Abstract
Objective
This study was conducted to explore the application of age-male-ALBI-platelets (aMAP) score for predicting late recurrence of hepatocellular carcinoma (HCC) following radiofrequency ablation (RFA) and develop an aMAP score based-nomogram to predict prognosis in Chinese population.
Materials and Methods
HCC patients who developed late recurrence following RFA at National Cancer Center (NCC) of China, First Hospital of Shanxi Medical University and Beijing Hospital of Traditional Chinese Medicine from January 2011 to December 2016 were included as a training cohort, and patients who were treated at Affiliated Cancer Hospital of Zhengzhou University between January 2012 and December 2016 were included as an external validation cohort. The optimal cut-off value for aMAP score was determined using X-tile software to discriminate the performance of recurrence-free survival (RFS).
Results
A total of 339 eligible patients were included in this study. Patients were grouped into low-risk (aMAP score ≤64.2), medium-risk (64.3 ≤aMAP score ≤68.6) and high-risk (aMAP score ≥68.7) groups by X-tile plots. The prognostic factors that affected RFS were the number of lesions and aMAP score. A nomogram was constructed to predict the RFS with a C-index of 0.793 (95% CI: 0.744–0.842). The time-dependent receiver operating characteristic curves (t-AUCs) of the nomogram to predict 3, 4 and 5-year RFS were 0.808, 0.820 and 0.764, respectively. The model was then tested with data from an external validation cohort. The calibration curve confirmed the optimal agreement between the predicted and observed values.
Conclusion
The aMAP score provided a well-discriminated risk stratification and is an independent prognostic factor for the late recurrence of HCC following RFA. The aMAP score-based nomogram could help to strengthen prognosis-based decision making and formulate adjuvant therapeutic and preventive strategies.
Keywords: hepatocellular carcinoma, radiofrequency ablation, recurrence-free survival, age-male-ALBI-platelets score
Introduction
In 2018, 392,868 new cases of liver cancer were diagnosed and 368,960 people died due to liver cancer in China.1 Hepatocellular carcinoma (HCC) is the most common primary liver cancer.2 The therapeutic option for HCC depends on the stage of the disease. For HCC within Milan criteria, liver transplantation (LT) and surgical resection (SR) are the first-line curative therapies.3 However, both SR and LT have some limitations.4
Local thermal ablation is one of the first-line therapies for very early-stage HCC (single tumor ≤2 cm) and selected patients with early-stage HCC (single tumor, or ≤ 3 tumors ≤3 cm) or with recurrence where SR is not feasible.5,6 A study7 found similar survival rates with SR and ablation for HCC within Milan criteria. The National Comprehensive Cancer Network (NCCN) guidelines recommend that HCC >5 cm should not receive palliative therapy (arterially directed or systemic therapy) instead of radical therapy (surgery or ablation), because radical therapy showed less favorable outcomes for HCC ≥5 cm.8 Radiofrequency ablation (RFA) is a commonly used thermal ablation technique for HCC within Milan criteria that has favorable oncological outcomes.9–13
However, there still be a high incidence of HCC recurrence following RFA.14,15 HCC recurrence following radical therapies could be defined as early recurrence (<2 years) and late recurrence (>2 years) based on the temporal distribution.15,16 Previous studies reported that early recurrence probably originated from primary tumors due to the aggressive biological behavior and it was commonly associated with various factors, such as the number of tumors, tumor size, pathological classification, albumin-bilirubin (ALBI) ratio, serum alpha-fetoprotein (AFP), and microvascular invasion (MVI).17–19 However, late recurrence is thought to arise from de novo carcinogenesis of a second primary HCC.15–18
Recently, Fan et al developed a novel HCC risk score, the age-male-ALBI-platelets (aMAP) score to assess 5-year HCC risk in patients with chronic hepatitis.20 Males have a 3–8 times higher risk of developing HCC, because of the influence of sex hormones.21,22 ALBI indicates the functional liver reserve in patients with HCC.23 In addition, platelet (PLT) count is correlated to liver function and fibrosis. The aMAP score has excellent calibration and discrimination to predict the 5-year HCC risk irrespective of etiology and ethnicity.20 We consider that there might exist certain degree of similarities between a chronic development process of primary HCC and late recurrence after RFA which may attribute to de novo carcinogenesis.
The significance of the aMAP score to assess late recurrence of HCC following RFA has not been reported. We hypothesize that the aMAP score might also provide a well-discriminated and calibrated risk stratification for HCC late recurrence after RFA. Therefore, this multicenter study will explore the prognostic value of the aMAP score in HCC patients with late recurrence following RFA, and then, an aMAP score based-nomogram will be developed to predict the probability of recurrence-free survival (RFS), which could help oncologists to formulate preventive and therapeutic strategies.
Materials and Methods
Patients
In this study, HCC patients that were treated at the National Cancer Center (NCC) of China, First Hospital of Shanxi Medical University and Beijing Hospital of Traditional Chinese Medicine between January 2011 and December 2016, were included in the training cohort and patients that were treated at the Affiliated Cancer Hospital of Zhengzhou University between January 2012 and December 2016, were included in the external validation cohort. The Institutional Review Boards of the four hospitals approved this retrospective study (NCC2019KZ-010). Informed consent for treatment was obtained from all patients. A separate consent to publish the data was waived by the Ethics Committees, since the personal details of these patients were kept confidential. This study was conducted in accordance with the Declaration of Helsinki. Patients who refused or were deemed unfit for surgery received RFA. The patients that developed early recurrence or died within 2 years after RFA were excluded, because this study mainly focused on late recurrence.15,24
In this study, patients with hepatitis (viral or alcohol-related) that had HCC within the Milan criteria, which was confirmed by histology or the European Association for the Study of Liver (EASL) proposed noninvasive diagnostic criteria, without any metastasis outside the liver or any major vascular invasion that underwent complete ablation by RFA were included. The Eastern Cooperative Oncology Group (ECOG) performance status of the included patients was zero or one. Patients with early recurrence, those who died <2 years following RFA from any cause, or those who were lost to follow-up were excluded. In addition, patients that received primary RFA at other centers, or underwent primary surgery were excluded.
