Abstract
Noninvasive, accurate measurement of pressures within the human body has long been an important but elusive clinical goal. Contrast agents for ultrasound imaging are gas-filled, encapsulated microbubbles (diameter < 10 μm) that traverse the entire vasculature and enhance signals by up to 30 dB. These microbubbles also produce nonlinear oscillations at frequencies ranging from the subharmonic (half of the transmit frequency) to higher harmonics. The subharmonic amplitude has an inverse linear relationship with the ambient hydrostatic pressure. Here an ultrasound system capable of performing real-time, subharmonic aided pressure estimation (SHAPE) is presented. During ultrasound contrast agent infusion, an algorithm for optimizing acoustic outputs is activated. Following this calibration, subharmonic microbubble signals (i.e., SHAPE) have the highest sensitivity to pressure changes and can be used to noninvasively quantify pressure. The utility of the SHAPE procedure for identifying portal hypertension in the liver is the emphasis here, but the technique has applicability across many clinical scenarios.
Keywords: Pressure estimation, contrast-enhanced ultrasound, subharmonic imaging, liver, hepatic venous pressure gradient, portal hypertension
SUMMARY:
A protocol for noninvasively, estimating ambient pressures utilizing subharmonic ultrasound imaging of infused contrast microbubbles (following appropriate calibration) is described with examples from human patients with chronic liver disease.
INTRODUCTION:
A number of different ultrasound contrast agents (UCAs) are approved for clinical use in cardiology (in particular left ventricular opacification) and radiology (in particular adult and pediatric liver lesion characterization) across the world.1 The sensitivity and specificity of ultrasound imaging can be improved by intravenous (IV) injection of gas-filled microbubbles (diameter < 10 μm) encapsulated by a lipid or protein shell as UCAs that traverse the entire vasculature and enhance signals by up to 30 dB.1 These UCAs not only enhance the backscattered ultrasound signals, but at sufficient acoustic pressures (> 200 kPa) they also act as nonlinear oscillators. Hence, significant energy components will be produced in the received echoes ranging from subharmonic and harmonic to ultraharmonic frequencies.1,2 These nonlinear signal components can be extracted from tissue and linear bubble echoes (e.g., using pulse inversion) and used to create contrast -specific imaging modalities such as subharmonic imaging (SHI), which receives at half the transmit frequency (i.e., at f0/2).3-Our group has demonstrated in human clinical trials that SHI can detect the blood flow in neovessels and arterioles associated with a variety of tumors and tissues.4–9
We have advocated the use of UCAs not as vascular tracers, but as sensors for noninvasive pressure estimation in the circulatory system by monitoring subharmonic contrast bubble amplitude variations.10 This innovative technique, called subharmonic-aided pressure estimation (SHAPE), relies on the inverse linear correlation between the amplitude of the subharmonic signals and hydrostatic pressure (up to 186 mmHg) measured for most commercial UCAs in vitro (r2 > 0.90) as summarized in Table 1.10,11 However, it should be noted that not all UCAs exhibit this behavior. Most notably, it has been shown that subharmonic signals from the UCA SonoVue (known as Lumason in the USA) initially rise with hydrostatic pressure increases, followed by a plateau and a decreasing phase.12 Nonetheless, SHAPE offers the possibility of allowing pressure gradients in the heart and throughout the cardiovascular system as well as interstitial fluid pressure in tumors to be obtained noninvasively.13–17 Recently, we implemented a real-time version of the SHAPE algorithm on a commercial ultrasound scanner and provided proof-of-concept that SHAPE can provide in vivo pressure estimates with errors of less than 3 mmHg in the left and right ventricles of patients.16,17
Table 1:
Subharmonic response (and correlation) of commercial UCAs to a pressure increase of approximately 185 mmHg.
| UCA | Manufacturer | Subharmonic signal reduction (dB) | Linear regression (r2) |
|---|---|---|---|
| Definity | Lantheus Medical Imaging, N Billerica, MA, USA | 11.0 ± 0.3 | 0.98 |
| Levovist | Schering AG, Berlin, Germany | 9.6 ± 0.2 | 0.98 |
| Lumason aka SonoVue | Bracco, Milan, Italy | 1.0 ± 1.3 | 0.20 |
| Optison | GE Healthcare, Princeton, NJ, USA | 10.1 ± 0.2 | 0.97 |
| Sonazoid | GE Healthcare, Oslo, Norway | 13.3 ± 0.2 | 0.99 |
The most experience with SHAPE has been gained diagnosing portal hypertension with more than 220 subjects enrolled to date and initial findings confirmed in a multi-center trial.13,14 Portal hypertension is defined as an increase in the pressure gradient between the portal vein and hepatic veins or the inferior vena cava exceeding 5 mmHg, while clinically significant portal hypertension (CSPH) requires a gradient or its equivalent, a hepatic venous pressure gradient (HVPG) ≥ 10 mmHg.18 CSPH is associated with an increased risk of gastroesophageal varices, ascites, hepatic decompensation, post-operative decompensation, and hepatocellular carcinoma.18,19 Patients who develop ascites have a 50% three-year mortality and those who develop spontaneous infection of the ascites fluid carry a 70% one-year mortality. Patients with cirrhosis have a 5–10% yearly incidence of gastroesophageal variceal formation, and a 4–15% yearly incidence of bleeding; each bleeding episode carries up to a 20% risk of death.18,19
This manuscript describes how to conduct a SHAPE study using commercially available equipment and UCAs with an emphasis on identifying portal hypertension in the liver of patients. The critical calibration procedure required to achieve the highest sensitivity to estimating pressure changes is explained in detail.
