Abstract
Zishen Yutai Pills (ZYP) is a safe and well quality-controlled TCM preparation with promising effects in many fields of reproduction, including prevention of miscarriage, increase of pregnancy rate during in vitro fertilization-embryo transfer (IVF-ET). The plasma of patients was collected from a clinical trial, namely, “Effect of Traditional Chinese Medicine vs placebo on live births among women undergoing in vitro fertilization, a multi-center randomized controlled trial.” Plasma samples were analyzed with metabonomics method. UPLC-MS technology was used to establish the plasma metabolic fingerprint. Multivariate statistical analysis was applied for comparing the differences of plasma metabolites between ZYP group and placebo group, 44 potential metabolites were screen out and identified. Pathway analysis was conducted with database mining. Compared with placebo, chemicals were found to be significantly down-regulated on HCG trigger day and 14 days after embryo transplantation, including trihexosylceramide (d18:1/26:1), glucosylceramide(d18:1/26:0), TG(22:6/15:0/22:6), TG(22:4/20:4/18:4). Compared with placebo, some chemicals were found to be significantly up-regulated on HCG trigger day and 14 days after embryo transplantation, i.e., PIP3(16:0/16:1), PIP2(18:1/18:1), tauroursodeoxycholic acid, L-asparagine, L-glutamic acid, kynurenic acid, 11-deoxycorticosterone, melatonin glucuronide, hydroxytyrosol. These metabolites were highly enriched in pathways including sphingolipid metabolism, alanine, aspartic acid and glutamic acid metabolism, aminoacyl tRNA biosynthesis, taurine and hypotaurine metabolism. This study revealed metabolic differences between subjects administered with ZYP and placebo. Relating metabolites were identified and pathways were enriched, providing basis on the exploration on the underlying mechanisms of ZYP combined with IVF-ET in the treatment of infertility.
Keywords: traditional Chinese medicine, Zishen Yutai Pills, metabonomics, infertility, embryo transfer
Introduction
Infertility has been one of the major conditions affecting the well-being of human worldwide (Inhorn and Patrizio, 2015). Nowadays, in vitro fertilization-embryo transfer (IVF-ET) is a common procedure, helping couples with fertility problems to achieve parenthood (Kissin et al., 2014). However, according to previous report from the European Annual Conference on Reproduction, the success rate of IVF-ET was only 30–40%, and the rate of pregnancy rate was even lower (Wyns et al., 2020). There is still a bottleneck in the improvement of pregnancy outcome.
In IVF-ET, oocytes quality and endometrial receptivity are the two most important factors affecting the outcome of embryo transfer (Bu et al., 2016; Kristensen et al., 2017). Prior to IVF-ET, multiple mature oocytes could be obtained through controlled ovarian hyperstimulation (COH). Poor ovarian response to gonadotropin leads to defects in both the quality and quantity of oocytes, and eventually lead to a low pregnancy rate (Vaiarelli et al., 2018). It is also well acknowledged that embryo implantation requires good endometrial receptivity. Although a clear definition on endometrial receptivity is still absent, many literatures have put forward that endometrial thickness could be an important indicator for endometrial receptivity (Mahajan and Sharma, 2016). A thickness of endometrium below 8 mm is a risk factor for pregnancy loss during IVF-ET (Bu et al., 2016).
Traditional Chinese medicine is an important complementary therapy in the IVF-ET (Smith et al., 2010). Zishen Yutai Pills (ZYP) is a safe and well quality-controlled TCM preparation with promising effects in many fields of reproduction, including prevention of miscarriage, increase of pregnancy rate (Zhu et al., 2002; Gao et al., 2015b; Ma et al., 2018; Cao et al., 2020).
ZYP contains 15 Chinese traditional medicine herbs, i.e., Cuscutae Semen (the ripe dried seed of Cuscuta Chinensis Lam.), Ginseng Radix et Rhizome (the dried root and rhizome of Panax ginseng C. A. Mey.), Dipsaci Radix (the dried root of Dipsacus asper Wall. ex DC.), Taxilli Herba (the dried leafy stem and branch of Taxillus chinensis (DC.) Danser), Eucommiae Cortex (the dried bark of Eucommia ulmoides Oilv.), Morindae Officinalis Radix (the dried root of Marinda officinalis How), Cervi Cornu Degelatinatum (the residue after water extraction of ossified antler of Cervus nippon Temminck), Codonopsis Radix (the dried root of Codonopsis pilosula (Franch.) Nannf.), Atractylodis Macrocephalae Rhizoma (the dried rhizome of Atractylodes macrocephala Koidz.), Asini Corii Colla (solid glue prepared by stewing and concentrating from the hide of Equus asinus L.), Lycii Fructus (the dried ripe fruit of Lycium barbarum L.), Rehmanniae Radix Praeparata (the steamed and dried root of Rehmannia glutinosa (Gaertn.) DC.), Polygoni Multiflori Radix Praeparata (the steamed and dried root of Polygonum multiflorum Thunb.), Artemisiae Argyi Folium (the dried leaf of Artemisia argyi Lévl. et Vant.), and Amomi Fructus (the dried fruit of Amomum villosum Lour.) (Cao et al., 2020). The formula of ZYP is listed as shown in Table 1, including detailed formula and the amounts of raw materials contained in the daily dose. In addition, production process of ZYP complies with the relevant requirements of law of China’s Drug Administration and GMP. Production process is normative and controllable to ensure the quality consistency of each batch of products.
TABLE 1.
Standard prescription of ZYP and raw material amount used in daily dose.
| Medicine material | Standard prescription amount/g | Raw material amount used in daily dose/g |
|---|---|---|
| Cuscutae semen | 800 | 9.60 |
| Ginseng radix et rhizoma | 50 | 0.60 |
| Dipsaci radix | 480 | 5.76 |
| Taxilli herba | 480 | 5.76 |
| Eucommiae cortex | 290 | 3.48 |
| Morindae officinalis radix | 190 | 2.28 |
| Cervi cornu degelatinatum | 140 | 1.68 |
| Codonopsis radix | 580 | 6.96 |
| Atractylodis macrocephalae rhizoma | 240 | 2.88 |
| Asini corii colla | 30 | 0.36 |
| Lycii fructus | 190 | 2.28 |
| Rehmanniae radix praeparata | 480 | 5.76 |
| Polygoni multiflori radix praeparata | 240 | 2.88 |
| Artemisiae argyi folium | 140 | 1.68 |
| Amomi fructus | 70 | 0.84 |
According to the clinical protocol, ZYP is given at a dose of 15 g/day.
According to previous research, different methods were applied in the quality control of ZYP. The contents of five components, namely, loganic acid, chlorogenic acid, loganin, sweroside, and asperosaponin Ⅵ, were determined in ZYP by high performance liquid chromatography (HPLC) (Ma et al., 2018). In another report, ultrahigh performance liquid chromatography coupled with charged aerosol detector (UPLC-CAD) fingerprint and multi-components quantitative analysis was developed and validated for quality evaluation of ZYP. Fifty-two characteristic peaks were selected to evaluate the similarities among different batches of ZYP (Cao et al., 2020). Both methods could be the proof of the stability of ZYP, due to the consistency in both contents of components and fingerprint chromatogram.
Previous reports have shown that during IVF-ET, the administration of ZYP on the third day of the menstrual cycle before COH could increase the thickness of endometrium and improve the quality of oocytes, leading to an increased pregnancy rate (Zhu et al., 2002). In vivo experiments also indicated that ZYP could increase the thickness of endometrium in peri-implantation mice, increase the expression of β3 mRNA, and promote the expression of HOXA10 gene in the endometrium of mice (Gao et al., 2015a). However, the mechanisms of ZYP, especially during the procedure of IVF-ET, still requires further exploration.
The mechanisms of traditional Chinese medicine are hard to decipher due to its complexity of chemical components and the pharmacological interactions among components (Kibble et al., 2015). However, metabonomics could be a useful tool under such conditions because this method focus on the biological effects of treatment from a holistic perspective, making it a promising tool in the exploration of traditional Chinese medicine (Wang et al., 2012; Li et al., 2020).
In this study, plasma samples were collected during a clinical trial entitled “Effect of Traditional Chinese Medicine vs placebo on live births among women undergoing in vitro fertilization, a multicenter randomized controlled trial” (registration number: ChiCTR-TRC-14004494, abbreviated as ZYP-RCT). UPLC-MS technology was used to establish the fingerprint of plasma metabolites, and metabonomics analysis was carried out on the plasma of patients from both the ZYP group and the placebo group with multivariate statistical analysis and pathway enrichment, providing evidence for its possible application in IVF-ET for the treatment of infertility.
Materials and Methods
Drug Used in ZYP-RCT
ZYP (China National Medical Products Administration Permit No. Z44020008) was obtained from Guangzhou Baiyunshan Zhongyi Pharmaceutical Co. Ltd. (Guangzhou, China). Four different batches of commercial product ZYP were used in the current ZYP-RCT (Batch No. 20130301, 20141101, 20150301, 20150802).
All the voucher specimens were deposited at Guangdong Provincial Hospital of Chinese Medicine (Guangzhou, China). Chemical profiles and quantitative determination were reported in Supplementary Material.
Plasma Sample Collection
A total of 94 patients participated in the ZYP-RCT, including 46 cases in placebo group and 48 cases in ZYP group. This RCT was registered on Clinical Trial Registration in China (http://www.chictr.org.cn), with a registration No. Chictr-TRC-14004494. The treatment protocol was based on best practice (Chen et al., 2019b). Elbow vein plasma (6 ml) was collected from all subjects after an overnight fast and placed in EDTA anticoagulant tube. After centrifugation at 3,000 rpm for 15 min, plasma was collected and stored under −80°C for further analysis (Li et al., 2020).
Standard protocol of ZYP-RCT was provided in Supplementary Material. The plasma samples were collected at four time points, i.e., baseline (T1), Gn-starting day (T2), HCG trigger day (T3) and 14 days after ET (T4). A total of 329 plasma samples were included in this metabonomics study.
According previous reports, ZYP has promising effects in the prevention of miscarriage. In addition, Zishen Yutai pill could also be applied in reproductive assistant technology as a complementary medicine, especially for those advanced maternal age women (≥35 yr old). Therefore, metabonomics study was also carried out in the following subgroups, namely, the advanced maternal age group (≥35 yr old, abbreviated as AMA group), and the abortion history group (abbreviated as AH group). The statistical results of clinical samples are shown in Table 2, with clinical characteristics from both groups provided as well.