Preoperative aMAP scores were calculated using age, gender, serum total bilirubin (TBIL), serum albumin (ALB), and PLT count() as follows:.20 All the relevant parameters based on previous studies were tested before RFA.12,24,25 The cut-off value of the parameters was set as the upper limit of the normal range at laboratory of NCC of China. Tumors at unfavorable locations were those located <0.5 cm from vital structures, such as major vessels (inferior vena cava, main hepatic artery, and the first and second branch of the portal vein), stomach, small bowel, colon, primary and secondary intrahepatic bile ducts, gallbladder, diaphragm, and pericardium.24
For patients with chronic hepatitis B virus (HBV) infection (positive hepatitis B surface antigen [HBsAg]) and a HBV DNA level of >1000 copies/mL, oral antiviral therapy with lamivudine, adefovir dipivoxil, or entecavir daily was prescribed. For patients with chronic hepatitis C virus (HCV) infection (positive serum HCV RNA), a combination therapy of interferon and ribavirin was given.
RFA Procedure
Abdominal ultrasound or computed tomography (CT) guided RFA was performed under local anesthesia using a radiofrequency generator system (S-1500, MedSphere International Inc.). We used either single electrode or expandable multi-tined electrode (MedSphere International Inc.) according to the power of the generator, tumor characteristics, temporal availability and manufacturer’s recommendations historically. Decision of electrode selection and ablative technique was discussed in our therapeutic group before operation. A single electrode will be used for diameter of tumor less than 2.0 cm. When the diameter of tumor was larger than 2.0 cm, we will adopt overlapping technique with 2 to 3 single electrodes or 1 to 2 expandable multi-tined electrode(s) to create a sufficient safety margin of at least 0.5cm as far as possible. For patients with multiple tumors, different electrodes were used in combination according the number, shape, diameter and positions of tumors. Respiratory and cardiovascular functions were monitored during the operation. If the tumor in an unfavorable location, artificial ascites was created if required. The ablation path was cauterized following tumor ablation to avoid tumor seeding and hemorrhage.
Follow-Up
The last date of follow-up for this study was 30 September 2019. The follow-up protocol and treatment were the same as described previously.24
Definitions
1. RFS: the time between the first RFA and tumor recurrence.
2. Overall survival (OS): the time between the first RFA and death from any cause.
3. Complete ablation: the minimal ablative margin of >0.5 cm beyond the tumor in all directions, or absence of medical imaging features, or both (arterial contrast enhancement and portal venous washout within the ablation zone) of residual tumor 1 month after RFA.26–28
4. Local tumor recurrence (LTP): the appearance of tumor foci at the edge of the ablation zones after RFA.29,30
5. Intrahepatic distant recurrence (IDR): the appearance of new intrahepatic tumors within different liver subsegments from the ablation zones, or the same liver subsegment that was not adjacent to the ablation zones after RFA.31–33
6. Extrahepatic recurrence (ER): the appearance of new extrahepatic metastasis after RFA.29,30
Statistical Analysis
Qualitative variables were compared using Fisher’s exact test or the Chi-squared test and quantitative data were compared using the Mann–Whitney U-test or t-test, as appropriate. The cumulative OS was estimated using the Kaplan-Meier method. The log rank test was used to evaluate the differences between groups. The factors that affected RFS were determined using Cox proportional hazards models. Variables with p<0.10 on the univariate analysis were included in the multivariate forward stepwise model. p<0.05 was deemed statistically significant. Bonferroni correction was used when the comparative subgroups (n) were >2 (α = 0.05/n). X-tile software (Yale University School of Medicine, New Haven, CT, USA) was used to determine the best cut-off value for the aMAP score.34 All statistical analyses were conducted with R software version 3.6.2. The nomograms were tested with 500 bootstrap resampling for internal validation. The performance of the prognostic models was tested by calculating the concordance index (C‐index). The nomograms for RFS were calibrated by comparing the observed and predicted survivals. A time-dependent receiver operating characteristic curve (t-ROC) analysis was carried out to calculate the area under the t-ROC (t-AUC).
Results
Patient Demographics
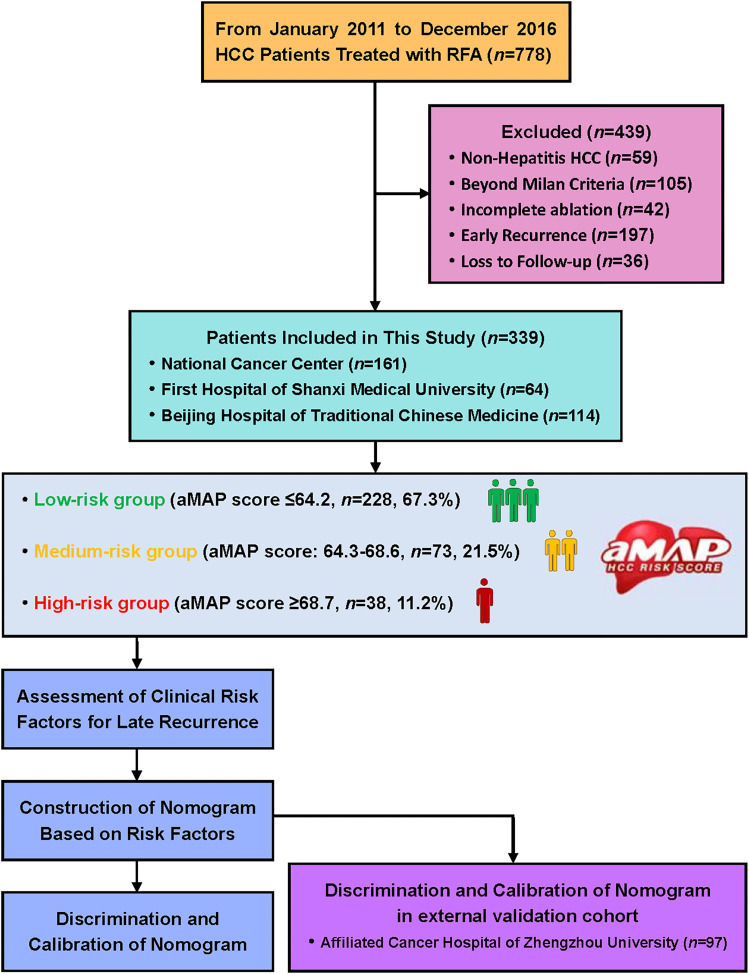
During this study, 778 patients with HCC received RFA at three Chinese hospitals. Among them, 439 patients were excluded based on the selection criteria shown in Figure 1. Finally, 339 patients were included (161 patients from NCC, 64 patients from First Hospital of Shanxi Medical University, and 114 patients from Beijing Hospital of Traditional Chinese Medicine). The demographic data were compared between late recurrence (n = 87) and no late recurrence groups (n = 252) as given in Table 1. The gender, ALB, number of tumors, cirrhosis maximum tumor size, and aMAP score were significantly different between the groups.