PROTOCOL:
1. Research approvals and support
1.1.
The institutional review boards of both Thomas Jefferson University and the Hospital of the University of Pennsylvania approved this protocol.
1.1.1.
The protocol is compliant with the Health Insurance Portability and Accountability Act.
1.2.
The United States Food and Drug Administration (FDA) issued an Investigational New Drug approval (IND # 124,465 to F. Forsberg) for this protocol.
1.3.
GE Healthcare (Oslo, Norway) provided the UCA used in this research (Sonazoid; Table 1).
NOTE: Sonazoid is not approved by the FDA for any clinical applications in the United Sates, which is why an IND was necessary. Other UCAs with FDA approval1 can be used off-label at the discretion of the treating physician if deemed potentially clinically useful.
1.4.
The full protocol and statistical analysis plan are available at https://clinicaltrials.gov/ct2/show/NCT02489045. Trial registration number: NCT # 02489045.
2. Subject preparation
2.1.
Review the subject’s known drug allergies or intolerances in particular any known allergy to the UCA being used.
2.2.
Exclude subjects with unstable cardiopulmonary conditions or who are generally medically unstable.
2.3.
Put the subject on a stretcher in the supine position.
2.4.
Place an 18 – 22 gauge cannula in a vein in the subject’s right or left arm for the UCA infusion.
2.5.
Make sure emergency services (e.g., a crash cart) will be available within the hospital in case of any acute adverse reactions.
NOTE: UCAs are very safe with serious anaphylactoid-type reactions reported at a rate of less than 0.01%.20
3. UCA preparation (Specific to Sonazoid)
3.1.
This UCA is supplied as a dry powder within 10 mL sealed vials. The headspace of the vials contains perfluorobutane. Three (3) vials with 48 μL of microbubbles (6 mL) will be prepared for each subject by resuspending according to the manufacturer’s instructions.
3.1.1.
Perforate the stopper of the UCA vial with a chemospike.
3.1.2.
Remove the protective cap from the syringe port of the chemospike and add 2 mL of sterile water.
3.1.3.
With the syringe remaining attached to the chemospike, immediately shake the product for 1 minute to ensure a homogeneous product.
3.1.4.
Withdraw the product into the syringe and re-inject the product back into the vial again. This is to avoid dilution of the product due to the dead-space volume in the chemospike.
3.1.5.
Remove the syringe from the syringe port and reattach the protective cap. The concentration of the reconstituted UCA is 8 μL microbubbles/mL.
3.1.6.
Repeat the reconstitution procedure for the other 2 vials.
3.2.
Use saline (0.9% NaCl solution) to fill up the connecting tubes before being connected to a 3-way stopcock. The stopcock will then be connected to the extension tubing leading to the cannula.
3.3.
Draw all three (3) vials of suspended UCA into a 10 mLl syringe, and place it in a syringe pump at the same level or below the patient, and connect directly to the stopcock.
3.4.
After the initial ultrasound imaging and after the stopcock has been opened, infuse the NaCl solution at a rate of 120 mL/hour, and co-infuse Sonazoid at a rate of 0.024 μL/ kg body weight/ minute (suspension infusion rate of 0.18 mL/kg/hour).
NOTE: This infusion rate was selected based on our group’s previous experiences with Sonazoid infusion in portal hypertension subjects undergoing SHAPE.13,14,21 The exact resuspension procedure and infusion method will vary depending on the UCA used.
4. Initial ultrasound imaging
4.1.
Power up a Logiq E10 ultrasound scanner and select the C1–6-D curvilinear probe.
4.2.
Select an abdominal preset on the ultrasound scanner and use a curvi-linear array (typically with a 1–6 or 2–8 MHz bandwidth) to acquire grayscale images of both the portal and a hepatic vein in the same imaging plane and at similar depths (Figure 1). This is generally best achieved via a subcostal approach.
Figure 1: Example of grayscale liver image for SHAPE initiation.
