Abstract
Background
Natalizumab is a highly efficacious treatment for relapsing-remitting multiple sclerosis (RRMS).
Objective
To assess the real-world long-term safety of natalizumab in RRMS.
Methods
This multicenter, 5-year prospective observational study, included adults with RRMS newly initiated on natalizumab as per the approved product label in the routine care in Greece. Safety was evaluated by collecting serious adverse events (SAEs) following study enrollment.
Results
Between 19-Apr-2012 and 18-Dec-2014, 304 eligible patients (median age at natalizumab initiation: 38.0 years; median disease duration: 6.2 years) were enrolled by 20 hospital-based neurologists. Over a median treatment duration period of 58.7 months, 50.7% of the patients discontinued natalizumab, mainly due to anti-JCV antibody detection (59.1%). The adverse event treatment discontinuation rate was 5.2%. The SAE incidence rate during the safety data collection period (median: 48.7 months) was 4.6%. The most common SAEs were infections (1.0%), including 2 cases (0.7%) of progressive multifocal leukoencephalopathy (PML), and no other opportunistic infections. PML diagnoses occurred 6.2-6.7 years after natalizumab initiation, and approximately 2 years after first detection of anti-JCV antibody for both patients. The incidence rate of malignancies was 0.7%.
Conclusion
In real-world settings in Greece, natalizumab displayed an acceptable safety profile, with no new safety signals emerging.
Keywords: adverse events, JC virus, long-term safety, multiple sclerosis, natalizumab, observational study, progressive multifocal leukoencephalopathy, relapsing-remitting
Introduction
Natalizumab is a humanized recombinant monoclonal antibody approved as monotherapy for the treatment of relapsing-remitting multiple sclerosis (RRMS). By binding to the α4 subunit of α4β1 and α4β7 integrins and blocking their binding to their endothelial receptors, natalizumab inhibits leukocyte transmigration across the blood-brain-barrier and pathological inflammation, and reduces the formation or enlargement of multiple sclerosis (MS) central nervous system (CNS) lesions.1–4
Two pivotal controlled studies, the phase III AFFIRM trial examining the efficacy of natalizumab (Tysabri®) as monotherapy, and the phase III SENTINEL trial examining its efficacy in combination with interferon-b1a, demonstrated that natalizumab is associated with a significant reduction in the clinical relapse and sustained disability progression rates, and in other imaging measures of MS disease activity.5,6 Following report of three cases of progressive multifocal leukoencephalopathy (PML), a rare opportunistic infection of the CNS caused by the John Cunningham virus (JCV), two in SENTINEL (which was early terminated) and one in a trial in patients with Crohn’s disease, natalizumab was withdrawn from the market. Following additional research and analyses of risks and benefits it was remarketed as monotherapy for highly active RRMS with the recommendation for monitoring for new cases of PML.4,7 Based on data from 2009 to 2018 the risk of PML has leveled-off in mid-2016, and stabilized thereon at at 4.14-4.18/1,000.8,9 The presence of anti-JCV antibodies in serum, long (>2 years) natalizumab therapy duration, and prior receipt of immunosuppressants have been identified as risk factors of PML occurrence.10–12 Risk stratification algorithms and PML management strategies are being implemented in the clinic, which facilitate personalized treatment-decision making and a safer use of natalizumab.4,7,12,13
In view of the above, a number of real-world studies initiated following natalizumab approval aimed to monitor the long-term clinical and safety outcomes of natalizumab. The present prospective 5-year observational study was designed to assess the long-term safety profile and impact of natalizumab on disease activity and progression, in patients treated under routine clinical care conditions in Greece. The baseline characteristics of the study population and safety outcomes are presented herein.
Patients and methods
Study design and patients
‘TOPICS (TYSABRI® Observational Program International data CollectionS) Greece’ was a non-interventional, open label, multicenter, prospective, observational study, conducted in Greece, which included patients with RRMS who were therapy-naïve to natalizumab (TYSABRI®) and who met the criteria for initiating therapy according to the locally approved label.
The decision to treat the patient with natalizumab had been taken prior to enrollment. Eligible patients ought to have received ≤3 natalizumab infusions before study enrollment, unless they were enrolled during the 6-month periods after approval of the original protocol and protocol amendment I or during the 3-month period after approval of protocol amendment II by the Greek Regulatory Authority and the Scientific Committees of the participating hospitals (the purpose of these periods was to facilitate recruitment). During these three periods, which included retrospective data collection (other than of safety data) patients could be enrolled irrespective of the number of prior natalizumab infusions they had received, as long as they met all other eligibility criteria. Moreover, patients from Greece enrolled in the Tysabri Observation Program (TOP) study could be transferred to the TOPICS Greece database.