Figure 1.
Flowchart for patient selection and study design.
Table 1.
Patient Demographics of Training Cohort
| Characteristics | Total Patients | No Late Recurrence | Late Recurrence | p-value |
|---|---|---|---|---|
| n | 339 | 252 (74.34%) | 87 (25.66%) | |
| aMAP Score | <0.001 | |||
| ≤64.2 | 228 | 200 (79.37%) | 28 (32.18%) | |
| 64.3–68.6 | 73 | 47 (18.65%) | 26 (29.89%) | |
| ≥68.7 | 38 | 5 (1.98%) | 33 (37.93%) | |
| Age (years) | 0.169 | |||
| ≤60 | 217 | 156 (61.90%) | 61 (70.11%) | |
| >60 | 122 | 96 (38.10%) | 26 (29.89%) | |
| Gender | <0.001 | |||
| Female | 54 | 52 (20.63%) | 2 (2.30%) | |
| Male | 285 | 200 (79.37%) | 85 (97.70%) | |
| Max Size (cm) | 0.019 | |||
| ≤3.0 | 266 | 190 (75.40%) | 76 (87.36%) | |
| >3.0 | 73 | 62 (24.60%) | 11 (12.64%) | |
| Number | <0.001 | |||
| Solitary | 247 | 213 (84.52%) | 34 (39.08%) | |
| Multiple | 92 | 39 (15.48%) | 53 (60.92%) | |
| Ablation sessions | 0.855 | |||
| 1 | 298 | 222 (88.10%) | 76 (87.36%) | |
| 2 | 41 | 30 (11.90%) | 11 (12.64%) | |
| Location | 0.148 | |||
| Favorable | 309 | 233 (92.46%) | 76 (87.36%) | |
| Unfavorable | 30 | 19 (7.54%) | 11 (12.64%) | |
| Etiology | 0.909 | |||
| HBV | 302 | 225 (89.28%) | 77 (88.50%) | |
| HCV | 31 | 23 (9.13%) | 8 (9.20%) | |
| Alcoholic | 6 | 4 (1.59%) | 2 (2.30%) | |
| Antiviral Therapy | 0.013 | |||
| No | 141 | 95 (37.70%) | 46 (52.87%) | |
| Yes | 198 | 157 (62.30%) | 41 (47.13%) | |
| Cirrhosis | 0.002 | |||
| No | 38 | 36 (14.29%) | 2 (2.30%) | |
| Yes | 301 | 216 (85.71%) | 85 (97.70%) | |
| Child-Pugh class | 0.311 | |||
| A | 178 | 131 (51.98%) | 47 (54.02%) | |
| B | 161 | 121 (48.02%) | 40 (45.98%) | |
| ALBI grade | 0.743 | |||
| 1 | 178 | 131 (51.98%) | 47 (54.02%) | |
| 2 | 161 | 121 (48.02%) | 40 (45.98%) | |
| AFP (ng/mL) | 0.347 | |||
| ≤400 | 307 | 226 (89.68%) | 81 (93.10%) | |
| >400 | 32 | 26 (10.32%) | 6 (6.90%) | |
| ALB (g/L) | 0.073 | |||
| ≤35 | 37 | 32 (12.70%) | 5 (5.75%) | |
| >35 | 302 | 220 (87.30%) | 82 (94.25%) | |
| AST (U/L) | 0.897 | |||
| ≤40 | 279 | 207 (82.14%) | 72 (82.76%) | |
| >40 | 60 | 45 (17.86%) | 15 (17.24%) | |
| ALT (U/L) | 0.656 | |||
| ≤40 | 76 | 55 (21.83%) | 21 (24.14%) | |
| >40 | 263 | 197 (78.17%) | 66 (75.86%) | |
| PLT (109/L) | 0.241 | |||
| ≤100 | 134 | 95 (37.70%) | 39 (44.83%) | |
| >100 | 205 | 157 (62.30%) | 48 (55.17%) | |
| TBIL (μmol/L) | 0.452 | |||
| ≤17.1 | 214 | 162 (64.29%) | 52 (59.77%) | |
| >17.1 | 125 | 90 (35.71%) | 35 (40.23%) | |
| γ-GT (U/L) | 0.769 | |||
| ≤60 | 238 | 178 (70.63%) | 60 (68.97%) | |
| >60 | 101 | 74 (29.37%) | 27 (31.03%) |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; ALBI, albumin-bilirubin; AFP, alpha-fetoprotein; ALB, albumin; AST, aspartic transaminase; ALT, alanine aminotransferase; TBIL, total bilirubin; PLT, platelet; γ-GT, γ-glutamyl transpeptidase.
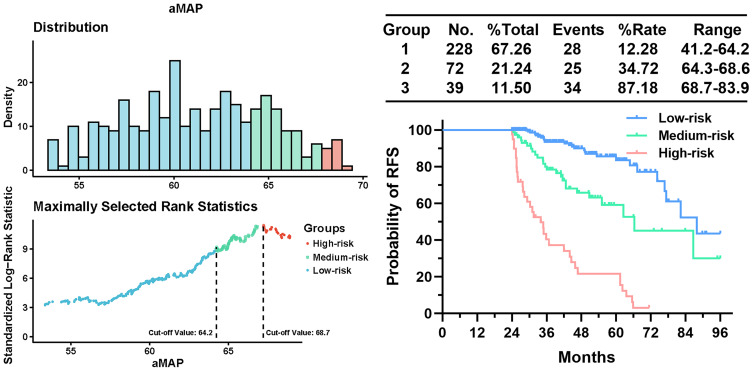
The Cut-off Value for aMAP Score
Two optimal cut-off values (aMAP score = 64.2 and 68.7) that demonstrated the best discriminative performance for RFS to separate the training cohort into low-risk (aMAP score ≤64.2), medium-risk (64.3 ≤aMAP score ≤68.6) and high-risk (aMAP score ≥68.7) groups groups were determined using X-tile plots as shown in Figure 2.
