SEP were set at its baseline level. The NIE is the difference between the relevant TE and NDE. We estimated these effects using the g-computation procedure in STATA, version 15 (StataCorp LLC, College Station, Texas) (56) (see Web Appendix 2). This procedure allowed us to estimate the effects of interest while allowing for confounders of the relationship between adult SEP and frailty that are influenced by early-life SEP.
We conducted sensitivity analysis to determine whether any single item or one of the 8 domains in the FI was particularly important for examined relationships. We systematically removed one item (or domain) at a time from the FI and examined associations between 1) FI50y (minus the single item/domain) and subsequent mortality and 2) early-life SEP and FI50y (minus the single item/domain).
The prevalence of missing data ranged from 2.8% (early-life SEP) to 21.0% (problem drinking at age 45 years). To minimize data loss, missing information for confounders, early-life SEP, and adult SEP was imputed using chained equations. Following guidelines (57), the imputation models included all substantive variables and the main predictors of missingness (childhood internalizing and externalizing behaviors and cognitive ability) (36). Linear regression analyses were carried out across 20 imputed data sets; overall estimates were obtained. For the g-computation procedure, a single imputation was used (bootstrapped standard errors were used to calculate 95% confidence intervals).
RESULTS
As expected, the FI50y was right-skewed, with a median value of 0.07 for both men and women, corresponding to an expression of approximately 2.4 (34 × 0.07) health-related deficits (Table 2). While 20% of the cohort had fathers in the highest occupational category (I/II) when they were born, 42% were themselves in this occupational category at age 42 years.
Associations between early-life SEP, FI50y, and mortality
Sex-adjusted Kaplan-Meier curves indicated that mortality generally increased progressively with increasing levels of frailty (Figure 2). For example, compared with the least frail adults, the sex-adjusted hazard ratio was 1.66 (95% CI: 1.01, 2.74) for adults in the fourth-highest quintile of frailty and 4.07 (95% CI: 2.64, 6.25) for the most frail adults (Web Table 2). Associations between FI and mortality remained stable when we systematically excluded a single item or domain from the FI. For example, per increase in frailty quintile, the sex-adjusted hazard ratio for mortality was 1.48 (95% CI: 1.32, 1.67); removing a single item from the index resulted in hazard ratios ranging from 1.40 (95% CI: 1.28, 1.54) to 1.54 (95% CI: 1.38, 1.71) when “self-reported health” and “number of animals named in 1 minute” were removed, respectively. When the domains were removed, hazard ratios ranged from 1.38 (95% CI: 1.24, 1.54) for cognitive function to 1.50 (95% CI: 1.33, 1.69) for comorbidity (Web Figure 2A).
Figure 2.
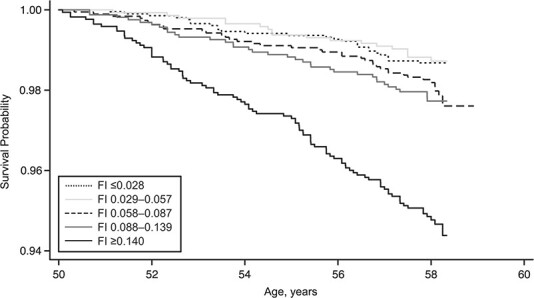
Sex-adjusted Kaplan-Meier survival probabilities according to quintile of frailty index (FI) score in the 1958 British birth cohort (n = 8,711; 209 deaths), 2008–2016. Higher FI scores represent higher levels of frailty.
Lower early-life SEP was associated with greater percent differences in FI50y. For example, each decrease (on a 4-point scale) in early-life SEP was associated with a 14.2% (95% CI: 12.1, 16.3) increase in FI. Associations between early-life SEP and FI50y were broadly stable when a single item or domain was removed from the FI. For example, percent increases in FI ranged from 11.2% (95% CI: 9.48, 13.1) to 14.8% (95% CI: 12.7, 16.9) when “self-reported health” and “expect health to get worse” were removed, respectively. Associations ranged from 14.2% (95% CI: 12.1, 16.4) to 10.2% (95% CI: 7.56, 12.5) when the domains “sensory functioning” and “cognitive function” were respectively removed (Web Figure 2B).