Eligible patients had a documented diagnosis of RRMS and highly active disease at the time of initiating treatment with natalizumab. Concomitant receipt of immunomodulatory or immunosuppressive therapy was not allowed. Female participants were postmenopausal for at least one year, surgically sterile, or willing to practice effective contraception while receiving treatment. Women who were breastfeeding or pregnant were excluded from study participation.
The observation period for each patient, i.e., the time between informed consent signing and completion of study participation, was 5 years. Data were collected at enrollment and at clinical visits performed at 6 (±1)-month intervals, and during any visits taking place in order to evaluate new or worsening neurological symptoms or serious adverse events (SAEs). Patients who discontinued natalizumab were encouraged to remain in the study, in order to collect data, including SAEs, for an additional 6 months, where possible.
Only prospective safety data collection took place, i.e., adverse events (AEs) experienced prior to informed consent signing were not recorded. AEs handled as solicited reports and reported in the context of this study included: all SAEs, suspected transmission of infectious agent via a medicinal product, and drug exposure and outcomes of use during pregnancy. Non-serious AEs, drug exposure and adverse reactions during breastfeeding, off-label use, overdose, abuse, misuse, medication errors, occupational exposure, and lack of efficacy, were not handled as solicited AEs. MS relapses meeting criteria of SAE were not be considered as SAEs for the purposes of this study.
The study protocol and its amendments were approved by the competent national regulatory authority [National Organization for Medicines (EOF)] and the scientific committees of the participating hospitals. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and all applicable local regulations. All participating patients provided written informed consent.
Study objectives
The primary study objective was the long-term SAE incidence rate in patients receiving natalizumab. Secondary endpoints included the assessment of MS disease activity, as determined by the occurrence of clinical relapses, the assessment of disability progression as determined by the Expanded Disability Status Scale (EDSS), the evaluation of baseline disease characteristics as prognostic indicators for disease activity and disability progression over time, and the evaluation of short-term disease outcomes as prognostic indicators for disease activity and disability progression over time. In the present publication, only baseline patient characteristics and results pertaining to the primary objective are presented. Secondary outcomes are presented in a separate publication.
Sample size
The sample size was not based on statistical power considerations; between 300 to 500 patients were considered adequate to examine the primary objective of the study in a descriptive manner.
Statistical methods
Statistical analysis was performed using SAS software (v9.4; SAS Institute, Carry, NC). Baseline was defined as the time closest prior to or at natalizumab treatment onset. Data are presented as median [interquartile range (IQR)] since they did not follow a normal distribution. The incidence of SAEs was assessed by calculating the percentage of patients experiencing at least one SAE as well as the exposure-adjusted incidence rates (EAIRs) along with the respective 95% confidence intervals (CIs). The EAIR was defined as the number of patients with a SAE divided by the total exposure-time in subject-years. Recorded SAEs were mapped by system organ class (SOC) and preferred term (PT) according to the Medical Dictionary for Regulatory Activities (MedDRA). No imputation of missing data was applied with the exception of partial start or end dates.
Results
Patient enrollment and disposition
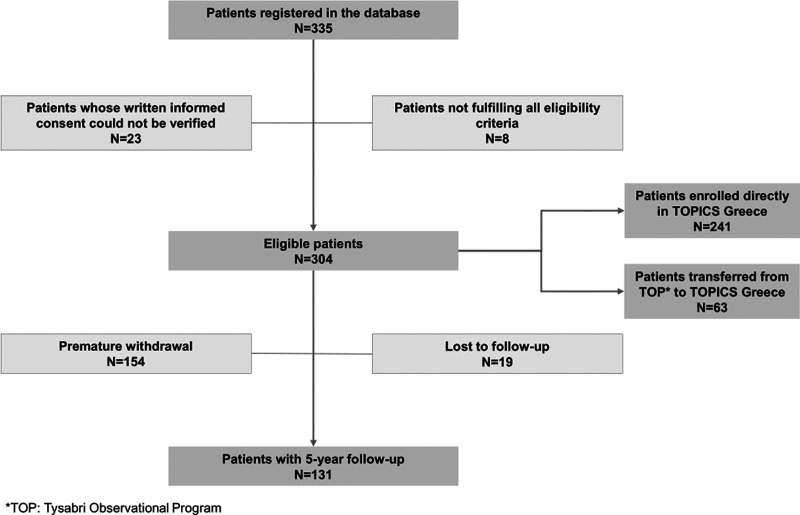
Between 19-April-2012 and 18-December-2014, 304 eligible patients [median (IQR) age at enrollment: 39.5 (31.0-47.0) years] were enrolled in the study by 20 study sites distributed across eight of the 13 administrative regions of Greece. The median (IQR) duration of study participation was 48.7 (23.0-58.9) months.