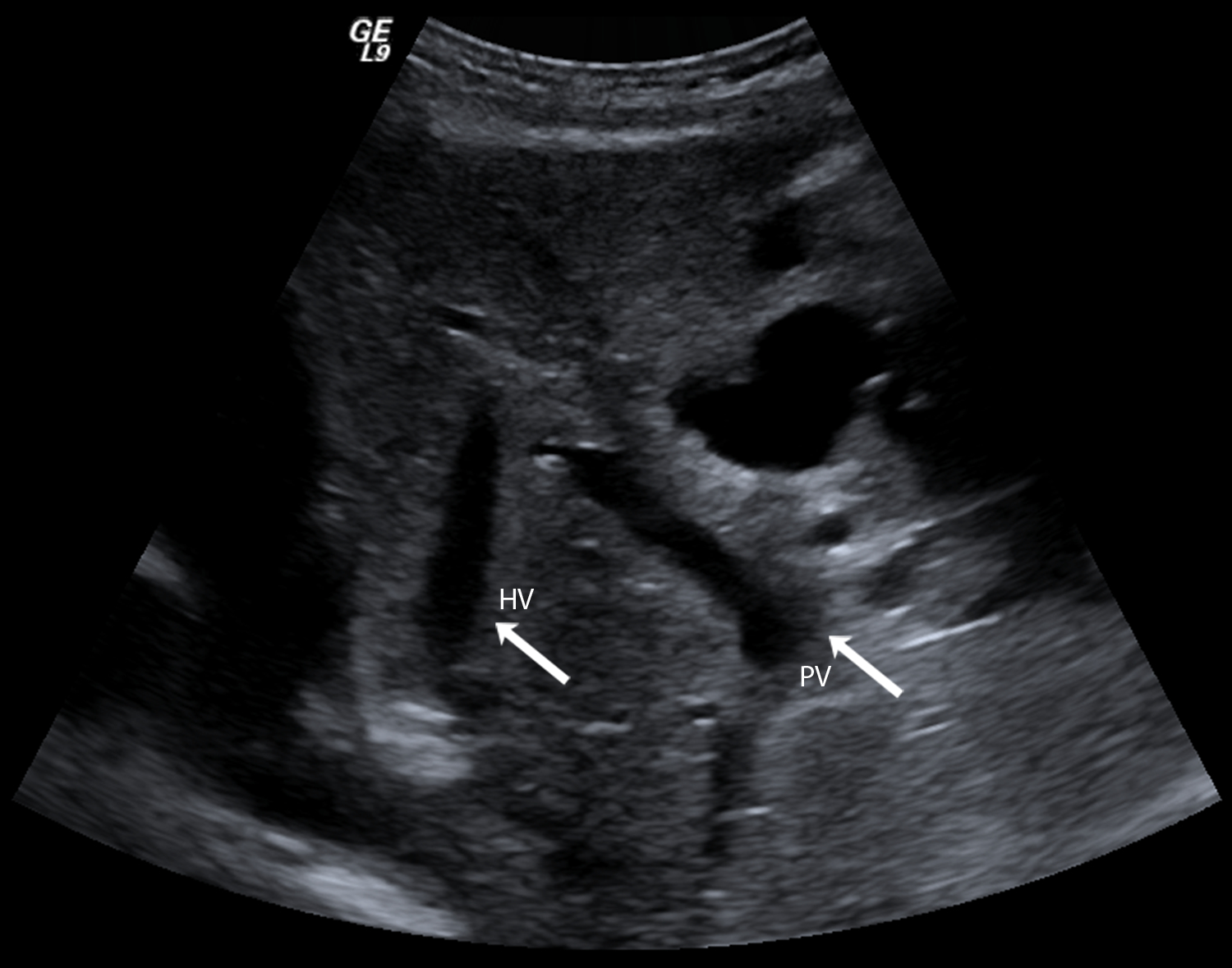
Arrows indicate the portal vein (PV) and a hepatic vein (HV).
4.3.
Optimize the images based on Good Clinical Practice and take care to select the hepatic vein region away from the inferior vena cava to avoid the influence of retrograde flow.
5. SHI and SHAPE imaging
5.1.
Activate the SHI contrast imaging mode in dual display mode (i.e., running real-time B-mode and SHI simultaneously) using the “Subharmonic Contrast” touch panel button and activate Contrast mode. Then select “SUBH-AM” on the rotary control.
5.1.1.
SHI will be performed at a transmit frequency of 2.5 MHz and the received signals will be obtained at 1.25 MHz.
5.1.2.
Use pulse-shaping to maximizing the generation of subharmonic microbubble signals, such as a Gaussian windowed binomial filtered square wave with Sonazoid,21 but this is scanner and UCA dependent.17
NOTE: The choice of imaging frequency and pulse shape may not be available to end-users.
5.2.
Confirm the patency of the portal and the hepatic vein as well as the presence of microbubbles, which can take up to 1–2 minutes from the start of the infusion.
5.3.
Activate the SHAPE automated optimization code to optimize SHAPE by compensating for varying depth and attenuation.22,23 Select “TIC Analysis” on the touch panel followed by F6 and then the “k” button.
5.4.
The SHAPE optimization algorithm will acquire subharmonic data for every acoustic output level. Once data acquisition is complete, position an ROI on the portal vein in the contrast sample window (top left on the TIC Analysis screen).
5.4.1.
The average subharmonic data within the ROI is plotted as a function of acoustic output and a logistic curve is fitted to the data. Select the inflection point of this curve (or rather the peak in the derivative curve shown underneath) as the optimized power, as this has been shown to be the point of greatest SHAPE sensitivity.22,23 One such set of curves is shown in Figure 2.
Figure 2: Calibration curve for SHAPE optimization.
