Abstract
Background:
The optimal treatment for gastric cancer with peritoneal metastasis (GCPM) remains debatable. This study aimed to compare the efficacy and safety of neoadjuvant intraperitoneal and systemic chemotherapy (NIPS) versus neoadjuvant systemic chemotherapy (NSC) for GCPM.
Methods:
Patients of GCPM received neoadjuvant chemotherapy with docetaxel, oxaliplatin and S-1 between January 2011 and June 2019 were retrospectively evaluated. Propensity score matched (PSM) analysis was carried out to reduce the selection bias. Multivariate Cox regression model was applied to screen the prognostic factors.
Results:
After PSM processing, 71 patients in each group were matched among the 186 GCPM patients included. NIPS yielded a better ascites and cytology response to chemotherapy, higher conversion resection rate and R0 resection rate than NSC. The overall survival (OS) rate in NIPS group was better than that in NSC group. Multivariate analysis revealed that the P stage, ascites response, conversion surgery rate and R0 resection rate were independent prognostic factors. Subgroup analysis indicated that NIPS showed a survival benefit over NSC only in patients with cT3-4a, P1-2, whose cytology turned negative, and who received conversion surgery; while not in patients with cT4b, P0 or P3, whose cytology did not turn negative, or who did not receive conversion surgery.
Conclusions:
NIPS is a safe and feasible treatment for GCPM, which showed more benefit than NSC.
Keywords: gastric cancer, neoadjuvant chemotherapy, peritoneal metastasis, conversion gastrectomy, propensity score matched analysis
Introduction
Gastric cancer (GC) is one of the most common malignancies and a leading cause of cancer mortality worldwide. 1 In China, both the incidence and the mortality of GC are estimated to rank the second highest among all cancers. 2 Peritoneal metastasis (PM) is a common manifestation of primary dissemination or recurrence in patients with GC, which often accompanies oral intake deficiency, bowel obstruction, malignant ascites and overconsumption. 3 Despite the better understanding of the underlying molecular mechanisms on the development of PM in the past decade, the overall survival (OS) of gastric cancer with peritoneal metastasis (GCPM) remains very poor. 4
GCPM is a theoretically incurable disease and the widely accepted management of GCPM for patients with good performance status (PS) scores is palliative systemic chemotherapy. 5,6 However, despite major advances in the treatment of GC with the introduction of a variety of novel chemotherapeutic and targeted agents, 7,8 the overall therapeutic effect of GCPM is far from satisfactory under systemic chemotherapy alone. On the other hand, some investigators indicated that GCPM should be considered a locoregional disease, 9 and elimination of visible lesions might postpone progression or possibly cure some patients. 10 Based on this theory, the effectiveness of complete cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) was explored worldwide. 11 -13 Multimodal treatment with gastrectomy, CRS, and HIPEC improved patient survival in GCPM in comparison to gastrectomy and palliative chemotherapy alone. 14 However, recurrence developed subsequently in all patients. 14 In addition, CRS plus HIPEC was associated with high incidences of complications and mortality, 10,15 which further limited its popularity in clinical practice.
Conversion therapy is referred to a surgical treatment with the aim of R0 resection after chemotherapy for tumors that were initially unresectable, and it is currently believed to be a promising modality in the multidisciplinary treatment of GCPM. 16 However, an intensive chemotherapy is usually needed in conversion therapy setting in order to maximize tumor shrinkage and eliminate peritoneal dissemination before surgery. Since the peritoneum-plasma barrier might impede drug permeation from the systemic circulation into the peritoneal cavity, 17 the intraperitoneal (IP) administration of chemotherapeutic agents becomes a reasonable method for treating PM. Because either paclitaxel (PTX) or docetaxel (DTX) is absorbed slowly through the lymphatic system due to its large molecular weight and lipophilic nature, a high concentration and a long drug acting duration within the peritoneal cavity is readily achieved when administered intraperitoneally. 18 Therefore, taxane is believed to be the most ideal agent for IP administration which is extensively explored as the neoadjuvant intraperitoneal and systemic chemotherapy (NIPS) for the treatment of GCPM. 19 However, the optimal NIPS regimen for the treatment of GCPM remains debatable.
Studies of NIPS for the treatment of GCPM were mostly conducted in Japan, with the combination of taxane (paclitaxel or docetaxel) and 5-FU (capecitabine or S-1) as the most commonly used regimens. 20 -22 However, such a doublet protocol was believed to be much less effective for a primary tumor than for peritoneal metastases, as was supported by the fact that the PM had been effectively controlled for years but the primary tumor progressed in a short time in many patients. 23 On the other hand, the triplet docetaxel, oxaliplatin, and S-1 (DOS) regimen has been used as a neoadjuvant systemic chemotherapy (NSC) for the treatment of locally advanced GC and a conversion therapy for metastatic GC in our department since 2011. On the basis of these concepts, we attempted to transfer some dosages of docetaxel from IV route to IP route to develop a novel triplet NIPS regimen with IP docetaxel, IV docetaxel + oxaliplatin, and oral S-1 for the conversion therapy of GCPM since the year 2016. The aim of the present study was to evaluate the efficacy and safety of NIPS versus NSC with DOS as conversion therapy for GCPM.
Methods
Study Design
This retrospective cohort study was aimed to compare the efficacy and safety of neoadjuvant intraperitoneal and systemic chemotherapy (NIPS) versus neoadjuvant systemic chemotherapy (NSC) for GCPM. Specifically, the NIPS chemotherapy included intraperitoneal (IP) docetaxel, intravenous (IV) docetaxel + oxaliplatin, and oral S-1, while the NSC chemotherapy included IV docetaxel + oxaliplatin, and oral S-1. Clinical data were retrieved from the medical records as we described previously. 24 Written informed consent was obained from all included patients. Propensity score matched (PSM) was used to match the 2 groups on a 1:1 ratio.