Of the patients, 50.7% (154/304) were prematurely withdrawn from the study after a median (IQR) of 24.7 (15.1-42.2) months of participation (Figure 1). The main reasons for premature study withdrawal, reported in >10.0% of the patients (multiple reasons could apply), were positive anti-JCV antibody serostatus (reported for 54.5% of the patients who were prematurely withdrawn), patient’s decision (43.5%), physician’s decision (31.2%), and concern about natalizumab treatment duration (12.3%). In addition, two patients discontinued study participation due to death, one due to PML, and one due to pregnancy (without an associated SAE).
Figure 1.
Patient enrollment and disposition.
Baseline disease and prior treatment characteristics
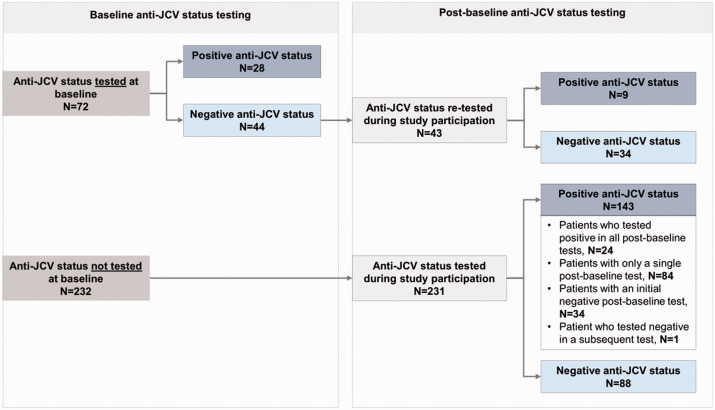
The patients’ median age at baseline (i.e., at natalizumab treatment initiation) was 38.0 years, while the median disease duration was 6.2 years. Patients had experienced a median (IQR) of 2 (1-2) relapses (1-year annualized relapse rate: 1.859) in the year prior to treatment initiation. The median baseline EDSS score was 3.5. Moreover, anti-JCV status was tested in 72 (23.7%) patients at baseline, of which 38.9% were seropositive (Table 1).
Table 1.
Patient, disease and prior treatment characteristics.
| Patient and disease characteristics | |
|---|---|
| Females (N = 304), n (%) | 192 (63.2) |
| Age at baseline (N = 304), median (IQR), years | 38.0 (29.0–45.5) |
| Disease duration at baseline (N = 299), median (IQR), years | 6.2 (3.1–10.7) |
| Relapses in the year prior to natalizumab initiation | |
| Patients with ≥ 1 relapse (N = 304), n (%) | 302 (99.3) |
| Total number of relapses (N = 302), n | 548 |
| Median (IQR) number of relapses (N = 302) | 2.0 (1.0–2.0) |
| Patients with ≥ 1 relapse requiring steroids (N = 299), n (%) | 288 (96.3) |
| Patients with ≥ 1 relapse requiring hospitalization (N = 294), n (%) | 234 (79.6) |
| Relapses in the 2 years prior to natalizumab initiation | |
| Patients with ≥ 1 relapse (N = 304), n (%) | 303 (99.7) |
| Total number of relapses (N = 303), n | 776 |
| Median (IQR) number of relapses (N = 303) | 2.0 (1.0–3.0) |
| ARR in the year prior to natalizumab onset (95% CI), (N = 304) | 1.859 (1.708–2.023) |
| ARR in the two years prior to natalizumab onset (95% CI), (N = 304) | 1.355 (1.261–1.455) |
| Baseline EDSS score (N = 286), median (IQR) | 3.5 (2.0–5.0) |
| MRI findings in the 180-day period prior to natalizumab initiation | |
| At least one T1-Gadolinium (Gd) enhancing lesion (N = 227), n (%) | 137 (60.4) |
| At least 9 T2-hyperintense lesions (N = 233), n (%) | 196 (84.1) |
| Positive anti-JCV serostatus at baseline (N = 72), n (%)a | 28 (38.9) |
| Prior DMT (N = 304), n (%) | 267 (87.8) |
| 1 DMT (N = 304), n (%) | 139 (45.7) |
| ≥2 DMTs (N = 304), n (%) | 128 (42.1) |
| Prior DMTs (N = 304), n (%) | |
| Interferon beta 1-a (Rebif) | 130 (42.8) |
| Interferon beta 1-b | 108 (35.5) |
| Interferon beta 1-a (Avonex) | 102 (33.6) |
| Glatiramer acetate | 68 (22.4) |
| Fingolimod | 24 (7.9) |
| Dimethyl fumarate | 2 (0.7) |
| Daclizumab HYP/Interferon beta 1-a | 1 (0.3) |
| Prior steroids/immunoglobulins (N = 304), n (%) | 148 (48.7) |
| Prior antineoplastic/immunosuppressive therapy (i.e., mitoxantrone, azathioprine, cyclophosphamide) (N = 304), n (%) | 20 (6.6) |
Note: ARR, annualized relapse rate; CI, confidence interval; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; IQR, interquartile range; JCV, John Cunningham virus; MRI, magnetic resonance imaging
aAnti-JCV testing at baseline was not a prerequisite during the enrolment period of the study.
