Abstract
From December 2005 to April 2007, we enrolled 60 adults starting antiretroviral therapy (ART) in a health district of Lima, Peru to receive community-based accompaniment with supervised antiretroviral (CASA). Paid community health workers performed twice-daily home visits to directly observe ART and offered additional medical, social and economic support to CASA participants. We matched 60 controls from a neighboring district by age, CD4 and primary referral criteria (TB status, female, neither). Using validated instruments at baseline and 12 months (time of DOT-HAART completion) we measured depression, social support, quality of life, HIV-related stigma and self-efficacy. We compared 12 month clinical and psychosocial outcomes among CASA versus control groups. CASA participants experienced better clinical and psychosocial outcomes at 12 months, including proportion with virologic suppression, increase in social support and reduction in HIV-associated stigma.
Keywords: Adherence, HIV, Resource-poor setting, Poverty, DOT-HAART
Introduction
One of the strongest predictors of HIV clinical outcomes, nonadherence to high active antiretroviral therapy (HA-ART) is associated with excess morbidity and mortality (Mills et al. 2006). From the public health perspective, nonadherence results in a costly, chronic pool of patients who contribute to primary transmission of drug-resistant strains (Wainberg and Friedland 1998). Although HAART programs in resource-poor settings have reported excellent adherence rates (Byakika-Tusiime et al. 2003; Mills et al. 2006; Muyingo et al. 2008), in reality achieving excellent adherence to HIV treatment requires both retention on HAART as well as HAART adherence among those who stay on treatment. In resource-poor settings where individuals face socioeconomic instability and limited antiretroviral options, sustained HIV treatment adherence is all the more crucial and challenging. Unfortunately, emerging data on rising rates of virologic failure and loss to follow-up reflect the challenges of both HAART adherence and retention as national programs in such settings expand (Larsson et al. 2007; Rosen et al. 2007; Spacek et al. 2006; Tuboi et al. 2005; van Oosterhout et al. 2005).
The socio-ecological model posits that behavior is influenced by embedded, dynamic, and interconnected social spheres, from personal factors to close social networks to larger fields such as cultural, community and policy factors (Bronfenbrenner 1979). We have argued that the “accompagnateur” model addresses adherence barriers within a socio-ecological model (Castro 2005). We define the term accompaniment as medical, social and economic support delivered by paid community-based health workers (Farmer et al. 2001). Community-based directly observed antiretroviral therapy (DOT-HAART), an integral component of accompaniment, helps address daily barriers to pill-taking, provides emotional and informational support, and serves as a liaison with formal health services (Mukherjee et al. 2006). We now provide community-based accompaniment with supervised antiretrovirals (CASA) to over 12,000 patients in Boston, rural Haiti, Rwanda and elsewhere, with excellent adherence and clinical outcomes (Behforouz et al. 2004; Koenig et al. 2004; Partners In Health 2008). Nonetheless, whether DOT-HAART should be implemented in resource-poor settings remains controversial. Some speculate about paradoxically increasing drug resistance, worry that stigma poses an insurmountable barrier to DOT-HAART in poor communities, and question whether DOT-HAART is even needed given higher overall adherence rates in such settings (Liechty and Bangsberg 2003). Unfortunately, emerging data likely marks the end of our “honeymoon” period of high adherence in early treatment cohorts in resource-poor settings. Low patient retention and trends of rising nonadherence serve as a “wake-up call” for the need to improve adherence in these regions (Bisson et al. 2008; Chen et al. 2008; Gill et al. 2005; Phillips et al. 2007; Wakabi 2008).
In 1996, we started a partnership between our Peru-based organization, Socios En Salud, with the Peruvian Ministry of Health to provide community-based directly observed therapy of multidrug-resistant tuberculosis (MDR-TB). Based on this strong community network and public-private alliance, we initiated a pilot in 2005 to provide 12 months of CASA support to patients starting antiretroviral therapy in a single health district of Lima, Peru. Here, we describe the clinical and psychosocial outcomes among HIV-positive adults at 12 months receiving CASA, compared with a matched control group selected from a neighboring health district.
Methods
Setting and Recruitment
Peru has an HIV prevalence of 0.6% and an estimated 60,000–80,000 HIV-positive individuals as of 2005 (Population Reference Bureau 2006). In collaboration with the National HIV Program, we enrolled 95 adult patients between December 2005 and April 2007. All patients were referred from a tertiary public hospital, which provides HIV care and antiretroviral therapy to the health region of Lima Este (total population 1,856,514 inhabitants). Providers referred patients living in poverty and who were starting or had recently started HAART. In response to the needs cited by local providers and our community-based team, enrollment priority was given to individuals who were co-infected with TB or female. However, males without TB co-infection were not excluded. For each patient enrolled in DOT-HAART, we sought a matched control in tertiary hospital serving a neighboring health region (Lima Ciudad). Because of limited funds, we enrolled the first 60 controls that could be successfully matched to our cohort. Controls were chosen among those eligible for HAART during the same period and were matched to cases by baseline characteristics of age (±5 years), primary referral criteria (TB, woman, neither), and CD4 cell count (≤ or >200 cells/ml3). All patients provided informed consent. Patients were excluded if they lived outside their respective hospitals’ catchment area.