Inclusion and Exclusion Criteria
Patients aged 18-75 ys with pathologically proven primary gastric cancer between January 2011 and June 2019 were eligible for inclusion. The eligibility criteria were as follows: 1) the presence of PM confirmed by laparoscopy or open laparotomy, including positive peritoneal cytology without detectable lesions within the peritoneal cavity; 2) sufficient liver, renal, cardiac, lung, and bone marrow function; 3) adequate oral intake; and 4) Eastern Cooperative Oncology Group (ECOG) score 0-1 points.
Exclusion criteria included: 1) concurrent or a previous history of other malignancies; 2) prior treatment including surgery, chemotherapy, targeted therapy, or radiotherapy; 3) existence of non-curative factors other than peritoneal or ovarian metastasis (e.g. distant lymph nodes, liver, lung or bone metastasis); and 4) uncontrolled systemic diseases.
Preoperative Evaluation and Peritoneal Metastasis Grading
Each patient underwent abdominal and pelvic contrast-enhanced computed tomography (CT) and/or endoscopic ultrasonography to determine clinical tumor and regional lymph node staging. Laparoscopic exploration was routinely performed to confirm and classify the extent of PM according to the Japanese classification of gastric carcinoma 12th edition. 22 The amount of ascites was estimated by radiologists using CT according to the Japanese conventional 5-point method, 25 and quantified as <500ml (+), 500-2000ml (++), and >2000ml (+++).
Neoadjuvant Chemotherapy
NIPS regimen: Docetaxel (30mg/m2) was administered through the IP catheter on day 1. Docetaxel (45 mg/m2) and oxaliplatin (130 mg/m2) were administered IV on day 1, and S-1 was administered orally depending on the patient’s body surface area (80 mg/d if ≤ 1.25 m2; 100mg/d if 1.25-1.5 m2; and 120mg/d if ≥ 1.5 m2) on day 1-14 followed by a 1-week rest.
NSC regimen: Docetaxel (75 mg/m2) and oxaliplatin (130 mg/m2) were administered IV on day 1, and S-1 was administered orally depending on the patient’s body surface area (80 mg/d if ≤ 1.25 m2; 100mg/d if 1.25-1.5 m2; and 120 mg/d if ≥ 1.5 m2) on day 1-14 followed by a 1-week rest.
An IP catheter was implanted into the pelvic cavity during the laparoscopic exploration for each patient. NAC was initiated within 7 days after surgery.
Assessment of Response to NAC
The tumor response was assessed every 3 cycles of NAC or whenever necessary by serum CEA, CA19-9, CA125, peritoneal cytology examination, and contrast-enhanced CT scan according to the Response Evaluation Criteria in Solid Tumors (RECIST) guideline 1.1. 26 Inspired by RECIST guideline, we defined ascites response in this study as disappearance (undetectable under CT scan), decreased (>30% decrease in volume), progression (>20% increase in volume), and stable (unable to qualify “decreased” nor “progression” in volume). A second laparoscopic exploration would be performed when all the following criteria were met: 1) tolerance of the resection surgery; 2) negative peritoneal cytology; and 3) primary tumor assessed as resectable based on the second CT scan.
Conversion Surgery
A subsequential curative-intent surgery, including radical gastrectomy with standardized D2 lymph node dissection, would be performed if no unresectable metastasis was identified for P0CY1 and if the disappearance of peritoneal nodules or a decrease in number and size of the peritoneal nodules with negative frozen pathological results was achieved for P1-3, during the second laparoscopic exploration. Instead, the non-surgical candidates would continue with the original NAC when the overall evaluation was stable disease or better. These patients would be treated and evaluated as the same scheme described above until disease progression, intolerable toxicity, or consent withdrawal. The patients evaluated as PD were switched to a second-line treatment and were defined as “PD” regardless of the subsequent outcome.
Toxicity Evaluation
NAC-related toxicity was evaluated every course of treatment according to the National Cancer Institute Common Toxicity Criteria version 3.0, 27 and the most severe toxicity record for each patient among the entire NAC course was used in the analysis.
Postoperative Treatment and Follow-Up
Patients achieving R0 resection were given priority to adjuvant chemotherapy with oxaliplatin and S-1 for 3 cycles in both groups. S-1 monotherapy was reserved for patients not tolerating 2-drug therapy. All patients were followed up regularly as we described previously. 24 OS was calculated from date of diagnosis.
Statistical Analysis
PSM was performed to reduce selection bias between NIPS and NSC groups. Propensity scores were estimated by using a logistic-regression model and a nearest neighbor matching algorithm. The caliper used in the present study was 0.08.
Chi-square test and student’s t-test were applied to assess categorical and continuous data respectively between the 2 groups. The Kaplan-Meier method was used to estimate the survival rates. The univariate and multivariate analyses of various clinicopathologic variables were performed to identify independent factors that might predict prognosis. A P values lower than 0.05 was considered statistically significant. All statistical calculations were performed by using SPSS software (Version 22.0).
Results
Patient Characteristics
A total 186 patients of GCPM received NAC with docetaxel, oxaliplatin and S-1 in our department between January 2011 and June 2019, including 78 in NIPS group and 108 in NSC group. Their baseline demographics and clinical characteristics are shown in Table 1. The 2 groups were not balanced with regard to clinical peritoneal (P) stage and initial ascites volume variables, and the disparities were completely resolved after PSM manipulation (Table 1).
Table 1.
