Toggle switches and oscillations emerging in cellular networks have been in the limelight of scientific interest since late nineties (1-4). Synthetic biologists strived to build similar input-output responses by constructing DNA-RNA-Protein circuits (1). Engineering genetic circuits has seen successes, including a synthetic mammalian circadian clock with oscillation periods of hours (5) and genetic bistable toggle switches operating on timescales of tens of minutes to hours (6). On page 75 of this issue, Mishra et al. (REF 7) report the design of a much faster regulatory network in yeast cells comprising several synthetic protein phosphorylation circuits that act as logical gates. Furthermore, the authors identified instances of similar network motifs across all known endogenous signaling pathways in yeast.
Phosphorylation-encoded switches and oscillators in protein networks operate much quicker than genetic switches but the attempts to build these circuits synthetically have so far incorporated slower DNA logical operations (8). To attain rapid responses, optogenetics manipulations have been extensively employed. Indeed, different periods of light exposure triggered distinct enzyme activation kinetics, dynamics-dependent gene expression, and cell differentiation that are normally induced by growth factors and receptors in mammalian cells (9). However, no synthetic system has ever reproduced a logic gate that responds to signals as fast as in seconds.
In digital electronics, logical gates are combined into integrated circuits to perform chains of operations that enable computer calculations. Mishra et al. constructed artificial phosphorylation circuits that operate as logical gates (OR, NOT and BUFFER) used in electronics. Each gate is built with several fusion proteins. The upstream protein receives an input signal and binds a downstream protein, which is the gate output (see the figure). The input protein effector domain is a protein phosphorylating enzyme (kinase) for an OR gate or a dephosphorylating enzyme (phosphatase) for a NOT gate. In an engineered, two distinct fusion proteins receive different input signals, and both proteins share the same output protein. The synthetic NOT gate has a single input protein and a single output protein. Following phosphorylation of an input fusion protein that has a phosphatase domain (PTP), the initially phosphorylated output protein is dephosphorylated, which eliminates signaling.
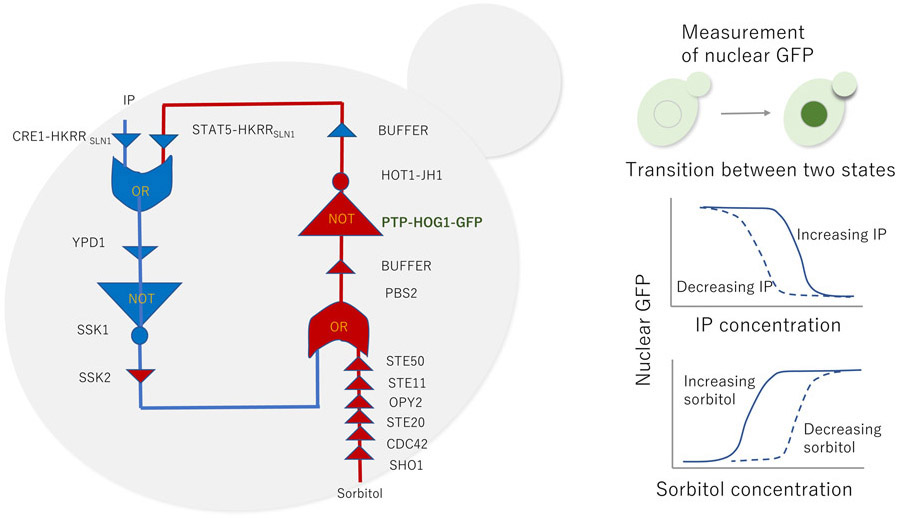
A fast, protein-only synthetic toggle network
A toggle bistable switch that contains two OR, BUFFER, or NOT gates creates a mutual repression topology in yeast cells (left). Changes in the concentration of input signals (sorbitol and isopentenyl adenine) rapidly control switches between the two stable states, which is monitored by the nuclear translocation of a fusion green fluorescent protein (right).