Latent classes representing adult SEP
We considered 2–5 latent classes. The 4-class solution was our chosen solution, because it had the smallest Akaike and Bayesian information criteria and a satisfactory entropy score (Web Table 3). The predicted probabilities for each of the 4 latent class memberships for adult occupational class, education, and tenure are shown in Web Table 4.
Mediation analysis
The estimated TE of early-life SEP on FI50y was 10.3% (95% CI: 3.0, 17.7) for participants with fathers in the class III nonmanual SEP category, 27.8% (95% CI: 22.2, 33.3) for those with fathers in the class III manual category, and 42.0% (95% CI: 35.5, 48.4) for those with fathers in the classes IV/V category when compared with participants with fathers in the classes I/II category (Table 3). When partitioned, compared with participants with fathers in classes I/II, the estimated NDEs for participants with fathers in the class III nonmanual, class III manual, and classes IV/V categories were 15.3% (95% CI: 7.9, 22.7), 32.4% (95% CI: 26.6, 38.1), and 23.0% (95% CI: 16.4, 29.7), respectively. Mediation by (the latent variable representing) adult SEP was negligible, except for participants with fathers in classes IV/V, where the proportion mediated was 0.45% (95% CI: 0.35, 0.55).
Table 3.
Total, Natural Direct, and Natural Indirect Effects of Socioeconomic Position at Birth on Frailty Index Score at Age 50 Yearsa and Proportion Mediatedb by Socioeconomic Position at Birthc in the 1958 British Birth Cohort (n = 8,711), 1958–2008d
| SEP at Birth e , f | ||||||
|---|---|---|---|---|---|---|
| Effect | Class III Nonmanual | Class III Manual | Classes IV/V | |||
|
Mean % Difference |
95% CI |
Mean % Difference |
95% CI |
Mean % Difference |
95% CI | |
| Total effect | 10.35 | 3.02, 17.67 | 27.75 | 22.23, 33.27 | 41.96 | 35.48, 48.44 |
| Natural direct effect (not via adult SEP) | 15.28 | 7.88, 22.69 | 32.36 | 26.59, 38.14 | 23.03 | 16.36, 29.70 |
| Natural indirect effect (via adult SEP) | −4.94 | −7.77, −2.10 | −4.61 | −7.28, −1.95 | 18.93 | 15.49, 22.38 |
| Proportion mediated (via adult SEP) | −0.48 | −20.39, 19.43 | −0.17 | −0.28, −0.06 | 0.45 | 0.35, 0.55 |
Abbreviations: CI, confidence interval; SEP, socioeconomic position.
a Mean percentage difference in frailty index score.
b The proportion mediated was the natural indirect effect divided by the total effect.
c Mediator: adult SEP.
d The analysis adjusted for sex and for adult physical activity, smoking, and problem alcohol drinking as confounders of the adult SEP–frailty relationship that are themselves influenced by early-life SEP (see text, Figure 1, and Web Appendix 1 for details).
e SEP at birth was measured as father’s occupational class at birth or, if missing, at age 7 years.
f Reference group: classes I/II.
DISCUSSION
Our study examining early-life SEP in relation to the accumulation of health-related deficits by midadulthood, carried out in a general population sample, is important for several reasons. First, we showed that by midlife, health deficits have begun to accumulate. For example, a median FI of 0.07 indicates that half the population at age 50 years had at least 2 of the considered health deficits, and similarly, one-quarter of the population had at least 4 deficits. Second, it is noteworthy that this accumulation of deficits at a relatively young age was strongly associated with mortality up to 8 years later. For example, morality hazards were 4 times higher when comparing adults with the most deficits (≥5) to those with the fewest (0–1). Third, lower SEP in early life was associated with higher levels of frailty by midadulthood, such that the estimated TE on FI50y was 42% greater for participants born in the lowest SEP category as compared with the highest. Finally, compared with those born in the highest SEP category, the effect of early-life SEP on frailty for participants born in the lowest SEP category was partly explained by continuities in disadvantage into adulthood.