Of the eligible population, 95.7% had received prior treatment for MS. Disease-modifying treatments (DMTs) had been received by 87.8%, and immunosuppressants (i.e. mitoxantrone, azathioprine, and cyclophosphamide) by 6.6% of the patients (Table 1).
Natalizumab treatment characteristics
Treatment with natalizumab was initiated due to prior insufficient response to DMT and/or due to rapidly evolving severe RRMS for 97.0% of the patients. During a median (IQR) natalizumab treatment duration period of 58.7 (30.7-78.0) months, 94 temporary treatment interruptions of a median (IQR) length of 2.0 (1.0-4.0) months were recorded for 14.8% of the patients (Table 2).
Table 2.
Natalizumab treatment characteristics.
| Natalizumab treatment characteristics | |
|---|---|
| Treatment duration (N = 304), median (IQR), months | 58.7 (30.7–78.0) |
| Temporary interruptions | |
| Patients with interruptions (N = 304), n (%) | 45 (14.8) |
| Number of interruptions (N = 45), median (IQR) | 1.0 (1.0–3.0) |
| Length of interruptions (N = 45), median (IQR), months | 2.0 (1.0–4.0) |
| Treatment permanent discontinuation | |
| Patients with treatment discontinuation (N = 304), n (%) | 154 (50.7) |
| Reasons for treatment discontinuation (N = 154)a, n (%) | |
| Anti-JCV antibody positive | 91 (59.1) |
| Patient’s decision | 62 (40.3) |
| Physician’s decision | 49 (31.8) |
| Natalizumab treatment duration concern | 17 (11.0) |
| Pregnancy desire | 11 (7.1) |
| Insufficient efficacy | 8 (5.2) |
| Adverse event | 5 (3.2) |
| Medication change from natalizumab | 5 (3.2) |
| Tolerability problem other than adverse events | 4 (2.6) |
| Prior immunosuppressive use | 3 (1.9) |
| Tysabri antibody positive | 3 (1.9) |
| PML | 2 (1.3) |
| Moved out of the area | 2 (1.3) |
| Malignancy/cancer | 1 (0.6) |
| Death | 1 (0.6) |
| Consent withdrawal | 1 (0.6) |
| Patients receiving concomitant therapy for MS management (N = 304), n (%) | 59 (19.4) |
| Methylprednisolone | 33 (10.9) |
| Prednisone | 21 (6.9) |
| Systemic corticosteroid (not specified) | 5 (1.6) |
| Prednisolone | 1 (0.3) |
Note: IQR, interquartile range; JCV, John Cunningham virus; MS, multiple sclerosis; PML, progressive multifocal leukoencephalopathy
aMultiple reasons could apply.
In addition, natalizumab was permanently discontinued in 50.7% of the patients. Of these, 92.9% prematurely discontinued study participation (either on the same date of treatment discontinuation or after treatment discontinuation), 4.5% were lost-to follow-up, while 2.6% completed study participation. The main reason for natalizumab discontinuation included anti-JCV antibody seropositivity, alone or in addition to physician/patient decision or reasons related to the anti-JCV positive serostatus (i.e., prior use of immunosuppressants, and treatment duration concern), reported for 53.9% of the patients. The treatment discontinuation rate due to AE occurrence (including PML and cancer) was 5.2% (Table 2).
