The urological community seems to have decided that multiparametric magnetic resonance imaging (mpMRI) before prostate biopsy is routinely indicated in the work-up for a patient with elevated prostate specific-antigen (PSA). The 2019 European Association of Urology guidelines on prostate cancer recommend that clinicians perform MRI before prostate biopsy for both biopsy-naïve patients and those with a prior negative biopsy [1]. In the USA, the American Urological Association (AUA) guidelines state that “Current evidence now supports the use of mpMRI in men at risk of harboring prostate cancer prior to their first biopsy” [2].
The clear impetus for the recent adoption of prebiopsy prostate MRI in guidelines was the high-profile publication of the PROMIS and PRECISION trials. In the nonrandomized PROMIS trial [3], all patients received MRI followed by transperineal template mapping biopsy and standard (10–12 core) transrectal ultrasound (TRUS)-guided biopsy. In the PRECISION trial [4], men were randomized to undergo MRI-guided or standard TRUS biopsy. Men randomized to the MRI group underwent biopsy targeted to the MRI lesions only, and did not undergo biopsy if no lesion was identified. The authors of the PROMIS trial declared that MRI had resulted in fewer biopsies, reduced detection of indolent disease, and identified more high-grade cancers. The PRECISION trial [4] authors reported essentially the same findings, with 12 more high-grade cancers per 100 found among men randomized to receive biopsy only of MRI lesions compared to those undergoing TRUS biopsy. As the AUA guidelines put it, results from the PRECISION trial have “provided level 1 data to support the recommendation of mpMRI prior to biopsy for all men” [5].
MRI as a triage test finds more aggressive cancers while reducing unnecessary biopsy rates and overdiagnosis, apparently by improving both the sensitivity and specificity of the biopsy procedure for high-grade cancer. MRI may miss some high-grade cancers in patients without MRI-visible lesions, the argument goes, but this is more than compensated for by identification of lesions that would be missed by systematic biopsy.
Yet it only makes sense to compare the number of high-grade cancers found by two biopsy approaches if the cancers found by each are, grade-for-grade, of equivalent oncologic risk. There are several lines of evidence against this position. First, there are anatomic considerations. Figure 1 shows a Gleason 3 + 4 lesion. A single biopsy needle randomly placed in the region of a suspicious lesion will, on average, find 3 + 4 disease (eg, needle A or B). A targeted biopsy involves the use of multiple needles, with the final Gleason score determined by the needle with the highest grade. A targeted biopsy using needles B–E would give a score of 4 + 4.
Fig. 1 –
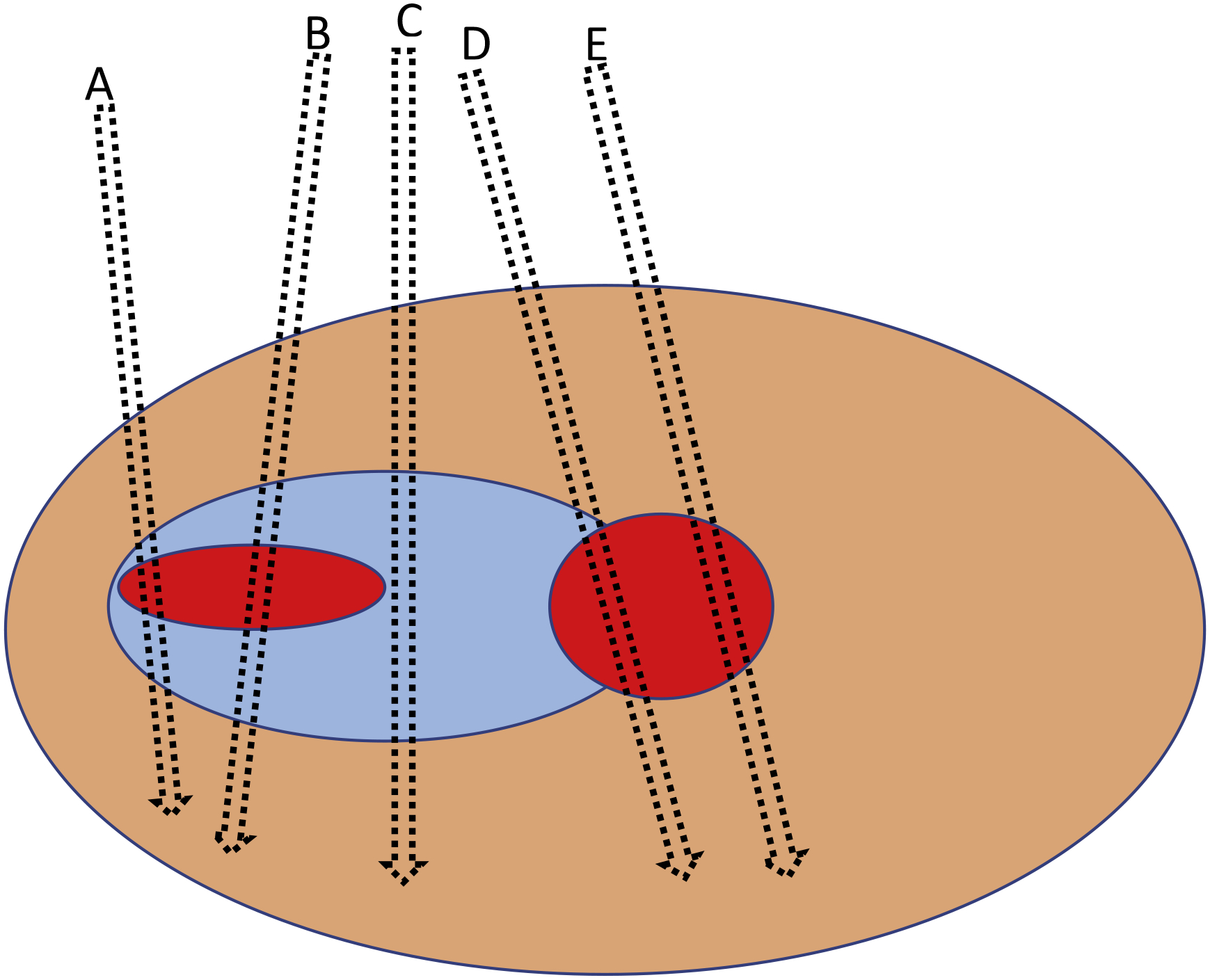
Schematic illustration of a prostate gland (orange) with needle biopsies (A–E) directed to an area with prostate cancer with Gleason pattern 3 (blue) and Gleason pattern 4 (red). Adapted from Varma et al [9].