A major strength of this study over previous work was the examination of an age-homogenous sample. Age is strongly associated with frailty (7), and this influence could be eliminated in our study. Further strengths included examination of a large general-population sample with data collected prospectively from birth, a validated measure of frailty capturing multiple health domains, and analysis that accounted for confounders of the adult SEP–frailty relationship. We acknowledge that there is no single best indicator of SEP (52). We used father’s occupation at birth to represent early-life SEP because it is a commonly used measure, reflecting a wide range of early-life social and economic indicators, including household educational attainment, income level, and social standing. In addition, rather than using a single measure of SEP in adulthood, we constructed a latent variable capturing 3 different well-established aspects of adult SEP (52). Health deficits accumulate at a slower rate in midlife than at older ages (34), and frailty measured in younger populations might be clinically and biologically different from that measured in older populations (1). Nonetheless, our measure of frailty is particularly suited to midlife because it has demonstrated good construct validity at this life stage (58) and it provides a continuous score for the range of fitness to frailty (6), allowing detection of small differences in health as compared with other measures (33). Regarding temporality, our indicators of adult SEP were captured prior to age 50 years, when the FI was constructed. This provides some evidence supporting the temporal sequence of events, which is further bolstered by evidence from several cohort studies showing associations between adult SEP and a range of subsequent health conditions (59). However, we do acknowledge that health can also influence subsequent SEP (30). Our mediation analysis relied on several assumptions, including no unmeasured confounding and accurate parametric modeling; but required assumptions were less stringent than those for conventional alternative analyses. Finally, as with all long-term studies, attrition occurred over time. Although participants in this study had more favorable early-life and adult SEP compared with those not included, in general the sample remained broadly representative of the original cohort (36). We prevented further sample reductions due to missing data by using imputation.
Our findings that health deficits have already begun to accumulate by age 50 years and are associated with subsequent mortality agree with the literature on the accumulation of health deficits in midlife (47, 60). Although the implications of frailty in clinical practice may vary by age, we and other researchers (18, 60) have demonstrated the utility of measuring frailty earlier in the life course. Thus, our findings emphasize that measuring frailty at a particular age is meaningful in identifying persons at risk of adverse health outcomes and, because frailty is progressive, beginning with a preclinical stage, there are opportunities for early prevention (1). Furthermore, our finding that the early-life SEP–frailty and frailty–mortality associations were not largely driven by any single item or health domain suggests that the value of the FI exceeds any single item/domain.
Our results are consistent with previous studies showing that lower early-life SEP is associated with greater risk of frailty in adulthood (13, 24, 25) such that, compared with persons born in the highest SEP category, those born in the lowest category had a 42% higher FI at age 50 years. However, we found the estimated effect of early-life SEP on frailty mediated by adult SEP to be noteworthy only for the lowest (compared with the highest) SEP category at birth. Since adult SEP did not fully explain early-life SEP associations in this cohort, other explanatory pathways may be involved. Evidence suggests that early-life socioeconomic disadvantage may lead to poor adult health via biological embedding (61). For example, abnormal biological changes have been observed in adults who experienced early-life socioeconomic disadvantage in this cohort (62) and elsewhere (63), which have been proposed to lead to accelerated aging (64). In addition, pathways operating via obesity and physical activity may be relevant and warrant further research. Findings that the estimated direct effects of the 2 intermediate early-life SEP categories were larger than the corresponding estimated TEs were surprising and contrasted with previous studies in which adult SEP fully explained early-life SEP–frailty associations. Discrepancies may be due to the single-aged sample examined here as compared with the broad age ranges previously examined (24, 25) or the younger age of adults in this study compared with others (13). Notwithstanding this difference, our finding that adult SEP is an important intermediary factor through which early-life disadvantage is associated with midlife frailty agrees with other studies. Therefore, our findings, together with other evidence, suggest that interventions to improve the adult socioeconomic circumstances of persons from disadvantaged backgrounds may reduce the burden of frailty in midlife and beyond.