TABLE 2.
Clinical characteristic of subjects in ZYP-RCT on baseline and sample grouping.
| Placebo | ZYP | ||||
|---|---|---|---|---|---|
| Subjects in whole (n) | 46 | 48 | |||
| Subjects in AMA (n) | 22 | 20 | |||
| Subjects in AH (n) | 31 | 31 | |||
| Characteristics of Placebo | Characteristics of ZYP | p | |||
| Age (yr) | 33.35 ± 4.44 | 33.38 ± 4.29 | 0.976 | ||
| AMA | 37.27 ± 2.00 | 37.60 ± 1.88 | 0.589 | ||
| AH | 33.16 ± 4.73 | 33.06 ± 4.15 | 0.932 | ||
| Height (cm) | 157.83 ± 3.92 | 158.17 ± 5.12 | 0.717 | ||
| AMA | 157.77 ± 2.84 | 157.42 ± 5.16 | 0.784 | ||
| AH | 158.26 ± 4.37 | 159.27 ± 5.07 | 0.408 | ||
| Weight (kg) | 53.7 ± 7 | 53.7 ± 6.75 | 0.996 | ||
| AMA | 54.41 ± 5.94 | 51.74 ± 5.79 | 0.154 | ||
| AH | 54.68 ± 6.77 | 54.93 ± 7.09 | 0.886 | ||
| BMI | 21.53 ± 2.46 | 21.46 ± 2.48 | 0.897 | ||
| AMA | 21.85 ± 2.20 | 20.86 ± 1.86 | 0.133 | ||
| AH | 21.81 ± 2.34 | 21.65 ± 2.54 | 0.797 | ||
| Thickness of endometrium (mm) | 7.8 ± 2.2 | 7.56 ± 2.94 | 0.672 | ||
| AMA | 7.95 ± 2.55 | 7.54 ± 3.24 | 0.647 | ||
| AH | 7.87 ± 2.31 | 7.27 ± 2.75 | 0.361 | ||
| Duration of infertility (yr) | 5.11 ± 4.09 | 5.23 ± 3.53 | 0.877 | ||
| AMA | 6.44 ± 4.71 | 7.20 ± 3.79 | 0.573 | ||
| AH | 4.81 ± 4.2 | 4.94 ± 3.33 | 0.901 | ||
| Sampling numbers | |||||
| T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | Total | ||
| Whole | 36 | 45 | 43 | 38 | 32 | 50 | 43 | 43 | 329 | |
| AMA | 16 | 20 | 21 | 18 | 14 | 21 | 16 | 18 | 144 | |
| AH | 27 | 37 | 35 | 28 | 20 | 36 | 31 | 31 | 245 |
AMA, the advanced maternal age group (≥35 yr old); AH, patients with abortion history.
The plasma samples were collected at four time points, i.e., baseline (T1), Gn-starting day (T2), HCG trigger day (T3) and 14 days after ET (T4).
Instruments and Reagents
Ultimate™ 3000 high performance liquid chromatograph was applied in LC analysis. Mass spectrometry was carried out in Q Exactive™ Plus Mass Spectrometry with ESI ionization source (Thermo Fisher Scientific Ultimate™). Acetonitrile and methanol (HPLC grade) were purchased from Merck (Darmstadt, Germany), and formic acid from FLUKA company (Buchs, Switzerland). All other reagents were commercially available. Ultrapure water (18.2 mΩ) was prepared using Millipore-Q ultra-pure water system (Millipore, France).
Plasma Sample Preparation
Before metabonomics analysis, samples were placed and thawed under 4°C. Plasma (100 μl) was then transferred into EP tube (1.5 ml). After adding acetonitrile (400 μl), sample was vortexed for 2 min and then centrifuged at 13,000 rpm at 4°C for 20 min. Supernatant (400 μl) was collected and then transferred into EP tube thereafter. Water (200 μl) was added in EP tube. The sample was vortexed for 30 min and centrifuged for 20 min at 13,000 rpm under 4°C. Finally, the sample was placed in an injection vial (2 ml) for sample analysis (Li et al., 2020).
LC-MS Analysis and Methodology Investigation
Detailed conditions for LC-MS analysis were reported in Supplementary Material. Methodology investigation was also conducted, including precision, stability and repeatability (Supplementary Material).
Data Acquisition and Processing
Metabonomics raw data were collected with Xcalibur™ software (Thermo Scientific), and the profilings of metabolic fingerprints were obtained. The raw data were processed by Progenesis QI software. The quantitative information of all metabolites in each sample was obtained after chromatographic peak recognition, peak alignment, and normalization.
Multivariate Statistical Analysis
After data processing, the data of each sample was imported into Simca-p14.1 software. Unsupervised analysis method was performed in the present study, i.e., principal component analysis (PCA). Supervised analysis methods were also performed, including partial least-squares discrimination analysis (PLS-DA) and orthogonal partial least-squares discrimination analysis (OPLS-DA). The contribution of different metabolites in the sample clustering was obtained. The differences of plasma metabolites between the subjects in ZYP group and placebo group were compared.
Identification of Metabolites and Pathway Analysis
The compounds were ranked according to their variable importance in projection (VIP) value in multivariate statistical analysis, and those with significant difference (p < 0.05) were screened out. These metabolites were identified according to the isotope matching results of tandem mass spectrometry, and searched from different database, including HMDB, METLIN, KEGG.
Identification of characteristic metabolites was carried out using Thermo Scientific™ Compound Discoverer™. Potential compounds and pathway analysis were also performed on online and local databases, including mzCloud™, ChemSpider™ and KEGG.
Receiver Operating Characteristic (ROC) Curve
MetaboAnalyst 3.0 (www.metaboanalyst.ca), a metabonomics data analysis platform, was applied to draw the ROC curves and multivariate exploratory ROC of the potential biomarkers (Xia et al., 2015). ROC curves were analyzed using metabolites in T3, in all subjects, distinguish the ZYP and the placebo. T3 was selected because at this time points, embryos would be collected. The value of area under the curve (AUC) above 0.7 suggested good predict ability of potential biomarkers.
Results
Metabolic Fingerprint of Plasma
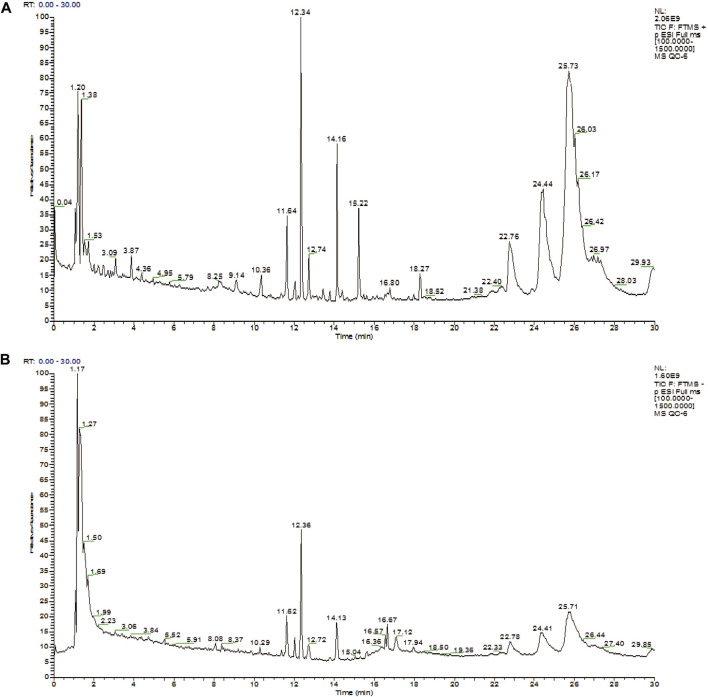
The positive ion mode and negative ion mode were used to establish the plasma metabolic fingerprint profiling of ZYP-RCT, as shown in Figure 1.
FIGURE 1.
Total ion chromatography (TIC) profiling of plasma sample (A: positive ion mode, B: negative ion mode).
Methodology Investigation
The quality control (QC) sample obtained by mixing 20 μl serum of each group of samples, processed as sample preparation. One QC sample was injected six times continuously to test the precision of the instrument, 10 m/z peaks with the highest peak intensity were selected to calculate the relative standard deviations (RSD) value, the RSD value was less than 10% (n = 6). One QC sample was injected into LC-MS after every 10 serum samples running to ensure the stability in the metabonomics raw data acquisition, the RSD value of 10 m/z peaks with the highest peak intensity was less than 10% in the 26 injections. The results showed that the metabonomics method had a good precision, the instrument stability was excellent, and the acquired data were reliable as well (Supplementary Material).
Multivariate Statistical Analysis in Whole Dataset
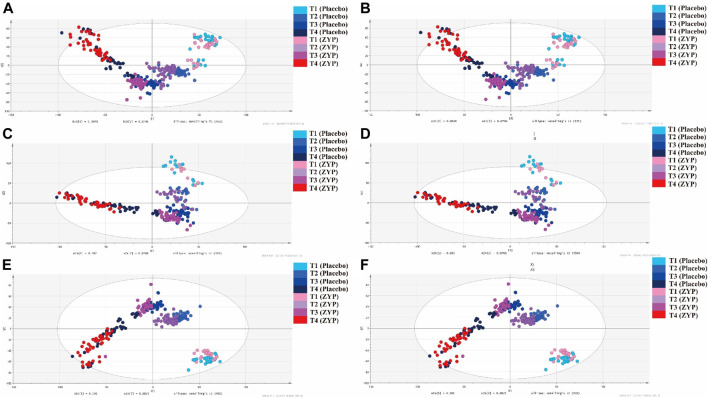
PCA, PLS-DA and OPLS-DA analysis were performed on 329 plasma samples and QC samples. Metabolic status of ZYP group and placebo group were well clustered at each time point (Figures 2A, B, 3). In Figures 2A,B, within-group distributions of metabolites were observed using PCA. In Figure 3, the plasma metabolites of ZYP group and placebo group achieved good overlap on baseline, which showed that there was no significant difference between the two groups before treatment, making these two sets of data comparable. After treatment, the distribution of each time point was different.
FIGURE 2.