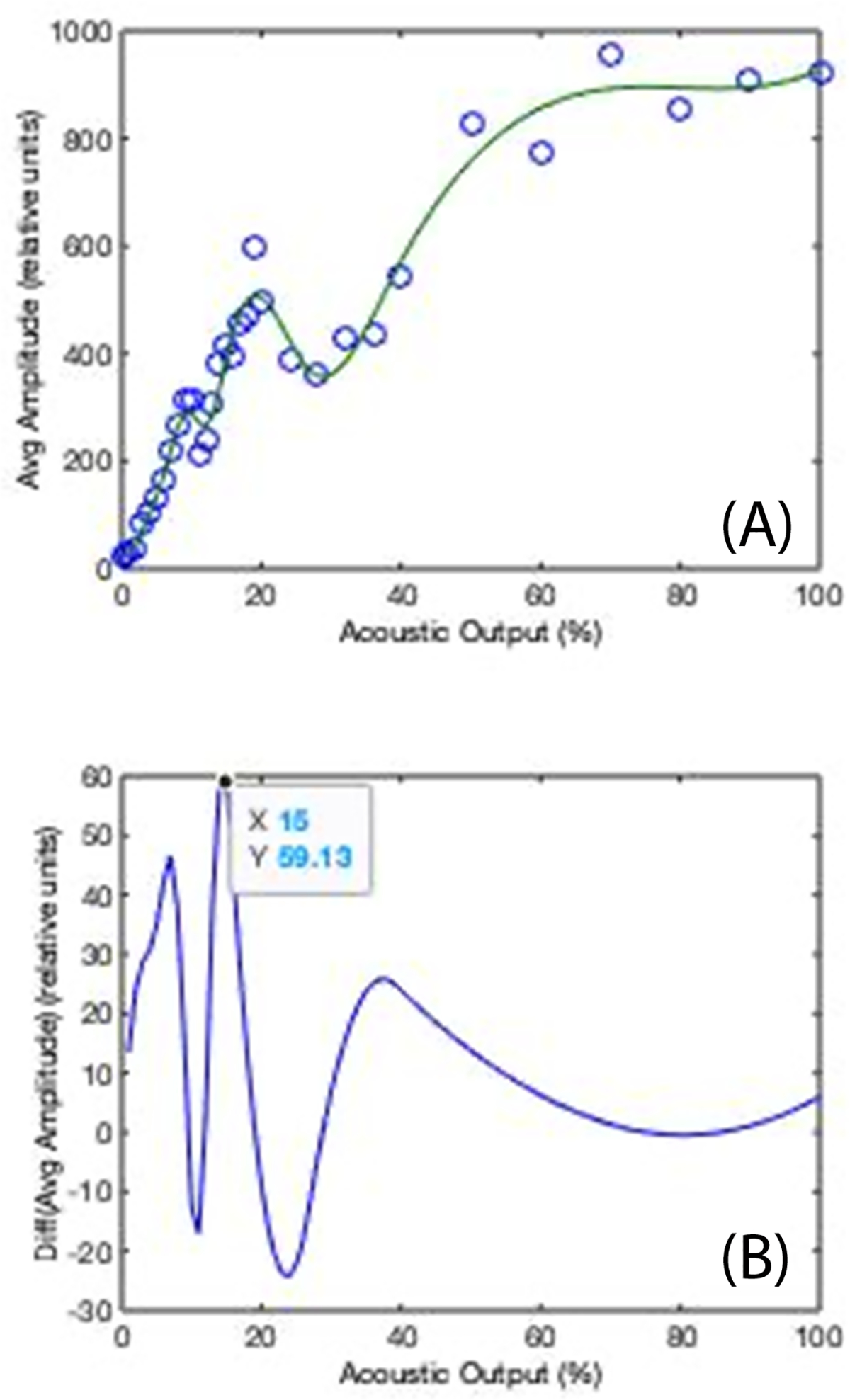
(A) Subharmonic amplitude (in dB) as a function of acoustic output power (in %) showing the characteristic S-curve behavior. (B) The derivative of the S-curve for output power selection (arrow indicates selected peak and, thus, power).
5.5.
Adjust the acoustic output power to the value identified in step 5.4.1, which will ensure the maximum change in subharmonic amplitudes a function of ambient pressure (i.e., maximizing the sensitivity of SHAPE).
5.6.
Acquire subharmonic data from the microbubbles (i.e., SHAPE) in 5–15 s segments during the infusion of the UCA suspension (Figure 3).
Figure 3: Dual Imaging with B-mode (black and white) and subharmonic imaging (gold) on the left and right respectively of each image.