The median (IQR) time elapsed from natalizumab initiation to enrollment in TOPICS (including the treatment period prior to enrollment for patients transferred from TOP to TOPICS and that for patients enrolled during the periods during which receipt of >3 doses of natalizumab prior to enrollment was permitted) was 241.5 (1.0-1232) days.
JCV antibody status throughout study participation
Of the 44 patients negative for anti-JCV at baseline, 43 were re-tested during study participation of which 20.9% switched to being seropositive. Moreover, of the 231 patients first tested between baseline and the end of their study participation, 61.9% were found to be anti-JCV positive (Figure 2).
Figure 2.
Anti-JCV status at baseline and throughout study participation.
Safety
During the prospective study observation period, a total of 20 SAEs experienced by 4.6% of the patients were recorded with a corresponding EAIR of 1.4 per 100 patient-years (95% CI: 0.8-2.3). The most common group of SAEs by MedDRA SOC was ‘infections and infestations’ (1.0%; EAIR: 0.3; 95% CI: 0.1-0.8). The incidence of serious opportunistic infections was 0.7%, comprising two events of PML, and the incidence of malignancies was also 0.7%, including one event each of breast cancer (diagnosed 1.5 years post-treatment onset), and renal oncocytoma (diagnosed 5 years post-treatment onset) (Table 3).
Table 3.
Serious adverse events during the prospective study observation period.
| Serious adverse events during the prospective observation perioda | |||
|---|---|---|---|
| MedDRA system organ class | Events | n (%) | EAIR (95% CI) |
| MedDRA preferred term | |||
| At least one serious adverse event | 20 | 14 (4.6) | 1.4 (0.8–2.3) |
| Infections and infestations | 4 | 3 (1.0) | 0.3 (0.1–0.8) |
| Progressive multifocal leukoencephalopathy | 2 | 2 (0.7) | 0.2 (0.0–0.7) |
| Appendicitis | 1 | 1 (0.3) | |
| Respiratory tract infection | 1 | 1 (0.3) | |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 2 | 2 (0.7) | 0.2 (0.0–0.7) |
| Breast cancer | 1 | 1 (0.3) | |
| Renal oncocytoma | 1 | 1 (0.3) | |
| Pregnancy, puerperium and perinatal conditions | 2 | 2 (0.7) | 0.2 (0.0–0.7) |
| Imminent abortion | 1 | 1 (0.3) | |
| Pregnancy | 1 | 1 (0.3) | |
| Respiratory, thoracic and mediastinal disorders | 2 | 2 (0.7) | 0.2 (0.0–0.7) |
| Acute respiratory failure | 1 | 1 (0.3) | |
| Bronchospasm | 1 | 1 (0.3) | |
| Vascular disorders | 2 | 2 (0.7) | 0.2 (0.0–0.7) |
| Circulatory collapse | 1 | 1 (0.3) | |
| Hypotension | 1 | 1 (0.3) | |
| Cardiac disorders | 1 | 1 (0.3) | 0.1 (0.0–0.5) |
| Atrial fibrillation | 1 | 1 (0.3) | |
| General disorders and administration site conditions | 1 | 1 (0.3) | 0.1 (0.0–0.5) |
| Gait disturbance | 1 | 1 (0.3) | |
| Hepatobiliary disorders | 1 | 1 (0.3) | 0.1 (0.0–0.5) |
| Hepatic function abnormal | 1 | 1 (0.3) | |
| Immune system disorders | 1 | 1 (0.3) | Unknown |
| Immune reconstitution inflammatory syndrome (IRIS) | 1 | 1 (0.3) | |
| Injury, poisoning and procedural complications | 1 | 1 (0.3) | 0.1 (0.0–0.5) |
| Fall | 1 | 1 (0.3) | |
| Metabolism and nutrition disorders | 1 | 1 (0.3) | 0.1 (0.0–0.5) |
| Hyponatremia | 1 | 1 (0.3) | |
| Skin and subcutaneous tissue disorders | 1 | 1 (0.3) | 0.1 (0.0–0.5) |
| Rash | 1 | 1 (0.3) | |
| Surgical and medical procedures | 1 | 1 (0.3) | 0.1 (0.0–0.5) |
| Large intestinal polypectomy | 1 | 1 (0.3) | |
Note: CI, confidence interval; EAIR, exposure-adjusted incidence rate per 100 patient-years; MedDRA, Medical Dictionary for Regulatory Activities.
a With the exception of the event of respiratory tract infection, which occurred over 6 months after the last natalizumab infusion, all remaining events occurred between informed consent obtainment and up to 6 months after the last natalizumab infusion; the event of IRIS had an unknown date of occurrence. The median safety data collection period was 48.7 months, while SAEs that occurred during a median period of 0.7 months between natalizumab initiation and enrollment, were not recorded in the database.