PCA analysis on plasma samples in whole dataset and two subgroups. (A) PCA analysis of whole dataset in positive mode. (B) PCA analysis of whole dataset in negative mode. (C) PCA analysis of AMA subgroup in positive mode. (D) PCA analysis in AMA subgroup in negative mode. (E) PCA analysis in AH subgroup in positive mode. (F) PCA analysis in AH subgroup in negative mode.  : T1 (Placebo),
: T1 (Placebo),  : T2 (Placebo),
: T2 (Placebo),  : T3 (Placebo),
: T3 (Placebo),  : T4 (Placebo),
: T4 (Placebo),  : T1 (ZYP),
: T1 (ZYP),  : T2 (ZYP),
: T2 (ZYP),  : T3 (ZYP),
: T3 (ZYP),  : T4 (ZYP).
: T4 (ZYP).
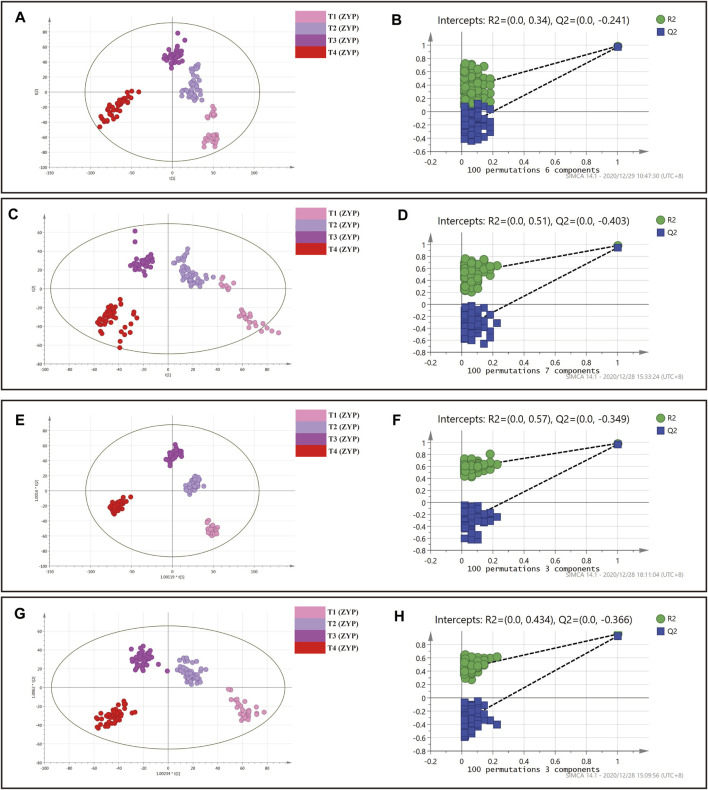
FIGURE 3.
PLS-DA and OPLS-DA analysis on plasma samples in whole dataset. (A) PLS-DA analysis in positive mode. (B) Permutation test of PLS-DA analysis in positive mode. (C) PLS-DA analysis in negative mode. (D) Permutation test of PLS-DA analysis in negative mode. (E) OPLS-DA analysis in positive mode. (F) Permutation test of OPLS-DA analysis in positive mode. (G) OPLS-DA analysis in negative mode. (H) Permutation test of OPLS-DA analysis in negative mode.  : T1 (Placebo),
: T1 (Placebo),  : T2 (Placebo),
: T2 (Placebo),  : T3 (Placebo),
: T3 (Placebo),  : T4 (Placebo),
: T4 (Placebo),  : T1 (ZYP),
: T1 (ZYP),  : T2 (ZYP),
: T2 (ZYP),  : T3 (ZYP),
: T3 (ZYP),  : T4 (ZYP).
: T4 (ZYP).
As can be seen in Figures 2A, B, 3A,C,E,G, each group was also well distinguished and clustered, with the changes in each group were well distinguished among different sampling times. The values of R2X(cum), R2Y(cum), Q2(cum) were 0.453, 0.923 and 0.674, respectively in PLS-DA (positive ion mode). The values of R2X(cum), R2Y(cum), Q2(cum) were 0.520, 0.933 and 0.673, respectively in PLS-DA (negative ion mode). The values of R2X(cum), R2Y(cum), Q2(cum) were 0.418, 0.830 and 0.600, respectively in OPLS-DA (positive ion mode). The values of R2X(cum), R2Y(cum), Q2(cum) were 0.400, 0.715 and 0.544, respectively in OPLS-DA (negative ion mode). The statistical parameters demonstrated both regression methods were satisfying. No over fitting was observed by permutation test with 100 iterations (Figures 3B,D,F,H), implying that all models were reliable.
The results showed that both the placebo group and the ZYP group achieved good clustering in the four observation time points, and the metabolic fingerprints could effectively distinguish the differences of the changes at each observation time point, and the ZYP group was more closely clustered than the placebo group, suggesting that the ZYP group had a strong specificity of metabolic changes.
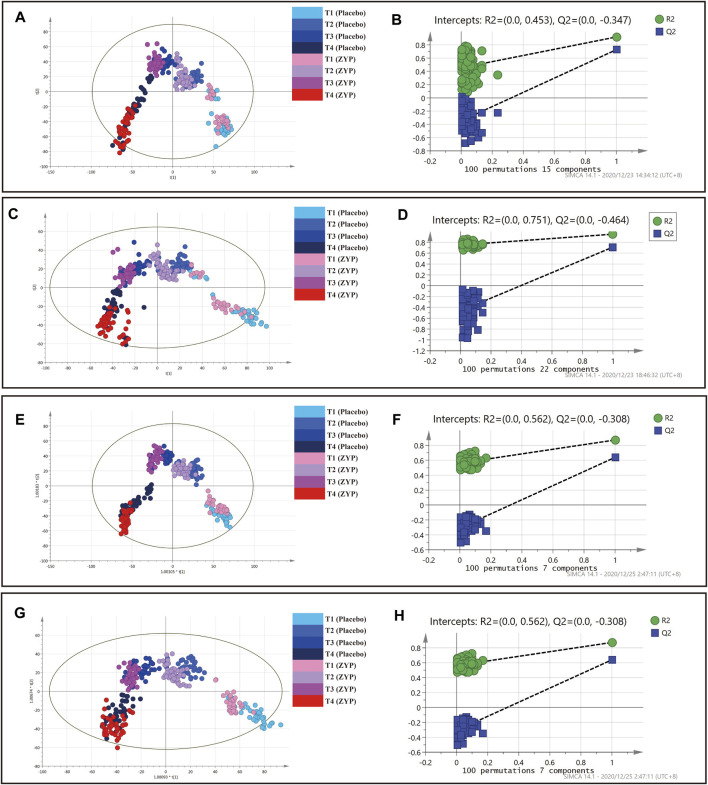
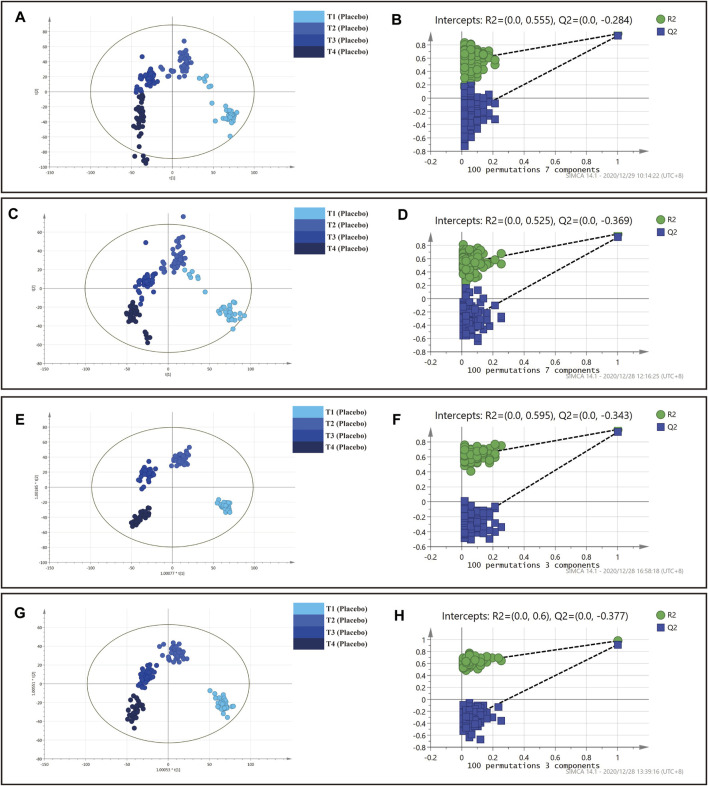
Multivariate Statistical Analysis of ZYP and Placebo Group
In this section, multivariate statistical analysis of ZYP and placebo group was conducted respectively. Detailed information of grouping was shown in Table 2. Score plots showed that the metabolic status of plasma samples in ZYP group was well distinguished (Figures 4, 5), indicating that the plasma metabolic status had changed significantly at each time point. The values of R2X(cum), R2Y(cum), Q2(cum) were 0.331, 0.969 and 0.900, respectively in PLS-DA (positive ion mode). The values of R2X(cum), R2Y(cum), Q2(cum) were 0.376, 0.958 and 0.900, respectively in PLS-DA (negative ion mode). The values of R2X(cum), R2Y(cum), Q2(cum) were 0.315, 0.953 and 0.886, respectively in OPLS-DA (positive ion mode). The values of R2X(cum), R2Y(cum), Q2(cum) were 0.377, 0.950 and 0.890, respectively in OPLS-DA (negative ion mode). No overfitting was observed as shown in the permutation test results in Figures 4, 5 in 100 iterative tests, implying that the models were reliable.
FIGURE 4.
PLS-DA and OPLS-DA analysis on plasma samples in placebo group. (A) PLS-DA analysis in positive mode. (B) Permutation test of PLS-DA analysis in positive mode. (C) PLS-DA analysis in negative mode. (D) Permutation test of PLS-DA analysis in negative mode. (E) OPLS-DA analysis in positive mode. (F) Permutation test of OPLS-DA analysis in positive mode. (G) OPLS-DA analysis in negative mode. (H) Permutation test of OPLS-DA analysis in negative mode.  : T1 (Placebo),
: T1 (Placebo),  : T2 (Placebo),
: T2 (Placebo),  : T3 (Placebo),
: T3 (Placebo),  : T4 (Placebo).
: T4 (Placebo).
FIGURE 5.
PLS-DA and OPLS-DA analysis on plasma samples in ZYP group. (A) PLS-DA analysis in positive mode. (B) Permutation test of PLS-DA analysis in positive mode. (C) PLS-DA analysis in negative mode. (D) Permutation test of PLS-DA analysis in negative mode. (E) OPLS-DA analysis in positive mode. (F) Permutation test of OPLS-DA analysis in positive mode. (G) OPLS-DA analysis in negative mode. (H) Permutation test of OPLS-DA analysis in negative mode.  : T1 (ZYP),
: T1 (ZYP),  : T2 (ZYP),
: T2 (ZYP),  : T3 (ZYP),
: T3 (ZYP),  : T4 (ZYP).