The Baseline Demographics and Clinical Characteristics.
| Variable | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| NIPS | NSC | P | NIPS | NSC | P | |
| n = 78 (%) | n = 108 (%) | n = 71 (%) | n = 71 (%) | |||
| Gender | 0.673 | 0.389 | ||||
| Male | 46 (59.0) | 67 (62.0) | 46 (64.8) | 41 (57.7) | ||
| Female | 32 (41.0) | 41 (38.0) | 25 (35.2) | 30 (42.3) | ||
| Age (ys) | 0.913 | 0.977 | ||||
| <40 | 16 (20.5) | 21 (19.4) | 15 (21.1) | 16 (22.5) | ||
| 41-59 | 39 (50.0) | 52 (48.1) | 37 (52.1) | 36 (50.7) | ||
| 60-75 | 23 (29.5) | 35 (32.4) | 19 (26.8) | 19 (26.8) | ||
| PS score | 0.324 | 0.609 | ||||
| 0 | 45 (57.7) | 70 (64.8) | 43 (60.6) | 40 (56.3) | ||
| 1 | 33 (42.3) | 38 (35.2) | 28 (39.4) | 31 (43.7) | ||
| cT stage | 0.483 | 0.790 | ||||
| T3 | 15 (19.2) | 29 (26.9) | 15 (21.1) | 14 (19.7) | ||
| T4a | 51 (65.4) | 64 (59.3) | 46 (64.8) | 44 (62.0) | ||
| T4b | 12 (15.4) | 15 (13.9) | 10 (14.1) | 13 (18.3) | ||
| cN stage | 0.768 | 0.870 | ||||
| N0 | 12 (15.4) | 20 (18.5) | 11 (15.5) | 8 (11.3) | ||
| N1 | 26 (33.3) | 36 (33.3) | 25 (35.2) | 26 (36.6) | ||
| N2 | 31 (39.7) | 36 (33.3) | 27 (38.0) | 27 (38.0) | ||
| N3 | 9 (11.5) | 16 (14.8) | 8 (11.3) | 10 (14.1) | ||
| P stage | 0.012 | 0.752 | ||||
| P0CY1 | 6 (7.7) | 23 (21.3) | 6 (8.5) | 3 (4.2) | ||
| P1 | 28 (35.9) | 44 (40.7) | 27 (38.0) | 29 (40.8) | ||
| P2 | 31 (39.7) | 34 (31.5) | 30 (42.3) | 32 (45.1) | ||
| P3 | 13 (16.7) | 7 (6.5) | 8 (11.3) | 7 (9.9) | ||
| Measurable lesion | 0.137 | 0.731 | ||||
| No | 29 (37.2) | 52 (48.1) | 29 (40.8) | 27 (38.0) | ||
| Yes | 49 (62.8) | 56 (51.9) | 42 (59.2) | 44 (62.0) | ||
| Ascites volume | 0.010 | 0.581 | ||||
| None | 7 (9.0) | 23 (21.3) | 7 (9.9) | 6 (8.5) | ||
| + | 29 (37.2) | 49 (45.4) | 28 (39.4) | 32 (45.1) | ||
| ++ | 34 (43.6) | 33 (30.6) | 29 (40.8) | 30 (42.3) | ||
| +++ | 8 (10.3) | 3 (2.8) | 7 (9.9) | 3 (4.2) | ||
Abbreviations: PSM, propensity score matching; NIPS, neoadjuvant intraperitoneal and systemic chemotherapy; NSC, neoadjuvant systemic chemotherapy; PS, performance status; P stage, peritoneal stage; +, <500 ml; ++, 500∼2000 ml; +++, >2000 ml.
Response to NAC
In the PSM dataset, the average numbers of NAC cycles administered per patient were 6.8 ± 2.0 (range 2-12) and 6.6 ± 1.7 (range 3-11) for NIPS and NSC group respectively (P = 0.500). The overall tumor response to NAC was not statistically significant between the 2 groups (P = 0.824), and the response rate (RR, P = 0.841) and the disease control rate (DCR, P = 0.522) were also comparable. However, NIPS was superior to NSC with respect to ascites response (P < 0.001) and cytology response (P < 0.001). Disappearance of ascites was witnessed in 45.3% patients under NIPS treatment compared with 10.8% under NSC treatment. Meanwhile, 73.2% patients achieved negative results of cytology examination after NIPS treatment compared with 43.7% after NSC treatment. The detailed data of response to NAC was shown in Table 2.
Table 2.
Response to NAC and Surgical Outcomes.
| Variable | NIPS | NSC | P |
|---|---|---|---|
| n (%) | n (%) | ||
| NAC cycle | 6.8 ± 2.0 | 6.6 ± 1.7 | 0.500 |
| Tumor response | 42 (100) | 44 (100) | 0.824 |
| CR | 3 (7.1) | 2 (4.5) | |
| PR | 19 (45.2) | 22 (50.0) | |
| SD | 16 (38.1) | 14 (31.8) | |
| PD | 4 (9.5) | 6 (13.6) | |
| RR (CR+PR) | 22 (52.4) | 24 (54.5) | 0.841 |
| DCR (CR+PR+SD) | 38 (90.5) | 38 (86.4) | 0.522 |
| Ascites response | 64 (100) | 65 (100) | <0.001 |
| Disappearance | 29 (45.3) | 7 (10.8) | |
| Decreased | 23 (35.9) | 29 (44.6) | |
| Stable | 8 (12.5) | 21 (32.3) | |
| Progression | 4 (6.3) | 8 (12.3) | |
| Cytology response | 71 (100) | 71 (100) | <0.001 |
| Negative | 52 (73.2) | 31 (43.7) | |
| Positive | 19 (26.8) | 40 (56.3) | |
| Conversion surgery | 71 (100) | 71 (100) | 0.012 |
| No | 31 (43.7) | 46 (64.8) | |
| Yes | 40 (56.3) | 25 (35.2) | |
| R0 resection | 71 (100) | 71 (100) | 0.039 |
| No | 37 (52.1) | 49 (69.0) | |
| Yes | 34 (47.9) | 22 (31.0) | |
| Complication | 40 (100) | 25 (100) | 0.425 |
| No | 29 (72.5) | 19 (76.0) | |
| Yes | 11 (27.5) | 6 (24.0) |
Abbreviations: NAC, neoadjuvant chemotherapy; NIPS, neoadjuvant intraperitoneal and systemic chemotherapy; NSC, neoadjuvant systemic chemotherapy; CR, complete response; PR, partial response; SD, stable disease; PD, progression disease; RR, response rate; DCR, disease control rate.