Figure 2.
X-tile plots used to generate optimal cut-off values of aMAP.
Risk Factors for Late Recurrence
The univariate analysis identified various clinical risk factors for late recurrence following RFA is given in Table 2. Subsequently, on multivariate analyses, aMAP score (64.3–68.6: HR = 2.537, 95% CI: 1.463–4.398; p<0.001; ≥68.7: HR = 6.193, 95% CI: 3.512–10.922; p<0.001), and multiple tumors (HR: 2.874, 95% CI: 1.822–4.535; p<0.001) were identified as independent predictors for late recurrence (Table 2).
Table 2.
Risk Factors for Late HCC Recurrence
| Variables (RFS) | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| aMAP Score | ||||||
| ≤64.2 | ||||||
| 64.3–68.6 | 2.757 | 1.615–4.705 | <0.001 | 2.537 | 1.463–4.398 | <0.001 |
| ≥68.7 | 9.691 | 5.792–16.213 | <0.001 | 6.193 | 3.512–10.922 | <0.001 |
| Max Size (cm) | ||||||
| ≤3.0 | 0.663 | 0.352–1.249 | 0.204 | NA | NA | NA |
| >3.0 | ||||||
| Number | ||||||
| Solitary | 1.806 | 0.957–3.411 | <0.001 | 2.874 | 1.822–4.535 | <0.001 |
| Multiple | ||||||
| Ablation sessions | ||||||
| 1 | 1.827 | 0.964–3.460 | 0.064 | 1.469 | 0.562–3.836 | 0.433 |
| 2 | ||||||
| Location | ||||||
| Favorable | 1.806 | 0.957–3.411 | 0.068 | 1.788 | 0.683–4.683 | 0.237 |
| Unfavorable | ||||||
| Etiology | ||||||
| HBV | NA | NA | NA | |||
| HCV | 1.320 | 0.636–2.741 | 0.456 | |||
| Alcoholic | 1.519 | 0.372–6.199 | 0.600 | |||
| Antiviral Therapy | ||||||
| No | 0.651 | 0.425–0.996 | 0.048 | 0.815 | 0.518–1.282 | 0.376 |
| Yes | ||||||
| Cirrhosis | ||||||
| No | 5.146 | 1.265–20.927 | 0.022 | 3.450 | 0.828–14.380 | 0.090 |
| Yes | ||||||
| Child-Pugh class | ||||||
| A | 0.536 | 0.074–3.856 | 0.535 | NA | NA | NA |
| B | ||||||
| AFP (ng/mL) | ||||||
| ≤400 | 0.664 | 0.289–1.526 | 0.335 | NA | NA | NA |
| >400 | ||||||
| ALB (g/L) | ||||||
| ≤35 | 1.697 | 0.688–4.188 | 0.251 | NA | NA | NA |
| >35 | ||||||
| AST (U/L) | ||||||
| ≤40 | 0.760 | 0.435–1.328 | 0.335 | NA | NA | NA |
| >40 | ||||||
| ALT (U/L) | ||||||
| ≤40 | 1.341 | 0.818–2.197 | 0.245 | NA | NA | NA |
| >40 | ||||||
| γ-GT (U/L) | ||||||
| ≤60 | 1.244 | 0.786–1.968 | 0.352 | NA | NA | NA |
| >60 | ||||||
Abbreviations: NA, not applicable; HBV, hepatitis B virus; HCV, hepatitis C virus; ALBI, albumin-bilirubin; AFP, alpha-fetoprotein; ALB, albumin; AST, aspartic transaminase; ALT, alanine aminotransferase; γ-GT, γ-glutamyl transpeptidase.
Survival Analysis
The median follow-up of 339 patients was 45.6 months (range: 24.1–96.0). In total, 46 patients died during follow-up, 87 patients developed late recurrence and were treated by SR (n = 6), repeat RFA (n = 39), transarterial chemo-embolization (TACE) (n = 29), TACE + sorafenib (n = 9), and FOLFOX4 regimen (n = 4), which depended on the extent of the disease.
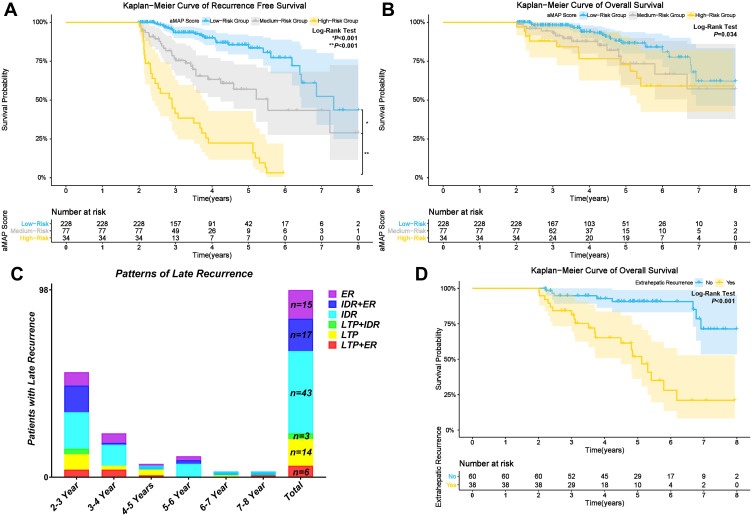
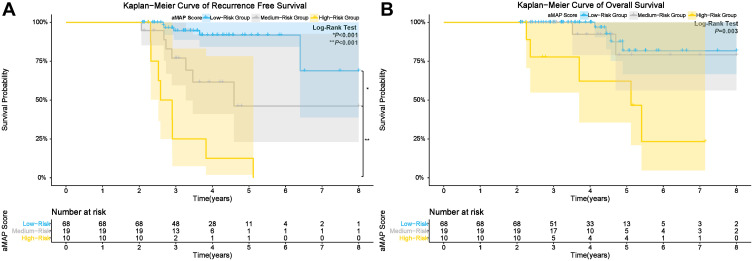
The 3, 4, and 5-year RFS rates of the low-risk group (median RFS: 87.9 months) were 93.4%, 90.0%, and 83.5%, respectively. The 3, 4, and 5-year RFS rates of the medium-risk group (median RFS time: 66.3 months) were 75.4%, 63.3%, and 56.9%, respectively. The 3, 4, and 5-year RFS rates of the high-risk group (median RFS time: 34.8 months) were 41. 6%, 22.4%, and 22.4%, respectively. The cumulative RFS rates of the low-risk group were the highest and that of the high-risk group was lowest (p<0.001) (Figure 3A).