Second, experience from routine clinical practice over the years does not suggest that systematic TRUS has been missing many high-grade cancers. PRECISION reported that MRI leads to a 2.8% absolute increase in the risk of highest risk, grade group 5 tumors [4]. Given the US incidence of prostate biopsy, this suggests we have been missing approximately 10000 grade group 5 cancers every year for decades. If that were so, we would expect that it would be common in our clinics for patients to present with metastatic disease in the years following a negative biopsy or to show signs of rapid progression on active surveillance. But such cases are extremely rare.
Third, there have been studies of men with negative biopsy followed up for many years. In Danish cancer registry data following men with negative TRUS biopsy and PSA <10 ng/ml—similar to the majority of those in the PRECISION trial—the cumulative incidence of prostate cancer–specific death was 0.7% at 15 yr [6], comparable to the population average. Even lower rates have been reported in the European study of PSA screening (ERSPC), in which patients underwent repeat PSA testing after negative biopsy [7]. Moreover, the Danish and ERSPC results were based on sextant biopsy rather than the contemporary 10–14-core technique and thus probably overestimate risk.
The fourth line of evidence that MRI-detected lesions are not oncologically equivalent to those detected by systematic biopsy comes from studies comparing biopsy grade with surgical pathology. Studies have shown that patients with low-grade tumors on biopsy that are found to have high-grade cancer on surgical pathology have recurrence and death rates close to those for low-risk patients [8]. Thus, missing, or delaying, the detection of a high-grade cancer on TRUS biopsy raises risk, but not by very much.
There are clear clinical indications for MRI-guided biopsy, such as repeated negative biopsy in the setting of high PSA. However, the randomized trial evidence for MRI in the prebiopsy work-up for biopsy-naïve men with moderately elevated PSA is based on the assumption that a tumor of a given grade is of equivalent risk whether detected via MRI or systematic biopsy. This is almost undoubtedly false. If a trial of, for example, adjuvant therapy used a different definition of recurrence in the adjuvant group compared to the control group, we would be cautious about claims of effectiveness. The same should hold true for trials of MRI for prostate biopsy. Therefore, routine use of MRI in biopsy-naïve men for detection of prostate cancer is not justified at present.
Acknowledgments:
This work was supported in part by the National Institutes of Health/National Cancer Institute (NIH/NCI) via a Cancer Center Support Grant to Memorial Sloan Kettering Cancer Center (P30 CA008748), a SPORE grant in Prostate Cancer to Dr. H. Scher (P50-CA92629), the Sidney Kimmel Center for Prostate and Urologic Cancers, and David H. Koch through the Prostate Cancer Foundation.
Conflicts of interest:
Andrew Vickers is named on a patent for a statistical method to detect prostate cancer that has been commercialized by OPKO Health and receives royalties from sales of the test and has stock options in OPKO Health. Sigrid Carlsson has received a lecture honorarium and travel support from Astellas Pharma (unrelated to the current study). Matthew Cooperberg has nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mottet N, van den Bergh RCN, Briers E, et al. European Association of Urology guidelines on prostate cancer. https://uroweb.org/guideline/prostate-cancer/
- 2.Bjurlin MA, Carroll PR, Eggener S, et al. Update of the standard operating procedure on the use of multiparametric magnetic resonance imaging for the diagnosis, staging and management of prostate cancer. J Urol. 2020;203:706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815–22. [DOI] [PubMed] [Google Scholar]
- 4.Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018;378:1767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fulgham PF, Rukstalis DB, Rubenstein JN, et al. Standard operating procedure for multiparametric magnetic resonance imaging in the diagnosis, staging and management of prostate cancer: a collaborative initiative by the American Urological Association and the Society of Abdominal Radiology Prostate Disease Focus Panel. American Urological Association/Society of Abdominal Radiology; 2019. www.auanet.org/guidelines/mri-ofthe-prostate-sop [Google Scholar]
- 6.Klemann N, Roder MA, Helgstrand JT, et al. Risk of prostate cancer diagnosis and mortality in men with a benign initial transrectal ultrasound-guided biopsy set: a population-based study. Lancet Oncol 2017;18:221–9. [DOI] [PubMed] [Google Scholar]
- 7.Schroder FH, van den Bergh RC, Wolters T, et al. Eleven-year outcome of patients with prostate cancers diagnosed during screening after initial negative sextant biopsies. Eur Urol 2010;57:256–66. [DOI] [PubMed] [Google Scholar]
- 8.Kovac E, Vertosick EA, Sjoberg DD, Vickers AJ, Stephenson AJ. Effects of pathological upstaging or upgrading on metastasis and cancer-specific mortality in men with clinical low-risk prostate cancer. BJU Int 2018;122:1003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varma M, Berney D, Oxley J, Trpkov K. Gleason score assignment is the sole responsibility of the pathologist. Histopathology 2018;73:5–7. [DOI] [PubMed] [Google Scholar]