Intervention Description
DOT-HAART participants were assigned a DOT worker. The community-based team also included a team of 12 field supervisors and two nurses. Nurses coordinated patient care with National and District program leaders and health professionals, monitored clinical and psychosocial follow-up, and supervised team activities and training. Field supervisors consisted of lay individuals who often had prior community leadership roles. Responsible for ~20 patients 3–4 DOT volunteers, field supervisors visited health establishments to arrange appointments and inform providers of patient-related issues; monitored DOT; provided clinical and social support through home and hospital visits and patient accompaniment to outpatient appointments, reported activities and events to nurses and providers; and conducted household contact screening for risk factors and/or symptoms of HIV and TB. DOT workers were selected by Ministry of Health providers, and were usually individuals living in the community who had prior experience working as community health workers for local health establishments. Caseload was usually 3–4 patients who lived near the worker’s residence. DOT workers were responsible for supervising all outpatient HAART doses in patients’ homes or elsewhere if by the patient, for which they were compensated with monthly food baskets. Workers also supervised ingestion of other medications (e.g., for opportunistic infections, diabetes, psychiatric disorders, tuberculosis, etc.), and reported any missed doses, adverse events and/or psychosocial crises to nurses and providers. During DOT encounters, workers provided patients and family members with emotional support, health education and screening for HIV- and/or medication-related symptoms. The team helped coordinate follow-up appointments, communicated patient issues to providers, and carried out physician indications by helping the patient obtain laboratory testing and medications. The entire team underwent 4 days of initial training, reviewing Ministry HIV services; HIV and antiretroviral medications; principles of adherence and the role of DOT; and issues in mental health, domestic violence, and substance use. The support lasted 12 months, with tapered DOT visits during the last 2 months. Patients also received comprehensive support, including financial aide for diagnostic tests and medications to treat opportunistic infections and adverse reactions, transportation and nutritional support, as needed. Phase two of the pilot will provide matched social support (i.e., peer group therapy and/or microfinance assistance based on a needs assessment) in year two and assess outcomes at 24 months (See Fig. 1).
Fig. 1.
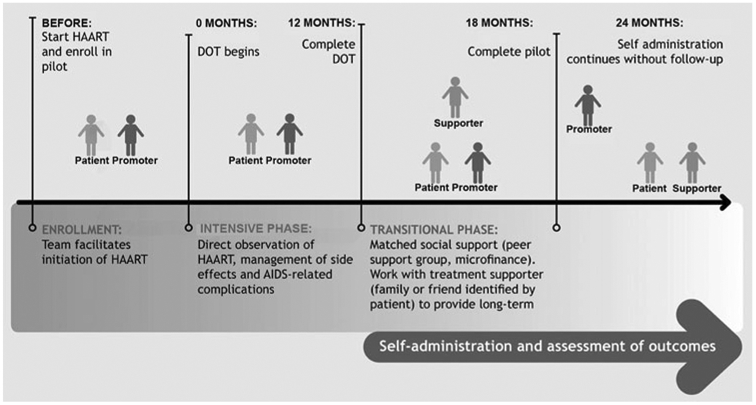
Description and time-line of community-based intervention
Routine Care
Patients are evaluated for HIV when they present to health services with clinical suggestions and/or risk factors for HIV. Voluntary counseling and testing is offered at health centers and hospitals. Free CD4 count and HIV viral load are performed upon diagnosis. Patients are evaluated for opportunistic infections, including TB by sputum smear microscopy, culture and chest radiograph. A psychosocial evaluation includes a peer counseling session and evaluation by both a psychologist and social worker. HAART is initiated based on WHO criteria (World Health Organization 2003). In order to initiate HAART, patients are required to identify a treatment supporter (“agente de soporte”) who is responsible for providing emotional support around medication adherence and accompanying patients to their medical visits. The treatment supporter does not receive training or compensation and is not expected to supervise medication doses. Patients are seen every week for the first month of HAART and then every 2–4 weeks. At each visit, patients are dispensed medications until their next appointment. Self-administration is the norm. Monthly laboratory monitoring for toxicity includes complete cell count and liver function tests. CD4 count and HIV load are performed every 6 months. Patients have access to psychiatric and psychological consultation, peer educators, and social work assistance. For patients lost to clinic follow-up, social workers may perform a home visit, if resources permit.
Data Collection and Measures
A separate, non-blinded team was trained to collect data using standardized forms from medical charts and patient interview. In addition to the variables described below, we collected sociodemographic and clinical characteristics, including TB status and treatment outcomes, baseline and follow-up weight, and substance use per physician assessment. We assessed depression, stigma, social support, quality of life and self-efficacy using standardized instruments that were administered in a one-on-one interview by a trained native Spanish speaker. We used the Hopkins Symptom Checklist to measure depression, the Berger Stigma Instrument, the Duke University of North Carolina Social Support Scale, and the Medical Outcomes Study HIV Quality of Life questionnaires (Berger et al. 2001; Broadhead et al. 1988; Derogatis et al. 1974; Wu et al. 1991). For self-efficacy, we used a scale derived by our sister organization, PACT, which was adapted from the HIV Self-Efficacy questionnaire (Shively et al. 2002), as well as the Confidence in Diabetes Self-Care Scale (Van Der Ven et al. 2003) and HIV self-management items specific to medication adherence developed and tested by Smith et al. (2003). The validation and internal reliability of these instruments in our cohort has been described elsewhere (Shin et al. 2008). We conducted interviews at baseline and 12 months and derived a change score (12 month score minus baseline score) for each scale.
We measured adherence as the number of days HAART was not taken over the total number of days in which HAART was prescribed within the last month of observation, based on patient self-report (Bangsberg et al. 2000). One of the most commonly used self-report tools was developed by the AIDS clinical trials group (ACTG) (Chesney et al. 2000). While the ACTG instrument measures adherence in the previous 4 days, recent data suggests greater intervention effect may be identified if adherence is measured over the past month (Simoni et al. 2006). We modified the ACTG to recall adherence over the past month. Individuals who were taking HAART were considered to have 0% adherence.
Clinical outcomes were virologic suppression; clinical status (on HAART, died, stopped HAART); TB treatment outcomes; and mean change in CD4 count at 12 months compared with baseline. Our primary endpoint, virologic suppression, was defined as having a viral load of <400 copies/ml at 1 year. As per intent to treat analysis, individuals who died or were missing viral load data were counted as unsuppressed.