Conversion Surgery Outcomes
In the unmatched study, 42 out of 78 patients (54%) in NIPS group and 48 out of 108 patients (44%) in NSC group underwent conversion surgery (P = 0.205). 35 out of 78 patients (45%) in NIPS group and 43 out of 108 patients (40%) in NSC group received R0 resection (P = 0.490). After PSM, 40 patients (56%) in NIPS group underwent conversion surgery, of whom 34 patients (48%) received R0 resection; of the 25 patients (35%) in NSC group who underwent conversion surgery, 22 patients (31%) received R0 resection, indicating that NIPS was associated with higher conversion rate (P = 0.012) and R0 resection rate (P = 0.039). No perioperative death occurred in either group. No perceptible differences were observed in terms of the incidence of surgical complication (NIPS 27.5% vs NSC 24.0%, P = 0.425; Table 2).
NAC-Related Adverse Events
As shown in Table 3, the overall incidence of Grade 3/4 NAC-related adverse events was high in both groups (NIPS 73.2% vs NSC 69.0%), but no statistically significant difference was observed between the 2 groups (P = 0.579). Even though, there was no NAC-related death in either group. The most common Grade 3/4 adverse events were leukopenia (NIPS 38.0% vs NSC 31.0%, P = 0.377) and neutropenia (NIPS 57.7% vs NSC 53.5%, P = 0.612) in both groups. It is noteworthy that patients in NIPS group experienced a higher incidence of diarrhea (25.4% vs 11.3%, P = 0.030) as compared with SOX group.
Table 3.
Grade 3/4 NAC-Related Adverse Events (n = 142).
| Event | NIPS | NSC | P |
|---|---|---|---|
| N = 71 (%) | N = 71 (%) | ||
| Overall toxicity | 52 (73.2) | 49 (69.0) | 0.579 |
| Related death | 0 (0) | 0 (0) | NA |
| Leukopenia | 27 (38.0) | 22 (31.0) | 0.377 |
| Neutropenia | 41 (57.7) | 38 (53.5) | 0.612 |
| Thrombocytopenia | 16 (22.5) | 20 (28.2) | 0.440 |
| Anorexia | 11 (15.5) | 15 (21.1) | 0.385 |
| Vomitting | 13 (18.3) | 16 (22.5) | 0.532 |
| Neurotoxicity | 19 (26.8) | 17 (23.9) | 0.700 |
| Diarrhea | 18 (25.4) | 8 (11.3) | 0.030 |
Abbreviations: NAC, neoadjuvant chemotherapy; NIPS, neoadjuvant intraperitoneal and systemic chemotherapy; NSC, neoadjuvant systemic chemotherapy; NA, not applicable.
Survival Analyses
The median length of follow-up was 43.0 (95%CI 37.6-48.4) months and 59.0 (95% CI 29.1-88.9) months for NIPS and NSC group respectively (P = 0.004). The median survival time for patients in NIPS group and NSC group was 21.0 (95%CI 15.5-26.5) months and 16.0 (95%CI 14.6-17.4) months respectively, and Kaplan-Meier analysis revealed that NIPS yielded better OS than NSC (P < 0.001) (Figure 1A). Accordingly, the 1-year survival rates in NIPS group and NSC group was 88.7% and 73.2%, respectively (P = 0.019); the 2-year survival rates was 44.7% and 25.4%, respectively (P = 0.014); and the 3-year survival rates was 21.9% and 4.5%, respectively (P = 0.001).
Figure 1.
