Abstract
The Fitness Versus Body Fat Hypothesis argues that cardiorespiratory fitness (CRF) plays a more important role in cardiovascular health than adiposity. It remains poorly understood whether CRF or adiposity accounts for a greater amount of variation in measures of brain health. We examined the contribution of CRF, adiposity, and their interaction with hippocampal structure. This study included 124 sedentary adults (M = 44.34) with overweight/obesity (Body Mass Index mean = 32.43). FMRIB’s Integrated Registration and Segmentation Tool was used to segment the hippocampus. Using hierarchical regression, we examined whether CRF, assessed via a submaximal graded exercise test, or adiposity, assessed as percent body fat using dual-energy x-ray absorptiometry (DXA) was associated with left and right hippocampal volume. Vertex-wise shape analysis was performed to examine regional shape differences associated with CRF and adiposity. Higher CRF was significantly associated with greater left hippocampal volume (p = .031), with outward shape differences along the surface of the subiculum and CA1 regions. Adiposity was not associated with left or right hippocampal volume or shape. The interaction between adiposity and CRF was not significant. Neither CRF nor adiposity were associated with thalamus or caudate nucleus volumes or shapes, two control regions. Higher CRF, but not adiposity, was related to greater left hippocampal volume, with outward shape differences along the surface of the subiculum and CA1 regions in a midlife sample with overweight/obesity. These findings indicate that, within the context of obesity, CRF is an important contributor to hippocampal structure, highlighting the importance of interventions targeting CRF.
Keywords: anatomical, fitness, hippocampus, middle-aged adults, obesity
1 |. INTRODUCTION
The prevalence of obesity in the United States has been rapidly increasing over the last 15 years (Hales, Carroll, Fryar, & Ogden, 2017). It was estimated that approximately 70% of American adults in 2016 had an overweight or obese Body Mass Index (BMI), with the highest rates of obesity (40%) among adults aged 40–59 years (Hales et al., 2017). A BMI in the obese range at any age is associated with an 18% increase in all-cause mortality risk (Flegal, Kit, Orpana, & Graubard, 2013). However, higher cardiorespiratory fitness (CRF) significantly attenuates the increased risk for mortality associated with obesity (McAuley & Beavers, 2014). In fact, a meta-analytical review found the risk for mortality to be equivalent among adults with higher CRF who are normal-weight, overweight, or obese (Barry et al., 2014). Additionally, adults with lower CRF had twice the risk for mortality as adults with higher CRF, irrespective of BMI (Barry et al., 2014). These results lend support for the importance of fitness over body fat, a hypothesis that posits that CRF plays a more important role in cardiovascular health than adiposity (Fogelholm, 2010; Hainer, Toplak, & Stich, 2009). Yet, whether the dominance of CRF over adiposity also extends to measures of brain health remains unknown.
The integrity of the hippocampus, one of the neural structures responsible for supporting episodic and relational memory, is more susceptible to deterioration than other brain regions among several patient populations, including Alzheimer’s disease (Frisoni, Fox, Jack, Scheltens, & Thompson, 2010), multiple sclerosis (Sicotte et al., 2008), schizophrenia (Heckers & Konradi, 2002), depression (Liu et al., 2017), and older adults (Raz et al., 2005). Therefore, a greater understanding of the various health factors (i.e., adiposity versus. CRF) that promote the integrity of the hippocampus is crucial for maximizing brain health and cognition among these patient populations, as well as for the optimal development of approaches for preventing or treating hippocampal atrophy. One health factor, greater CRF, is unequivocally associated with greater integrity of the hippocampus across the life span, with more consistent and robust positive associations reported in the anterior and subiculum sections of the hippocampus (Boots et al., 2015; Chaddock et al., 2010; Erickson et al., 2011; Esteban-Cornejo et al., 2017; Herting & Nagel, 2012; Hillman, Erickson, & Hatfield, 2017; Jonasson et al., 2016; Maass et al., 2015; Morris et al., 2017; Niemann, Godde, & Voelcker-Rehage, 2014; Ortega et al., 2019; Pajonk et al., 2010; Stillman et al., 2018; Szabo et al., 2011; Varma, Tang, & Carlson, 2016). Although BMI was controlled for in many prior studies, BMI cannot differentiate adipose tissue from lean mass, leading to significant intersubject variability in body fat percentage for any given BMI value (Romero-Corral et al., 2008). Studies using instruments that account for individual variability in body composition, such as dual-energy x-ray absorptiometry (DXA), have found higher percent body fat to be linked with reduced gray matter volume in the left temporal lobe (Weise, Thiyyagura, Reiman, Chen, & Krakoff, 2013). In addition, the majority of these studies focused on normal-weight adults, highlighting the need for a greater understanding of whether CRF can offset the detrimental effects of adiposity on hippocampal structure in adults with overweight/obesity.
