Abstract
Background:
Failed back surgery syndrome (FBSS) has a high incidence following spinal surgery, is notoriously refractory to treatment, and results in high health care utilization. Spinal cord stimulation (SCS) is a well-accepted modality for pain relief in this population; however, until recently magnetic resonance imaging (MRI) was prohibited due to risk of heat conduction through the device.
Objectives:
We examined trends in imaging use over the past decade in patients with FBSS to determine its impact on health care utilization and implications for patients receiving SCS.
Study Design:
Retrospective.
Setting:
Inpatient and outpatient sample.
Methods:
We identified patients from 2000 to 2012 using the Truven MarketScan database. Annual imaging rates (episodes per 1000 patient months) were determined for MRI, computed tomography (CT) scan, x-ray, and ultrasound. A multivariate Poisson regression model was used to determine imaging trends over time, and to compare imaging in SCS and non-SCS populations.
Results:
A total of 311,730 patients with FBSS were identified, of which 5.17% underwent SCS implantation (n = 16,118). The median (IQR) age was 58.0 (49.0 – 67.0) years. Significant increases in imaging rate ratios were found in all years for each of the modalities. Increases were seen in the use of CT scans (rate ratio [RR] = 3.03; 95% confidence interval [CI]: 2.79 – 3.29; P < 0.0001), MRI (RR = 1.73; 95% CI: 1.61 – 1.85; P < 0.0001), ultrasound (RR = 2.00; 95% CI: 1.84 – 2.18; P < 0.0001), and x-ray (RR = 1.10; 95% CI: 1.05 – 1.15; P < 0.0001). Despite rates of MRI in SCS patients being half that in the non-SCS group, these patients underwent 19% more imaging procedures overall (P < 0.0001). SCS patients had increased rates of x-ray (RR = 1.27; 95% CI: 1.25 – 1.29), CT scans (RR = 1.32; 95% CI: 1.30 – 1.35), and ultrasound (RR = 1.10; 95% CI: 1.07 – 1.13) (all P < 0.0001).
Limitations:
This study is limited by a lack of clinical and historical variables including the complexity of prior surgeries and pain symptomatology. Miscoding cannot be precluded, as this sample is taken from a large nationwide database.
Conclusions:
We found a significant trend for increased use of advanced imaging modalities between the years 2000 and 2012 in FBSS patients. Those patients treated with SCS were 50% less likely to receive an MRI (as expected, given prior incompatibility of neuromodulation devices), yet 32% and 27% more likely to receive CT and x-ray, respectively. Despite the decrease in the use of MRI in those patients treated with SCS, their overall imaging rate increased by 19% compared to patients without SCS. This underscores the utility of MR-conditional SCS systems. These findings demonstrate that imaging plays a significant role in driving health care expenditures. This is the largest analysis examining the role of imaging in the FBSS population and the impact of SCS procedures. Further studies are needed to assess the impact of MRI-conditional SCS systems on future trends in imaging in FBSS patients receiving neuromodulation therapies.
Keywords: Failed back surgery syndrome, spinal cord stimulation, imaging, health care utilization, MRI, chronic pain, back pain, neuromodulation
Chronic low back pain (LBP) is reported to affect as much as 37% of the adult population, with a lifetime prevalence between 60% and 85%, and is estimated to cost between 12.2 and 90.6 billion U.S. dollars each year (1–3). Failed back surgery syndrome (FBSS) refers to the persistence of pain following surgical treatment of these patients. This umbrella term has a variety of root causes, and establishing the diagnosis is important for management as the conditions can be both surgical and non-surgical (4). The most common diagnoses are foraminal stenosis, painful disc, pseudoarthrosis, neuropathic pain, recurrent disc herniation, facet joint pain, and sacroiliac joint pain (5). The incidence of FBSS among patients undergoing back surgery is reported to be between 10% and 40% (6). These patients with FBSS fail to obtain adequate pain relief and have a lower quality of life compared to other chronic pain syndromes (7). Initially, FBSS patients are treated with conventional medical management (CMM) such as physical rehabilitation and other nonsurgical interventions (8). In those in whom CMM has failed, spinal cord stimulation (SCS) is recommended over an additional spine operation (9,10).
There is a paucity of studies analyzing changes in imaging rates in patients with FBSS. Overall rates of imaging have been shown to be escalating in recent years in various populations (11–15), including both Medicare and private insurance patients. Use of lumbar spine magnetic resonance imaging (MRI) was found to increase by 307% in the Medicare population during a 12-year interval from 1994 to 2006 (11). Moreover, data from a large California private insurer showed that rates of MRI and computed tomography (CT) increased by more than 50%, and the rate of positron emission tomography (PET) increased by almost 400% between 2000 and 2004 (12).
We hypothesized that a major source of health care utilization in this population is an increased use of imaging in the diagnosis and management of these patients. The primary objective of this study was to determine whether the rates of various imaging modalities have increased in FBSS over a recent twelve-year period (2000 to 2012). Furthermore, we sought to determine the impact of SCS during this time period on differences in the rates of imaging for FBSS patients.
Methods
Data Source
We utilized the Truven Reuters MarketScan® Database, containing information on more than 200 million unique patients in the United States since 1995. This database contains fully integrated patient-level data, including inpatient, outpatient, drug, and lab information from commercial, Medicare Supplemental, and Medicaid populations. We performed a retrospective review of imaging rates in patients with FBSS from the years 2000 to 2012.