In conclusion, our findings have several practical and policy-relevant implications. They emphasize the value of using previously collected health data to identify persons who may be vulnerable to accelerated aging earlier in the life course. Derivations of the FI are widely used in clinical and primary-care settings in England (6, 65) to systematically identify the extent of frailty in adults aged 65 years and over. Our findings suggest that similar assessments could be valuable in midadulthood and suggest that in a primary-care setting, in addition to considering single health deficits in midlife, the accumulation of deficits is also important. Identifying adults in midlife who could benefit from early interventions might reduce the burden of frailty at older ages, improving quality of life and reducing health-care costs (18, 60). We highlight the importance of improving socioeconomic conditions over the whole life course in order to reduce health inequalities. Thus, a potential intervention focus could be on improving socioeconomic opportunities available in adulthood for those disadvantaged in childhood. Moreover, relative child poverty in the United Kingdom is projected to rise from 29.7% to 36.6% between 2018 and 2022 (66); thus, our findings underscore the importance of much-needed policies to redress socioeconomic inequalities in childhood, because they have the potential to improve health in midadult life and beyond.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Institute of Sport, Exercise and Health, Division of Surgery and Interventional Science, Faculty of Medical Sciences, University College London, London, United Kingdom (Nina T. Rogers, Snehal M. Pinto Pereira); MRC Unit for Lifelong Health and Aging at UCL, University College London, London, United Kingdom (Joanna M. Blodgett, Daniel H. J. Davis); Department of Medicine (Geriatrics), Faculty of Medicine, Dalhousie University, Halifax, Nova Scotia, Canada (Samuel D. Searle); and Department of Sport and Exercise Sciences, Musculoskeletal Science and Sports Medicine Research Centre, Manchester Metropolitan University, Manchester, United Kingdom (Rachel Cooper).
This work was supported by a Medical Research Council Career Development Award (award MR/P020372/1) to S.M.P.P.
We are grateful to the Centre for Longitudinal Studies, UCL Institute of Education, University College London, for use of the 1958 cohort data and to the UK Data Service for making them available.
The cohort data used in this study comply with the data-sharing policies of the Economic and Social Research Council; readers can access these data via the UK Data Archive (http://www.data-archive.ac.uk/).
The views expressed in this publication are those of the authors and not necessarily those of the funders. The funders had no input into the study design; data collection, analysis, and interpretation; the writing of the report; or the decision to submit the article for publication. Neither the Centre for Longitudinal Studies nor the UK Data Service bears any responsibility for the analysis or interpretation of these data.
Conflict of interest: none declared.