Figure 3.
Kaplan-Meier curves for: (A) RFS; and (B) OS of patients in low, medium, and high-risk groups; Patterns of late recurrence in the study (C); Kaplan-Meier curves for: (D) OS of patients with and without ER.
The 3, 5, and 8-year OS rates of the low-risk group were 98.0%, 86.5%, and 62.1%, respectively. The 3, 5, and 8-year OS rates of the medium-risk group were 93.0%,73.3%, and 57.1%, respectively. The 3, 5, and 8-year OS rates of the high-risk group were 87.9%, 72.8%, and 59.0%, respectively. The cumulative OS rates were significantly different between the low, medium, and high-risk groups (p = 0.034) (Figure 3B).
Three types of late recurrence based on the location of the recurrence with six patterns were identified (Figure 3C): LTP (n = 14, 14.29%), IDR (n = 43, 43.88%), ER (n = 15, 15.31%), IDR + ER (n = 17, 17.35%), LTP + IDR (n = 3, 3.06%), and LTP + ER (n = 6, 6.12%). IDR was the most common recurrence pattern, and more than 60% of patients (n = 60, 61.22%) developed late recurrence with IDR. Subgroup analysis revealed that patients with ER (ER, IDR+ER) had significant poorer cumulative OS rates compared to patients without ER (LTP, IDR, LTP+IDR) (p<0.001, Figure 3D).
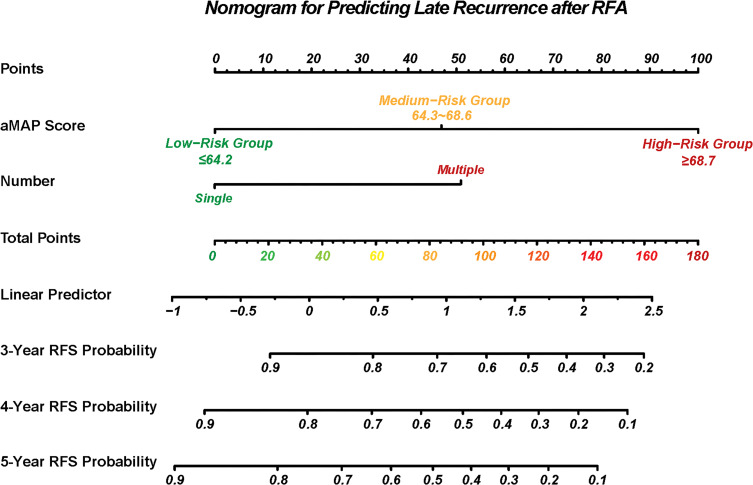
Nomogram for Late Recurrence
A nomogram was constructed to predict the RFS probabilities for HCC patients after RFA (Figure 4). In this nomogram, the independent risk factors for RFS that were identified by the multivariate analysis (aMAP score and number of tumors) were included. Each factor was assigned a score, and the sum of two scores was located on the total points axis, to obtain RFS probabilities.
Figure 4.
Nomogram to predict the RFS after RFA.
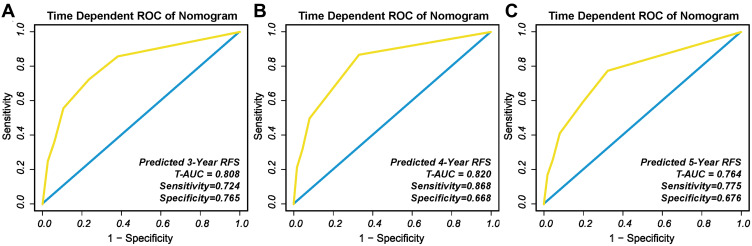
The C-index of the nomogram to predict RFS probabilities was 0.793 (95% CI: 0.744–0.842). The t-AUCs to predict 3, 4, and 5-year RFS were 0.808 (Figure 5A), 0.820 (Figure 5B), and 0.764 (Figure 5C), respectively.
Figure 5.
The t-ROC of the nomogram to predict: (A) 3-year; (B) 4-year; and (C) and 5-year RFS.