Patient Population
International Classification of Diseases, Ninth Revision [ICD-9], codes were used to select patients with a diagnosis of FBSS. The following codes were used: 72283, 3382, and 3384. FBSS patients were defined as having the ICD code 77283, or a chronic pain diagnosis code of 3382 or 3384 with a prior lumbar spine surgery procedure code of 63005, 63012, 63017, 63030, 63042, or 63047. Patients with a history of SCS were defined by the presence of the procedure code 63685 and one of the following codes: 63650 or 63655. Only patients with a minimum of one year of continuous data were included. “Patient months” were based on the patient’s enrollment period with partial months rounded to the next whole month.
Data Collection
Data were collected on patient age, gender, race, employment status, geographical region, and date of claim. The yearly total imaging count was determined for all imaging, as well as separately for MRI, CT, x-ray, and ultrasound. Imaging counts were collected based on the number of imaging episodes. An imaging episode was defined as one outpatient visit per day for outpatients and as one overall hospital admission with associated imaging for inpatients. The number of months of data recorded in the database varied by patients. To account for this, imaging rates were expressed per number of patient months within the database rather than per total number of patients. All rates of imaging were determined annually as the number of imaging episodes per 1000 patient months within the database.
Statistical Analysis
Observations from 311,730 unique patients with FBSS were evaluated to count the annual number of each individual and overall imaging procedures. Annual imaging rates were then computed as the total number of imaging episodes divided by the total number of patient months within the given year. The rates and counts are presented by year from 2000 to 2012. All rates are expressed per 1000 patient months. A multivariate Poisson regression model was used to determine whether rates were increasing with time. This regression method uses the count of a procedure type in a given year as the dependent variable with the log of patient months as the offset term. Each model includes gender, age, employment status, SCS history, and calendar year as the independent variables, among which gender, employment status, SCS history, and year were evaluated as categorical variables. This analysis uses an auto regressive correlation structure for patients with multiple years of data. All analyses and data processing were conducted using SAS software, V9.4, SAS Institute Inc., Cary, NC, USA.
Results
Demographics
Demographic data for FBSS patients both with and without a history of SCS are presented in Table 1. A total of 311,730 patients were identified, of which 5.17% underwent SCS implantation (n = 16,118). There were more women (55.3%) than men in the overall cohort. The median (interquartile range [IQR]) age was 58.0 (49.0 – 67.0) years. The total number of SCS implantations was recorded. In the patient cohort with a history of SCS implantation, 90.4% of patients underwent one implantation.
Table 1.
Demographics of FBSS patients with and without history of SCS.
| All patients | No SCS History | SCS History | |
|---|---|---|---|
| Total – no. (col%, row%) | 311,730 (100.0, 100.0) | 295,612 (100.0, 94.83) | 16,118 (100.0, 5.17) |
| Gender - no. (col%, row%) | |||
| Male | 139,466 (44.74, 100.0) | 132,860 (44.94, 95.26) | 6,606 (40.99, 4.74) |
| Female | 172,264 (55.26, 100.0) | 162,752 (55.06, 94.48) | 9,512 (59.01, 5.52) |
| Age of Patient | |||
| mean (SD) | 58.1 (14.17) | 58.2 (14.23) | 56.9 (12.92) |
| median (IQR) | 58.0 (49.0 – 67.0) | 58.0 (49.0 – 67.0) | 56.0 (48.0 – 64.0) |
| SCS Count - no. (col%, row%) | |||
| 0 | 295,612 (94.83, 100.0) | 295,612 (100.0, 100.0) | |
| 1 | 14,572 (4.67, 100.0) | 14,572 (90.41, 100.0) | |
| 2 | 1,442 (0.46, 100.0) | 1,442 (8.95, 100.0) | |
| 3 | 91 (0.03, 100.0) | 91 (0.56, 100.0) | |
| 4 | 12 (0.00, 100.0) | 12 (0.07, 100.0) | |
| 8 | 1 (0.00, 100.0) | 1 (0.01, 100.0) | |
Imaging Rates and Rate Ratios in Patients with FBSS
The overall annual rates of imaging for patients with FBSS are shown in Table 2. The total imaging rate, defined as imaging episodes per 1,000 patient-months, increased over this 12-year period in patients with FBSS. In 2000, the imaging rate was 332 episodes per 1,000 patient-months. The imaging rate reached 2 peaks in 2009 and 2012 with 736 and 734 episodes per 1,000 patient-months, respectively.
Table 2.
Overall imaging rates for FBSS patients by year.
| Year | Unique patients | Patient months enrolled | Imagining count | Rate (episodes per 1000 patient-months) |
|---|---|---|---|---|
| 2000 | 3,177 | 36,002 | 11,960 | 332 |
| 2001 | 8,016 | 81,169 | 36,539 | 450 |
| 2002 | 16,118 | 1,48,669 | 77,103 | 519 |
| 2003 | 27,166 | 2,37,596 | 1,35,884 | 572 |
| 2004 | 41,013 | 3,40,701 | 2,13,300 | 626 |
| 2005 | 54,664 | 4,15,645 | 2,92,828 | 705 |
| 2006 | 64,018 | 4,79,788 | 3,43,588 | 716 |
| 2007 | 73,122 | 5,40,569 | 3,84,388 | 711 |
| 2008 | 94,644 | 6,99,818 | 5,05,908 | 723 |
| 2009 | 1,13,702 | 8,31,260 | 6,12,064 | 736 |
| 2010 | 1,28,587 | 9,76,127 | 6,45,827 | 662 |
| 2011 | 1,54,827 | 11,01,803 | 7,28,804 | 661 |
| 2012 | 1,69,191 | 10,55,959 | 7,75,306 | 734 |
Treating years as categorical variables, the annual rates of imaging were compared to the year 2000 as a reference year to determine imaging rate ratios in patients with FBSS. Significant increases in overall imaging use were found in all subsequent years (Table 3). The greatest increase in overall imaging was seen in 2009 with a rate ratio of 1.58 (95% confidence interval [CI]: 1.51 – 1.65; P < 0.0001). The rate ratios for the various imaging modalities in the year 2012 compared to 2000 as the reference year are shown in Table 4. The use of all modalities increased across this interval. The greatest increase was seen with CT scan use (rate ratio [RR] = 3.03; 95% CI: 2.79 – 3.29; P < 0.0001). The use of MRI (RR = 1.73; 95% CI: 1.61 – 1.85; P < 0.0001) and ultrasound (RR = 2.00; 95% CI: 1.84 – 2.18; P < 0.0001) increased as well. A modest increase in x-ray use was also seen in this time period (RR = 1.10; 95% CI: 1.05 – 1.15; P < 0.0001).