Data Analysis
We provided baseline descriptions as proportions and means (with standard deviations, STD). To test significance, we reported the Chi-square for categorical variables, unless there were <5 predicted participants per cell where Fisher’s exact test was used. For continuous variables, we reported t tests or, for if not normally distributed, the Kruskal–Wallis test. We assessed the effect of our intervention on proportion with virologic suppression using univariate and multivariable analysis. For multivariable analysis, logistic regression analyses were performed on datasets multiply imputed using Markov Chain Monte Carlo methods (Schafer 1997). We included in this model all significantly different baseline characteristics between the intervention and control group as well as baseline characteristics associated with favorable response on univariate analysis. We assessed for effect modification of the two primary enrollment criteria (TB co-infection, female gender) upon the association of our intervention on outcome. We also excluded collinear variables, defined as those with a Pearson correlation coefficient of >0.60. We calculated post-hoc that our cohort size would enable us to detect a difference of 20% with a power of 80% and α = 0.05.
Results
As shown in Fig. 2, 95 adults were enrolled to receive CASA support. We matched 60 individuals to control subjects. These matched pairs (N = 120) comprise the cohort for analysis. Nine patients died before starting HAART. At 12 months of follow-up, 21 patients had died, and six abandoned HAART. None of the participants refused or quit the CASA intervention.
Fig. 2.

Intervention and control group flowchart
The CASA and control groups had some baseline differences, shown in Tables 1 and 2. Because the control region was somewhat less impoverished, the CASA group tended to have worse indicators of low socioeconomic status (food scarcity, lack of basic services, one-room households) and fewer had a history of substance abuse. Due to the time required to match and enroll controls, individuals in the intervention arm had a slightly longer time on HAART. Baseline CD4, viral load and weight did not differ significantly. Baseline psychosocial differences were notable for lower perceived HAART benefit among CASA participants.
Table 1.
Baseline sociodemographic and clinical characteristics (N = 120)
| Variable N, if not 120 | CASA cohort, N = 60 N (%) or Mean ± STDa |
Control group, N = 60 N (%) or Mean ± STDa |
X2, unless otherwise specified |
P value | Degrees of freedom |
|---|---|---|---|---|---|
| Sex | 0.00 | 1.00 | 1 | ||
| Male | 28 (46.7) | 28 (46.7) | |||
| Female | 32 (53.3) | 32 (53.3) | |||
| Age | 31.7 ± 7.8 | 31.9 ± 7.1 | −0.16 (t test) | 0.87 | 118 |
| Civil status, 117 | 2.11 | 0.15 | 1 | ||
| Married or living together | 29 (48.3) | 20 (35.1) | |||
| Single, separated, divorced, widowed | 31 (51.7) | 37 (64.9) | |||
| Socioeconomic status | |||||
| Limited education,b 116 | 7 (11.9) | 5 (8.8) | 0.21 (Fisher’s exact) | 0.76 | 1 |
| Unemployed, 115 | 44 (75.9) | 32 (56.1) | 4.99 | 0.03* | 1 |
| Lacks basic services,c 119 | 20 (33.3) | 7 (11.9) | 7.82 | 0.005** | 1 |
| Food insecurity,d 106 | 34 (56.7) | 16 (34.8) | 5.00 | 0.03* | 1 |
| Difficulty accessing health services in past 3 months, 102 | 35 (62.5) | 29 (63.0) | 0.003 | 0.96 | |
| HIV status | |||||
| Months from diagnosis to HAART,a 111 | 3.2 [1.6, 13.0] | 3.3 [2.1, 11.1] | 0.64 (Kruskal–Wallis) | 0.43 | 1 |
| Months on HAART at enrollmenta | 1.3 [0.2, 3.9] | 2.3 [0.0, 6.6] | 3.27 (Kruskal–Wallis) | 0.07 | 1 |
| Weight (kg), 116 | 53.9 ± 10.0 | 53.4 ± 11.9 | 0.17 (t test) | 0.86 | 93 |
| CD4 (cells/ml3), 111 | 114.8 ± 87.2 | 109.7 ± 97.9 | 0.29 (t test) | 0.77 | 109 |
| Viral load (copies/ml),a 106 | 130,000 [29,000, 230,000] | 72,000 [26,000, 284,000] | 0.62 (Kruskal–Wallis) | 0.43 | 1 |
| Substance abuse (drug or alcohol) | 12 (20.0) | 24 (40.0) | 5.71 | 0.02* | 1 |
| Documented drug abuse | 3 (5.0) | 10 (16.7) | 0.03 (Fisher’s exact) | 0.07 | 1 |
| Documented alcohol abuse | 11 (18.3) | 22 (36.7) | 5.06 | 0.02* | 1 |
| TB co-infection | 33 (55.0) | 35 (58.3) | 0.14 | 0.71 | 1 |
| Suspected MDR TB | 8 (13.3) | 8 (13.3) | 0.00 | 1.00 | 1 |
If non-normal, median [1st & 3rd quartiles]
Illiterate or no education beyond primary level
Home lacks at least one of the following: electricity, running water, or plumbing
Patient reported at least a day without food in the past 3 months due to poverty
P < .05
P < .01
Table 2.