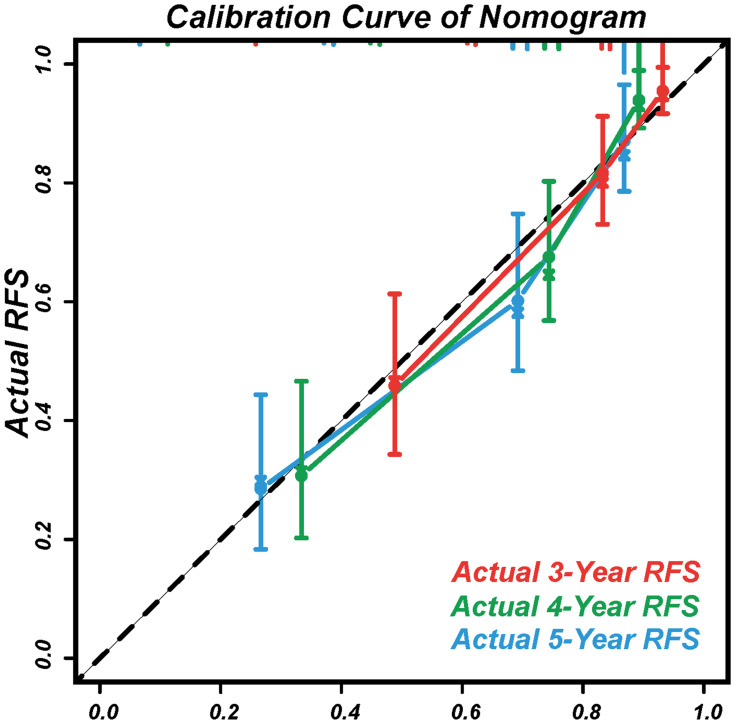
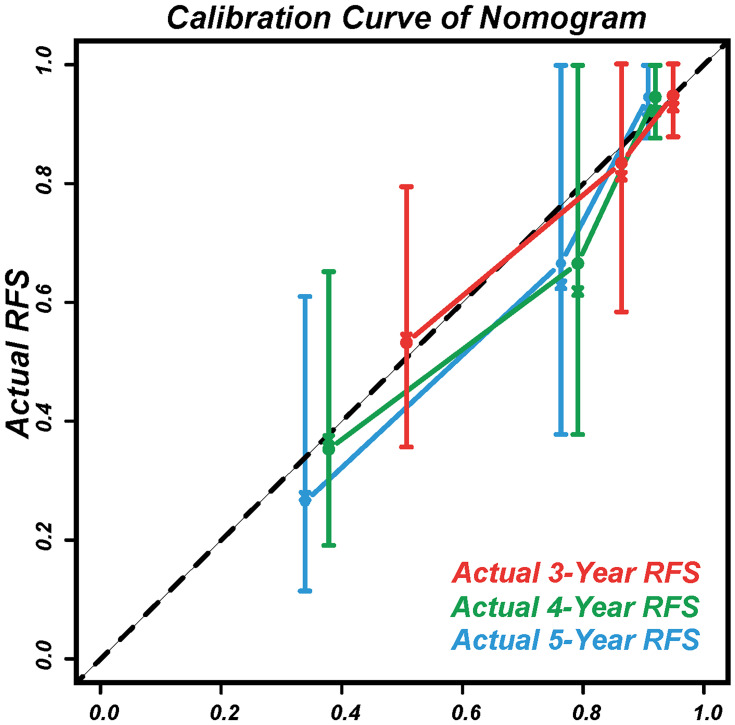
The calibration curves displayed agreement between the nomogram-predicted RFS (x-axis) and the actual RFS rates (y-axis) (Figure 6).
Figure 6.
Calibration curves to predict the 3, 4, and 5-year RFS.
Performance of the Nomogram in the External Validation Cohort
In total, 97 HCC patients were included in the validation cohort and their baseline characteristics are summarized in Supplementary Table 1.
The cumulative RFS rates of the low-risk group were highest and that of the high-risk group was lowest (p<0.001) (Figure 7A). The cumulative OS rates were significantly different between the low, medium, and high-risk groups of the validation cohort (p = 0.003) (Figure 7B).
Figure 7.
Kaplan-Meier curve for: (A) RFS; and (B) OS of patients in low, medium, and high-risk groups of external validation cohort.
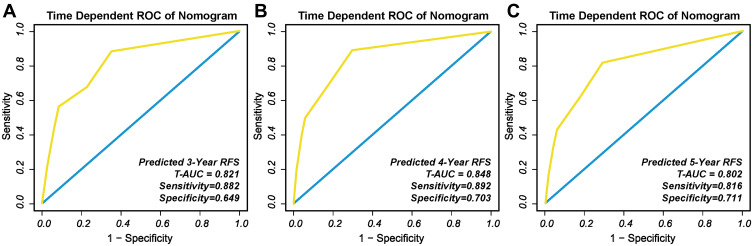
The t-AUC to predict 3, 4, and 5-year RFS using the nomogram was 0.821 (Figure 8A), 0.848 (Figure 8B), and 0.802 (Figure 8C), respectively. In addition, the calibration curves for the nomograms demonstrated good agreement (Figure 9).
Figure 8.
T-ROC of the nomogram to predict: (A) 3-year; (B) 4-year; and (C) 5-year RFS in external validation cohort.
Figure 9.
Calibration curves to predict the 3, 4, and 5-year RFS in external validation cohort.
Discussion
High incidance of tumor recurrence is a key factor that adversely affects the long-term survival of HCC patients following RFA.16 HCC recurrence can be defined as early recurrence and late recurrence based on the temporal distribution; however, a clear scientific cut-off time for early and late recurrence remains unclear.16,35 In this study, 2 years was adopted as the cut-off point to differentiate between early and late recurrence based on previous studies,15,16,36,37 because we considered that 2 years might be enough time for metastasis to appear in a patient with HCC with aggressive biological behavior. Early recurrence after ablation therapy has been widely studied, but few studies focused on late recurrence. Our previous study investigated the late recurrence of HCC following RFA, which showed that the presence of multiple tumors, male gender, and cirrhosis were risk factors for late recurrence and constructed a well-discriminated and calibrated nomogram (hereafter called nomogram 1.0) based on risk factors of late recurrence with a C-index of 0.763 (95% CI: 0.740–0.786) to predict the probability of RFS. The t-AUCs of the nomogram for predicting 3-, 4-, and 5-year RFS were 0.813, 0.781, and 0.723, respectively.13
In present study, we try an exploratory application of aMAP score on late recurrence of HCC patients after RFA, in which we found the aMAP score could not only provide a well-discriminated risk stratification for late recurrence as the low-risk group (aMAP score ≤64.2, median RFS time: 87.9 months), medium-risk group (64.3≤aMAP score ≤68.6, median RFS time: 66.3 months) and high-risk group (aMAP score≥ 68.7, median RFS time: 34.8 months) but also be identified as an independent risk factor of late recurrence. Therefore, we conducted a novel nomogram (hereafter called nomogram 2.0) based on risk factors (aMAP score and multiple tumors) with a C-index of 0.793 (95% CI: 0.744–0.842) to predict the probability of RFS. The t-AUCs of the nomogram 2.0 for predicting 3-, 4-, and 5-year RFS were 0.808, 0.820, and 0.764, respectively. The discrimination and calibration of nomogram 2.0 obviously improved compared with nomogram 1.0. The components of nomogram 2.0 were more abundant and reasonable than nomogram 1.0 with the supplement of ALBI grade (log10 bilirubin × 0.66) + (albumin × −0.085) which is an important liver functional predictor that influences prognosis in patients with HCC.23,38
Our nomogram 2.0 could better help interventional oncologists determine patient prognosis, strengthen decision making, and formulate adjuvant therapeutic and preventive strategies. For example, a male patient with multiple tumors and aMAP score of 70 has <30% and <20% probability of achieving 3 and 4-year RFS after RFA, respectively according to the nomogram, which meant that this patient has >70% and >80% probability of developing recurrence 3–4 years after RFA. Therefore, this patient should receive adjuvant therapies, such as etiological therapy39–41 and aggressive surveillance even after 2 years.15 In further studies, we will try to modify existing prognostic scores and develop novel prognostic scores for HCC patients after interventional therapy.