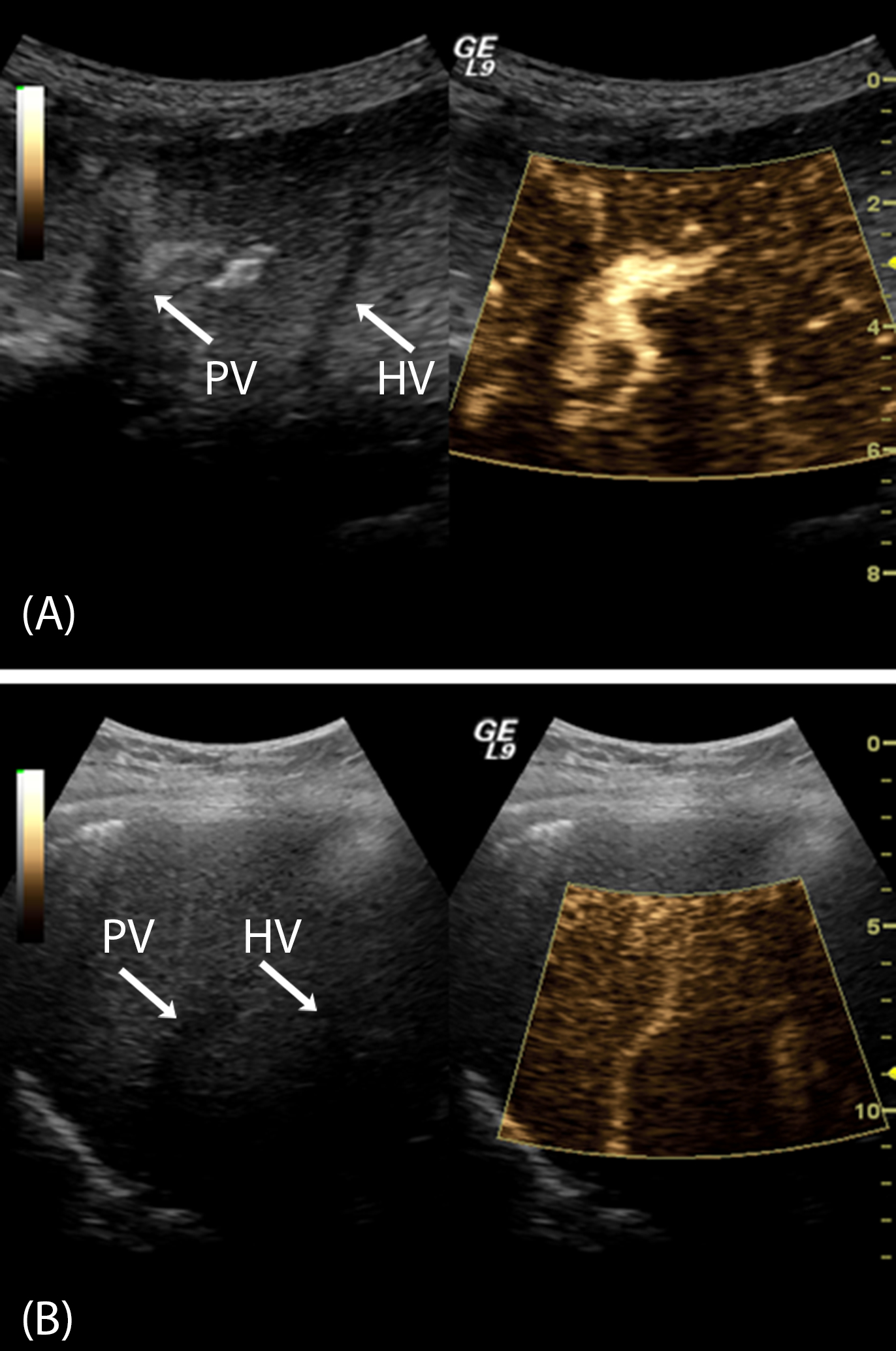
(A): A patient with normal HVPG values (3 mmHg) with a bright subharmonic signal from the portal vein (PV) and inadequate signal from the hepatic vein (HV). (B): A patient with CSPH and a HVPG of 15 mmHg demonstrating considerable subharmonic signals in both portal and hepatic veins.
6. SHAPE data processing
6.1.
Once the optimized SHI cine-loop has been acquired (step 5.6) select “TIC Analysis” on the touch panel.
6.1.1.
Make sure “Motion Tracking” is activated on the touch panel, which adjusts the ROI position for each frame to compensate for any breathing or other motion.
6.1.2.
Make sure dB is selected as the unit for the Y-axis on the traces in the analysis window.
6.2.
In the contrast sample window (top left on the screen) select identical ROIs (elliptical regions are default) within the hepatic and portal veins. In the analysis window (to the right) the subharmonic signal (in dB) within each vessel is averaged over all the frames in a 0.5 MHz bandwidth around 1.25 MHz.
6.3.
The final SHAPE gradient (in dB) is calculated as the difference in the mean subharmonic signal between the hepatic and the portal vein ROIs. Based on our current studies, the optimal operating point for identifying CSPH is −0.11 dB and the linear regression equation is HVPG = 0.81 × SHAPE + 9.43.14 It is important to note that this cutoff and equation iare both scanner and UCA dependent.
REPRESENTATIVE RESULTS:
As with all ultrasound imaging examinations, the first consideration for liver SHAPE is to obtain the best possible baseline grayscale images of the target region and to ensure (using Doppler imaging) that there are no intrahepatic portal venous shunts or other vascular abnormalities present. In the case of liver imaging for diagnosing portal hypertension the key is to visualize both the portal vein and a hepatic vein at the same depth in order to minimize the impact of attenuation (Figure 1).
Even though UCA concentration is not considered a critical factor in SHAPE procedures,10,23 it is nonetheless recommended to infuse the UCA to minimize all sources of variability. The UCA should be reconstituted and infused (preferably through a 20 or 22 gauge needle24) according to the manufacturer’s specific instructions. Once equilibrium enhancement is reached the optimization algorithm should be activated and an ROI in the portal vein selected, which will produce curves such as those shown in Figure 2. Once the optimal acoustic output power has been selected calibrated SHI data (i.e., SHAPE) can be acquired.