Table 3.
Rate ratios for overall imaging use in FBSS patients.
| Year | Rate Ratio (5% – 95% CI) | Z Stat | P-value |
|---|---|---|---|
| 2000 | 1 | ||
| 2001 | 1.2 (1.14 – 1.26) | 7.38 | < 0.0001 |
| 2002 | 1.31 (1.25 – 1.38) | 11.4 | < 0.0001 |
| 2003 | 1.33 (1.27 – 1.39) | 12.4 | < 0.0001 |
| 2004 | 1.39 (1.33 – 1.45) | 14.5 | < 0.0001 |
| 2005 | 1.45 (1.39 – 1.52) | 16.5 | < 0.0001 |
| 2006 | 1.48 (1.42 – 1.55) | 17.5 | < 0.0001 |
| 2007 | 1.51 (1.44 – 1.58) | 18.3 | < 0.0001 |
| 2008 | 1.55 (1.48 – 1.62) | 19.5 | < 0.0001 |
| 2009 | 1.58 (1.51 – 1.65) | 20.6 | < 0.0001 |
| 2010 | 1.45 (1.39 – 1.52) | 16.7 | < 0.0001 |
| 2011 | 1.37 (1.32 – 1.44) | 14.3 | < 0.0001 |
| 2012 | 1.39 (1.33 – 1.45) | 14.6 | < 0.0001 |
Table 4.
Rate ratios for each of the individual imaging modalities in 2012.
| Modality | Year | Rate Ratio (5% – 95% CI) | Z Stat | P-value |
|---|---|---|---|---|
| MRI | 2000 | 1 | ||
| 2012 | 1.73 (1.61–1.85) | 15.5 | < 0.0001 | |
| X-ray | 2000 | 1 | ||
| 2012 | 1.1 (1.05–1.15) | 4.18 | < 0.0001 | |
| CT Scan | 2000 | 1 | ||
| 2012 | 3.03 (2.79–3.29) | 26.2 | < 0.0001 | |
| Ultrasound | 2000 | 1 | ||
| 2012 | 2.00 (1.84–2.18) | 16 | < 0.0001 |
Comparison of Imaging in FBSS Patients with and without SCS
Rate ratios were determined to compare imaging rates in FBSS patients with and without a history of SCS implantation over the 12-year interval. These results are displayed in Table 5. The imaging rates for patients without a history of SCS implantation were normalized to one. The overall imaging rate across this period was higher in FBSS patients with a history of SCS (RR = 1.19; 95% CI: 1.17 – 1.21; P < 0.0001). The rates of x-ray, CT scan, and ultrasound were all higher in SCS patients (Table 5). The rate of MRI was decreased for FBSS patients who had undergone SCS implantation (RR = 0.50; 95% CI: 0.49 – 0.52; P < 0.0001). These trends in MRI use over the study interval are displayed in Fig. 1. While rates of MRI steadily increased in both cohorts across the study interval, rates were consistently higher in the non-SCS cohort. A comparison of MRI rate by SCS status in 2012 is shown in Fig. 2.
Table 5.
Imaging rate ratios for FBSS patients with and without SCS. Rates for patients without a history of SCS were normalized to one.
| Imaging Modality | Variable | Rate Ratio (5% – 95% CI) | Z Stat | P-value |
|---|---|---|---|---|
| Overall Imaging | SCS History | 1.19 (1.17–1.21) | 23.6 | |
| No SCS History | 1 | < 0.0001 | ||
| MRI | SCS History | 0.50 (0.49–0.52) | −41 | |
| No SCS History | 1 | < 0.0001 | ||
| X-ray | SCS History | 1.27 (1.25–1.29) | 30.6 | |
| No SCS History | 1 | < 0.0001 | ||
| CT scan | SCS History | 1.32 (1.30–1.35) | 29.3 | |
| No SCS History | 1 | < 0.0001 | ||
| Ultrasound | SCS History | 1.10 (1.07–1.13) | 6.47 | |
| No SCS History | 1 | < 0.0001 |
Fig. 1.