Baseline psychosocial characteristics (N = 120)
| Variable N, if not 120 | CASA cohort, N = 60 N (%) or Mean ± STD |
Control group, N = 60 N (%) or Mean ± STD |
T-test, unless otherwise specified |
P value | Degrees of freedom |
|---|---|---|---|---|---|
| Perceives HAART benefit, 102 | 44 (78.6) | 44 (95.7) | 0.01 (Fisher’s exact) | 0.02* | 1 |
| Quality of life, 102 | |||||
| Quality of life | 40.7 ± 9.1 | 40.0 ± 8.9 | 0.58 | 0.69 | 100 |
| Physical health (PHS) | 40.1 ± 10.0 | 40.8 ± 11.3 | −0.11 | 0.77 | 100 |
| Mental health (MHS) | 41.3 ± 10.2 | 39.2 ± 9.8 | 1.17 | 0.30 | 100 |
| Depression | |||||
| HSC depression, 102 | 2.00 ± 0.55 | 1.96 ± 0.42 | 0.41 | 0.68 | 100 |
| Suicidal ideation in past month, 102 | 14 (25.0) | 9 (19.6) | 0.43 | 0.51 | 1 |
| Stigma score, 102 | 51.6 ± 14.4 | 46.0 ± 16.4 | 1.83 | 0.07 | 100 |
| Social support, 102 | |||||
| Social support | 62.3 ± 18.5 | 65.4 ± 20.7 | −0.58 | 0.43 | 100 |
| Emotional social support | 69.4 ± 19.4 | 67.6 ± 21.4 | 0.60 | 0.65 | 100 |
| Instrumental social support | 51.6 ± 27.8 | 62.1 ± 30.7 | −1.60 | 0.07 | 100 |
| Self-efficacy | |||||
| Self-efficacy | 64.8 ± 19.3 | 66.0 ± 14.5 | −0.37 | 0.71 | 100 |
P < .05
We evaluated psychosocial wellbeing among those who were on HAART at 12 months, shown in Table 3. The CASA group experienced significantly greater improvements in stigma (−10.4 vs. −1.7, t test (91, N = 93) = −2.70, P < 0.01), social support (+12.7 vs. −9.8, t test (91, N = 93) = 3.90, P < 0.01) and self-efficacy (+25.4 vs. +10.7, t test (91, N = 93) = 3.24, P < 0.01). Fewer CASA participants reported difficulty accessing health services (42.6% vs. 69.2%, Fisher’s (1, N = 93) = 7.37, P < 0.05). Both groups reported comparable improvements in quality of life and depressive symptoms, including a marked reduction in suicidal ideation. The control group reported almost minimal change in stigma and in fact reported decreased social support (in particular instrumental) compared with baseline. Clinical outcomes at 1 year are shown in Table 4. CASA participants were more likely to remain on HAART at 12 months (90.0% vs. 65.0%, Fisher’s (2, N = 120) = −0.0001, P < 0.01), be cured of TB (83.8% vs. 51.6%, Fisher’s (5, N = 120) = −0.0001, P < 0.05), and adhere to HAART (80.0% vs. 61.7%, X2 (1, N = 120) = 4.88, P < 0.05). Mean adherence (with standard deviations) were 86.0% (STD 29.1) vs. 62.5% (STD 46.3), Kruskal–Wallis (1, N = 120) = 6.50, P < 0.05. Participants were more likely to have a suppressed viral load at 12 months if they received CASA support (76.7% vs. 58.3%, X2 (1, N = 120) = 4.60, P < 0.05).
Table 3.
Psychosocial outcomes among those on HAART at 1 year (N = 93)
| Variable | CASA cohort, N = 54 N (%) or Mean ± STD |
Control group, N = 39 N (%) or Mean ± STD |
T-test, unless otherwise specified |
P value | Degrees of freedom |
|---|---|---|---|---|---|
| Health attitudes and behavior | |||||
| Perceived HAART benefit | 52 (96.3) | 33 (84.6) | 0.05 (Fisher’s exact) | 0.06 | 1 |
| Difficulty accessing health services | 23 (42.6) | 27 (69.2) | 7.37 | 0.01** | 1 |
| Quality of life (QOL) | |||||
| Change in total score | +14.8 ± 11.1 | +11.5 ± 12.3 | 1.03 | 0.19 | 90 |
| Change in physical QOL | +17.2 ± 11.8 | +12.0 ± 14.1 | 1.58 | 0.06 | 90 |
| Change in mental QOL | +12.2 ± 13.0 | +10.9 ± 12.9 | 0.28 | 0.66 | 90 |
| Depression | |||||
| Change in depression | −0.42 ± 0.63 | −0.21 ± 0.46 | −1.68 | 0.07 | 91 |
| Suicidal ideation in past month | 4 (7.4) | 3 (7.8) | 0.31 (Fisher’s exact) | 1.00 | 1 |
| Stigma score | |||||
| Change in stigma score | −10.4 ± 13.6 | −1.7 ± 16.3 | −2.70 | 0.005** | 91 |
| Social support | |||||
| Change in social support | +12.7 ± 25.0 | −9.8 ± 24.8 | 3.90 | <0.0001** | 91 |
| Change in emotional social support | +4.0 ± 24.4 | −6.4 ± 25.3 | 1.72 | 0.05* | 91 |
| Change in instrumental social support | +25.6 ± 35.6 | −15.0 ± 33.3 | 5.10 | <0.0001** | 91 |
| Self-efficacy | |||||
| Change in self-efficacy | +25.4 ± 22.1 | +10.7 ± 21.0 | 3.24 | 0.002** | 91 |
P ≤ .05
P ≤ .01
Table 4.