In addition to the human studies described above that document associations between CRF, adiposity, and hippocampal structure, there have also been numerous animal studies that provide a mechanistic foundation for explaining associations between CRF and adiposity on brain health in humans. For example, rodent studies have documented that exercise positively influences numerous cellular and molecular cascades (e.g., cell proliferation) in the hippocampus (Christie et al., 2008; Cotman & Berchtold, 2002; Neeper, Gómez-Pinilla, Choi, & Cotman, 1995; van Praag, Shubert, Zhao, & Gage, 2005; Vaynman, Ying, & Gomez-Pinilla, 2004). In contrast, higher rates of obesity, along with related physiological effects of adiposity (i.e., insulin resistance), attenuate many of these same cellular and molecular pathways (Stranahan, 2015). Obesity is also associated with chronic low-grade inflammation in peripheral tissues and in the brain (Miller & Spencer, 2014), which contributes to neuroinflammation and eventual neuronal damage and death (Spielman, Little, & Klegeris, 2014). In contrast, exercise reduces pro-inflammatory conditions, which, in turn, stimulates plasticity, neurogenesis, and cerebrovascular perfusion (Cotman, Berchtold, & Christie, 2007). Thus, there is a solid theoretical basis, and several possible molecular and cellular mechanisms, for predicting that CRF may offset the detrimental effects of adiposity on hippocampal volume.
The current study examined the contributions of CRF and adiposity to individual variability in hippocampal volume and shape in sedentary midlife adults with overweight or obesity. Based on prior research of cardiovascular health and mortality, we predicted that CRF would be more strongly associated with hippocampal volume and shape than measures of adiposity. We also examined an interaction between adiposity and CRF on hippocampal volume to test whether: (a) higher adiposity would amplify the association between lower CRF and reduced hippocampal volume, or (b) higher CRF would mitigate the association between greater adiposity and reduced hippocampal volume. Although at first glance these two hypotheses seem like an inverse of one another, it is conceptually and statistically possible that both, one, or neither, is operating. This is the first study to extend the fitness versus body fat debate to hippocampal structure in sedentary midlife adults with overweight and obesity.
2 |. METHOD
2.1 |. Participants
One-hundred twenty-five sedentary participants were recruited from a parent study examining cardiac changes following a 12-month diet and exercise intervention at the University of Pittsburgh (PI: Jakicic) to take part in an ancillary neuroimaging study (PI: Erickson). The baseline data from the intervention was used for this analysis. Participants were considered eligible if they were between 18 and 55 years of age, had a BMI in the overweight or obese range (BMI = 25.0–39.9 kg/m2), and were able to provide informed consent. Participants were excluded if they met any of the following criteria: current medical condition that could affect body weight; current symptoms indicative of an increased risk for a cardiovascular event; hypertension, characterized by a resting systolic blood pressure of ≥160 mmHg or a diastolic blood pressure of ≥90 mmHg; eating disorder; alcohol or substance abuse; current treatment for a psychological disorder; self-reporting ≥60 min per week of structured moderate-to-vigorous intensity physical activity; and magnetic resonance imagining (MRI) contraindications. Additional exclusionary criteria for the ancillary study included any form of traumatic brain injury and prior diagnosis of a neurological disorder, including dementia, Parkinson’s disease, multiple sclerosis, schizophrenia or schizotypal disorder, epilepsy, autism spectrum disorder, and narcolepsy. One participant did not have neuroimaging data, and thus, the current study focused on analyzing the baseline data of 124 individuals from the sample.