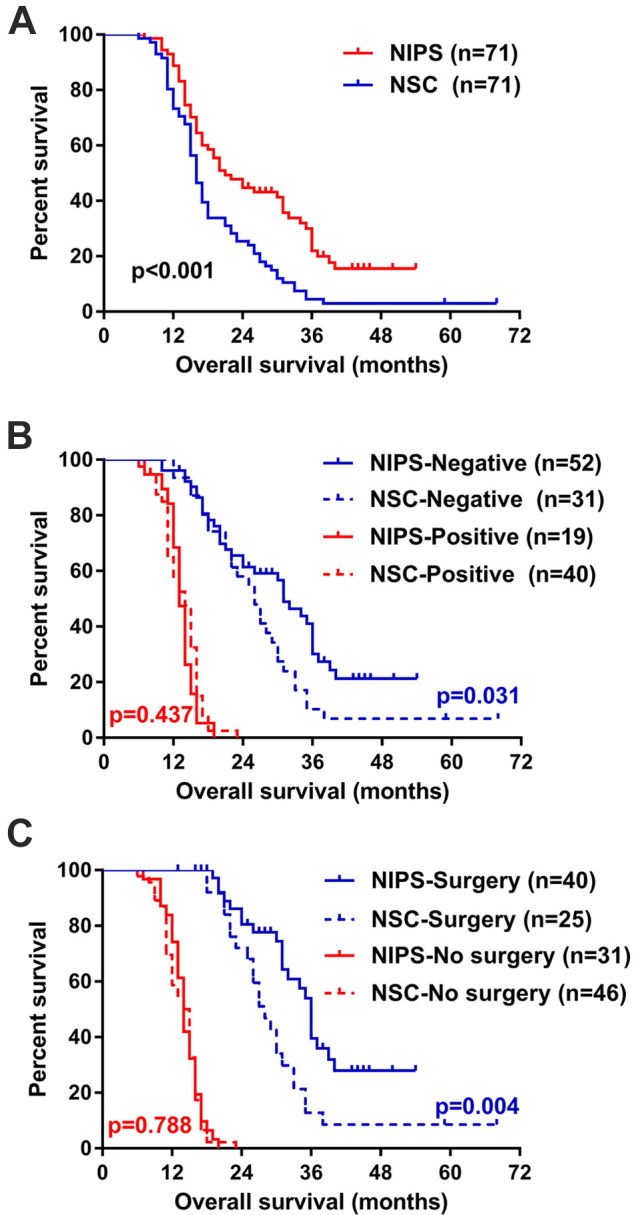
Kaplan-Meier overall survival analysis. A, NIPS group (n = 71) versus NSC group (n = 71) after PSM. B, Stratified by cytology. C, Stratified by conversion surgery. NIPS indicates neoadjuvant intraperitoneal and systemic chemotherapy; NSC, neoadjuvant systemic chemotherapy.
Prognostic Analyses
As shown in Table 4, univariate analysis indicated that both patient-related and treatment-related factors might be the predictors for patient survival. Specifically, the patient-related prognostic factors included performance status (P < 0.001), cT stage (P < 0.001), cN stage (P < 0.001), P stage (P < 0.001), and ascites volume (P < 0.001), while the treatment-related prognostic factors included NAC regimen (P < 0.001), tumor response (P < 0.001), ascites response (P < 0.001), cytology response (P < 0.001), conversion surgery rate (P < 0.001), R0 resection rate (P < 0.001), and the occurrence of surgical complication (P = 0.006). However, multivariate analysis showed that only the P stage (P = 0.001), ascites response (P < 0.001), conversion surgery rate (P = 0.010) and R0 resection rate (P = 0.026) were independent prognostic factors (Table 5).
Table 4.
Univariate Analysis for Prognostic Factors in PSM Analysis (n = 142).
| Variable | HR | 95% CI | P | Variable | HR | 95% CI | P |
|---|---|---|---|---|---|---|---|
| NAC regimen | <0.001 | Age (ys) | 0.271 | ||||
| NIPS | 0.520 | 0.360-0.750 | <40 | 0.653 | 0.388-1.098 | ||
| NSC | Ref | 41-59 | 0.817 | 0.540-1.238 | |||
| Gender | 0.844 | 60-75 | Ref | ||||
| Male | 0.964 | 0.669-1.390 | cT stage | <0.001 | |||
| Female | Ref | T3 | 0.106 | 0.053-0.210 | |||
| PS score | <0.001 | T4a | 0.361 | 0.220-0.592 | |||
| 0 | 0.522 | 0.362-0.752 | T4b | Ref | |||
| 1 | Ref | P stage | <0.001 | ||||
| cN stage | <0.001 | P0CY1 | 0.020 | 0.004-0.089 | |||
| N0 | 0.160 | 0.073-0.349 | P1 | 0.152 | 0.081-0.287 | ||
| N1 | 0.351 | 0.198-0.625 | P2 | 0.433 | 0.244-0.770 | ||
| N2 | 0.917 | 0.530-1.589 | P3 | Ref | |||
| N3 | Ref | Tumor response | <0.001 | ||||
| Ascites volume | <0.001 | CR | 0.060 | 0.018-0.199 | |||
| None | 0.137 | 0.054-0.346 | PR | 0.156 | 0.073-0.336 | ||
| + | 0.246 | 0.122-0.497 | SD | 0.279 | 0.130-0.597 | ||
| ++ | 0.265 | 0.131-0.533 | PD | Ref | |||
| +++ | Ref | Ascites response | <0.001 | ||||
| Cytology response | <0.001 | Disappearance | 0.018 | 0.007-0.043 | |||
| Negative | 0.096 | 0.058-0.161 | Decreased | 0.053 | 0.024-0.118 | ||
| Positive | Ref | Stable | 0.321 | 0.156-0.662 | |||
| Conversion surgery | <0.001 | Progression | Ref | ||||
| No | Ref | R0 resection | <0.001 | ||||
| Yes | 0.031 | 0.015-0.063 | No | Ref | |||
| Complication | 0.006 | Yes | 0.017 | 0.006-0.046 | |||
| No | 0.402 | 0.211-0.767 | |||||
| Yes | Ref | ||||||
Abbreviations: PSM, propensity score matching; NAC, neoadjuvant chemotherapy; NIPS, neoadjuvant intraperitoneal and systemic chemotherapy; NSC, neoadjuvant systemic chemotherapy; PS, performance status; CR, complete response; PR, partial response; SD, stable disease; PD, progression disease; RR, response rate; DCR, disease control rate; HR, hazard ratio; Ref, reference; CI, confidence interval; +, <500 ml; ++, 500∼2000 ml; +++, >2000 ml.
Table 5.