Clinical outcomes at 1 year (N = 120)
| Variable N, if not 120 | CASA cohort, N = 60 N (%) or Mean ± STD |
Control group, N = 60 N (%) or Mean ± STD |
X2, unless otherwise specified |
P value | Degrees of freedom |
|---|---|---|---|---|---|
| HAART status | −0.0001 (Fisher’s exact) | 0.001** | 2 | ||
| On HAART | 54 (90.0) | 39 (65.0) | |||
| Abandoned HAART | 0 (0) | 6 (10.0) | |||
| Died | 6 (10.0) | 15 (25.0) | |||
| TB outcomes among TB patients, 68 | −0.0001 | 0.02* | 5 | ||
| Cure | 26 (83.8) | 17 (51.6) | |||
| Treatment completed | 0 (0.0) | 2 (6.5) | |||
| Failure | 3 (9.7) | 4 (12.9) | |||
| Death | 1 (3.3) | 7 (22.6) | |||
| Default | 0 (0.0) | 2 (6.5) | |||
| In treatment | 1 (3.2) | 0 (0) | |||
| CD4 cell count, 89 | 269.7 ± 115.0 | 264.0 ± 161.0 | 0.06 | 0.87 | 87 |
| Change CD4 cell count, 89 | 143.5 ± 120.9 | 148.9 ± 129.6 | −0.34 | 0.84 | 87 |
| HAART adherence (≥95% doses in past month) | 48 (80) | 37 (61.7) | 4.88 | 0.03* | 1 |
P < .05
P < .01
We performed univariate analysis to identify factors associated with achieving virologic suppression at 12 months, shown in Table 5. Self-efficacy was associated with greater chance of achieving virologic suppression. For logistic regression analysis, we controlled for baseline differences in the control and intervention groups (unemployment, lacking basic services, food scarcity, substance abuse, and perceived benefit of HAART), in addition to self-efficacy. None of the covariates in the model were collinear and we detected no effect modification. CASA support was associated with a 2.75-fold increased chance of achieving a suppressed viral load at 1 year (adjusted OR and 95% CI: 2.75, 1.03–7.33, degrees of freedom = 7). The only other factor significantly associated with 12 month outcome was food scarcity (adjusted odds ratio 0.25, 95% CI 0.19, 0.61, degrees of freedom = 7).
Table 5.
Baseline characteristics associated with suppressed viral load at 12 months on univariate analysis (N = 113)
| Variable | Suppressed viral load, N = 76 N (%)/Mean ± STD |
Death or unsuppressed viral load, N = 44 N (%)/Mean ± STD |
X2, unless otherwise specified |
P value | Degrees of freedom |
|---|---|---|---|---|---|
| Male gender | 37 (45.7) | 19 (48.7) | 0.10 | 0.75 | 1 |
| Age | 31.7 ± 7.9 | 32.0 ± 6.4 | 0.81 | ||
| Unemployed | 53 (66.3) | 23 (65.7) | 0.003 | 0.96 | 1 |
| Lacks basic services | 19 (23.5) | 8 (21.1) | 0.09 | 0.77 | 1 |
| Food insecurity | 35 (43.2) | 15 (60.0) | 2.16 | 0.14 | 1 |
| Substance abuse | 21 (25.9) | 15 (38.5) | 1.97 | 0.16 | 1 |
| TB co-infection | 43 (53.1) | 25 (64.1) | 1.30 | 0.25 | 1 |
| Perceives HAART benefit at baseline | 70 (86.4) | 18 (85.7) | 0.27 (Fisher’s exact) | 1.00 | |
| Difficulty accessing health services | 52 (64.2) | 12 (57.1) | 0.36 | 0.55 | 1 |
| Depression | 1.96 ± 0.53 | 2.05 ± 0.32 | −1.01 | 0.44 | 52 |
| Stigma | 48.5 ± 15.5 | 51.4 ± 15.7 | −0.78 | 0.32 | 100 |
| Social support | 65.2 ± 19.6 | 57.9 ± 18.3 | 1.53 | 0.13 | 100 |
| Self-efficacy | 67.6 ± 20.5 | 56.7 ± 20.2 | 2.65 | 0.009** | 100 |
| CD4 ≤ 200 | 64 (79.0) | 25 (83.3) | 0.19 (Fisher’s exact) | 0.79 | 1 |
P < .01
Discussion
This pilot experience suggests that comprehensive community-based accompaniment with DOT-HAART may be instrumental in improving clinical and psychosocial outcomes within the first year of antiretroviral therapy. We observed a significant association between the intervention and suppressed viral load at 12 months. Our data add to the scant literature of controlled studies of community-based DOT-HAART in resource-poor settings. Idoko et al. (2007) compared daily and modified DOT by community and family members with self-administration and observed greater rates of virologic suppression at 48 weeks. In a recent RCT providing modified DOT-HAART (m-DOT-HAART) in Kenya, Sarna et al. (2008) observed short-term benefits on adherence in the entire cohort, and a significant long-term effect of m-DOT-HAART on virologic suppression among individuals suffering from depression. Pearson et al. (2007) conducted an RCT of m-DOT-HAART in Mozambique and observed improved rates of adherence at 6 and 12 months. Although viral load was not assessed, mean CD4 cell count did not differ significantly at both study endpoints. Compared with these studies, we may have observed greater impact because of the community-based long-term nature of our intervention, as both of these studies required patients to attend health centers daily to receive m-DOT-HAART and their interventions lasted between 6 and 24 weeks. However, perhaps more importantly, our outcomes were assessed just as individuals were completing DOT-HAART and do not reflect the durability of the intervention, unlike data reported by Sarna et al. (2008) and Pearson et al. (2007). We hope that additional outcomes assessments of our cohort at 24 months, i.e., 12 months after DOT-HAART, will be informative.
To our knowledge, our study is the first quantitative assessment of the psychosocial impact of community-based DOT-HAART. Several studies support the importance of psychosocial support provided by DOT-HAART. Nachega et al. (2006) have eloquently described the impact of patient-selected treatment supporters, identifying the importance of trust, emotional support and instrumental support provided by supporters and other family members. Bradley-Ewing et al. (2008) have also described the psychosocial benefits among m-DOT-HAART participants in an ongoing US-based trial. Modified DOT-HAART participants described greater motivation to adhere to HAART and other medications, strengthened ability to communicate with providers, and improved overall quality of life, in part through decreased loneliness and hopelessness, and better mood. These data support our own findings that community-based accompaniment has an important impact beyond direct medication supervision. Among these, our findings rebut concerns that community-based accompaniment may not be acceptable to HIV-positive individuals due to stigma concerns. Similar to our Haiti experience (Castro and Farmer 2005), we have found that CASA support does not increase but rather reduces stigma, through the act of receiving emotional support from a community peer, which in turn, cultivates behavior changes among family members and providers. Further, the improvements in psychosocial indicators in our cohort at 12 months support a possible mediating mechanism by which CASA support could achieve long-term HAART adherence: that of increased social support, which in turn decreases perceived stigma and bolsters self-efficacy.