2.2 |. Adiposity assessment
Whole-body DXA scans were performed using a GE iDXA with subjects lying in a supine position with their legs together, arms by their sides, and palms facing the hips/thighs. Adiposity was determined as percent body fat using a ratio of fat mass to total body mass. Females completed a urine pregnancy test prior to DXA assessment to confirm non-pregnancy. DXA is considered to be an accurate and reliable measure of percent body fat, and has high test–retest reliability (ICC = 0.99) (Abe, Thiebaud, Loenneke, & Young, 2015; Kennedy, Shea, & Sun, 2009).
2.3 |. CRF assessment
CRF (VO2submax) was determined using a graded submaximal exercise test using a motor-driven treadmill, as previously described (Rogers et al., 2020). VO2submax was defined as the oxygen consumption (ml/kg/min) achieved at 85% of the age-predicted maximal heart rate. Age-predicted maximal heart was computed as (220 − age) × 0.85.
2.4 |. Image acquisition and processing
Magnetic resonance images were collected on a Siemens 3T Verio MRI scanner with a 32-channel phased-array head coil. High-resolution anatomical MPRAGE (1 mm3 voxels, 256 slices, TE = 2.93 ms, TR = 1,900 ms) images were used for volumetric analyses. FMRIB’s Integrated Registration and Segmentation Tool (FIRST), a semi-automated model-based segmentation tool in FMRIB’s Software Library (FSL), was used to register and segment the hippocampus and two regions that have served as negative control regions: the thalamus and the caudate nucleus (Patenaude, Smith, Kennedy, & Jenkinson, 2011). Scan–rescan reliability and inter-scanner reliability has been evaluated extensively for FIRST segmentation data and indicates excellent reliability (ICC = 0.95) (Amann et al., 2015). A priori information about the shape and location of each subcortical structure, as well as the boundaries between gray matter, white matter, and cerebro-spinal fluid, were used to segment left and right subcortical volumes. Visual inspection in the coronal plane was conducted to ensure accuracy. For secondary analyses, the center of gravity was used to divide the hippocampus into anterior and posterior sections for each subject (Erickson et al., 2009).
Vertex-wise shape analysis was performed using the hippocampal, thalamus, and caudate nucleus volumes calculated with FIRST. Average left and right subcortical structure meshes were created for the sample in Montreal Neurological Institute (MNI) standard space. This allows for the normalization of brain size, negating the need to control for total intracranial volume in our shape analysis models. The vertex locations at each surface point were projected onto the average mesh for each subject to calculate localized shape differences. Statistically significant results indicate regional positive (outward shape) or negative (inward shape) differences associated with our variables of interest.
2.5 |. Analyses
Preliminary analyses were conducted to ensure that assumptions of normality, linearity, and homoscedasticity were not violated. Correlations between variables were examined for multicollinearity. For the volumetric analysis, three-step hierarchical multiple regression analyses were conducted with left and right hippocampal volume as the outcome variables. Sex and intracranial volume were entered into the first step of the regression models as covariates. CRF (VO2submax) was entered into step two and adiposity (percent body fat from the DXA assessment) was entered into step three. Analyses were also conducted with CRF and adiposity entered into the model in the reverse order. In addition, we tested the interactive effects of adiposity and CRF on hippocampal volume by including an interaction term (DXA × VO2submax) in the model. Significance was set a priori as p < .05. All data were analyzed using SPSS Statistics v24.
For the shape analysis, we examined the relationship of: (a) CRF with the shape of the left and right hippocampus while controlling for sex and adiposity, and (b) adiposity with the shape of the left and right hippocampus while controlling for sex and CRF. In order to account for multiple comparisons, we used a 2D-optimized threshold-free cluster enhancement for multiple comparison correction.