The first case of PML concerned a female patient initiated on natalizumab at the age of 38 years; at baseline her disease duration was 24.7 years and her EDSS score was 6. The patient had received prior treatment with interferon beta 1a, and beta 1 b and prednisone and was found to be anti-JCV positive approximately 4.1 years after natalizumab initiation; PML was diagnosed 2.2 years later. The second patient concerned a male patient who was 34 years-old at natalizumab initiation, had not received any prior MS-related treatments, and who at baseline had an EDSS score of 3 and a 7.2-year disease duration. The patient first tested positive for anti-JCV approximately 4.5 years following natalizumab initiation, and PML was diagnosed 2.2 years later. The patient also developed immune reconstitution inflammatory syndrome; he died about 1.5 months after PML diagnosis, approximately 7 years after natalizumab initiation, due to PML.
In addition to the aforementioned case, one more patient died during the study (overall incidence of death 0.7%). This case concerned a male patient who had initiated natalizumab at the age of 31. The patient died due to ‘acute respiratory failure’ and ‘circulatory collapse’, which he experienced approximately 2.5 years after treatment initiation and while he was on treatment. Details on the patient’s medical history were not collected, as in the context of the study only selected medical conditions were recorded, pertaining to history of malignancies, opportunistic infection and organ transplant or HIV infection, none of which has been reported for the specific participant.
Discussion
TOPICS Greece collected long-term safety data over a median period of 48.7 months in 304 patients with RRMS treated with natalizumab in the routine care in Greece. The results confirm natalizumab’s established safety profile and suggest that adoption of careful monitoring of potential risks in the routine care helps lower the incidence of SAEs.
With respect to the main study objective, the SAE incidence rate in TOPICS Greece was 4.6%, including both natalizumab-related and non-related events, lower than the 13.5-15.3% rates reported in other real-world studies.14,15 An important factor to take into consideration when comparing this rate with that of other studies is the duration of the safety data collection period. Safety data collection in TOPICS Greece occurred over a median of 4 years, a period longer than the 3.3-year treatment period in the 10-year analysis of TOP. 15 In both studies, SAE collection extended to 6 months post natalizumab discontinuation. It should, however, also be taken into consideration that in TOPICS Greece only prospective SAE collection took place, and therefore, SAEs that may have occurred post-treatment initiation but prior to enrollment (median: 0.7 years) have not been included in the analyses. In agreement with the other studies, the most common class of SAEs reported in TOPICS Greece was ‘infections and infestations’, though with a lower incidence rate than in other reports (1.0% vs 3.2-4.1%).5,15 The 0.7% incidence of malignancies in the present study is similar to the 0.8-1.1% rate reported elsewhere.5,15 Regarding their timing, the two events of malignancy in TOPICS Greece were diagnosed at 1.5 and at 5 years post treatment onset, within the range (2 months-8 years) reported in TOP. 15 The corresponding EAIR of malignancy in TOPICS Greece was 0.2 per 100 patient-years, lower than the 449.0 per 100,000 patient-years reported in TYGRIS. 14 In the aforementioned studies, breast cancer was the most common malignancy reported, which accounted for one of the two cases of malignancies reported herein.
The 0.7% incidence of PML in TOPICS Greece is the same as that reported in TYGRIS, STRATA (over a 240-week period) and, when adjusted for exposure, also the same as that reported in TOP.15–17 With respect to the characteristics of the cases diagnosed with PML in TOPICS Greece, both patients were diagnosed with PML more than 6 years after their first natalizumab infusion and tested positive for anti-JCV antibody after treatment initiation and about 2 years prior to PML diagnosis. Neither patient had been previously exposed to immunosuppressive therapy. Notably, in the present study, only 6.6% of the patients had previously received immunosuppressive therapy, a rate lower compared to other cohorts (16.0-20.4%).15–17 According to recent guidelines, MRI and anti-JCV testing are recommended before starting treatment with natalizumab as well as at regular intervals following treatment initiation, in order to estimate the patient’s risk of developing PML.13,18 The frequency of MRI and anti-JCV testing is determined by the patients’ risk, which has been associated with natalizumab treatment duration (especially >2 years), anti-JCV seropositivity, and prior use of immunosuppressants.10,19,20 As these guidelines were not in effect at the time of study design and index testing had not been incorporated in clinical practice, the anti-JCV status was only qualitatively recorded in TOPICS Greece, even though study physicians gradually adopted such guidelines in their clinic.