Interestingly, the control group also experienced dynamic changes in psychosocial status during the first year of HAART. Depression, suicidal ideation, and quality of life improved by surviving the first year of HAART; on the other hand, individuals in the control group reported less social support (in particular instrumental support) at 1 year. We have frequently observed the tendency for family members to withdraw support once the patient is no longer critically ill, as limited emotional and material resources are exhausted over time.
We also found that food insecurity was associated with failure to achieve virologic control at 12 months. Food insecurity is known to impact HIV outcomes in numerous ways. HIV-positive individuals with competing priorities of poverty and hunger are less likely to utilize health services and adhere to antiretroviral therapy (Hardon et al. 2007; Nachega et al. 2006). Malnutrition and micronutrient deficiencies increase vertical transmission, accelerate HIV progression and increase mortality among HIV-positive individuals (Semba et al. 1995). Conversely, physical illness and decreased productivity from HIV/AIDS further feeds the cycle of economic stress and food insecurity (Bukusuba et al. 2007; Kadiyala and Gillespie 2004). Given the profound impact of poverty and food insecurity on HIV outcomes, we have argued that adherence interventions for HIV-positive individuals in resource-poor settings must address economic instability and food insecurity in order to achieve long-term success.
Our study is limited in its small size and differences between intervention and control groups. Because this study is not randomized, there may be additional confounders that have not been taken into account and bias study results. Further, we prioritized TB co-infected and female patients, in response to the community’s perception of greatest need. That the outcomes of our control group are worse than the outcomes of the general cohort of HIV-positive patients treated at the control hospital demonstrate that we have indeed identified vulnerable groups at risk of doing poorly during the first year of HAART. While this limits the generalizability of our findings, we did not detect effect modification by either primary enrollment category. Based on our understanding of the HIV-affected population in Peru and elsewhere, we feel that this cohort likely represents the most vulnerable groups who may most benefit from comprehensive accompaniment. TB patients and women with HIV/AIDS are not only among the most socioeconomically marginalized individuals in Peruvian society; further, they are groups that must often negotiate between multiple “vertical” health systems (e.g., TB and obstetrics in addition to HIV programs) and often fall through the cracks of coordinated care.
Conclusions
Despite its limitations, this pilot experience invites further exploration of CASA support and its potential impact on early and long-term physical and psychosocial recovery of individuals starting HAART in resource-poor settings. Long-term follow-up data as well as an assessment of cost and health service utilization are needed to fully understand the effectiveness of CASA in addition to matched support.
Acknowledgments
We would like to acknowledge the Office for AIDS Research at the National Institutes for Health; the Eleanor and Miles Shore Fellowship at Harvard Medical School; David Rockefeller Center for Latin American Studies at Harvard University, and Partners In Health for support of this project.
Contributor Information
Maribel Muñoz, Socios En Salud Sucursal Perú, Lima, Peru.
Karen Finnegan, Rollins School of Public Health, Emory University, Atlanta, GA, USA.
Jhon Zeladita, Socios En Salud Sucursal Perú, Lima, Peru.
Adolfo Caldas, Division of Global Health Equity, Brigham and Women’s Hospital, FXB Building, 7th Floor, 651 Huntington Avenue, Boston, MA 02115, USA.
Eduardo Sanchez, Hospital Nacional Hipólito Unanue, Lima, Peru.
Miriam Callacna, Hospital Nacional Hipólito Unanue, Lima, Peru.
Christian Rojas, Hospital Nacional Hipólito Unanue, Lima, Peru.
Jorge Arevalo, Hospital Dos de Mayo, Lima, Peru.
Jose Luis Sebastian, Peruvian HIV Program, Ministerio de Salud, Lima, Peru.
Cesar Bonilla, Peruvian TB Program, Ministerio de Salud, Lima, Peru.
Jaime Bayona, Socios En Salud Sucursal Perú, Lima, Peru.
Sonya Shin, Socios En Salud Sucursal Perú, Lima, Peru; Division of Global Health Equity, Brigham and Women’s Hospital, FXB Building, 7th Floor, 651 Huntington Avenue, Boston, MA 02115, USA; Harvard Medical School, Boston, MA, USA.