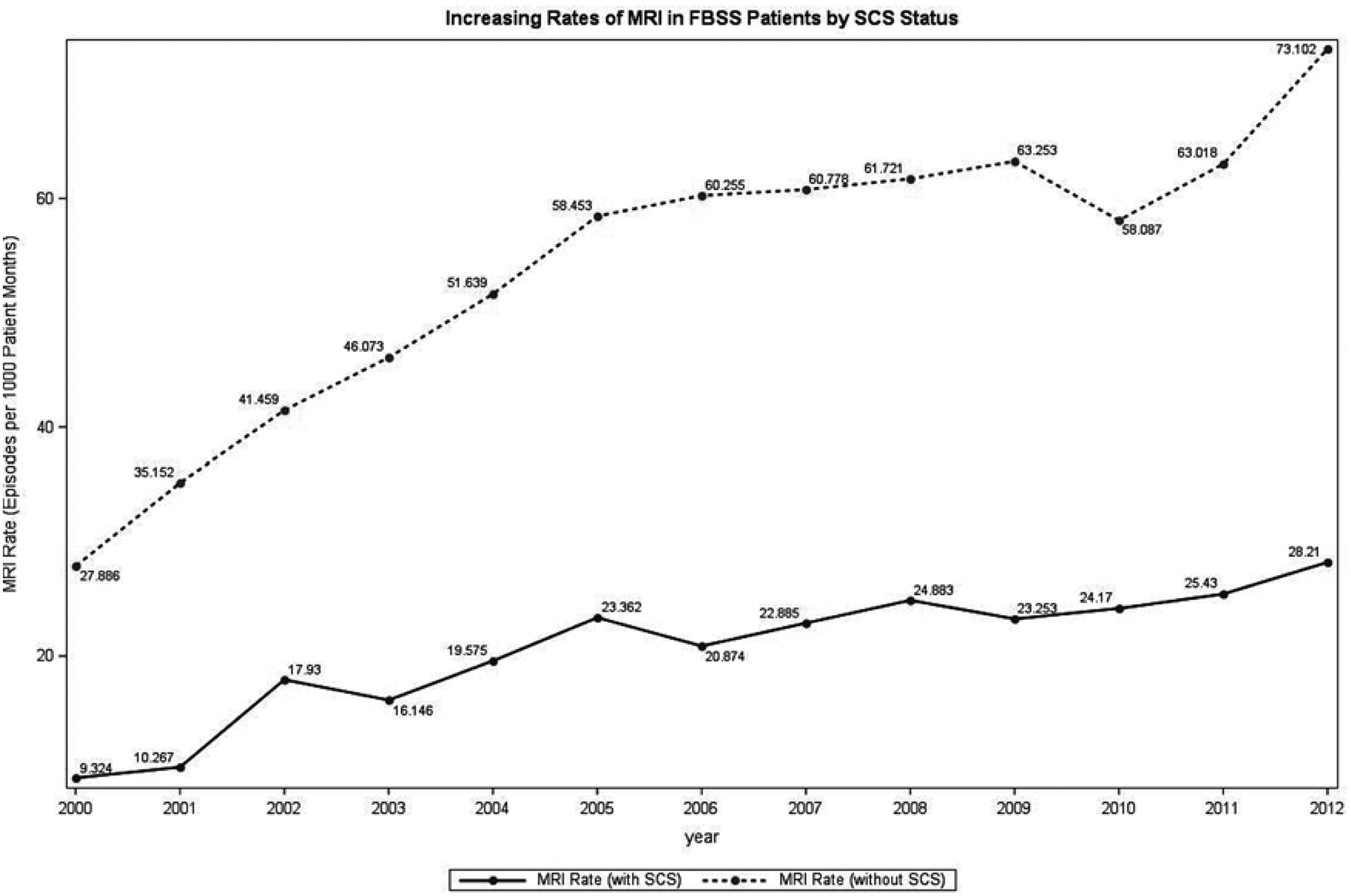
Increasing rates of MRI by SCS status in FBSS patients between 2000 and 2012.
Fig. 2.
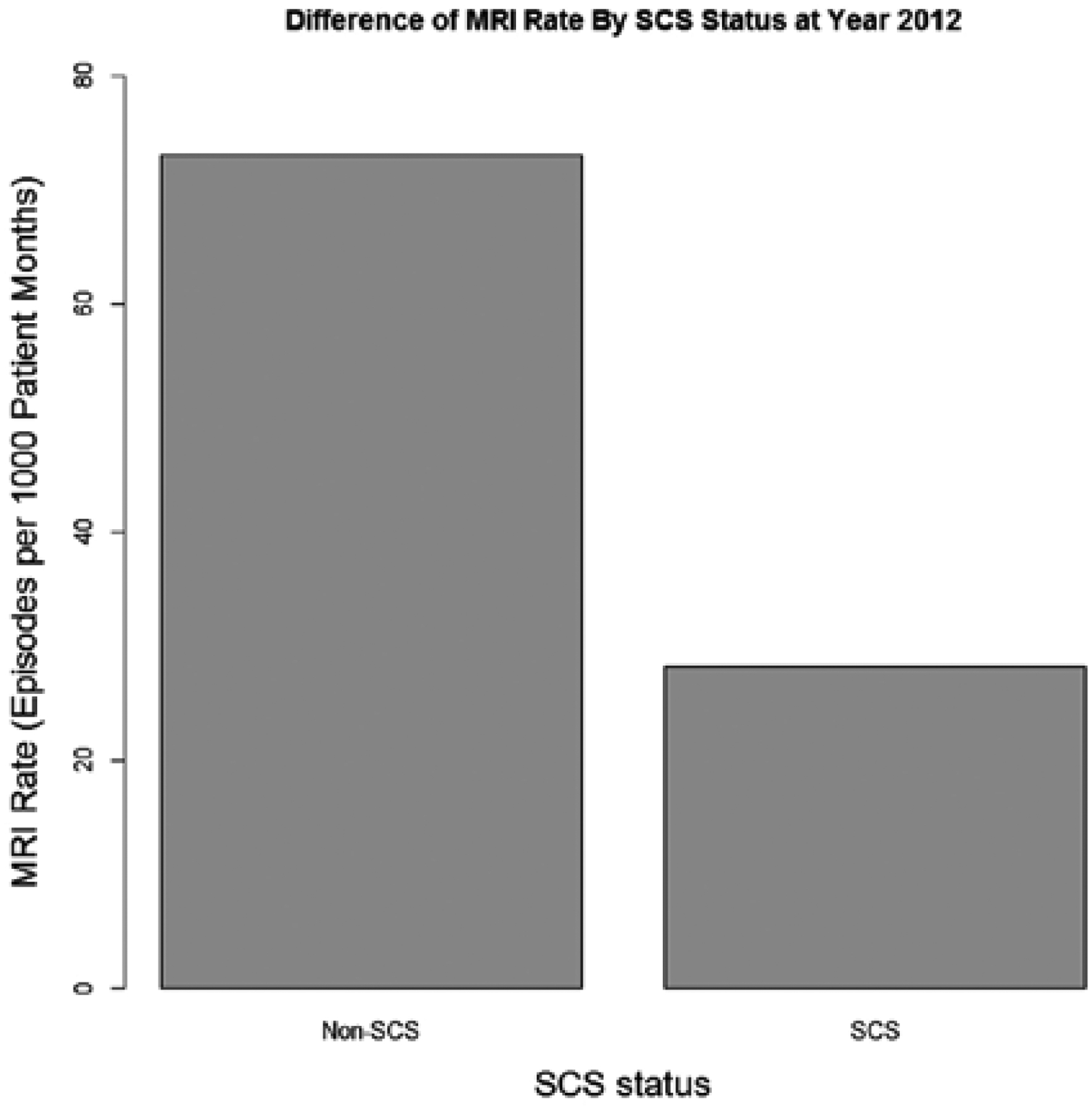
Difference in MRI rate by SCS status in 2012.
Discussion
Several studies have shown that rates of imaging have escalated steeply over the past decade for various patient and disease-specific populations. A retrospective analysis of 6 large integrated health systems in the United States identified increased use of advanced diagnostic imaging during the 1996 – 2010 study period, with annual increases of 7.8%, 10%, 3.9%, and 57% for CT, MRI, ultrasound, and PET scan, respectively (16). Several reasons have been posited to account for this trend, among which include improved imaging technology and modalities (17), broadened clinical applications, expanded recipient populations, and increasingly defensive practice of medicine by physicians (18,19). Payments to physicians for diagnostic imaging have had the highest rate of growth among all physician services over the last decade, and changes to policy have been scrutinized and enacted to address the high volume of imaging services relative to their value towards patient outcomes (20). Here, we show similar increases in the use of advanced imaging in FBSS patients, which differ based on whether the patient also has a SCS device implanted.
There are no specific guidelines regarding utilization of advanced imaging in the FBSS patient population. These patients generally receive imaging as part of their diagnostic workup, interventional treatments such as fluoroscopy-guided spinal injections or nerve blocks, preoperative planning, and postoperative follow-up (21). Although there is reasonable evidence demonstrating a relationship between imaging and symptomatology, this is not consistent across all back pain symptoms and many patterns of pathology are associated with equivocal imaging results (21). Rohde and colleagues (22) found that among patients with failed lumbar disc surgery, a correct diagnosis of etiology could be made in just 57% of cases, suggesting a limited value of radiological workup in identifying the underlying cause of FBSS. Due to the heterogeneity of patient anatomy, disease processes, and surgical procedures, assessment of the lumbosacral spine