Close patient monitoring throughout the study, has likely contributed both to the low incidence of PML but also to the low SAE incidence rate across all categories in TOPICS Greece compared to other observational studies, particularly since baseline patient and disease characteristics do not show any marked differences between studies.5,14,15,17,21 This is also supported by the fact that anti-JCV seropositivity was reported as the reason for natalizumab discontinuation in more than half of the patients in the study, and for many patients, physicians reported also taking into consideration concerns about natalizumab treatment duration and prior use of immunosuppressants in their decision to discontinue treatment. The 50.7% overall treatment discontinuation rate in TOPICS Greece is within the range of 17.78-52.2% reported in other observational studies of natalizumab in RRMS.14,15,17,21,22 The treatment discontinuation rate due to AE occurrence (including PML and cancer) was 5.2%.
The percentage of patients tested for anti-JCV at baseline in TOPICS Greece was 23.7%, lower than the 70.5% reported at enrollment in TOP. 15 In all but one patient not tested at baseline, anti-JCV status was examined at least once during the study observation period. Among patients with known anti-JCV status at baseline, 38.9% were seropositive, which is lower than the 48.4% and 57.7% rates among patients with known and available data reported in other studies.15,17 However, the anti-JCV positive rate was 61.9% in patients first tested between baseline and the end of their study participation. Among the patients known to be anti-JCV antibody negative at baseline, the seroconversion rate was 20.9%. This rate is slightly lower than the 25.3% rate reported in TOP. However, comparison of this rate to that of other studies requires adjustment for the person-years of exposure to natalizumab until seroconversion, which has not been estimated in TOPICS Greece. For example, in TOP this rate is reported to be 5.7% per person-year, lower than the annual rates of 13% or 26.7% reported elsewhere.15,21,23
With respect to baseline patient and disease characteristics, the cohort in TOPICS Greece does not display any pronounced deviations from the cohorts described in the other studies of natalizumab mentioned above. In particular, at baseline, the median patient age in the present study was 38.0 years, median disease duration was 6.2 years, and median EDSS score was 3.5. Similarly, in other studies, median patient age at baseline ranges from 32.9 to 44.0 years, median disease duration from 7.3 to 15.1 years, and median EDSS from 2.5 to 4.0.14–17,21,22,24
The main limitations of the present study have been previously discussed and include the fact that no retrospective SAE collection during a median treatment period of 0.7 years prior to enrollment took place, and also that the study has not recorded data regarding anti-JCV index and type of assay utilized, which would have assisted in comparison of these findings with those of other studies. Notably, the latter did not present an objective of the study. Strengths of the study include its multicentric nature, and geographic diversity of study sites, which allow for reflection of variations in medical practice paradigms and aid the generalizability of the findings.
In conclusion, the results of TOPICS Greece offer complementary real-world evidence to the well-characterized safety profile of natalizumab in RRMS patients receiving long-term treatment in routine clinical care settings of Greece. No new safety signals emerged, while the AE treatment discontinuation rate was low.
Conflict of Interests
The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Mastorodemos received speaker’s fees, honoraria for participation to advisory boards and travel support from Genesis Pharma (Biogen), Novartis, Bayer, Merck-Serono, Sanofi-Genzyme, Roche and Specifar-Teva. Dr Karanasios received honoraria for participation to advisory boards and research support from Merck, Genesis Pharma, Specifar-Teva, Novartis, Sanofi-Aventis and Allergan. George Karachalios, Alexopoulou Tania, Rania Gourgioti are employees of Genesis Pharma SA. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
The authors would like to thank Qualitis Ltd. for medical writing support funded by Genesis Pharma S.A.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Genesis Pharma SA.
ORCID iD: Rania Gourgioti https://orcid.org/0000-0002-7421-9035
Contributor Information
Panagiotis Karanasios, Department of Neurology, “Saint Andrew’s” General Hospital of Patras Agios Andreas, Patras, Greece.
Antonia Alexopoulou, Genesis Pharma S.A, CNS Department, Athens, Greece.
TOPICS Study Group, Department of Neurology, Medical School, University of Crete, University General Hospital of Heraklion, Heraklion, Greece.
References
- 1.Tysabri. Summary of product characteristics, www.ema.europa.eu/en/documents/product-information/tysabri-epar-product-information_en.pdf (2020, accessed 08 February 2021).