References
- Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner L, et al. (2000). Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS (London, England), 14(4), 357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- Behforouz HL, Kalmus A, Scherz CS, Kahn JS, Kadakia MB, & Farmer PE (2004). Directly observed therapy for HIV antiretroviral therapy in an urban US setting. Journal of Acquired Immune Deficiency Syndromes, 36(1), 642–645. doi: 10.1097/00126334-200405010-00016. [DOI] [PubMed] [Google Scholar]
- Berger BE, Ferrans CE, & Lashley FR (2001). Measuring stigma in people with HIV: Psychometric assessment of the HIV stigma scale. Research in Nursing & Health, 24(6), 518–529. doi: 10.1002/nur.10011. [DOI] [PubMed] [Google Scholar]
- Bisson GP, Gaolathe T, Gross R, Rollins C, Bellamy S, Mogorosi M, et al. (2008). Overestimates of survival after HAART: Implications for global scale-up efforts. PLoS ONE, 3(3), e1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley-Ewing A, Thomson D, Pinkston M, & Goggin KJ (2008). A qualitative examination of the indirect effects of modified directly observed therapy on health behaviors other than adherence. AIDS Patient Care and STDs, 22(8), 663–668. doi: 10.1089/apc.2007.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhead WE, Gehlbach SH, de Gruy FV, & Kaplan BH (1988). The Duke-UNC functional social support questionnaire. Measurement of social support in family medicine patients. Medical Care, 26(7), 709–723. doi: 10.1097/00005650-198807000-00006. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U (1979). The ecology of human development: Experiments by nature and design. Cambridge: Harvard University Press. [Google Scholar]
- Bukusuba J, Kikafunda JK, & Whitehead RG (2007). Food security status in households of people living with HIV/AIDS (PLWHA) in a Ugandan urban setting. The British Journal of Nutrition, 98(1), 211–217. doi: 10.1017/S0007114507691806. [DOI] [PubMed] [Google Scholar]
- Byakika-Tusiime J, Oyugi JH, Tumwikirize WA, Katabira ET, Mugyenyi PN, & Bangsberg DR (2003). Ability to purchase and secure stable therapy are significant predictors of nonadherence to antiretroviral therapy in Kampala, Uganda. Presented February 10–14, 2003 at the 10th Conference on Retroviruses and Opportunistic Infections (CROI), Boston, MA. Abstract #170 retrieved from http://www.retroconference.org/2003/cd/Abstract/170.htm. [Google Scholar]
- Castro A (2005). Adherence to antiretroviral therapy: Merging the clinical and social course of AIDS. PLoS Medicine, 2(12), e338. doi: 10.1371/journal.pmed.0020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro A, & Farmer P (2005). Understanding and addressing AIDS-related stigma: From anthropological theory to clinical practice in Haiti. American Journal of Public Health, 95(1), 53–59. doi: 10.2105/AJPH.2003.028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC, Yu JK, Harries AD, Bong CN, Kolola-Dzimadzi R, Tok TS, King CC, & Wang JD (2008). Increased mortality of male adults with AIDS related to poor compliance to antiretroviral therapy in Malawi. Tropical Medicine & International Health. doi: 10.1111/j.1365-3165.2008.02029.x. [DOI] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. (2000). Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. Patient care committee & adherence working group of the outcomes committee of the adult AIDS clinical trials group (AACTG). AIDS Care, 12(3), 255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, & Covi L (1974). The hopkins symptom checklist (HSCL): A self-report symptom inventory. Behavioral Science, 19(1), 1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- Farmer P, Leandre F, Mukherjee JS, Claude M, Nevil P, Smith-Fawzi MC, et al. (2001). Community-based approaches to HIV treatment in resource-poor settings. Lancet, 358(9279), 404–409. doi: 10.1016/S0140-6736(01)05550-7. [DOI] [PubMed] [Google Scholar]
- Gill CJ, Hamer DH, Simon JL, Thea DM, & Sabin LL (2005). No room for complacency about adherence to antiretroviral therapy in sub-Saharan Africa. AIDS (London, England), 19(12), 1243–1249. doi: 10.1097/01.aids.0000180094.04652.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardon AP, Akurut D, Comoro C, Ekezie C, Irunde HF, Gerrits T, et al. (2007). Hunger, waiting time and transport costs: Time to confront challenges to ART adherence in Africa. AIDS Care, 19(5), 658–665. doi: 10.1080/09540120701244943. [DOI] [PubMed] [Google Scholar]
- Idoko JA, Agbaji O, Agaba P, Akolo C, Inuwa B, Hassan Z, et al. (2007). Direct observation therapy-highly active antiretroviral therapy in a resource-limited setting: The use of community treatment support can be effective. International Journal of STD & AIDS, 18(11), 760–763. doi: 10.1258/095646207782212252. [DOI] [PubMed] [Google Scholar]
- Kadiyala S, & Gillespie S (2004). Rethinking food aid to fight AIDS. Food and Nutrition Bulletin, 25(3), 271–282. [DOI] [PubMed] [Google Scholar]
- Koenig SP, Leandre F, & Farmer PE (2004). Scaling-up HIV treatment programmes in resource-limited settings: The rural Haiti experience. AIDS (London, England), 18(Suppl. 3), S21–S25. doi: 10.1097/00002030-200406003-00005. [DOI] [PubMed] [Google Scholar]
- Larsson EC, Okong P, Thorson A, & Ekstrom AM (2007). Antiretroviral treatment of HIV in Uganda: A comparison of three different delivery models in a single hospital. Transactions of the Royal Society of Tropical Medicine and Hygiene, 101(9), 885–892. doi: 10.1016/j.trstmh.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Liechty CA, & Bangsberg DR (2003). Doubts about DOT: Antiretroviral therapy for resource-poor countries. AIDS (London, England), 17(9), 1383–1387. doi: 10.1097/00002030-200306130-00013. [DOI] [PubMed] [Google Scholar]
- Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, et al. (2006). Adherence to antiretroviral therapy in sub-Saharan Africa and North America: A meta-analysis. Journal of the American Medical Association, 296(6), 679–690. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- Mukherjee JS, Ivers L, Leandre F, Farmer P, & Behforouz H (2006). Antiretroviral therapy in resource-poor settings: Decreasing barriers to access and promoting adherence. Journal of Acquired Immune Deficiency Syndromes, 43(Suppl. 1), S123–S126. doi: 10.1097/01.qai.0000248348.25630.74. [DOI] [PubMed] [Google Scholar]
- Muyingo SK, Walker AS, Reid A, Munderi P, Gibb DM, Ssali F, et al. (2008). Patterns of individual and population-level adherence to antiretroviral therapy and risk factors for poor adherence in the first year of the DART trial in Uganda and Zimbabwe. Journal of Acquired Immune Deficiency Syndromes, 48(4), 468–475. [DOI] [PubMed] [Google Scholar]
- Nachega JB, Knowlton AR, Deluca A, Schoeman JH, Watkinson L, Efron A, et al. (2006). Treatment supporter to improve adherence to antiretroviral therapy in HIV-infected South African adults. A qualitative study. Journal of Acquired Immune Deficiency Syndromes, 43(Suppl. 1), S127–S133. doi: 10.1097/01.qai.0000248349.25630.3d. [DOI] [PubMed] [Google Scholar]
- Partners In Health. (2008). Partners in health 2008 annual report. Available from http://www.pih.org/inforesources/annual/PIH2008_annualreport.pdf.