REFERENCES
- 1. Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381(9868):752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing. 2018;47(2):193–200. [DOI] [PubMed] [Google Scholar]
- 3. Theou O, Brothers TD, Mitnitski A, et al. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61(9):1537–1551. [DOI] [PubMed] [Google Scholar]
- 4. Theou O, Walston J, Rockwood K. Operationalizing frailty using the frailty phenotype and deficit accumulation approaches. Interdiscip Top Gerontol Geriatr. 2015;41:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. [DOI] [PubMed] [Google Scholar]
- 6. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–727. [DOI] [PubMed] [Google Scholar]
- 8. Buckinx F, Rolland Y, Reginster J-Y, et al. Burden of frailty in the elderly population: perspectives for a public health challenge. Arch Public Health. 2015;73(1):Article 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Government Office for Science, United Kingdom Department for Business, Energy and Industrial Strategy . Future of an Ageing Population. London, United Kingdom: Government Office for Science; 2016. https://www.gov.uk/government/publications/future-of-an-ageing-population. Accessed February 5, 2021. [Google Scholar]
- 10. Rockwood K, Blodgett JM, Theou O, et al. A frailty index based on deficit accumulation quantifies mortality risk in humans and in mice. Sci Rep. 2017;7:Article 43068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ding YY, Kuha J, Murphy M. Multidimensional predictors of physical frailty in older people: identifying how and for whom they exert their effects. Biogerontology. 2017;18(2):237–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hajek A, Brettschneider C, Posselt T, et al. Predictors of frailty in old age—results of a longitudinal study. J Nutr Health Aging. 2016;20(9):952–957. [DOI] [PubMed] [Google Scholar]
- 13. Gale CR, Booth T, Starr JM, et al. Intelligence and socioeconomic position in childhood in relation to frailty and cumulative allostatic load in later life: the Lothian Birth Cohort 1936. J Epidemiol Community Health. 2016;70(6):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitnitski AB, Graham JE, Mogilner AJ, et al. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2:Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gill TM, Gahbauer EA, Allore HG, et al. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166(4):418–423. [DOI] [PubMed] [Google Scholar]
- 16. Lee JSW, Auyeung T-W, Leung J, et al. Transitions in frailty states among community-living older adults and their associated factors. J Am Med Dir Assoc. 2014;15(4):281–286. [DOI] [PubMed] [Google Scholar]
- 17. Mitnitski A, Rockwood K. The rate of aging: the rate of deficit accumulation does not change over the adult life span. Biogerontology. 2016;17(1):199–204. [DOI] [PubMed] [Google Scholar]
- 18. Hanlon P, Nicholl BI, Jani BD, et al. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health. 2018;3(7):e323–e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuh D. A life course approach to healthy aging, frailty, and capability. J Gerontol Ser A Biol Sci Med Sci. 2007;62(7):717–721. [DOI] [PubMed] [Google Scholar]
- 20. Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62(7):731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alvarado BE, Zunzunegui M-V, Béland F, et al. Life course social and health conditions linked to frailty in Latin American older men and women. J Gerontol A Biol Sci Med Sci. 2008;63(12):1399–1406. [DOI] [PubMed] [Google Scholar]
- 22. Herr M, Robine J-M, Aegerter P, et al. Contribution of socioeconomic position over life to frailty differences in old age: comparison of life-course models in a French sample of 2350 old people. Ann Epidemiol. 2015;25(9):674–680.e1. [DOI] [PubMed] [Google Scholar]
- 23. Li Y, Xue Q-L, Odden MC, et al. Linking early life risk factors to frailty in old age: evidence from the China Health and Retirement Longitudinal Study. Age Ageing. 2020;49(2):208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van der Linden BWA, Cheval B, Sieber S, et al. Life course socioeconomic conditions and frailty at older ages. J Gerontol Ser B Psychol Sci Soc Sci. 2020;75(6):1348–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haapanen MJ, Perälä MM, Salonen MK, et al. Early life determinants of frailty in old age: the Helsinki Birth Cohort Study. Age Ageing. 2018;47(4):569–575. [DOI] [PubMed] [Google Scholar]