- 2.Rudick RA, Sandrock A. Natalizumab: alpha4-integrin antagonist selective adhesion molecule inhibitors for MS. Expert Rev Neurother 2004; 4: 571–580. [DOI] [PubMed] [Google Scholar]
- 3.Ransohoff RM. Natalizumab for multiple sclerosis. N Engl J Med 2007; 356: 2622–2629. [DOI] [PubMed] [Google Scholar]
- 4.Rudick R, Polman C, Clifford D, et al. Natalizumab: bench to bedside and beyond. JAMA Neurol 2013; 70: 172–182. [DOI] [PubMed] [Google Scholar]
- 5.Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006; 354: 899–910. [DOI] [PubMed] [Google Scholar]
- 6.Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 2006; 354: 911–923. [DOI] [PubMed] [Google Scholar]
- 7.Shirani A, Stüve O. Natalizumab for multiple sclerosis: a case in point for the impact of translational neuroimmunology. J Immunol 2017; 198: 1381–1386. [DOI] [PubMed] [Google Scholar]
- 8.Cortese I, Reich DS, Nath A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat Rev Neurol 2021; 17: 37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giovannoni G, Kappos L, Berger J, et al. Updated incidence of natalizumab-associated progressive multifocal leukoencephalopathy (PML) and its relationship with natalizumab exposure over time. Neurology 2020; 94: 2815. [Google Scholar]
- 10.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012; 366: 1870–1880. [DOI] [PubMed] [Google Scholar]
- 11.Toboso I, Tejeda-Velarde A, Alvarez-Lafuente R, et al. New algorithms improving PML risk stratification in MS patients treated with natalizumab. Front Neurol 2020; 11: 579438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sørensen PS, Bertolotto A, Edan G, et al. Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Mult Scler 2012; 18: 143–152. [DOI] [PubMed] [Google Scholar]
- 13.McGuigan C, Craner M, Guadagno J, et al. Stratification and monitoring of natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry 2016; 87: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foley J, Carrillo-Infante C, Smith J, et al. The 5-year tysabri global observational program in safety (TYGRIS) study confirms the long-term safety profile of natalizumab treatment in multiple sclerosis. Mult Scler Relat Disord 2019; 39: 101863. [DOI] [PubMed] [Google Scholar]
- 15.Butzkueven H, Kappos L, Wiendl H, et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J Neurol Neurosurg Psychiatry 2020; 91: 660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Connor P, Goodman A, Kappos L, et al. Long-term safety and effectiveness of natalizumab redosing and treatment in the STRATA MS study. Neurology 2014; 83: 78–86 [Erratum in. Neurology 2014; 83: 773]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Outteryck O, Ongagna JC, Brochet B, et al. A prospective observational post-marketing study of natalizumab-treated multiple sclerosis patients: clinical, radiological and biological features and adverse events. The BIONAT cohort. Eur J Neurol 2014; 21: 40–48. [DOI] [PubMed] [Google Scholar]
- 18. European Medicines Agency, EMA confirms recommendations to minimise risk of brain infection PML with Tysabri. www.ema.europa.eu/en/documents/referral/tysabri-article-20-procedure-ema-confirms-recommendations-minimise-risk-brain-infection-pml-tysabri_en.pdf (2016, accessed March 2021).
- 19.Ho PR, Koendgen H, Campbell N, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol 2017; 16: 925–933. [DOI] [PubMed] [Google Scholar]
- 20.Plavina T, Subramanyam M, Bloomgren G, et al. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol 2014; 76: 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krämer J, Tenberge JG, Kleiter I, et al. Is the risk of progressive multifocal leukoencephalopathy the real reason for natalizumab discontinuation in patients with multiple sclerosis? PLoS One 2017; 12: e0174858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evdoshenko E, Stepanova A, Shumilina M, et al. Real-world study of efficacy, risk management and reasons for discontinuation of natalizumab for treatment of multiple sclerosis in Russia. PLoS One 2019; 14: e0217303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Outteryck O, Zéphir H, Salleron J, et al. JC-virus seroconversion in multiple sclerosis patients receiving natalizumab. Mult Scler 2014; 20: 822–829. [DOI] [PubMed] [Google Scholar]
- 24.Guger M, Enzinger C, Leutmezer F, et al. Austrian MS treatment registry (AMSTR). Long-term outcome and predictors of long-term disease activity in natalizumab-treated patients with multiple sclerosis: real life data from the Austrian MS treatment registry. J Neurol. Epub ahead of print 2021. DOI: 10.1007/s00415-021-10559-w. [DOI] [PMC free article] [PubMed]