: T4 (ZYP).
Identification of Metabolites and Metabolic Pathway Analysis
According to the methods mentioned above, 44 metabolites were screened out and identified. The identification results of each metabolite are shown in Table 4.
TABLE 4.
Identification of metabolites.
| No. | Retention Time | m/z | HMDB ID | Compound name | Ion | Formula | Class | Biological Process | KEGG ID |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.34 | 293.0855 | HMDB28755 | Aspartyl-Histidine | M+Na | C10H14N4O5 | Carboxylic acids and derivatives | NA | NA |
| 2 | 10.27 | 133.0649 | HMDB0000168 | L-Asparagine | M+H | C4H8N2O3 | Carboxylic acids and derivatives | Aspartate Metabolism | C00152 |
| 3 | 10.71 | 308.2198 | HMDB13250 | Myristoylglycine | M+Na | C16H31NO3 | Carboxylic acids and derivatives | NA | NA |
| 4 | 29.35 | 228.0076 | HMDB0001228 | L-Glutamic acid 5-phosphate | M+H | C5H10NO7P | Carboxylic acids and derivatives | NA | C03287 |
| 5 | 7.00 | 1,046.5402 | HMDB0001035 | Angiotensin II | M+H | C50H71N13O12 | Carboxylic acids and derivatives | Angiotensin Metabolism | C02135 |
| 6 | 1.06 | 148.1162 | HMDB0000148 | L-Glutamic acid | M+H | C5H9NO4 | Carboxylic acids and derivatives | Glutamate Metabolism | C00025 |
| 7 | 3.73 | 205.0972 | HMDB00929 | L-Tryptophan | M+H | C11H12N2O2 | Indoles and derivatives | Transcription/Translation;Tryptophan Metabolism | C00078 |
| 8 | 29.40 | 593.3307 | HMDB0001926 | 17α-Ethynylestradiol | 2M+H | C20H24O2 | Steroids and steroid derivatives | Lipid metabolism pathway | C07534 |
| 9 | 7.10 | 367.2692 | HMDB0000526 | 5α-Tetrahydrocortisol | M+H | C21H34O5 | Steroids and steroid derivatives | Lipid metabolism pathway | |
| 10 | 8.48 | 500.3798 | HMDB0000874 | Tauroursodeoxycholic acid | M+H | C26H45NO6S | Steroids and steroid derivatives | Lipid metabolism pathway | |
| 11 | 29.66 | 331.2091 | HMDB0000016 | 11-Deoxycorticosterone | M+H | C21H30O3 | Steroids and steroid derivatives | Steroidogenesis; Lipid metabolism pathway | C03205 |
| 12 | 6.11 | 343.2859 | HMDB0004666 | 2-Arachidonylglycerol | M+H-2H2O | C23H38O4 | Endocannabinoids | Fatty acid metabolism | C13856 |
| 13 | 12.41 | 253.2164 | HMDB00477 | 7Z,10Z-Hexadecadienoic acid | M+H | C16H28O2 | Fatty Acyls | Lipid metabolism pathway | |
| 14 | 21.89 | 340.3572 | HMDB00583 | Docosanamide | M+H | C22H45NO | Fatty Acyls | Lipid metabolism pathway | |
| 15 | 10.86 | 187.1119 | HMDB30931 | (E)-2-Tridecene-4,6,8-triyn-1-ol | M+H | C13H14O | Fatty Acyls | Lipid metabolism pathway | |
| 16 | 0.00 | 271.2634 | HMDB02259 | Heptadecanoic acid | M+H | C17H34O2 | Fatty Acyls | Lipid metabolism pathway | |
| 17 | 12.36 | 308.2796 | HMDB0002250 | Dodecanoylcarnitine | M+H-2H2O | C19H37NO4 | Fatty Acyls | Fatty acid metabolism | |
| 18 | 25.10 | 469.3782 | HMDB0055895 | TG(22:6/15:0/22:6) | M+2H | C62H96O6 | Glycerolipids | Lipid metabolism pathway | NA |
| 19 | 23.68 | 857.7542 | HMDB0010427 | TG(18:0/14:0/18:0) | M+Na | C53H102O6 | Glycerolipids | Lipid metabolism pathway | |
| 20 | 28.94 | 343.9054 | HMDB0046910 | TG(22:0/20:5/18:1) | M+3H | C63H110O6 | Glycerolipids | Lipid metabolism pathway | NA |
| 21 | 10.71 | 498.3277 | HMDB0054868 | TG(22:4/20:4/18:4) | M+Na | C63H98O6 | Glycerolipids | Lipid metabolism pathway | NA |
| 22 | 6.26 | 576.3234 | HMDB0011499 | LysoPE(0:0/24:6) | M+Na | C29H48NO7P | Glycerophospholipids | Lipid metabolism pathway | NA |
| 23 | 26.05 | 948.4617 | HMDB0116105 | CDP-DG(a-17:0/i-13:0) | M+Na | C42H77N3O15P2 | Glycerophospholipids | Cardiolipin Biosynthesis | NA |
| 24 | 23.84 | 536.1655 | HMDB0010148 | PIP3(16:0/16:1) | M+H+Na | C41H80O22P4 | Glycerophospholipids | Lipid metabolism pathway | C00626 |
| 25 | 21.94 | 775.5645 | HMDB0010618 | PG(18:1/18:1) | M+H | C42H79O10P | Glycerophospholipids | Lipid metabolism pathway | NA |
| 26 | 10.07 | 440.2858 | HMDB0011472 | LysoPE(0:0/15:0) | M+H | C20H42NO7P | Glycerophospholipids | Lipid metabolism pathway | NA |
| 27 | 26.20 | 1,023.5250 | HMDB0010089 | PIP2(18:1/18:1) | M+H | C45H85O19P3 | Glycerophospholipids | Glycerophospholipid metabolism; Lipid metabolism pathway | C00626 |
| 28 | 21.22 | 889.6357 | HMDB0009528 | PE(22:1/20:2) | M+ACN+Na | C47H88NO8P | Glycerophospholipids | Lipid metabolism pathway | C00350 |
| 29 | 11.66 | 702.4977 | HMDB0056388 | CL(16:0/16:0/16:0/18:0) | M+H+Na | C75H146O17P2 | Glycerophospholipids | Cardiolipin Biosynthesis | NA |
| 30 | 25.40 | 520.5090 | HMDB0004949 | Ceramide (d18:1/16:0) | M+H-H2O | C34H67NO3 | Sphingolipids | Lipid metabolism pathway | C00195 |
| 31 | 29.25 | 302.8993 | HMDB04977 | Glucosylceramide (d18:1/26:0) | M+3Na | C50H97NO8 | Sphingolipids | Lipid metabolism pathway | C01190 |
| 32 | 29.82 | 700.8428 | HMDB04938 | Ganglioside GM2 (d18:1/16:0) | M+2Na | C65H117N3O26 | Sphingolipids | Lipid metabolism pathway | C04884 |
| 33 | 20.30 | 410.8958 | HMDB0004884 | Trihexosylceramide (d18:1/26:1) | M+3Na | C62H117NO18 | Sphingolipids | Lipid metabolism pathway | C04737 |
| 34 | 1.63 | 224.0630 | HMDB0060830 | Melatonin glucuronide | M+H+K | C19H24N2O8 | Nucleoside and nucleotide analogues | NA | NA |
| 35 | 0.19 | 445.1205 | HMDB33041 | 1-(1-Propenylthio)propyl propyl disulfide | 2M+H | C9H18S3 | Organic disulfides | NA | NA |
| 36 | 1.15 | 148.0040 | HMDB0000251 | Taurine | M+Na | C2H7NO3S | Organic sulfonic acids and derivatives | Bile acid biosynthesis; Taurine and hypotaurine metabolism | C00245 |
| 37 | 28.99 | 314.9288 | HMDB0011625 | Dimethylarsinic acid | 2M+K | C2H7AsO2 | Organometalloid compounds | NA | C07308 |
| 38 | 28.58 | 100.1127 | HMDB0013648 | Palmitoleoyl ethanolamide | M+3H | C18H35NO2 | Organonitrogen compounds | NA | NA |
| 39 | 10.16 | 302.3056 | HMDB0000269 | Sphinganine | M+H | C18H39NO2 | Organonitrogen compounds | Sphingolipid Metabolism | C00836 |
| 40 | 7.39 | 155.0703 | HMDB05784 | Hydroxytyrosol | M+H | C8H10O3 | Phenols | NA | C01479 |
| 41 | 28.94 | 205.0173 | HMDB0000779 | Phenyllactic acid | M+K | C9H10O3 | Phenylpropanoic acids | NA | NA |
| 42 | 7.10 | 553.3839 | HMDB0033685 | α-Tocopherol succinate | M+Na | C33H54O5 | Prenol lipids | Lipid metabolism pathway | NA |
| 43 | 7.20 | 183.1381 | HMDB32050 | α-Terpineol formate | M+H | C11H18O2 | Prenol lipids | NA | NA |
| 44 | 12.96 | 190.0499 | HMDB00715 | Kynurenic acid | M+H | C10H7NO3 | Quinolines and derivatives | Tryptophan Metabolism | C00152 |
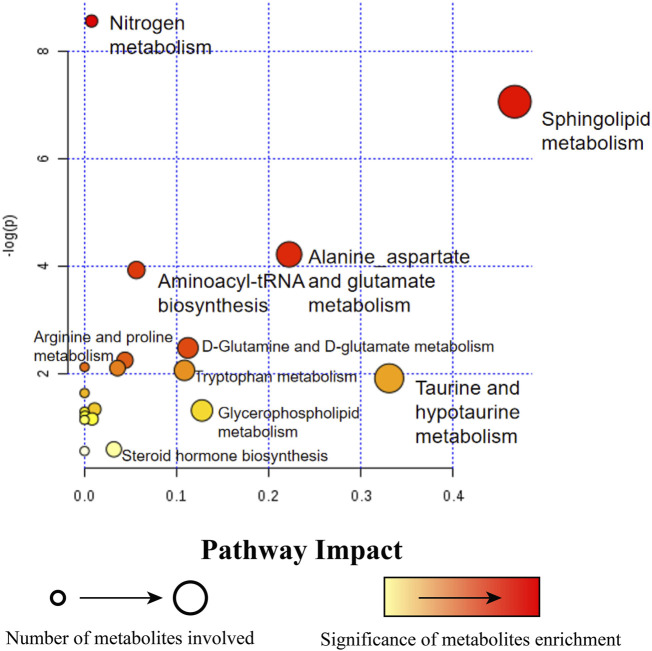
Metabolites with significant changes were analyzed by metabolic pathway impact index, including sphingolipid metabolism, alanine, aspartic acid and glutamic acid metabolism, aminoacyl tRNA biosynthesis, taurine and hypotaurine metabolism, nitrogen metabolism, glutamine and glutamic acid metabolism, arginine and proline metabolism, tryptophan metabolism, steroid hormone biosynthesis, glycerin and phospholipid metabolism pathway (Figure 6).