- 26. Marshall A, Nazroo J, Tampubolon G, et al. Cohort differences in the levels and trajectories of frailty among older people in England. J Epidemiol Community Health. 2015;69(4):316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bäckman K, Joas E, Falk H, et al. Changes in the lethality of frailty over 30 years: evidence from two cohorts of 70-year-olds in Gothenburg Sweden. J Gerontol A Biol Sci Med Sci. 2017;72(7):945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mousa A, Savva GM, Mitnitski A, et al. Is frailty a stable predictor of mortality across time? Evidence from the Cognitive Function and Ageing Studies. Age Ageing. 2018;47:721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. VanderWeele TJ, Valeri L, Ogburn EL. The role of measurement error and misclassification in mediation analysis: mediation and measurement error. Epidemiology. 2012;23(4):561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harrison S, Davies AR, Dickson M, et al. The causal effects of health conditions and risk factors on social and socioeconomic outcomes: Mendelian randomization in UK Biobank. Int J Epidemiol. 2020;49(5):1661–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kojima G, Iliffe S, Walters K. Smoking as a predictor of frailty: a systematic review. BMC Geriatr. 2015;15(1):Article 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mckenzie SK, Carter KN, Blakely T, et al. Effects of childhood socioeconomic position on subjective health and health behaviours in adulthood: how much is mediated by adult socioeconomic position? BMC Public Health. 2011;11(1):Article 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jang I-Y, Jung H-W, Lee HY, et al. Evaluation of clinically meaningful changes in measures of frailty. J Gerontol A Biol Sci Med Sci. 2020;75(6):1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kulminski A, Ukraintseva SV, Akushevich I, et al. Accelerated accumulation of health deficits as a characteristic of aging. Exp Gerontol. 2007;42(10):963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Power C, Elliott J. Cohort profile: 1958 British birth cohort (National Child Development Study). Int J Epidemiol. 2006;35(1):34–41. [DOI] [PubMed] [Google Scholar]
- 36. Atherton K, Fuller E, Shepherd P, et al. Loss and representativeness in a biomedical survey at age 45 years: 1958 British birth cohort. J Epidemiol Community Health. 2008;62(3):216–223. [DOI] [PubMed] [Google Scholar]
- 37. Searle SD, Mitnitski A, Gahbauer EA, et al. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:Article 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Centre for Longitudinal Studies, UCL Institute of Education, University College London . National Child Development Study Deaths Dataset, 1958–2016: Special Licence Access [data set]. 3rd ed. (SN: 7717). Colchester, United Kingdom: UK Data Service; 2018. https://beta.ukdataservice.ac.uk/datacatalogue/doi/?id=7717#!%233. Accessed June 28, 2020. [Google Scholar]
- 39. Hossin MZ, Björk J, Koupil I. Early-life social and health determinants of adult socioeconomic position: associations and trends across generations. J Epidemiol Community Health. 2020;74(5):412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Broer M, Bai Y, Fonseca F. A review of the literature on socioeconomic status and educational achievement. In: Socioeconomic Inequality and Educational Outcomes: Evidence From Twenty Years of TIMSS. (IEA Research for Education vol. 5). New York, NY: Springer Publishing Company; 2019:7–18. [Google Scholar]
- 41. Hoogendijk EO, van Hout HPJ, Heymans MW, et al. Explaining the association between educational level and frailty in older adults: results from a 13-year longitudinal study in the Netherlands. Ann Epidemiol. 2014;24(7):538–544.e2. [DOI] [PubMed] [Google Scholar]
- 42. Rogers NT, Marshall A, Roberts CH, et al. Physical activity and trajectories of frailty among older adults: evidence from the English Longitudinal Study of Ageing. PLoS One. 2017;12(2):e0170878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pinto Pereira SM, Li L, Power C. Early-life predictors of leisure-time physical inactivity in midadulthood: findings from a prospective British birth cohort. Am J Epidemiol. 2014;180(11):1098–1108. [DOI] [PubMed] [Google Scholar]
- 44. Strandberg AY, Trygg T, Pitkälä KH, et al. Alcohol consumption in midlife and old age and risk of frailty. Age Ageing. 2018;47(2):248–254. [DOI] [PubMed] [Google Scholar]