Examples of SHAPE images in subjects with and without CSPH are presented in Figure 3. The main visual difference is the marked subharmonic signal present in the hepatic vein in the subject with CSPH (Figure 3B) and absent in the other case (Figure 3A). Quantitative, relative pressure estimates can be calculated from the difference between the average subharmonic signals of ROIs placed in the hepatic and portal veins (i.e., the SHAPE gradient). However, in approximately 10% of cases studied so far, the subharmonic signal was too close to the scanner’s noise floor and had to be discarded. This could be due to inadequate contrast enhancement. Moreover, there are patients who present with clinical and laboratorial signs of portal hypertension, but who have HVPG values that are normal or zero. This can be attributed to a number of anatomical and/or vascular variations, such as one subject with a fistula between the portal and hepatic vein leading to no difference between the free and wedged pressures and, thus, an incorrect SHAPE diagnosis (Figure 4).
Figure 4: Unsuccessful SHAPE study in subject with a fistula between the hepatic veins.
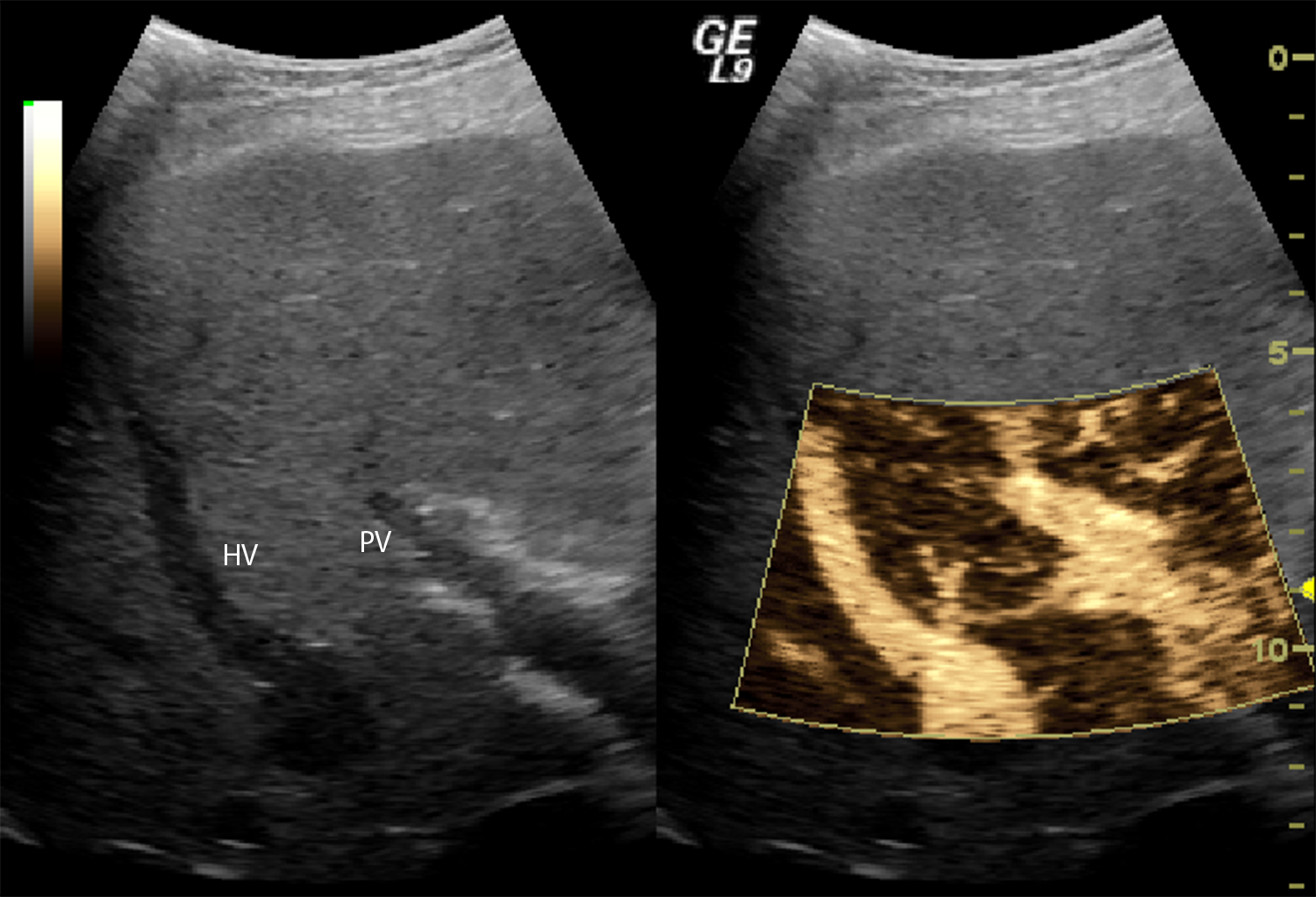
This anatomical variation resulted in an HVPG of 0 mmHg even though the gradient pressures (referred to as the free and wedged pressures18,19) were both 39 mmHg (i.e., a difference of 0 mmHg), while the SHAPE gradient was −15.33 dB.