FIGURE 6.
Pathway enrichment analysis of 44 metabolites. A circle with a bigger diagram means more metabolites are in volved in this pathway. Circle in light yellow ( ) is regarded as a lower significance than those in red (
) is regarded as a lower significance than those in red ( ).
).
Changes of Metabolites in the AMA Subgroup
A total of 245 plasma samples were selected from all samples in AMA subgroup, and the detailed sampling information was reported in Table 2. In Figures 2C, D, within-group distributions of metabolites were observed using PCA.
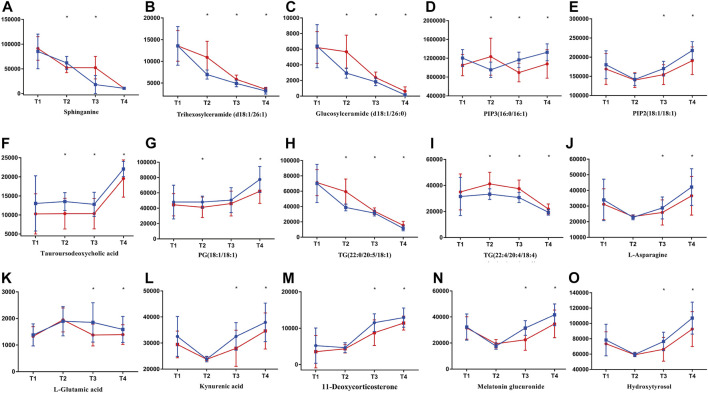
As can be seen from Figure 7, metabolites including sphinganine, trihexosylceramide (d18:1/26:1), glucosylceramide (d18:1/26:0) had undergone a decline during the whole process. For PIP3(16:0/16:1), PIP2(18:1/18:1) and tauroursodeoxycholic acid, increased levels of these metabolites in subjects administered with ZYP were observed on T3 and T4, compared with placebo group. Significant decline was also observed in TGs throughout the whole IVF-ET procedure. And the contents of TGs in ZYP group were significantly lower than that of placebo group, at T2, T3 and T4. In T3 and T4, subjects treated with ZYP had elevated levels of L-asparagine and L-glutamic acid compared with placebo group. A similar trend was observed in four metabolites including kynurenic acid, 11-deoxycorticosterone, melatonin glucuronide, and hydroxytyrosol. In addition, elevated levels of these metabolites in subjects administered with ZYP were observed in T3 and T4, compared with placebo group.
FIGURE 7.
Typical metabolites with significant changes in AMA subgroup. Lines in blue ( ) represent ZYP group while in red (
) represent ZYP group while in red ( ) represent placebo group. Contents of metabolites were assessed with t test between ZYP and placebo group. Contents of metabolites are shown in this figure, i.e., (A) sphinganine, (B) trihexosylceramide (d18:1/26:1), (C) glucosylceramide (d18:1/26:0), (D) PIP3(16:0/16:1), (E) PIP2(18:1/18:1), (F) tauroursodeoxycholic acid, (G) PG(18:1/18:1), (H) TG(22:0/20:5/18:1), (I) TG(22:4/20:4/18:4), (J) L-asparagine, (K) L-glutamic acid, (L) kynurenic acid, (M) 11-deoxycorticosterone, (N) melatonin glucuronide, (O) hydroxytyrosol. *p < 0.05 were regarded as statistically significant, compared with placebo group.
) represent placebo group. Contents of metabolites were assessed with t test between ZYP and placebo group. Contents of metabolites are shown in this figure, i.e., (A) sphinganine, (B) trihexosylceramide (d18:1/26:1), (C) glucosylceramide (d18:1/26:0), (D) PIP3(16:0/16:1), (E) PIP2(18:1/18:1), (F) tauroursodeoxycholic acid, (G) PG(18:1/18:1), (H) TG(22:0/20:5/18:1), (I) TG(22:4/20:4/18:4), (J) L-asparagine, (K) L-glutamic acid, (L) kynurenic acid, (M) 11-deoxycorticosterone, (N) melatonin glucuronide, (O) hydroxytyrosol. *p < 0.05 were regarded as statistically significant, compared with placebo group.
Metabolic status of ZYP group and placebo group were well clustered at each time point, and permutation test showed that the model is good without any overfitting (Supplementary Material).
Changes of Metabolites in the AH Subgroup
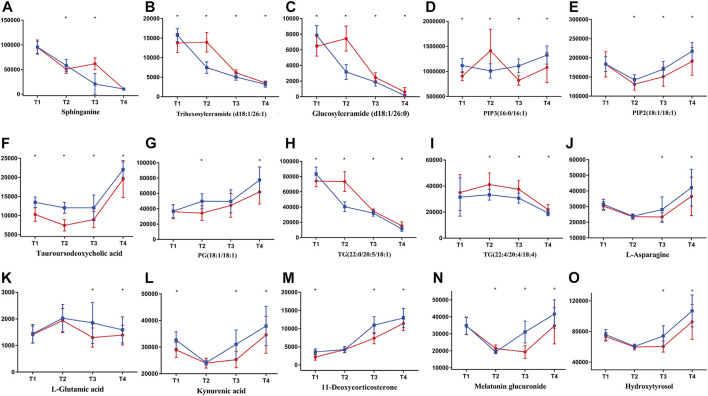
A total of 245 plasma samples were selected from all samples in AH subgroup, and the detailed sampling information was reported in Table 2. In Figures 2E,F, within-group distributions of metabolites were observed using PCA. As can be seen from Figure 8, similar changes were observed in AH subgroup as those of AMA subgroup.
FIGURE 8.
Typical metabolites with significant changes in AH subgroup. Lines in blue ( ) represent ZYP group while in red (
) represent ZYP group while in red ( ) represent placebo group. Contents of metabolites were assessed with t test between ZYP and placebo group. Contents of metabolites are shown in this figure, i.e., (A) sphinganine, (B) trihexosylceramide (d18:1/26:1), (C) glucosylceramide (d18:1/26:0), (D) PIP3(16:0/16:1), (E) PIP2(18:1/18:1), (F) tauroursodeoxycholic acid, (G) PG(18:1/18:1), (H) TG(22:0/20:5/18:1), (I) TG(22:4/20:4/18:4), (J) L-asparagine, (K) L-glutamic acid, (L) kynurenic acid, (M) 11-deoxycorticosterone, (N) melatonin glucuronide, (O) hydroxytyrosol. *p < 0.05 were regarded as statistically significant, compared with placebo group.
) represent placebo group. Contents of metabolites were assessed with t test between ZYP and placebo group. Contents of metabolites are shown in this figure, i.e., (A) sphinganine, (B) trihexosylceramide (d18:1/26:1), (C) glucosylceramide (d18:1/26:0), (D) PIP3(16:0/16:1), (E) PIP2(18:1/18:1), (F) tauroursodeoxycholic acid, (G) PG(18:1/18:1), (H) TG(22:0/20:5/18:1), (I) TG(22:4/20:4/18:4), (J) L-asparagine, (K) L-glutamic acid, (L) kynurenic acid, (M) 11-deoxycorticosterone, (N) melatonin glucuronide, (O) hydroxytyrosol. *p < 0.05 were regarded as statistically significant, compared with placebo group.
Metabolic status of ZYP group and placebo group were well clustered at each time point, and permutation test showed that the model is good without any overfitting (Supplementary Material).
Receiver Operating Characteristic Curve
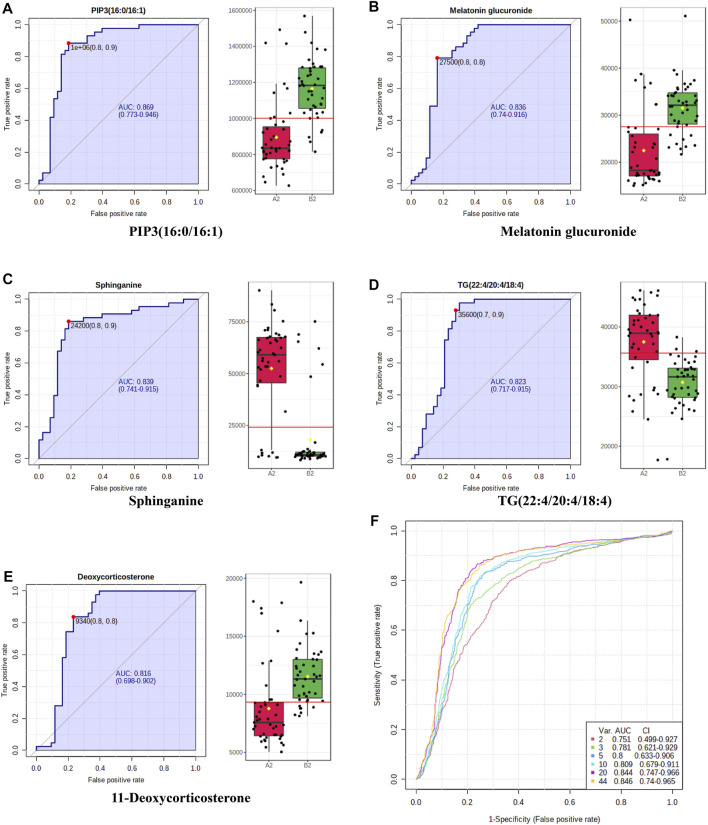
ROC curve analysis is generally considered to one important standard for the assessment of biomarker performance. The results of ROC curve analysis of the 44 differentiated metabolites guaranteed the reliability of potential biomarkers for wide and qualified independent validation. Typical ROC curves and AUC values were shown in Figures 9A–E. Multivariate exploratory ROC analysis overview was shown in Figure 9F. ROC curves for the AH and AMA subgroup were presented in, Supplementary Figures S6, S7. Representative ROC curves presented in Figure 9, Supplementary Figures S6, S7 had AUC values above 0.8.