following surgery can require a combination of imaging modalities in order to be clinically useful (23,24). Contrast-enhanced MRI is the diagnostic test of choice, able to most clearly differentiate epidural fibrosis from recurrent or residual disc herniation (25). The American College of Radiology recommends x-ray myelography with a post myelography CT spine for patients in whom MRI is contraindicated, such as those with non-MRI compatible SCS devices, or when radiological artifact obscures anatomy. CT is also suitable for evaluating graft and fusion integrity, and instrumentation. Plain radiographs with flexion and extension views are useful for assessing alignment and instability status post instrumentation and fusion (25). A summary of ACR recommendations for patients with new or progressive clinical symptoms of low back pain in the setting of prior lumbar surgery can be found in Table 6.
Table 6.
ACR recommendations for patients with new lumbar back pain symptomatology status post back surgery.
| Imaging | ACR Recommendation* | Comments | Relative Radiation Level** |
|---|---|---|---|
| MRI lumbar spine with and without contrast | Usually Appropriate (8) | Differentiate disc from epidural fibrosis | 0 mSv |
| CT lumbar spine with contrast | May be appropriate (6) | Most useful when MRI is contraindicated or indeterminate | 1 – 10 mSv |
| CT lumbar spine without contrast | May be appropriate (6) | Most useful when MRI is contraindicated or indeterminate | 1 – 10 mSv |
| MRI lumbar spine without contrast | May be appropriate (6) | Contrast is often necessary | 0 mSv |
| XR myelography and post myelography CT lumbar spine | May be appropriate (5) | 10 – 30 mSv | |
| XR lumbar spine | May be appropriate (5) | With flexion and extension views | 1 – 10 mSv |
| Tc-99m bone scan with SPECT spine | May be appropriate (5) | Identify and localize pseudoarthrosis | 1 – 10 mSv |
| XR discography and post-discography CT lumbar spine | May be appropriate (5) | 1 – 10 mSv | |
| XR discography | May be appropriate (4) | 0.1 – 1 mSv | |
| CT lumbar spine without and with contrast | Usually not appropriate (3) | 10 – 30 mSv | |
| XR myelography lumbar spine | Usually not appropriate (2) | 1 – 10 mSv |
Source: American College of Radiology ACR Appropriateness Criteria for Low Back Pain.