Mutual repression topology of two protein nodes or pathways in a circuit creates a toggle switch featuring two stable states - that is, either node is active when the other node is inactive (10-12). Mishra et al. built a bistable toggle switch using two paired OR and NOT gates and several BUFFER gates that formed mutual repression topology of the switch. This mutual repression occurs because one fusion protein (activated by sorbitol) phosphorylates a downstream protein, which was created as a fusion of a PTP domain and the yeast osmotic stress sensor HOG1 (a mitogen activated protein kinase) labeled with a green fluorescent protein (GFP). The activated phospho-PTP-HOG1-GFP dephosphorylates the other fusion protein, which comprises the high-osmolarity-induced transcription protein 1 (HOT1) and a catalytic domain of Janus kinase JAK 2 (JH1), HOT1-JH1. In turn, the isopentynyl adenine (IP)-stimulated protein CRE-HKRRSLN1 activates a downstream protein, MAPK kinase kinase (SSK1) through a chain of protein phosphorylations (BUFFER) and prevents the activation of sorbitol-stimulated protein (PBS2). The two external inputs, sorbitol and IP, activate their cognate fusion proteins through plasma membrane osmosensor SHO1 and histidine kinase receptor, respectively. Nuclear localized PTP-HOG1-GFP was measured as a read-out to characterize the system behaviors. These two inputs switched the system between two alternative stable states on the timescale of seconds.
Why does the engineered toggle switch have such a sophisticated design of paired OR, NOT and BUFFER gates? Increasing the number of elements in a circuit enables rapid and robust responses. An amplifier increases the speed of a logic gate, whereas an element with a sigmoidal input-output characteristic, implemented as a BUFFER gate, filters noise and increases robustness to internal and external noise. Synthetic biochemical devices are much noisier than electronic circuits because of internal noise in transcription-translation and cell heterogeneity. Future work is required to assess how speed and robustness of protein logic gates depend on the number and characteristics of individual elements (13). This will open endless possibilities of gate combinations into complex integrative circuits.
Mishra et al s developed a tool termed BoFENCE that allowed searching the Kyoto Encyclopedia of Genes and Genomes (KEGG) for all known signaling pathways in the yeast Saccahromyces cerevisiae and identified a number of toggled network motifs. Their analysis suggests that biological networks might inherently enable logical operations and computations. This emphasizes the importance of reconstructing causal, directional regulatory networks from “-omics” data; current statistical methods mostly infer correlative non-directional networks (14). Understanding how cells compute cell fate decisions will only be possible when network biology moves from correlation to causal. It will then be possible to deconstruct intracellular signaling networks into smaller parts to analyze and, more ambitiously, to reverse-engineer them by creating artificial cells with pre-programmed properties. It may not be long before the precise picture of signaling network abnormalities that cause diseases and the logic of potential treatments are identified from synthetic biology approaches.
Acknowledgment
This work was supported by NIH/NCI grant R01CA244660 and EU NanoCommons grant 731032 to B.N.K., JSPS KAKENHI grants (17H06299, 17H06302, and 18H04031), and JST-Mirai Program grant JPMJMI19G7 to M.O. The authors declare no conflict of interest.
REFERENCES AND NOTES
- 1.Bhalla US, Iyengar R, Science 283, 381–387 (1999). [DOI] [PubMed] [Google Scholar]
- 2.McClean MN, Mody A, Broach JR, Ramanathan S, Nat Genet 39, 409–414 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Kaimachnikov NP, Kholodenko BN, The FEBS journal 276, 4102–4118 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macarthur BD, Ma'ayan A, Lemischka IR, Nat Rev Mol Cell Biol 10, 672–681 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tigges M, Marquez-Lago TT, Stelling J, Fussenegger M, Nature 457, 309–312 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Lugagne JB et al. Nat Commun 8, 1671 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra et al. Science 372, (2021) [Google Scholar]
- 8.Chen S et al. Angew Chem Int Ed Engl 58, 18186–18190 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Toettcher JE, Weiner OD, Lim WA, Cell 155, 1422–1434 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolado-Carrancio A et al. Elife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mochida S, Rata S, Hino H, Nagai T, Novak B, Curr Biol 26, 3361–3367 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao G, Lee TJ, Mori S, Nevins JR, You L, Nature cell biology 10, 476–482 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Chen Z et al. Science 368, 78–84 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santra T, Rukhlenko O, Zhernovkov V, Kholodenko BN, Current Opinion in Systems Biology 9, 11–21 (2018). [Google Scholar]