FIGURE 9.
Representative ROC curve plots of potential biomarkers. (A–E) Representative ROC curves and AUC values of potential biomarkers with high-performance prediction. Box plots in green ( ) represent ZYP group while in red (
) represent ZYP group while in red ( ) represent placebo group. (F) Multivariate exploratory ROC analysis overview.
) represent placebo group. (F) Multivariate exploratory ROC analysis overview.
Discussion
In the present study, metabonomics analysis was conducted with plasma samples of ZYP-RCT, providing information on the mechanisms of ZYP. The MS data of plasma were subjected to multivariate data analysis including PCA, PLS-DA as well as OPLS-DA. All these methods were proved to be reliable, without overfitting. In addition, the conditions of metabolites of ZYP group on T2 (Gn starting day) and T3 (HCG trigger day) were closer to T4 (14 days after ET) compared to that of placebo group. Such findings could be an implication that ZYP is capable of adjusting the body to the state of pre-pregnancy. In addition, the thickness of endometrium on HCG day had shown a trend to increase after ZYP administration (Table 3), however without significance (p > 0.05). This may be accounted for the limited sample size. Metabolites that cause such differences were subjected to identification and enrichment analysis.
TABLE 3.
Thickness of endometrium on baseline (T1) and HCG trigger day (T3)
| Subgroup | Placebo group (mm) | ZYP group (mm) | p | |||
|---|---|---|---|---|---|---|
| Baseline (T1) | Whole | 7.80 ± 2.20 | 7.56 ± 2.94 | 0.672 | ||
| AMA | 7.95 ± 2.55 | 7.54 ± 3.24 | 0.647 | |||
| AH | 7.87 ± 2.31 | 7.27 ± 2.75 | 0.361 | |||
| HCG trigger day (T3) | Whole | 11.48 ± 2.61 | 12.16 ± 2.17 | 0.168 | ||
| AMA | 11.50 ± 2.78 | 11.97 ± 2.51 | 0.574 | |||
| AH | 11.10 ± 2.37 | 11.96 ± 2.20 | 0.144 | |||
p value was calculated between placebo group and ZYP group with t test.
According to statistical analysis, 44 metabolites were found to exhibit significant changes after administration of ZYP compared to those of placebo group. ZYP may exhibited its pharmacological effects, with such metabolites as its characteristics. As can be seen from Table 4, detailed information of 44 metabolites were presented. In addition, changes of representative metabolites from the both the AMA and AH subgroup were shown. Forty-four metabolites were found to show significant changes between ZYP and placebo group, including sphinganine, trihexosylceramide (d18:1/26:1), glucosylceramide (d18:1/26:0), PIP3(16:0/16:1), PIP2(18:1/18:1), tauroursodeoxycholic acid, PG(18:1)/(18:1), TGs, L-asparagine, L-glutamic acid, kynurenic acid, 11-deoxycorticosterone, melatonin glucuronide, hydroxytyrosol, providing us with insights into the mechanisms of ZYP.
Subsequently, pathway enrichment was also performed on these metabolites. As can be seen from Figure 6, the results of metabonomics in this study showed that among these metabolites, the metabolic pathways were mainly related to amino acid metabolism, lipid metabolism, steroid hormone synthesis and so on. Among those pathways enriched in amino metabolism, the following items were found to be altered after administrations of ZYP, including alanine, aspartate and glutamate metabolism, aminoacyl tRNA biosynthesis, glutamine and glutamate metabolism, arginine and proline metabolism, tryptophan metabolism. Lipid metabolism underwent significant changes as well, with alteration in sphingolipid metabolism, glycerophosphatidylcholine metabolism, etc. It could also be observed that ZYP exerted effects on metabolites relating to steroid hormone biosynthesis as well as taurine and hypotaurine metabolism. Detailed discussion on the pathways and related metabolites were reported as followed.
Lipid Metabolism
Sphingolipid Metabolism
Five metabolites, ceramide (d18:1/16:0), glucosylceramide (d18:1/26:0), ganglioside GM2 (d18:1/16:0), trihexosylceramide (d18:1/26:1) and sphinganine were found enriched in sphingolipids and their metabolic pathways (Figure 6).
As shown in Figures 7, 8, most of the metabolites in the ceramide family had undergone a significant decline during the whole IVF-ET process. In addition, after administration of ZYP, this type of metabolites had been decreased compared to those of the placebo group. ROC curve of sphinganine also validated such change (Figure 9). It is generally accepted that ceramide and sphingosine are pro-apoptotic and antigrowth factors via modulation of key intracellular signaling pathways (Arana et al., 2010). There is also evidence showing that an elevated level of sphingolipid metabolism could be risk factors of pregnancy loss (Mizugishi et al., 2007). Such findings agreed with our previous reports that ZYP could be a promising agent in the prevention of abortion, both in threatened abortion and spontaneous abortion (Li et al., 2015; Zhang et al., 2016).
Glycerophospholipids
Eight glycerophospholipids were found to exhibit significant changes in multivariate analysis, including lysoPE(0:0/24:6), CDP-DG(a-17:0/i-13:0), PIP3(16:0/16:1), PIP2(18:1/18:1), PG(18:1/18:1), lysoPE(0:0/15:0), PE(22:1/20:2) and CL(16:0/16:0/16:0/18:0).
Glycerophospholipids are the most abundant phospholipids in the body, which could play important roles in many biological processes, including formation of membrane, acting as surfactant, and participating in signal transduction. PIP3(16:0/16:1) and PIP2(18:1/18:1) are typical glycerophospholipids metabolites. After treatment of ZYP, elevated levels of both metabolites were observed as shown in Figures 7, 8. ROC curve of PIP3(16:0/16:1) also validated such change (Figure 9). Both metabolites are phosphatidylinositol derivatives, with different fatty acid moiety. The former metabolites possess a phosphatidylinositol triphosphate (PIP3) part, which is capable of activating a wide range of proteins, including protein kinase B (Akt) (Manna and Jain, 2015). Phosphatidylinositol biphosphates (PIP2) could be catalyzed by phosphatidylinositol 3-kinases (PI3K), adding of a phosphate group and resulting in formation of PIP3 (Lee et al., 2020). It is well acknowledged that PI3K/AKT signaling pathway is the regulatory center for many biological functions including protein synthesis, cell survival, differentiation, proliferation, and apoptosis (Maidarti et al., 2020). This signaling pathway is highly related to the proliferation and regeneration of endometrium. Previous studies reported that upregulation of PI3K/AKT pathway could lead to higher expression of HIF and VEGF pathways, and therefore, play important roles in endometrial angiogenesis, and eventually leading to improvements in endometrial receptivity (Gupta et al., 2018). It should also be noted that the development of oval cells is regulated by PI3K/AKT/mTOR pathway (Liu et al., 2018). There may be a potential relationship between elevated levels of both PIP3 and PIP2 and the therapeutic effects of ZYP. However, validation of this hypothesis still requires in vivo and in vitro experiments.
PG(18:1/18:1) belongs to the class of phosphatidylglycerol (PG), with cardiolipin (CL) as its precursor. Reports have shown that PG is the second most abundant phospholipid in lung surfactant (Tang et al., 2011). Numerous reports have shown that an elevated level of PG could be observed during pregnancy (Chen et al., 2019a).
Glycerides
Several triglycerides were identified in this study, including TG(22:6/15:0/22:6), TG(18:0/14:0/18:0), TG(22:0/20:5/18:1), TG(22:4/20:4/18:4). As can be seen in Figures 7, 8, significant reduction of TG levels was observed in AMA and AH subgroup. ROC curve of TG(22:4/20:4/18:4) also validated such change (Figure 9). Numerous reports had proved that an increased level of TG could cause alterations in mitochondrial activity and redox status in oocytes, and may further resulted in poor reproductive outcomes (Igosheva et al., 2010). ZYP may exerts its complementary effects in IVF-ET due to its possible role against lipid dysfunction.
Fatty Acid Metabolites
This kind of metabolites were fatty acids or derivatives that possess a moiety of fatty acid. Fatty acids are formed after lipolyzation from triglycerides, and exhibit dual roles in the development of oocytes. On one hand, the fatty acid oxidation is an important energy resource for the development of oocytes and early embryos (Prates et al., 2014). On the other hand, excessive fatty acids could also increase levels of reactive oxygen species (ROS), resulting in the dysfunction of mitochondria and endoplasmic reticulum, eventually impairing the subsequent oocyte development (Yang et al., 2012). In this study, some fatty acid metabolites were also found to exhibit significant changes after administration of ZYP, including 7Z,10Z-hexadecadienoic acid, docosanamide, (E)-2-tridecene-4,6,8-triyn-1-ol, heptadecanoic acid, dodecanoyl carnitine. However, due to the dual role of this kind of metabolites, more data, including results from targeted metabolomics and molecular biology experiments, are required to explore the roles of these metabolites in IVF-ET.
Amino Acids Metabolisms
Amino acids are important substances involved in every aspect of cell metabolism, including metabolisms of carbohydrates, maintenance of osmotic pressure and pH value, gene expressions and so on (Wu, 2013). Thus, amino acids play a central role in the biological processes of reproductive tissues. In particular, amino acids are important regulators in the growth of oocytes and embryos. Alterations in the amino acid metabolisms, including types of amino acids, concentrations of different amino acids, could be important factors affecting the outcomes of pregnancy (Brison et al., 2004; Wu, 2013).
Metabolism of Alanine, Aspartic Acid and Glutamic Acid
After administration of ZYP, L-asparagine and L-glutamic acid were significantly up-regulated in T2, T3 and T4 (Supplementary Tables S3–S5). Numerous reports from cell culture and animal studies shows that some of the important members in this pathway, for example, glutamine, glutamic acid, and arginine, play important roles in multiple signaling pathways, resulting in the regulations of gene expressions, intracellular protein turnover, nutrient metabolism, and oxidative defense (Bröer and Bröer, 2017; Mo et al., 2018). It is well acknowledged that the catabolism of L-glutamic acid could be a source of adenosine triphosphate (ATP). And L-glutamic acid is also an important secondary source of carbon and nitrogen for the re-synthesis of pyrimidines and hydrazines (Wu, 2013). Previous report had also proved that L-glutamic acid exhibited potential protective effects on embryos, and this might be related to the activation of mitogen-activated protein kinase (MAPK) pathway and cell proliferation (Boga Pekmezekmek et al., 2020). Another report had also proved that an elevated level of asparagine was significantly correlated with a clinical pregnancy and live birth (Brison et al., 2004).