ACR Rating scale from 1 – 9, 1 = least appropriate, 9 = most appropriate.
Adult effective dose estimate range
Neuromodulation in the form of SCS is accepted as an effective therapeutic option for patients with medically refractory lumbar back pain and radicular symptoms secondary to FBSS, for which all other management strategies have failed (21). There is a growing body of literature assessing the use of SCS in a variety of pain syndromes, with several retrospective and prospective studies examining the FBSS patient cohort in particular. Six-month mean health care costs for SCS patients have previously been shown to be significantly higher compared to patients receiving only conventional medical management (26), and total costs associated with SCS were only slightly lower compared to lumbar reoperation at 90-day follow-up (27). However, with several randomized clinical trials showing markedly improved gains in health-related quality of life (26,28,29), as well as results from other longer-term studies that show SCS to be in fact less expensive and more effective than both medical management (30,31) and reoperation (9,32,33), there is ample support for SCS as a viable first-line therapy and long-term option in appropriate FBSS patients. Cost neutrality for SCS compared to conventional pain therapy is achieved after the first 2.5 years (34). Moreover, not only does SCS obviate the need for reoperation, patients also report decreased requirement for opiate analgesics following the procedure (9,32) and higher rates of return to employment (34). Thus, current consensus guidelines support SCS for long-term relief in FBSS patients in terms of its efficacy compared to CMM, patient satisfaction vis-à-vis functional and quality of life improvement, cost effectiveness despite initial health care acquisition costs, and relative safety profile (21).
Here, we sought to characterize how rates of imaging have increased in FBSS patients over the 2000 – 2012 period, and to examine how SCS implantation contributes to selection of imaging and incurred costs throughout a patient’s treatment course. We hypothesized that utilization of imaging in the diagnosis and management of FBSS patients has increased overtime, and may significantly account for the high health care utilization in this population. We anticipated patients in the SCS implantation group would demonstrate lower rates of MRI compared to their control counterparts as devices were largely not compatible with MRIs during this timeframe. To our knowledge, this study is the first to determine wide-scale health care utilization concerning imaging modalities, and is also the most comprehensive analysis to date on this topic. We identified over 16,000 and 295,000 FBSS patients with and without SCS implantation, respectively. The overall imaging rate increased annually (with drop-offs between 2006 and 2007 and 2009 and 2011), with a total increase usage of 221% in the 12-year study period. CT scan use in 2012 was 3 times the rate of use in 2000, although MRI, x-ray, and ultrasound all saw increases in use over time as well. These trends mirror those seen in other patient populations.
When considering the impact of neuromodulation on utilization of imaging, FBSS patients who received SCS implantation were half as likely to receive an MRI compared to patients without a history of SCS; however, their overall imaging use was actually greater. This trend can be expected, as MRI-conditional stimulators were only made available in recent years, and so the majority of SCS implanters would have required alternative imaging modalities. This is also reflected in the rate ratio of CT scan use, which—following MRI—provides a similar level of anatomical detail, and was used almost a third more often in SCS patients. Barring other concerning symptomatology, x-ray is often the imaging modality of choice following SCS implant to assess stimulator placement and structural integrity during the postoperative follow-up period, and our results are consistent with the expectation that SCS implanters demonstrate increased use compared to controls. The difference between relative rates of ultrasound use is least notable, which is again expected, as ultrasound is not used in the management of spinal pain and is potentially more reflective of patient comorbidities rather than FBSS-associated pain.
Our study is not without limitations, the foremost of which is that it does not delineate subgroups of patients in terms of complexity of prior back surgery or surgeries (e.g., single versus multilevel, decompression with or without instrumentation, degree of residual stenosis or herniation, presence of technical failure or complication) or comorbid status. These clinical aspects contribute to the patient symptomatology and would direct subsequent management strategies of FBSS as well as the necessity of additional imaging. Components of patient history, such as predominance of axial back versus leg pain, and concordance with imaging results inform whether a particular intervention or surgery is appropriate and what kind would be most clinically appropriate. This would then impact subsequent SCS placement, given the anatomic architecture and pre-existing structural deficits that may prove the patient a better or worse candidate for neuromodulation therapies. It is logical to assume that successful lead placement in the ideal location would provide more effective pain coverage, which would result in decreased follow-up and necessitate less imaging. Conversely, a patient with an SCS implant who did not achieve successful attenuation of pain symptoms and subsequently elected to discontinue use or remove the device would also not be identified in this dataset. Such details could falsely elevate imaging rates in the SCS group.
Of note, our data does not take into account more recent developments in imaging technology. MR-conditional SCS systems have been made available for safe head, body, and extremity MRI scans, and recent developments in technology allow for total body MRI on a conditional basis (35,36). Thus, it is possible that the trajectory of imaging use after inclusion of more recent data would reflect this change. Moreover, new protocols and improved technology are being developed to accommodate implants. Mutter and colleagues (37) developed a protocol with a reduced specific energy absorption rate, which allowed safe spinal MRI examinations to be performed in patients with SCS. In 9/13 patients, the MRI detected new lesions that subsequently informed treatment in 8 of these individuals (35).
Lastly, we cannot preclude the possibility of miscoding across institutions. Miscoding is a limitation inherent to large administrative databases, which has been described previously (36,38). Additionally, our study is time-dependent, comprising patient data between 2000 and 2012; as MarketScan has expanded to include additional insurers in recent years, any attendant shift in patient demographics will require additional analysis. It would be beneficial in future studies to include additional patient demographic and clinical data to better categorize patient cohorts into high and low health care utilizers, and to characterize health care utilization for the purposes of targeting improved clinical care and resource use.