- 45. Gauffin K, Hemmingsson T, Hjern A. The effect of childhood socioeconomic position on alcohol-related disorders later in life: a Swedish national cohort study. J Epidemiol Community Health. 2013;67(11):932–938. [DOI] [PubMed] [Google Scholar]
- 46. Hoogendijk EO, Stenholm S, Ferrucci L, et al. Operationalization of a frailty index among older adults in the InCHIANTI Study: predictive ability for all-cause and cardiovascular disease mortality. Aging Clin Exp Res. 2020;32(6):1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Blodgett JM, Theou O, Howlett SE, et al. A frailty index from common clinical and laboratory tests predicts increased risk of death across the life course. GeroScience. 2017;39(4):447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Williams DM, Jylhävä J, Pedersen NL, et al. A frailty index for UK Biobank participants. J Gerontol A Biol Sci Med Sci. 2019;74(4):582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wong TY, Massa MS, O’Halloran AM, et al. Cardiovascular risk factors and frailty in a cross-sectional study of older people: implications for prevention. Age Ageing. 2018;47(5):714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cole TJ, Altman DG. Statistics notes: percentage differences, symmetry, and natural logarithms. BMJ. 2017;358:j3683. [DOI] [PubMed] [Google Scholar]
- 51. Gale CR, Westbury L, Cooper C. Social isolation and loneliness as risk factors for the progression of frailty: the English Longitudinal Study of Ageing. Age Ageing. 2018;47(3):392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Galobardes B, Shaw M, Lawlor DA, et al. Indicators of socioeconomic position (part 1). J Epidemiol Community Health. 2006;60(1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Akaike H. Information theory and an extension of the maximum likelihood principle. In: Csaki F, Petrov BN, eds. Information Theory: Proceedings of the 2nd International Symposium. Budapest, Hungary: Akadémiai Kiado; 1973:267–281. [Google Scholar]
- 54. Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6(2):461–464. [Google Scholar]
- 55. Asparouhov T, Muthén B. Auxiliary variables in mixture modeling: three-step approaches using Mplus. Struct Equ Modeling. 2014;21(3):329–341. [Google Scholar]
- 56. Daniel RM, De Stavola BL, Cousens SN. Gformula: estimating causal effects in the presence of time-varying confounding or mediation using the g-computation formula. Stata J. 2011;11(4):479–517. [Google Scholar]
- 57. Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kanters DM, Griffith LE, Hogan DB, et al. Assessing the measurement properties of a frailty index across the age spectrum in the Canadian Longitudinal Study on Aging. J Epidemiol Community Health. 2017;71(8):794–799. [DOI] [PubMed] [Google Scholar]
- 59. Kivimäki M, Batty GD, Pentti J, et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health. 2020;5(3):e140–e149. [DOI] [PubMed] [Google Scholar]
- 60. Pérez-Zepeda MU, Godin J, Armstrong JJ, et al. Frailty among middle-aged and older Canadians: population norms for the frailty index using the Canadian Longitudinal Study on Aging. Age Ageing. 2021;50(2):447–456. [DOI] [PubMed] [Google Scholar]
- 61. Hertzman C, Boyce T. How experience gets under the skin to create gradients in developmental health. Annu Rev Public Health. 2010;31(1):329–347. [DOI] [PubMed] [Google Scholar]
- 62. Power C, Atherton K, Strachan D, et al. Life-course influences on health in British adults: effects of socio-economic position in childhood and adulthood. Int J Epidemiol. 2007;36(3):532–539. [DOI] [PubMed] [Google Scholar]
- 63. Bush NR, Edgar RD, Park M, et al. The biological embedding of early-life socioeconomic status and family adversity in children’s genome-wide DNA methylation. Epigenomics. 2018;10(11):1445–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Danese A, Moffitt TE, Harrington H, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163(12):1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hood A, Waters T. Living Standards, Poverty and Inequality in the UK: 2017–18 to 2021–22. London, United Kingdom: Institute for Fiscal Studies; 2017. https://www.ifs.org.uk/publications/10028. Accessed January 1, 2021. [Google Scholar]
- 67. Babor TF, Higgins-Biddle JC, Saunders JB, et al. AUDIT: The Alcohol Use Disorders Identification Test. Guidelines for Use in Primary Health Care. 2nd ed. Geneva, Switzerland: World Health Organization; 2001. https://apps.who.int/iris/handle/10665/67205. Accessed February 14, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