Multivariate Analysis for Prognostic Factors (n = 142).
| Variable | HR | 95% CI | P |
|---|---|---|---|
| P stage | 2.008 | 1.340-3.011 | 0.001 |
| Ascites response | 2.545 | 1.782-3.635 | <0.001 |
| Conversion surgery | 0.229 | 0.075-0.705 | 0.010 |
| R0 resection | 0.131 | 0.022-0.787 | 0.026 |
Abbreviations: PSM, propensity score matching; HR, hazard ratio; CI, confidence interval.
Subgroup Analyses
Subgroup analysis was performed in the PSM population. Statistically significant interaction effect on survival was detected between the NAC regimen and cT stage (P = 0.021), conversion surgery rate (P = 0.032), and cytology response (P = 0.044), respectively. As shown in Figure 2, patients with cT3-4a benefited from the addition of intraperitoneal docetaxel, but those with cT4b did not. Also, NIPS showed a survival benefit over NSC in patients whose cytology turned negative (P = 0.031, Figure 1B), and in patients who received conversion surgery (Figure 1C); but no statistical significant difference was observed between NIPS and NSC treatment in patients whose cytology did not turn negative (P = 0.437, Figure 1B) or in patients who did not receive conversion surgery (Figure 1C). In addition, variations still existed between strata in P stage (Figure 2). NIPS was associated with better survival than NSC in P1 and P2 patients, while such difference was not statistically significant in P0 (HR 0.866, 95% CI 0.054-13.945; data not shown in Figure 2) or P3 patients.
Figure 2.
Subgroup analysis in the propensity score matched analysis. *P refers to P value for interaction analysis. NIPS indicates neoadjuvant intraperitoneal and systemic chemotherapy; NSC, neoadjuvant systemic chemotherapy.
Discussion
This is the first study to make a direct comparison of the efficacy and safety between NIPS and NSC as conversion therapy based on the same drug combination, and especially, of the same total drug dosage for the treatment of GCPM. The present study showed that NIPS was superior to NSC with respect to ascites response and cytology response, which further transformed into higher conversion surgery rate and R0 resection rate. Most importantly, NIPS yielded significantly better patient survival compared with NSC treatment. Although the overall chemotherapy toxicity was relatively high in both groups, especially leukopenia and neutropenia, NIPS did not bring a significant increase in the incidence of toxicity compared with NSC except for diarrhea. Therefore, the encouraging results from the present study could be considered as robust evidences to support the application of the novel triplet NIPS regimen in the treatment of GCPM.
Indeed, PM denotes worse outcomes than nonmetastatic disease and precludes consideration for upfront curative resection. 28 Therefore, palliative systemic chemotherapy with fluoropyrimidine and cisplatin has long been recommended in both the National Comprehensive Cancer Network (NCCN) guidelines 5 and the Japanese Gastric Cancer Treatment guidelines. 29 Given that IV administered docetaxel is able to penetrate ascites, 30 a triplet systemic chemotherapy regimen involving docetaxel, cisplatin, and S-1 was also used to treat GCPM. 31 Nevertheless, the overall prognosis remains extremely poor. A possible explanation for these frustrating results is that systemic chemotherapy alone is insufficient to eliminate PM. In contrast, IP administration of chemotherapeutic agents permits direct interaction of the drugs with the peritoneal disseminated nodules as well as free cancer cells in the peritoneal cavity, achieving significant effectiveness for both microscopic and macroscopic PM. 32
Taxanes are absorbed slowly through the lymphatic system after IP administration and rarely cause fibrotic adhesions in the abdomen even under repeated IP administration due to their physicochemical and pharmacokinetic properties, 18 rendering them to be the optimal agents for IP administration. In addition, as a representative of the third generation of platinum-based compound, oxaliplatin has a favorable toxicity profile compared with cisplatin. Therefore, we developed this triplet NIPS regimen on the basis of NSC with docetaxel, oxaliplatin, and S-1, by transferring partial dosages of docetaxel from IV route to IP route to balance the anti-tumor effect on both the primary tumor and the PM. Besides, we did not include patients who had undergone gastrectomy in this study to rule out the potential influence on the absorption of drugs caused by abdominal adhesions.
Accurate preoperative assessment of PM is essential for the determination of treatment options. However, peritoneal metastases are often only diagnosed intraoperatively due to the low sensitivity of CT for detection of peritoneal metastases in GC. Nevertheless, preoperative CT scan is still necessary as it may aid in determining the existence and volume of malignant ascites. 33 GC patients with malignant ascites tended to have a worse prognosis, 34 and a good response of malignant ascites was associated with improved OS in patients with PM. 35 However, unlike primary tumor, there is still no standard criterion on the quantity evaluation of the response of ascites. Cho et al 36 used CT scan based on the Japanese conventional 5-point method to quantify the volume of ascites, and set the cutoff value of ± 10% on the changes of ascites volume to make an assessment. Another, Ni et al 35 used an estimation of over 50% reduction in the depth of ascites based on ultrasound examination to evaluate the response of ascites. As CT is more accurate and objective than ultrasound in determining the ascites, we also used CT in the present study to estimate the ascites volume, with the cutoff of 30% reduction and 20% increase in the volume to quantify the response of ascites. Especially, multivariate analysis revealed that the response of ascites was an independent prognostic factor for patient survival, which is consistent with results from previous literatures. 35,36
Currently, increasing evidence has revealed that palliative gastrectomy after chemotherapy did not provide a survival benefit in comparison to chemotherapy alone. 37 On the other hand, conversion chemotherapy followed by curative surgery has been extensively investigated as a promising treatment for GCPM in the past decade. However, the specific therapeutic effect on GCPM is difficult to evaluate as distinction was seldom made between PM and other forms of metastasis in previous literatures. 16 In a retrospective study by Nakamura et al, 38 conversion surgery was performed in 28.8% (17/59) GC patients with CY1P0 or P1, and the median survival time was better in patients with conversion surgery than without it (CY1P0: 41 vs 11 months; P1: 31 vs 10 months). Yasufuku et al 39 reported that the doublet NSC regimen with platinum and fluorouracil yielded a 40.6% (13/32) conversion gastrectomy rate in GC patients with P0CY1, with a 3-year survival rate of 76.9%. Our present study showed that all patients with P0CY1 underwent conversion surgery in both groups, and the 3-year survival rate was 50.0% and 66.7% for NIPS (n = 6) and NSC (n = 3) group respectively (P = 0.635). These results seemed to indicate that NSC alone without IP administration was sufficient to achieve a satisfactory conversion rate for patients of GCPM who had no macroscopic peritoneal lesions. However, this finding should be interpreted with caution due to the very small sample size in this study. Therefore, we believe that patients of P0CY1 are also candidates for NIPS treatment as a conversion therapy.