It is clear that patient selection is an important component that contributes to the success of FBSS-related pain management and the role of SCS in pain relief (39). Our study shows that rates of imaging have increased dramatically since the turn of the century in FBSS patients across the US. Despite a 50% decrease in the use of MRI in those patients treated with SCS, their overall imaging rate increased by 19% compared to patients without SCS. MR-conditional SCS systems will likely play an important role in limiting the need for imaging use and thus health care expenditures in these patients.
Conclusion
This longitudinal, retrospective analysis found that the overall rates of imaging in patients with FBSS increased over the 12-year interval from 2000 to 2012 across the US. Those patients treated with SCS were 50% less likely to receive an MRI (as expected, given prior incompatibility of neuromodulation devices), yet 32% and 27% more likely to receive CT and x-ray, respectively. These findings demonstrate that imaging plays a significant role in driving health care expenditures. It also highlights the need for judicious use of imaging. This is the largest analysis examining the role of imaging in the FBSS population and the impact of SCS procedures. Further studies are needed to assess the impact of MRI-conditional SCS systems on future trends in imaging in FBSS patients receiving neuromodulation therapies.
Disclaimer:
Shivanand Lad, MD, PhD, has consulted for or received grant support from Medtronic Inc., Boston Scientific, and St. Jude Medical. He serves as Director of the Duke Neuro-Outcomes Center that has received research funding from NIH KM1 CA 156687.
Siyun Yang, MS, was partially supported by UL1TR001117 from the National Center for Advancing Translational Sciences (NCATS).
Footnotes
The remaining authors report no conflicts of interest or financial disclosures.
References
- 1.Schmidt CO, Raspe H, Pfingsten M, Hasenbring M, Basler HD, Eich W, Kohlmann T. Back pain in the German adult population: Prevalence, severity, and sociodemographic correlates in a multiregional survey. Spine (Phila Pa: 1976) 2007; 32:2005–2011. [DOI] [PubMed] [Google Scholar]
- 2.Patel AT, Ogle AA. Diagnosis and management of acute low back pain. Am Fam Physician 2000; 61:1779–1786, 1789–1790. [PubMed] [Google Scholar]
- 3.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J 2008; 8:8–20. [DOI] [PubMed] [Google Scholar]
- 4.Slipman CW, Shin CH, Patel RK, Isaac Z, Huston CW, Lipetz JS, Lenrow DA, Braverman DL, Vresilovic EJ. Etiologies of failed back surgery syndrome. Pain Medicine 2002; 3:200–214. [DOI] [PubMed] [Google Scholar]
- 5.Schofferman J, Reynolds J, Herzog R, Covington E, Dreyfuss P, O’Neill C. Failed back surgery: Etiology and diagnostic evaluation. Spine J 2003; 3:400–403. [DOI] [PubMed] [Google Scholar]
- 6.Chan CW, Peng P. Failed back surgery syndrome. Pain Med 2011; 12:577–606. [DOI] [PubMed] [Google Scholar]
- 7.Thomson S, Jacques L. Demographic characteristics of patients with severe neuropathic pain secondary to failed back surgery syndrome. Pain Pract 2009; 9:206–215. [DOI] [PubMed] [Google Scholar]
- 8.Taylor RS, Ryan J, O’Donnell R, Eldabe S, Kumar K, North RB. The cost-effectiveness of spinal cord stimulation in the treatment of failed back surgery syndrome. The Clinical Journal of Pain 2010; 26:463–469. [DOI] [PubMed] [Google Scholar]
- 9.North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for trial. Neurosurgery 2005; 56:98–106; discussion 106–107. [DOI] [PubMed] [Google Scholar]
- 10.Van Buyten J-P. Neurostimulation for chronic neuropathic back pain in failed back surgery syndrome. Journal of Pain and Symptom Management 2006; 31:S25–S29. [DOI] [PubMed] [Google Scholar]
- 11.Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: Time to back off? J Am Board Fam Med 2009; 22:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell JM. Utilization trends for advanced imaging procedures: Evidence from individuals with private insurance coverage in California. Med Care 2008; 46:460–466. [DOI] [PubMed] [Google Scholar]
- 13.Rao VM, Parker L, Levin DC, Sunshine J, Bushee G. Use trends and geographic variation in neuroimaging: Nationwide medicare data for 1993 and 1998. AJNR Am J Neuroradiol 2001; 22:1643–1649. [PMC free article] [PubMed] [Google Scholar]
- 14.Weiner DK, Kim YS, Bonino P, Wang T. Low back pain in older adults: Are we utilizing healthcare resources wisely? Pain Med 2006; 7:143–150. [DOI] [PubMed] [Google Scholar]
- 15.Commission. MPA. Med PAC Recommendations on Imaging Services: Statement of Glenn M. Hackbarth. Testimony before the Committee on Energy and Commerce, July 18, 2006. [Google Scholar]
- 16.Smith-Bindman R, Miglioretti DL, Johnson E, Lee C, Feigelson HS, Flynn M, Greenlee RT, Kruger RL, Hornbrook MC, Roblin D, Solberg LI, Vanneman N, Weinmann S, Williams AE. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996 – 2010. JAMA 2012; 307:2400–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhargavan M, Sunshine JH. Utilization of radiology services in the United States: Levels and trends in modalities, regions, and populations. Radiology 2005; 234:824–832. [DOI] [PubMed] [Google Scholar]