- Pearson CR, Micek MA, Simoni JM, Hoff PD, Matediana E, Martin DP, et al. (2007). Randomized control trial of peer-delivered, modified directly observed therapy for HAART in Mozambique. Journal of Acquired Immune Deficiency Syndromes, 46(2), 238–244. doi: 10.1097/QAI.0b013e318153f7ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AN, Leen C, Wilson A, Anderson J, Dunn D, Schwenk A, et al. (2007). Risk of extensive virological failure to the three original antiretroviral drug classes over long-term follow-up from the start of therapy in patients with HIV infection: An observational cohort study. Lancet, 370(9603), 1923–1928. doi: 10.1016/S0140-6736(07)61815-7. [DOI] [PubMed] [Google Scholar]
- Population Reference Bureau. (2006). 2006 World population data sheet. 2006, Available from http://www.prb.org/pdf06/06WorldDataSheet.pdf.
- Rosen S, Fox MP, & Gill CJ (2007). Patient retention in antiretroviral therapy programs in sub-Saharan Africa: A systematic review. PLoS Medicine, 4(10), e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarna A, Luchters S, Geibel S, Chersich MF, Munyao P, Kaai S, et al. (2008). Short- and long-term efficacy of modified directly observed antiretroviral treatment in Mombasa, Kenya: A randomized trial. Journal of Acquired Immune Deficiency Syndromes, 48(5), 611–619. [DOI] [PubMed] [Google Scholar]
- Schafer J (1997). Analysis of incomplete multivariate data. New York: Chapman and Hall. [Google Scholar]
- Semba RD, Caiaffa WT, Graham NM, Cohn S, & Vlahov D (1995). Vitamin A deficiency and wasting as predictors of mortality in human immunodeficiency virus-infected injection drug users. The Journal of Infectious Diseases, 171(5), 1196–1202. [DOI] [PubMed] [Google Scholar]
- Shin S, Munoz M, Espiritu B, Zeladita J, Sanchez E, Callacna M , et al. (2008). Psychosocial impact of poverty on antiretroviral nonadherence among HIV-TB coinfected patients in Lima, Peru. Journal of the International Association of Physicians in AIDS Care, 7(2), 74–81. doi: 10.1177/1545109708315326. [DOI] [PubMed] [Google Scholar]
- Shively M, Smith T, Bormann J, & Gifford A (2002). Evaluating self-efficacy for HIV disease management skills. AIDS and Behavior, 6, 371–379. doi: 10.1023/A:1021156914683. [DOI] [Google Scholar]
- Simoni JM, Pearson CR, Pantalone DW, Marks G, & Crepaz N (2006). Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. Journal of Acquired Immune Deficiency Syndromes, 43(Suppl. 1), S23–S35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SR, Rublein JC, Marcus C, Brock TP, & Chesney MA (2003). A medication self-management program to improve adherence to HIV therapy regimens. Patient Education and Counseling, 50(2), 187–199. [DOI] [PubMed] [Google Scholar]
- Spacek LA, Shihab HM, Kamya MR, Mwesigire D, Ronald A, Mayanja H, et al. (2006). Response to antiretroviral therapy in HIV-infected patients attending a public, urban clinic in Kampala, Uganda. Clinical Infectious Diseases, 42(2), 252–259. doi: 10.1086/499044. [DOI] [PubMed] [Google Scholar]
- Tuboi SH, Harrison LH, Sprinz E, Albernaz RK, & Schechter M (2005). Predictors of virologic failure in HIV-1-infected patients starting highly active antiretroviral therapy in Porto Alegre, Brazil. Journal of Acquired Immune Deficiency Syndromes, 40(3), 324–328. doi: 10.1097/01.qai.0000182627.28595.01. [DOI] [PubMed] [Google Scholar]
- Van Der Ven NC, Weinger K, Yi J, Pouwer F, Ader H, Van Der Ploeg HM, et al. (2003). The confidence in diabetes self-care scale: psychometric properties of a new measure of diabetes-specific self-efficacy in Dutch and US patients with type 1 diabetes. Diabetes Care, 26(3), 713–718. doi: 10.2337/diacare.26.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oosterhout JJ, Bodasing N, Kumwenda JJ, Nyirenda C, Mallewa J, Cleary PR, et al. (2005). Evaluation of antiretroviral therapy results in a resource-poor setting in Blantyre, Malawi. Tropical Medicine & International Health, 10(5), 464–470. doi: 10.1111/j.1365-3156.2005.01409.x. [DOI] [PubMed] [Google Scholar]
- Wainberg MA, & Friedland G (1998). Public health implications of antiretroviral therapy and HIV drug resistance. Journal of the American Medical Association, 279(24), 1977–1983. doi: 10.1001/jama.279.24.1977. [DOI] [PubMed] [Google Scholar]
- Wakabi W (2008). Low ART adherence in Africa. The Lancet Infectious Diseases, 8(2), 94. doi: 10.1016/S1473-3099(08)70010-0. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2003). Scaling up antiretroviral therapy in resource-limited settings: Treatment guidelines for a public health approach. Available from http://www.who.int/3by5/publications/documents/arv_guidelines/en/.
- Wu AW, Rubin HR, Mathews WC, Ware JE Jr, Brysk LT, Hardy WD, et al. (1991). A health status questionnaire using 30 items from the medical outcomes study. Preliminary validation in persons with early HIV infection. Medical Care, 29(8), 786–798. doi: 10.1097/00005650-199108000-00011. [DOI] [PubMed] [Google Scholar]