Surgery may result in the forced suspension of perioperative chemotherapy, a postoperative decrease of chemotherapy tolerance, and a postoperative decline in antitumor immunity, which brings about the progression of the occult residual tumor. Therefore, the selection of candidates and timing for the second-look laparoscopic exploration is of great importance. Appropriate reevaluation before surgical resection might help to avoid surgeries that would result in R1 or R2 resection. Meanwhile, radical gastrectomy should usually be performed when the peritoneal lesions are entirely eliminated, or sufficiently controlled at least, and excessive surgical stress and complications should be avoided. 23,39 In the present study, conversion gastrectomy was performed only in patients whose peritoneal lesions were well controlled and cytology turned negative. In addition, the post-operative adjuvant chemotherapy should be resumed as soon as the patients recovered from the surgery.
Some limitations existed in the present study. Firstly, Because NIPS was never used before the year 2016 in our department, the observation period in NIPS group was relatively short. During the follow-up, some patients survived for more than 3 years without recurrence, for whom an even longer survival may be expected. Therefore, the efficacy of NIPS might, to some extent, be underestimated. Secondly, all the subjects in this study are Chinese, the majority of whom are resided in East China or Jiangxi Province. All radical surgeries were performed by 3 proficient gastrointestinal surgeons in our department. Therefore, the generalizability of the conclusions to population with different geographical environments, ethnics, or races, or to surgeons with varied levels of surgical experience may be restricted. Finally, the present study was a retrospective, single-center cohort study, and the population was relatively small. Although a PSM analysis was carried out to reduce the selection bias as possible, a prospective randomized controlled trial is still warranted to further confirm the conclusion in the future.
Footnotes
Authors’ Note: Xin Zhang, Hejing Huang, and Dejun Yang contributed equally to this work. This study was conducted in accordance with the Declaration of Helsinki and the research protocol was approved by the ethics committee of Changzheng Hospital, Naval Medical University (No. C20180061).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the National Natural Science Foundation of China (81773049, 81402359).
ORCID iD: Qingping Cai  https://orcid.org/0000-0001-8088-0463
https://orcid.org/0000-0001-8088-0463
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 3. Bozzetti F, Yu W, Baratti D, Kusamura S, Deraco M. Locoregional treatment of peritoneal carcinomatosis from gastric cancer. J Surg Oncol. 2008;98(4):273–276. [DOI] [PubMed] [Google Scholar]
- 4. Kanda M, Kodera Y. Molecular mechanisms of peritoneal dissemination in gastric cancer. World J Gastroenterol. 2016;22(30):6829–6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Gastric Cancer. Version 2. 2019. Updated June 03, 2019. Accessed November 12, 2019. https://www.nccn.org/
- 6. Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet. 2016;388(10060):2654–2664. [DOI] [PubMed] [Google Scholar]
- 7. Knodler M, Korfer J, Kunzmann V, et al. Randomised phase II trial to investigate catumaxomab (Anti-EpCAM x anti-CD3) for treatment of peritoneal carcinomatosis in patients with gastric cancer. Br J Cancer. 2018;119(3):296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takashima A, Shitara K, Fujitani K, et al. Peritoneal metastasis as a predictive factor for nab-paclitaxel in patients with pretreated advanced gastric cancer: an exploratory analysis of the phase III ABSOLUTE trial. Gastric Cancer. 2019;22(1):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18(6):1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim DW, Park DG, Song S, Jee YS. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy as treatment options for peritoneal metastasis of advanced gastric cancer. J Gastric Cancer. 2018;18(3):296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manzanedo I, Pereira F, Rihuete Caro C, et al. Cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for gastric cancer with peritoneal carcinomatosis: multicenter study of Spanish group of peritoneal oncologic surgery (GECOP). Ann Surg Oncol. 2019;26(8):2615–2621. [DOI] [PubMed] [Google Scholar]
- 12. Wu HT, Peng KW, Ji ZH, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with lobaplatin and docetaxel to treat synchronous peritoneal carcinomatosis from gastric cancer: results from a Chinese center. Eur J Surg Oncol. 2016;42(7):1024–1034. [DOI] [PubMed] [Google Scholar]
- 13. Chia CS, You B, Decullier E, et al. Patients with peritoneal carcinomatosis from gastric cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is cure a possibility? Ann Surg Oncol. 2016;23(6):1971–1979. [DOI] [PubMed] [Google Scholar]
- 14. Boerner T, Graichen A, Jeiter T, et al. CRS-HIPEC prolongs survival but is not curative for patients with peritoneal carcinomatosis of gastric cancer. Ann Surg Oncol. 2016;23(12):3972–3977. [DOI] [PubMed] [Google Scholar]