Tryptophan Metabolism
Tryptophan is one of the essential amino acids for human, and the precursor of 5-hydroxytryptamine. Dietary tryptophan exhibited positive regulation on the size of follicles and fertilization rate (Jiang et al., 2018). In addition, kynurenic acid is an endogenous metabolite which could be synthesized after degradation of tryptophan. In previous reports, kynurenic acid exhibited potential antioxidative activities and may exert protective effects on endometrium and follicles (Lugo-Huitron et al., 2011). Another metabolite presented in this pathway is melatonin. According to metabonomics study, elevated level of melatonin glucuronide was observed after administration of ZYP (Figures 7, 8). ROC curve of melatonin glucuronide also validated such change (Figure 9). Reports have shown that melatonin treatment could improve the fertility of aged mice due to reduced ROS level in the oocytes, implying its possible therapeutic effects in the AMA subgroup (Zhang et al., 2019).
Steroid Metabolism
Five metabolites related to steroid metabolism were found in the metabonomics study, i.e., tauroursodeoxycholic acid, taurine, 17a-ethinylestradiol, 5α-tetrahydrocortisol, and 11-deoxycorticosterone.
Chemicals in taurine family are important metabolites, with a wide distribution in different organs and tissues. These metabolites could increase the solubility of lipids and cholesterol, playing an important role in lipid metabolism. In vivo and in vitro experiment had shown that this type of metabolites could inhibit inflammation mediators, reduce capillary permeability, and thus could exert beneficial effects on follicle growth, oocyte maturation, fertilization (Mu et al., 2019). Thus, the effects of ZYP might be achieved with the up-regulation of this pathway.
In addition, metabolites of the cortisol family, including 17α-ethynylestradiol, 5α-tetrahydrocortisol, and 11-deoxycorticosterone were found to significantly changed after administration of ZYP. ROC curve of 11-deoxycorticosterone also validated such change (Figure 9). These metabolites belong to the family of glucocorticoids, and are of great significance in the process of metabolism, which is a potential agent in the response to stress. Glucocorticoids affect the secretion of pituitary hormones and gonadal response to GnRH (Zavala et al., 2020). In addition, glucocorticoid can influence the production as well as the maturation of oocyte. Previous reports suggested that higher cortisol content may related to better pregnancy outcome during IVF-ET, because the cortisol content is an important factor in the development of oocytes (Keay et al., 2002). In addition, it was also observed that 17a-ethinylestradiol, as a component of contraceptives, perished in both groups after treatment.
Others
Significant changes were also observed in metabolites, including 2-arachidonylglycerol, hydroxytyrosol, α-tocopherol, α-tocopherol succinate, etc.
2-Arachidonoylglycerol is an endogenous cannabinomimetic lipid derivative. It has been well acknowledged that the exposure to cannabinoids, including 2-arachidonoylglycerol, could lead to adverse effects on reproductive functions, including retarded embryo development, fetal loss, pregnancy failure (Sun and Dey, 2009). The administration of ZYP could significantly reduce the level of 2-arachidonoylglycerol in serum, thus, exhibiting protective effects.
A previous report had shown that hydroxytyrosol was beneficial for pregnant women due to its antioxidative, metabolism-regulatory, anti-inflammatory and immuno-modulatory properties (Menichini et al., 2020).
In the present study, a statistically significant but modest increase was observed in both α-tocopherol succinate and α-terpineol formate. α-Tocopherol, a major composition moiety of vitamin E, is an important compound in the maintenance of reproduction activity and reputed for its antioxidative activity (Rimbach et al., 2010). It could influence the whole process of reproduction, including the health condition of pregnant women, pregnancy outcome, embryotic development, neonatal development, and so on (Cave et al., 2018). However, in the present study, only a modest increase was observed in alpha-tocopherol succinate. But still the alterations in tocopherol are critical in the exploration of mechanisms of ZYP.
A report had proved that α-terpineol possess relaxant effects on rat uterine (Ponce-Monter et al., 2008). In clinical use, ZYP is frequently used as a medicine for the treatment of threatened or spontaneous abortion (Li et al., 2015; Zhang et al., 2016). Thus, the change of this kind of metabolites could also be potential targets in further analysis, particularly in the use of ZYP, in the prevention of pregnancy loss.
Conclusion
In brief, this study provided metabonomics analysis between subjects administered with ZYP and placebo. Relating metabolites were identified and pathways were enriched, providing basis on the exploration on the underlying mechanisms of ZYP combined with IVF-ET in the treatment of infertility.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
Ethics approval has been sought from Ethics Committee at Sun Yat-Sen Memorial Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
LL was in charge of metabonomics research, G-aL, LH, and NN supervised the whole experiment. Q-LH, X-fP, and J-bZ participated in the clinical trial and data analysis relating to clinical trial. J-wZ revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Guangzhou International Science and Technology Cooperation Project (No: 201807010044), Secondary Development Projects of Traditional Chinese Herbal Formula Compound (No: 20174002), Guangdong Province Science and Technology Planning Project (2020B1212030006, 2017B030314166).
Conflict of Interest
Authors NN, Q-LH, X-fP, J-bZ, and J-wZ were employed by the company Guangzhou Baiyunshan Zhongyi Pharmaceutical Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.686133/full#supplementary-material
References
- Arana L., Gangoiti P., Ouro A., Trueba M., Gómez-Muñoz A. (2010). Ceramide and Ceramide 1-phosphate in Health and Disease. Lipids Health Dis. 9, 15. 10.1186/1476-511X-9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boga Pekmezekmek A., Emre M., Tunc E., Sertdemir Y. (2020). L-glutamic Acid Monosodium Salt Reduces the Harmful Effect of Lithium on the Development of Xenopus laevis Embryos. Environ. Sci. Pollut. Res. 27 (33), 42124–42132. 10.1007/s11356-020-10155-x [DOI] [PubMed] [Google Scholar]
- Brison D. R., Houghton F. D., Falconer D., Roberts S. A., Hawkhead J., Humpherson P. G., et al. (2004). Identification of Viable Embryos in IVF by Non-invasive Measurement of Amino Acid Turnover. Hum. Reprod. 19 (10), 2319–2324. 10.1093/humrep/deh409 [DOI] [PubMed] [Google Scholar]
- Bröer S., Bröer A. (2017). Amino Acid Homeostasis and Signalling in Mammalian Cells and Organisms. Biochem. J. 474 (12), 1935–1963. 10.1042/BCJ20160822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu Z., Wang K., Dai W., Sun Y. (2016). Endometrial Thickness Significantly Affects Clinical Pregnancy and Live Birth Rates in Frozen-Thawed Embryo Transfer Cycles. Gynecol. Endocrinol. 32 (7), 524–528. 10.3109/09513590.2015.1136616 [DOI] [PubMed] [Google Scholar]
- Cao J., Lei T., Wu S., Li H., Deng Y., Lin R., et al. (2020). Development of a Comprehensive Method Combining UHPLC-CAD Fingerprint, Multi-Components Quantitative Analysis for Quality Evaluation of Zishen Yutai Pills: A Step towards Quality Control of Chinese Patent Medicine. J. Pharm. Biomed. Anal. 191, 113570. 10.1016/j.jpba.2020.113570 [DOI] [PubMed] [Google Scholar]
- Cave C., Hanson C., Schumacher M., Lyden E., Furtado J., Obaro S., et al. (2018). A Comparison of Vitamin E Status and Associated Pregnancy Outcomes in Maternal-Infant Dyads between a Nigerian and a United States Population. Nutrients 10 (9), 1300. 10.3390/nu10091300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Wang J., Wang M., Lu J., Cai Y., Li B. (2019a). In Vitro fertilization Alters Phospholipid Profiles in Mouse Placenta. J. Assist. Reprod. Genet. 36 (3), 557–567. 10.1007/s10815-018-1387-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. L., Li L., Pan P., Yang D. Z. (2019b). Effects of Zishen Yutai Pill on the Outcomes of Assisted Reproductive Technique. J. Pract. Med. 35 (Suppl. 5), 42–44. [Google Scholar]
- Gao Q., Wang S., Tian H., Li X., La X. (2015b). Efficacy Studies on Zishen Yutai Pill of Different Courses In Vitro Fertilization-Embryo Transfer. J. Xinjiang Med. Univ. 38, 320–324. 10.3969/j.issn.1009-5551.2015.03.019 [DOI] [Google Scholar]
- Gao Q., Han L., Li X., Cai X. (2015a). Traditional Chinese Medicine, the Zishen Yutai Pill, Ameliorates Precocious Endometrial Maturation Induced by Controlled Ovarian Hyperstimulation and Improves Uterine Receptivity via Upregulation of HOXA10. Evidence-Based Complement. Altern. Med. 2015, 1–10. 10.1155/2015/317586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K., Sirohi V. K., Kumari S., Shukla V., Manohar M., Popli P., et al. (2018). Sorcin Is Involved during Embryo Implantation via Activating VEGF/PI3K/Akt Pathway in Mice. J. Mol. Endocrinol. 60 (2), 119–132. 10.1530/JME-17-0153 [DOI] [PubMed] [Google Scholar]