In present study, late recurrence occurred less in patients who received antiviral therapy than those did not receive antiviral therapy (41 of 198 [20.71%] vs 46 of 141 [32.62%]), but the difference was not significant (p=0.06), which is consistent with previous multicenter study (177 of 439 [40.3%] vs 108 of 232 [46.6%], p=0.12).2 We consider that antiviral therapy might bring preventive benefit for HCC patients with HBV or HCV infection after RFA therapy though there is none of approaches have been recommended as an universally accepted adjuvant therapy by current scientific guidelines, which still need more evidence of evidence-based medicine. Although the role of adjuvant therapeutic strategies to improve survival remains controversial, prospective clinical trials, such as targeted therapy combined with immunotherapy are being conducted to develop effective adjuvant therapeutic options for HCC patients.
Inflammation is considered to have a critical role in carcinogenesis.4 HCC is an inflammation-related cancer, and most patients with chronic hepatitis are at high risk for HCC.4 In this study, late recurrence occurred in 339 HCC patients with hepatitis after RFA. Among them, 302 had HBV infection (89.1%), 31 had HCV infection (9.1%), and 6 had alcoholic hepatitis (1.8%). Previous studies hypothesized that late recurrence after radical therapy probably occurred due to de novo HCC, especially in the presence of hepatitis and cirrhosis.15,24 In agreement with previous studies,15,24 multiple tumors were a risk factor for late recurrence in this study.
In this study’s cohort, approximately 36% of the patients were >60 years old, and elderly patients have a shorter lifespan than younger patients.42 In addition, males have a higher risk of developing HCC recurrence compared with females, because of the influence of sex hormones.21,22,43 This is similar to the higher incidence of HCC in males compared with females.
This study has some limitations. First, this study was retrospective in nature and included only Chinese patients. Second, the sample size was small, and most patients had HBV or HCV infection. Therefore, the role of aMAP score in patients with other etiologies, such as nonalcoholic fatty liver disease and primary biliary cirrhosis require further studies. Third, the results of this study require large prospective studies to validate the prognostic accuracy of the aMAP score in different races. Fourth, the cut-off time taken to distinguish between early and late recurrence in this study remains controversial.
In conclusion, the aMAP score is an independent prognostic factor for the late recurrence of HCC after RFA. The user-friendly nomogram based on the aMAP score and number of tumors accurately predicted RFS for HCC patients after RFA, which could help to provide individualized adjuvant therapy and surveillance strategies to improve the prognosis of HCC patients.
Funding Statement
National Natural Science Foundation of China (30970839&31170957), Research Project of Shanxi Province Health Commission (2017045), Youth Foundation of Pecking Union Medical College (2017320014), Natural Science Foundation of Henan Province (162300410270), Science and Technology Development Foundation of Henan Province (212102310115 &212102310135) Henan Provincial Medical Science and Technology Project (SBGJ202002025 &SBGJ202003009), Henan Medical Science and Technology Innovation Talent Project (YXKC2020045).
Disclosure
The authors of this manuscript declare that there are no relationships with any companies, whose products or services may be related to the subject matter of the article.
References
- 1.Zheng R, Qu C, Zhang S, et al. Liver cancer incidence and mortality in China: temporal trends and projections to 2030. Chin J Cancer Res. 2018;30(6):571–579. doi: 10.21147/j.issn.1000-9604.2018.06.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–700. doi: 10.1056/NEJM199603143341104 [DOI] [PubMed] [Google Scholar]
- 4.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Yang Y, Zhou B, et al. Challenges facing percutaneous ablation in the treatment of hepatocellular carcinoma: extension of ablation criteria. J Hepatocell Carcinoma. 2021;8:625–644. doi: 10.2147/JHC.S298709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruzzenente A, Guglielmi A, Sandri M, et al. Surgical resection versus local ablation for HCC on cirrhosis: results from a propensity case-matched study. J Gastrointest Surg. 2012;16(2):301–11; discussion 311. doi: 10.1007/s11605-011-1745-x [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. (NCCN) clinical practice guidelines in oncology. Hepatobiliary cancers, version 4; 2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed August16, 2020.