Our group conducted a first-in-humans, pilot study of SHAPE in 45 patients undergoing transjugular liver biopsy (providing HVPG measurements as the reference standard), which showed significantly higher SHAPE gradients between the portal and hepatic veins in subjects with CSPH (i.e., a HVPG ≥ 10 mmHg) than in those with lower HVPGs (1.37 ± 0.59 dB vs. −1.68 ± 0.27 dB, p < 0.001).13
Recently, we expanded on the concept of using SHAPE for portal pressure estimation in a large multi-center clinical trial. Results from 178 subjects across two sites using modified Logiq 9 systems established the utility of SHAPE for diagnosing CSPH with a sensitivity of 91% (95% confidence interval: 88–93%) and a specificity of 82% (95% confidence interval: 75–85%).14 The overall accuracy was 95% for diagnosing subjects with CSPH (95% confidence interval (CI): 89–99%) and these subjects had a higher SHAPE gradient than participants with lower HVPGs (0.27 ± 2.13 dB vs −5.34 ± 3.29 dB; p < 0.001) indicating SHAPE may indeed be a useful tool for the diagnosis of portal hypertension.14 Similarly, the sensitivity and specificity for diagnosing all subjects with portal hypertension (i.e., HVPG ≥ 5 mmHg) were 71% and 80%, respectively.
DISCUSSION:
Noninvasive, accurate measurement of pressures within the human body has long been an important but elusive clinical goal. The protocol for SHAPE measurements presented here achieves this goal. The most critical component of the SHAPE procedure is the optimization algorithm, since subharmonic data not acquired at the optimal acoustic power output will correlate poorly with hydrostatic pressures.17,22,23 The initial version of this software implemented on a Logiq 9 scanner was prone to displaying multiple peaks in the derivative of the S-curve (cf., Figure 2B) making the correct output power selection difficult.13,14 However, with improved motion correction on the Logiq E10 scanner this issue has been somewhat mitigated.23 Moreover, the SHAPE algorithm as currently implemented has a failure rate of approximately 10%, where the subharmonic signal-to-noise ratio is too low for reliable pressure estimates to be calculated.14 No differences in age, body-mass-index, depth of imaging or liver status have been identified between subjects with successful and with failed SHAPE studies.
In this protocol the UCA highlighted for SHAPE was Sonazoid, but a number of commercial UCAs can be used (cf., Table 1).11,13–16 The infusion setup and microbubble concentration required for any given UCA used with SHAPE should be adjusted based on the recommendations from the specific manufacturer.
Although this is not typically a user accessible parameter, using pulse-shaping to maximizing the generation of subharmonic microbubble signals is important for a successful SHAPE procedure. For the Logiq family of scanners (from GE Healthcare) a Gaussian windowed binomial filtered square wave with Sonazoid appears optimal,21 but this is scanner and UCA dependent.17 For the SonixTABLET scanner from BK Ultrasound both a square wave and a chirp pulse can be used (with different UCAs).17 Apart from the systems mentioned above, the only other commercial ultrasound scanners currently available with SHI and, thus, SHAPE are from MindRay.
This protocol focused on identifying portal hypertension in patients with chronic liver disease as the clinical application. A major reason is that existing noninvasive techniques, such as using CT, MRI or ultrasound imaging, are indirect and qualitative and results have been quite mixed.19 Noninvasive ultrasound measures such as elastography for liver stiffness are quantitative techniques that can identify patients at high risk of CSPH; especially when combined with measurement of spleen size and platelet count. Accuracies of 90–94% for the initial diagnosis of CSPH have been reported, but these methods are not sufficiently precise to allow therapeutic reductions in HVPG to be tracked.19 Improvement in clinical scoring systems, normalization of serum liver function tests, or reduction in ascites and varices qualitatively indicate improvement in portal hypertension.18 However, unlike SHAPE, none of these measures provides a quantitative measure of the portal pressure. Consequently, the only clinically accepted method for quantifying portal pressures is through the HVPG measured via an invasive pressure catheter.
Likewise, the SHAPE algorithm can provide cardiac pressure estimates with errors of less than 3 mmHg in patients.16 No quantitative, noninvasive alternatives to SHAPE exist in cardiology. This is nonetheless a challenging application, since absolute, real-time pressure estimates are required.16,17 Investigations into 3D SHAPE for monitoring interstitial fluid pressure as a measure of breast cancers response to neoadjuvant therapy have shown that at 10% completion of therapy (i.e., after one chemotherapy cycle) the SHAPE gradient between the tumor and the surrounding normal tissue can differentiate responders from partial/non responders (3.23 ± 1.41 dB vs. −0.88 ± 1.46 dB; p = 0.001).15 Other clinical areas, such as noninvasively estimating pressures in the bladder or the brain, are being pursued by researchers around the world demonstrating the wide applicability of the SHAPE technique.
In summary, this SHAPE protocol combines commercially available UCAs, an ultrasound scanner, and calibrated SHI to provide real time, noninvasive quantitative pressure estimates, thus, fulfilling a significant and hitherto unmet clinical need.
ACKNOWLEDGEMENTS:
Supported in part by the U.S. Army Medical Research Material Command under W81XWH-08-1-0503, and W81XWH-12-1-0066, by AHA grants no 0655441U and 15SDG25740015 as well as by NIH R21 HL081892, R21 HL130899, R21 HL119951, RC1 DK087365, R01 DK098526, R01 DK118964, R01 CA140338, R37 CA234428, by Lantheus Medical Imaging and by GE Healthcare, Oslo, Norway.