- 18.Studdert DM, Mello MM, Sage WM, DesRoches CM, Peugh J, Zapert K, Brennan TA. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA 2005; 293:2609–2617. [DOI] [PubMed] [Google Scholar]
- 19.Jena AB, Schoemaker L, Bhattacharya J, Seabury SA. Physician spending and subsequent risk of malpractice claims: Observational study. BMJ 2015; 351:h5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iglehart JK. Health insurers and medical- imaging policy: A work in progress. N Engl J Med 2009; 360:1030–1037. [DOI] [PubMed] [Google Scholar]
- 21.Manchikanti L, Boswell MV, Singh V, Benyamin RM, Fellows B, Abdi S, Buenaventura RM, Conn A, Datta S, Derby R, Falco FJ, Erhart S, Diwan S, Hayek SM, Helm S, Parr AT, Schultz DM, Smith HS, Wolfer LR, Hirsch JA, Asipp IPM. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician 2009; 12:699–802. [PubMed] [Google Scholar]
- 22.Rohde V, Mielke D, Ryang Y, Gilsbach JM. The immediately failed lumbar disc surgery: Incidence, aetiologies, imaging and management. Neurosurg Rev 2015; 38:191–195; discussion 195. [DOI] [PubMed] [Google Scholar]
- 23.Van Goethem JW, Parizel PM, Jinkins JR. Review article: MRI of the postoperative lumbar spine. Neuroradiology 2002; 44:723–739. [DOI] [PubMed] [Google Scholar]
- 24.Sanders WP, Truumees E. Imaging of the postoperative spine. Semin Ultrasound CT MR 2004; 25:523–535. [DOI] [PubMed] [Google Scholar]
- 25.Davis PC, Wippold FJ 2nd, Brunberg JA, Cornelius RS, De La Paz RL, Dormont PD, Gray L, Jordan JE, Mukherji SK, Seidenwurm DJ, Turski PA, Zimmerman RD, Sloan MA. ACR appropriateness criteria on low back pain. J Am Coll Radiol 2009; 6:401–407. [DOI] [PubMed] [Google Scholar]
- 26.Manca A, Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, Thomson S, O’Callaghan J, Eisenberg E, Milbouw G, Buchser E, Fortini G, Richardson J, Taylor RJ, Goeree R, Sculpher MJ. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). Eur J Pain 2008; 12:1047–1058. [DOI] [PubMed] [Google Scholar]
- 27.Lad SP, Babu R, Bagley JH, Choi J, Bagley CA, Huh BK, Ugiliweneza B, Patil CG, Boakye M. Utilization of spinal cord stimulation in patients with failed back surgery syndrome. Spine (Phila Pa: 1976) 2014; 39:E719–727. [DOI] [PubMed] [Google Scholar]
- 28.Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, Thomson S, O’Callaghan J, Eisenberg E, Milbouw G, Buchser E, Fortini G, Richardson J, North RB. The effects of spinal cord stimulation in neuropathic pain are sustained: A 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery 2008; 63:762–770; discussion 770. [DOI] [PubMed] [Google Scholar]
- 29.Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, Thomson S, O’Callaghan J, Eisenberg E, Milbouw G, Buchser E, Fortini G, Richardson J, North RB. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007; 132:179–188. [DOI] [PubMed] [Google Scholar]
- 30.Taylor RJ, Taylor RS. Spinal cord stimulation for failed back surgery syndrome: fectiveness analysis. Int J Technol Assess Health Care 2005; 21:351–358. [DOI] [PubMed] [Google Scholar]
- 31.Coleman SD, Mackey S. Spinal cord stimulation compared with medical management for failed back surgery syndrome. Curr Pain Headache Rep 2009; 13:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.North RB, Ewend MG, Lawton MT, Kidd DH, Piantadosi S. Failed back surgery syndrome: 5-year follow-up after spinal cord stimulator implantation. Neurosurgery 1991; 28:692–699. [PubMed] [Google Scholar]
- 33.North RB, Kidd D, Shipley J, Taylor RS. Spinal cord stimulation versus reoperation for failed back surgery syndrome: A cost effectiveness and cost utility analysis based on a randomized, controlled trial. Neurosurgery 2007; 61:361–368; discussion 368–369. [DOI] [PubMed] [Google Scholar]
- 34.Kumar K, Malik S, Demeria D. Treatment of chronic pain with spinal cord stimulation versus alternative therapies: Cost-effectiveness analysis. Neurosurgery 2002; 51:106–115; discussion 115–106. [DOI] [PubMed] [Google Scholar]
- 35.Buvanendran A, Lubenow TJ. Efficacy of transverse tripolar spinal cord stimulator for the relief of chronic low back pain from failed back surgery. Pain Physician 2008; 11:333–338. [PubMed] [Google Scholar]
- 36.Gologorsky Y, Knightly JJ, Lu Y, Chi JH, Groff MW. Improving discharge data fidelity for use in large administrative databases. Neurosurg Focus 2014; 36:E2. [DOI] [PubMed] [Google Scholar]
- 37.Mutter UM, Bellut D, Porchet F, Schuknecht B. Spinal magnetic resonance imaging with reduced specific absorption rate in patients harbouring a spinal cord stimulation device - A single-centre prospective study analysing safety, tolerability and image quality. Acta Neurochir 2013; 155:2327. doi: 10.1007/s00701-013-1885-8 [DOI] [PubMed] [Google Scholar]
- 38.O’Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring diagnoses: ICD code accuracy. Health Serv Res 2005; 40:1620–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dario A, Fortini G, Bertollo D, Bacuzzi A, Grizzetti C, Cuffari S. Treatment of failed back surgery syndrome. Neuromodulation 2001; 4:105–110. [DOI] [PubMed] [Google Scholar]