- Igosheva N., Abramov A. Y., Poston L., Eckert J. J., Fleming T. P., Duchen M. R., et al. (2010). Maternal Diet-Induced Obesity Alters Mitochondrial Activity and Redox Status in Mouse Oocytes and Zygotes. PLoS One 5 (4), e10074. 10.1371/journal.pone.0010074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inhorn M. C., Patrizio P. (2015). Infertility Around the Globe: New Thinking on Gender, Reproductive Technologies and Global Movements in the 21st Century. Hum. Reprod. Update 21 (4), 411–426. 10.1093/humupd/dmv016 [DOI] [PubMed] [Google Scholar]
- Jiang S. Q., Gou Z. Y., Lin X. J., Li L. (2018). Effects of Dietary Tryptophan Levels on Performance and Biochemical Variables of Plasma and Intestinal Mucosa in Yellow-Feathered Broiler Breeders. J. Anim. Physiol. Anim. Nutr. 102, e387–e394. 10.1111/jpn.12757 [DOI] [PubMed] [Google Scholar]
- Keay S. D., Harlow C. R., Wood P. J., Jenkins J. M., Cahill D. J. (2002). Higher Cortisol:cortisone Ratios in the Preovulatory Follicle of Completely Unstimulated IVF Cycles Indicate Oocytes with Increased Pregnancy Potential. Hum. Reprod. 17 (9), 2410–2414. 10.1093/humrep/17.9.2410 [DOI] [PubMed] [Google Scholar]
- Kibble M., Saarinen N., Tang J., Wennerberg K., Mäkelä S., Aittokallio T. (2015). Network Pharmacology Applications to Map the Unexplored Target Space and Therapeutic Potential of Natural Products. Nat. Prod. Rep. 32 (8), 1249–1266. 10.1039/c5np00005j [DOI] [PubMed] [Google Scholar]
- Kissin D. M., Jamieson D. J., Barfield W. D. (2014). Monitoring Health Outcomes of Assisted Reproductive Technology. N. Engl. J. Med. 371 (1), 91–93. 10.1056/NEJMc1404371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen S. G., Pors S. E., Andersen C. Y. (2017). Improving Oocyte Quality by Transfer of Autologous Mitochondria from Fully Grown Oocytes. Hum. Reprod. 32 (4), 1–8. 10.1093/humrep/dex043 [DOI] [PubMed] [Google Scholar]
- Lee S., Kim M.-G., Ahn H., Kim S. (2020). Inositol Pyrophosphates: Signaling Molecules with Pleiotropic Actions in Mammals. Molecules 25 (9), 2208. 10.3390/molecules25092208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Yao D.-n., Lu Y., Deng J.-w., Wei J.-a., Yan Y.-h., et al. (2020). Metabonomics Study on Serum Characteristic Metabolites of Psoriasis Vulgaris Patients with Blood-Stasis Syndrome. Front. Pharmacol. 11, 558731–558739. 10.3389/fphar.2020.558731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li X., Luo S. P. (2015). Effect of Recurrent Spontaneous Abortion on Pregnancy Outcomes in Sequent Successful Pregnancy Patients. J. Guangzhou Univ. Tradit Chin. Med. 32 (6), 979–983. 10.13359/j.cnki.gzxbtcm.2015.06.002 [DOI] [Google Scholar]
- Liu X., Zhang L., Liu Y., Cui J., Che S., An X., et al. (2018). Circ-8073 Regulates CEP55 by Sponging miR-449a to Promote Caprine Endometrial Epithelial Cells Proliferation via the PI3K/AKT/mTOR Pathway. Biochim. Biophys. Acta (Bba) - Mol. Cel Res. 1865 (8), 1130–1147. 10.1016/j.bbamcr.2018.05.011 [DOI] [PubMed] [Google Scholar]
- Lugo-Huitrón R., Blanco-Ayala T., Ugalde-Muñiz P., Carrillo-Mora P., Pedraza-Chaverrí J., Silva-Adaya D., et al. (2011). On the Antioxidant Properties of Kynurenic Acid: Free Radical Scavenging Activity and Inhibition of Oxidative Stress. Neurotoxicology and Teratology 33 (5), 538–547. 10.1016/j.ntt.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Ma H. W., Zou Q., Li C., Yin Z., Yi H., Tian L. C., et al. (2018). HPLC Fingerprint of Zishen Yutai Pills and Simultaneous Determination of 5 Index Components. China J. Chin. Mater. Med. 43 (14), 2878–2883. 10.19540/j.cnki.cjcmm.2018.0090 [DOI] [PubMed] [Google Scholar]
- Mahajan N., Sharma S. (2016). The Endometrium in Assisted Reproductive Technology: How Thin Is Thin? J. Hum. Reprod. Sci. 9 (1), 3–8. 10.4103/0974-1208.178632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidarti M., Anderson R. A., Telfer E. E. (2020). Crosstalk Between PTEN/PI3K/Akt Signalling and DNA Damage in the Oocyte: Implications for Primordial Follicle Activation, Oocyte Quality and Ageing. Cells 9 (1), 200. 10.3390/cells9010200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna P., Jain S. K. (2015). Phosphatidylinositol-3,4,5-triphosphate and Cellular Signaling: Implications for Obesity and Diabetes. Cell Physiol Biochem 35 (4), 1253–1275. 10.1159/000373949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichini D., Alrais M., Liu C., Xia Y., Blackwell S. C., Facchinetti F., et al. (2020). Maternal Supplementation of Inositols, Fucoxanthin, and Hydroxytyrosol in Pregnant Murine Models of Hypertension. Am. J. Hypertens. 33 (7), 652–659. 10.1093/ajh/hpaa041 [DOI] [PubMed] [Google Scholar]
- Mizugishi K., Li C., Olivera A., Bielawski J., Bielawska A., Deng C.-X., et al. (2007). Maternal Disturbance in Activated Sphingolipid Metabolism Causes Pregnancy Loss in Mice. J. Clin. Invest. 117 (10), 2993–3006. 10.1172/JCI30674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo W., Wu X., Jia G., Zhao H., Chen X., Tang J., et al. (2018). Roles of Dietary Supplementation with Arginine or N-Carbamylglutamate in Modulating the Inflammation, Antioxidant Property, and mRNA Expression of Antioxidant-Relative Signaling Molecules in the Spleen of Rats under Oxidative Stress. Anim. Nutr. 4 (3), 322–328. 10.1016/j.aninu.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu T., Feng Y., Che Y., Lv Q., Hu J., Yang Q., et al. (2019). Taurine Promotes Iin-Vvitro Follicle Development, Oocyte Maturation, Fertilization and Cleavage of Rats. Adv. Exp. Med. Biol. 1155, 197–203. 10.1007/978-981-13-8023-5_18 [DOI] [PubMed] [Google Scholar]
- Ponce-Monter H., Campos M. G., Pérez S., Pérez C., Zavala M., Macías A., et al. (2008). Chemical Composition and Antispasmodic Effect of Casimiroa Pringlei Essential Oil on Rat Uterus. Fitoterapia 79 (6), 446–450. 10.1016/j.fitote.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Prates E. G., Nunes J. T., Pereira R. M. (2014). A Role of Lipid Metabolism during Cumulus-Oocyte Complex Maturation: Impact of Lipid Modulators to Improve Embryo Production. Mediators Inflamm. 2014, 1–11. 10.1155/2014/692067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimbach G., Moehring J., Huebbe P., Lodge J. K. (2010). Gene-Regulatory Activity of α-Tocopherol. Molecules 15 (3), 1746–1761. 10.3390/molecules15031746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. F., Eisenberg M. L., Millstein S. G., Nachtigall R. D., Shindel A. W., Wing H., et al. (2010). The Use of Complementary and Alternative Fertility Treatment in Couples Seeking Fertility Care: Data from a Prospective Cohort in the United States. Fertil. Sterility 93 (7), 2169–2174. 10.1016/j.fertnstert.2010.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Dey S. K. (2009). Cannabinoid/Endocannabinoid Signaling Impact on Early Pregnancy Events. Curr. Top. Behav. Neurosci. 1, 255–273. 10.1007/978-3-540-88955-7_10 [DOI] [PubMed] [Google Scholar]
- Tang C.-H., Tsao P.-N., Chen C.-Y., Shiao M.-S., Wang W.-H., Lin C.-Y. (2011). Glycerophosphocholine Molecular Species Profiling in the Biological Tissue Using UPLC/MS/MS. J. Chromatogr. B 879 (22), 2095–2106. 10.1016/j.jchromb.2011.05.044 [DOI] [PubMed] [Google Scholar]
- Vaiarelli A., Cimadomo D., Ubaldi N., Rienzi L., Ubaldi F. M. (2018). What Is New in the Management of Poor Ovarian Response in IVF? Curr. Opin. Obstet. Gynecol. 30 (3), 155–162. 10.1097/GCO.0000000000000452 [DOI] [PubMed] [Google Scholar]
- Wang X., Yang B., Sun H., Zhang A. (2012). Pattern Recognition Approaches and Computational Systems Tools for Ultra Performance Liquid Chromatography-Mass Spectrometry-Based Comprehensive Metabolomic Profiling and Pathways Analysis of Biological Data Sets. Anal. Chem. 84 (1), 428–439. 10.1021/ac202828r [DOI] [PubMed] [Google Scholar]
- Wu G. (2013). Functional Amino Acids in Nutrition and Health. Amino Acids 45 (3), 407–411. 10.1007/s00726-013-1500-6 [DOI] [PubMed] [Google Scholar]
- Wyns C., Bergh C., Calhaz-Jorge C., De Geyter C., Kupka M. S., Motrenko T. (2020). ART in Europe, 2016: Results Generated from European Registries by ESHRE. Hum. Reprod. Open 2020 (3), 1–17. 10.1093/hropen/hoaa032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J., Sinelnikov I. V., Han B., Wishart D. S. (2015). MetaboAnalyst 3.0-making Metabolomics More Meaningful. Nucleic Acids Res. 43 (W1), W251–W257. 10.1093/nar/gkv380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Wu L. L., Chura L. R., Liang X., Lane M., Norman R. J., et al. (2012). Exposure to Lipid-Rich Follicular Fluid Is Associated with Endoplasmic Reticulum Stress and Impaired Oocyte Maturation in Cumulus-Oocyte Complexes. Fertil. Sterility 97 (6), 1438–1443. 10.1016/j.fertnstert.2012.02.034 [DOI] [PubMed] [Google Scholar]
- Zavala E., Voliotis M., Zerenner T., Tabak J., Walker J. J., Li X. F., et al. (2020). Dynamic Hormone Control of Stress and Fertility. Front. Physiol. 11, 598845. 10.3389/fphys.2020.598845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhang Z., Wang J., Lv D., Zhu T., Wang F., et al. (2019). Melatonin Regulates the Activities of Ovary and Delays the Fertility Decline in Female Animals via MT1/AMPK Pathway. J. Pineal Res. 66 (3), e12550. 10.1111/jpi.12550 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yan W., Ge P.-F., Li Y., Ye Q. (2016). Study on Prevention Effect of Zishen Yutai Pill Combined with Progesterone for Threatened Abortion in Rats. Asian Pac. J. Trop. Med. 9 (6), 577–581. 10.1016/j.apjtm.2016.04.002 [DOI] [PubMed] [Google Scholar]
- Zhu W., Li X., Chen X., Zhang L. (2002). Effect of Zishen Yutai Pill on Embryo Implantation Rate in Patients Undergoing Fertilization Embryo Transfer In Vitro. Chin. J. Integr. Med. 22, 729–737. 10.3321/j.issn:1003-5370.2002.10.002 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.