Footnotes
DISCLOSURES:
Drs. Forsberg, Gupta, Wallace and Eisenbrey have a patent pending on the SHAPE technology. Dr. Wallace is an employee of GE.
REFERENCES:
- 1.Lyshchik A (Editor). Fundamentals of CEUS. Elsevier. Manitoba, Canada. (2019). [Google Scholar]
- 2.Leighton TG The Acoustic Bubble. Academic Press: London, England, (1994). [Google Scholar]
- 3.Forsberg F, Shi WT, Goldberg BB Subharmonic imaging of contrast agents. Ultrasonics. 38 (1–8), 93–98, (2000). [DOI] [PubMed] [Google Scholar]
- 4.Forsberg F, Piccoli CW, Merton DA, Palazzo JP, Hall AL Breast lesions: imaging with contrast-enhanced subharmonic US - initial experience. Radiology. 244 (3), 718–726, (2007). [DOI] [PubMed] [Google Scholar]
- 5.Sridharan A, et al. Characterizing breast lesions using quantitative parametric 3D subharmonic imaging: a multi-center study. Academic Radiology. 27 (8), 1065–1074, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forsberg F, et al. Subharmonic and endoscopic contrast imaging of pancreatic masses: a pilot study. Journal of Ultrasound in Medicine. 37 (1), 123–129, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delaney LJ, et al. Characterization of adnexal masses using contrast-enhanced subharmonic imaging: a pilot study. Journal of Ultrasound in Medicine. 39 (5), 977–985, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenbrey JR, et al. Contrast-enhanced subharmonic and harmonic ultrasound of renal masses undergoing percutaneous cryoablation. Academic Radiology. 22 (7), 820–826, (2015). [DOI] [PubMed] [Google Scholar]
- 9.Gupta I, et al. Transrectal subharmonic ultrasound imaging for prostate cancer detection. Urology. 138 (4), 106–112, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi WT, Forsberg F, Raichlen JS, Needleman L, Goldberg BB Pressure dependence of subharmonic signals from contrast microbubbles. Ultrasound in Medicine and Biology. 25 (2), 275–283, (1999). [DOI] [PubMed] [Google Scholar]
- 11.Halldorsdottir VG, et al. Subharmonic contrast microbubble signals for noninvasive pressure estimation under static and dynamic flow conditions. Ultrasonic Imaging. 33 (3), 153–164, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nio AQX, et al. Optimal control of SonoVue microbubbles to estimate hydrostatic pressure. IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control. 67 (3), 557–567, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenbrey JR, et al. Chronic liver disease: noninvasive subharmonic aided pressure estimation of hepatic venous pressure gradient. Radiology. 268 (2), 581–588, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta I, et al. Diagnosing portal hypertension with noninvasive subharmonic pressure estimates from an ultrasound contrast agent. Radiology, (2020). In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nam K, et al. Monitoring neoadjuvant chemotherapy for breast cancer by using three-dimensional subharmonic aided pressure estimation and imaging with US contrast agents: preliminary experience. Radiology. 285 (1), 53–62, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dave JK, et al. Non-invasive intra-cardiac pressure measurements using subharmonic-aided pressure estimation: proof of concept in humans. Ultrasound in Medicine and Biology. 43 (11), 2718–2724 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esposito C, Dickie K, Forsberg F, Dave JK Developing an interface and investigating optimal parameters for real-time intra-cardiac subharmonic aided pressure estimation. IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control. doi: 10.1109/TUFFC.2020.3016264, (2020). Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosch J, Groszmann RJ, Shah VH Evolution in the understanding of the pathophysiological basis of portal hypertension: How changes in paradigm are leading to successful new treatments. Journal of Hepatology. 62 (1 Suppl) S121–S130, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Procopet B, Berzigotti A Diagnosis of cirrhosis and portal hypertension: imaging, noninvasive markers of fibrosis and liver biopsy. Gastroenterology Report (Oxford Academic). 5 (2),79–89, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dietrich CF, et al. Guidelines and good clinical practice recommendations for contrast-enhanced ultrasound (CEUS) in the liver-update 2020 WFUMB in cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound in Medicine and Biology. July 22:S0301–5629(20)30200–3, (2020). doi: 10.1016/j.ultrasmedbio.2020.04.030. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Gupta I, et al. Effect of pulse shaping on subharmonic aided pressure estimation in vitro and in vivo. Journal of Ultrasound in Medicine. 36 (1), 3–11, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dave JK, et al. On the implementation of an automated acoustic output optimization algorithm for subharmonic aided pressure estimation. Ultrasonics. 53 (4), 880–888, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta I, Eisenbrey JR, Machado P, Stanczak M, Wallace K, Forsberg F On factors impacting subharmonic- aided pressure estimation (SHAPE). Ultrasonic Imaging. 41 (1), 35–48, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenbrey JR, Daecher A, Kramer MR, Forsberg F Effects of needle and catheter size on commercially available ultrasound contrast agents. Journal of Ultrasound in Medicine. 34 (11), 1961–1968, (2015). [DOI] [PubMed] [Google Scholar]