- 9.Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252(6):903–912. doi: 10.1097/SLA.0b013e3181efc656 [DOI] [PubMed] [Google Scholar]
- 10.Liu W, Zheng Y, He W, et al. Microwave vs radiofrequency ablation for hepatocellular carcinoma within the Milan criteria: a propensity score analysis. Aliment Pharmacol Ther. 2018;48(6):671–681. doi: 10.1111/apt.14929 [DOI] [PubMed] [Google Scholar]
- 11.Shi F, Wu M, Lian SS, et al. Radiofrequency ablation following downstaging of hepatocellular carcinoma by using transarterial chemoembolization: long-term outcomes. Radiology. 2019;293(3):707–715. doi: 10.1148/radiol.2019181991 [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Ye F, Xin Y, et al. Prognostic significance of controlling nutritional status score-based nomogram for hepatocellular carcinoma within Milan criteria after radiofrequency ablation. J Gastrointest Oncol. 2020;11(5):1024–1039. doi: 10.21037/jgo-20-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Chen Y, Ye F, et al. Late recurrence of hepatocellular carcinoma after radiofrequency ablation: a multicenter study of risk factors, patterns, and survival. Eur Radiol. 2021;31(5):3053–3064. doi: 10.1007/s00330-020-07460-x [DOI] [PubMed] [Google Scholar]
- 14.Xia Y, Li J, Liu G, et al. Long-term effects of repeat hepatectomy vs percutaneous radiofrequency ablation among patients with recurrent hepatocellular carcinoma: a randomized clinical trial. JAMA Oncol. 2019;6(2):255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu XF, Xing H, Han J, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 2019;154(3):209–217. doi: 10.1001/jamasurg.2018.4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nault JC, Sutter O, Nahon P, et al. Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J Hepatol. 2018;68(4):783–797. doi: 10.1016/j.jhep.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 17.Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243(2):229–235. doi: 10.1097/01.sla.0000197706.21803.a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng J, Chou JF, Gonen M, et al. Prediction of hepatocellular carcinoma recurrence beyond Milan criteria after resection: validation of a clinical risk score in an international cohort. Ann Surg. 2017;266(4):693–701. doi: 10.1097/SLA.0000000000002360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan AWH, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69(6):1284–1293. doi: 10.1016/j.jhep.2018.08.027 [DOI] [PubMed] [Google Scholar]
- 20.Fan R, Papatheodoridis G, Sun J, et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol. 2020;73(6):1368–1378. doi: 10.1016/j.jhep.2020.07.025 [DOI] [PubMed] [Google Scholar]
- 21.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. [DOI] [PubMed] [Google Scholar]
- 22.White DL, Thrift AP, Kanwal F, et al. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology. 2017;152(4):812–820.e5. doi: 10.1053/j.gastro.2016.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinato DJ, Sharma R, Allara E, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol. 2017;66(2):338–346. doi: 10.1016/j.jhep.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Chen Y, Ye F, et al. Late recurrence of hepatocellular carcinoma after radiofrequency ablation: a multicenter study of risk factors, patterns, and survival. Eur Radiol. 2020;31(5):3053–3064. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Yang Y, Dou J, et al. Cholecystectomy is associated with higher risk of recurrence after microwave ablation of hepatocellular carcinoma: a propensity score matching analysis. Cancer Biol Med. 2020;17(2):478–491. doi: 10.20892/j.issn.2095-3941.2019.0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hocquelet A, Trillaud H, Frulio N, et al. Three-dimensional measurement of hepatocellular carcinoma ablation zones and margins for predicting local tumor progression. J Vasc Interv Radiol. 2016;27(7):1038–1045 e2. doi: 10.1016/j.jvir.2016.02.031 [DOI] [PubMed] [Google Scholar]
- 27.Nakazawa T, Kokubu S, Shibuya A, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007;188(2):480–488. doi: 10.2214/AJR.05.2079 [DOI] [PubMed] [Google Scholar]
- 28.Laimer G, Schullian P, Jaschke N, et al. Minimal ablative margin (MAM) assessment with image fusion: an independent predictor for local tumor progression in hepatocellular carcinoma after stereotactic radiofrequency ablation. Eur Radiol. 2020;30(5):2463–2472. doi: 10.1007/s00330-019-06609-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273(1):241–260. doi: 10.1148/radiol.14132958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262(1):43–58. doi: 10.1148/radiol.11110144 [DOI] [PubMed] [Google Scholar]
- 31.Maruyama H, Takahashi M, Shimada T, et al. Pretreatment microbubble-induced enhancement in hepatocellular carcinoma predicts intrahepatic distant recurrence after radiofrequency ablation. AJR Am J Roentgenol. 2013;200(3):570–577. doi: 10.2214/AJR.12.8999 [DOI] [PubMed] [Google Scholar]
- 32.Okuwaki Y, Nakazawa T, Kokubu S, et al. Repeat radiofrequency ablation provides survival benefit in patients with intrahepatic distant recurrence of hepatocellular carcinoma. Am J Gastroenterol. 2009;104(11):2747–2753. doi: 10.1038/ajg.2009.414 [DOI] [PubMed] [Google Scholar]
- 33.Okuwaki Y, Nakazawa T, Shibuya A, et al. Intrahepatic distant recurrence after radiofrequency ablation for a single small hepatocellular carcinoma: risk factors and patterns. J Gastroenterol. 2008;43(1):71–78. doi: 10.1007/s00535-007-2123-z [DOI] [PubMed] [Google Scholar]
- 34.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi: 10.1158/1078-0432.CCR-04-0713 [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Xin Y, Ye F, et al. Early recurrence after radiofrequency ablation for hepatocellular carcinoma: a multicenter retrospective study on definition, patterns and risk factors. Int J Hyperthermia. 2021;38(1):437–446. doi: 10.1080/02656736.2020.1849828 [DOI] [PubMed] [Google Scholar]
- 36.Cheng Z, Yang P, Qu S, et al. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB (Oxford). 2015;17(5):422–427. doi: 10.1111/hpb.12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsilimigras DI, Bagante F, Moris D, et al. Recurrence patterns and outcomes after resection of hepatocellular carcinoma within and beyond the Barcelona clinic liver cancer criteria. Ann Surg Oncol. 2020;27(7):2321–2331. doi: 10.1245/s10434-020-08452-3 [DOI] [PubMed] [Google Scholar]
- 38.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi: 10.1200/JCO.2014.57.9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie DY, Ren ZG, Zhou J, et al. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi: 10.21037/hbsn-20-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. [DOI] [PubMed] [Google Scholar]
- 41.European Association for the Study of the Liver; Pawlotsky JM, Negro F, Aghemo A, et al. EASL recommendations on treatment of hepatitis C: final update of the series. J Hepatol. 2020;73(5):1170–1218. [DOI] [PubMed] [Google Scholar]
- 42.Kao WY, Chiou YY, Hung HH, et al. Younger hepatocellular carcinoma patients have better prognosis after percutaneous radiofrequency ablation therapy. J Clin Gastroenterol. 2012;46(1):62–70. doi: 10.1097/MCG.0b013e31822b36cc [DOI] [PubMed] [Google Scholar]
- 43.Lee CM, Lu SN, Changchien CS, et al. Age, gender, and local geographic variations of viral etiology of hepatocellular carcinoma in a hyperendemic area for hepatitis B virus infection. Cancer. 1999;86(7):1143–1150. doi: [DOI] [PubMed] [Google Scholar]