- 15. Rudloff U, Langan RC, Mullinax JE, et al. Impact of maximal cytoreductive surgery plus regional Heated Intraperitoneal Chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol. 2014;110(3):275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fukuchi M, Ishiguro T, Ogata K, et al. Prognostic role of conversion surgery for unresectable gastric cancer. Ann Surg Oncol. 2015;22(11):3618–3624. [DOI] [PubMed] [Google Scholar]
- 17. Jacquet P, Sugarbaker PH. Peritoneal-plasma barrier. Cancer Treat Res. 1996;82:53–63. [DOI] [PubMed] [Google Scholar]
- 18. Yamaguchi H, Kitayama J, Ishigami H, et al. Breakthrough therapy for peritoneal carcinomatosis of gastric cancer: intraperitoneal chemotherapy with taxanes. World J Gastrointest Oncol. 2015;7(11):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ishigami H, Fujiwara Y, Fukushima R, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC Trial. J Clin Oncol. 2018;36(19):1922–1929. [DOI] [PubMed] [Google Scholar]
- 20. Fujiwara Y, Takiguchi S, Nakajima K, et al. Intraperitoneal docetaxel combined with S-1 for advanced gastric cancer with peritoneal dissemination. J Surg Oncol. 2012;105(1):38–42. [DOI] [PubMed] [Google Scholar]
- 21. Chan DY, Syn NL, Yap R, et al. Conversion surgery post-intraperitoneal paclitaxel and systemic chemotherapy for gastric cancer carcinomatosis peritonei. Are we ready? J Gastrointest Surg. 2017;21(3):425–433. [DOI] [PubMed] [Google Scholar]
- 22. Kitayama J, Ishigami H, Yamaguchi H, et al. Salvage gastrectomy after intravenous and intraperitoneal paclitaxel (PTX) administration with oral S-1 for peritoneal dissemination of advanced gastric cancer with malignant ascites. Ann Surg Oncol. 2014;21(2):539–546. [DOI] [PubMed] [Google Scholar]
- 23. Ishigami H, Yamaguchi H, Yamashita H, Asakage M, Kitayama J. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer. 2017;20(suppl 1):128–134. [DOI] [PubMed] [Google Scholar]
- 24. Zhang X, Huang H, Wei Z, et al. Comparison of docetaxel + oxaliplatin + S-1 vs oxalipatin + S-1 as neoadjuvant chemotherapy for locally advanced gastric cancer: a propensity score matched analysis. Cancer Manag Res. 2020;12:6641–6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oriuchi N, Nakajima T, Mochiki E, et al. A new, accurate and conventional five-point method for quantitative evaluation of ascites using plain computed tomography in cancer patients. Jpn J Clin Oncol. 2005;35(7):386–390. [DOI] [PubMed] [Google Scholar]
- 26. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 27. Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. [DOI] [PubMed] [Google Scholar]
- 28. Dahdaleh FS, Turaga KK. Evolving treatment strategies and outcomes in advanced gastric cancer with peritoneal metastasis. Surg Oncol Clin N Am. 2018;27(3):519–537. [DOI] [PubMed] [Google Scholar]
- 29. Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tamegai H, Kaiga T, Kochi M, et al. Pharmacokinetics of docetaxel in gastric cancer patients with malignant ascites. Cancer Chemother Pharmacol. 2013;71(3):727–731. [DOI] [PubMed] [Google Scholar]
- 31. Okabe H, Hata H, Hosogi H, et al. A phase 2 study of induction chemotherapy using docetaxel, cisplatin, and S-1 for gastric cancer with peritoneal metastasis (KUGC06). Ann Surg Oncol. 2019;26(6):1779–1786. [DOI] [PubMed] [Google Scholar]
- 32. Yamaguchi H, Kitayama J, Ishigami H, Emoto S, Yamashita H, Watanabe T. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer. 2013;119(18):3354–3358. [DOI] [PubMed] [Google Scholar]
- 33. Kim SH, Choi YH, Kim JW, et al. Clinical significance of computed tomography-detected ascites in gastric cancer patients with peritoneal metastases. Medicine (Baltimore). 2018;97(8):e9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zheng LN, Wen F, Xu P, Zhang S. Prognostic significance of malignant ascites in gastric cancer patients with peritoneal metastasis: a systemic review and meta-analysis. World J Clin Cases. 2019;7(20):3247–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ni X, Wu P, Wu J, et al. Hyperthermic intraperitoneal perfusion chemotherapy and response evaluation in patients with gastric cancer and malignant ascites. Oncol Lett. 2017;14(2):1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cho H, Ryu MH, Kim KP, et al. Phase I/II study of a combination of capecitabine, cisplatin, and intraperitoneal docetaxel (XP ID) in advanced gastric cancer patients with peritoneal metastasis. Gastric Cancer. 2017;20(6):970–977. [DOI] [PubMed] [Google Scholar]
- 37. Fujitani K, Yang HK, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17(3):309–318. [DOI] [PubMed] [Google Scholar]
- 38. Nakamura M, Ojima T, Nakamori M, et al. Conversion surgery for gastric cancer with peritoneal metastasis based on the diagnosis of second-look staging laparoscopy. J Gastrointest Surg. 2019;23(9):1758–1766. [DOI] [PubMed] [Google Scholar]
- 39. Yasufuku I, Nunobe S, Ida S, et al. Conversion therapy for peritoneal lavage cytology-positive type 4 and large type 3 gastric cancer patients selected as candidates for R0 resection by diagnostic staging laparoscopy. Gastric Cancer. 2019;23(2):319–327. [DOI] [PubMed] [Google Scholar]