A number of secondary analyses were conducted to investigate the regional specificity of the observed associations, including examining: (a) anterior and posterior hippocampal volumes separately, as previous research has found stronger associations between the anterior portions of the hippocampus and CRF (Erickson et al., 2011; Stillman et al., 2018), and (b) associations between CRF and adiposity with the thalamus and the caudate nucleus, two regions that have served as control regions in prior studies (Erickson et al., 2011; Ortega et al., 2019; Stillman et al., 2018). Previous research investigating the brain regions affected by an exercise intervention of older adults found an increase in volume of the hippocampus, but not the thalamus or the caudate nucleus, suggesting that all brain regions are not uniformly influenced by exercise (Erickson et al., 2011). This nonuniformity of effects suggests that the caudate nucleus and thalamus could serve as negative control areas for this study. Secondary shape analyses of the left and right thalamus and caudate nucleus were also conducted to determine whether the results were specific to the hippocampus.
3 |. RESULTS
3.1 |. Characteristics of the sample
Means and standard deviations of adiposity, CRF, and demographic variables are presented in Table 1.
TABLE 1.
Sample characteristics (N = 124)
| Variable | Mean (±SD) |
|---|---|
| Age (years) | 44.34 (±8.63) |
| Sex (% female) | 79.0 |
| Education (years) | 16.40 (±2.66) |
| Race (% Caucasian) | 76.6 |
| Body mass index (kg/m2) | 32.43 (±3.95) |
| Adiposity (% body fat) | 42.97 (±5.84) |
| CRF (ml/kg/min) | 22.94 (±4.37) |
Note: All values (except sex and race) represent means ± standard deviations.
The participants were on average 44.34 years old (±8.63) with 16.40 years (±2.66) of education. Females made up 79.0% of the sample. Average BMI was in the obese range (32.43 ± 3.95 kg/m2), consisting of 40 participants with overweight (BMI = 25.0–29.9 kg/m2) and 84 participants with obesity (BMI = 30.0–39.9 kg/m2). CRF ranged between 14.5 and 37.3 ml/kg/min and adiposity ranged between 26.5% and 55.8% body fat. Thus, given the broad ranges of both CRF and adiposity, these measures possess enough variation to test for associations. Nonetheless, we cannot make inferences about how the associations might differ if a normal BMI range were included.
As depicted in Table 2, age and years of education were not significantly correlated with CRF, adiposity, or hippocampal volume, and thus, were not included as covariates in the hierarchical regression models. Figure 1 depicts scatter plots of age by CRF, adiposity, and hippocampal volume as a function of sex. Although participants were excluded for hypertension, there still remained a large range of blood pressure values that could have been associated with hippocampal volume. When examining systolic and diastolic blood pressure, we found that they were not significantly correlated with left or right hippocampal volume (ranging between r = −.029, p = .750 and r = .133, p = .139). Sex and intracranial volume were significantly correlated with CRF, adiposity, and hippocampal volume (ranging between r = −.512, p < .01 and r = .683, p < .01), and therefore, were entered into all hierarchical regression models as covariates. CRF and adiposity were correlated (r = −.582, p < .001), such that 33.9% of the variance was shared by both variables. Age and years of education were not significantly associated with left or right hippocampal shape, and thus, were not included as covariates in any shape analyses. Sex was significantly associated with left and right hippocampal shape, and therefore, was included as a covariate in all shape analyses.
TABLE 2.
Pearson’s correlations for all variables of interest (N = 124)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. Age | – | −.090 | −.088 | −.069 | −.095 | .004 | .003 | −.023 |
| 2. Years of Education | – | −.060 | −.069 | −.008 | .113 | .120 | .055 | |
| 3. Sex | – | .683** | −.512** | −.543** | −.215* | −.301** | ||
| 4. Adiposity | – | −.582** | −.400** | −.191* | −.240** | |||
| 5. CRF | – | .373** | .302** | .235** | ||||
| 6. IV | – | .529** | .568** | |||||
| 7. Left Hippo. Vol. | – | .700** | ||||||
| 8. Right Hippo. Vol. | – |
Notes: Sex = male: 1, female: 2; CRF = cardiorespiratory fitness; IV = intracranial volume; Hippo. Vol. = hippocampal volume.
Correlation is significant at the p < .05 level.
Correlation is significant at the p < .01 level.
FIGURE 1.
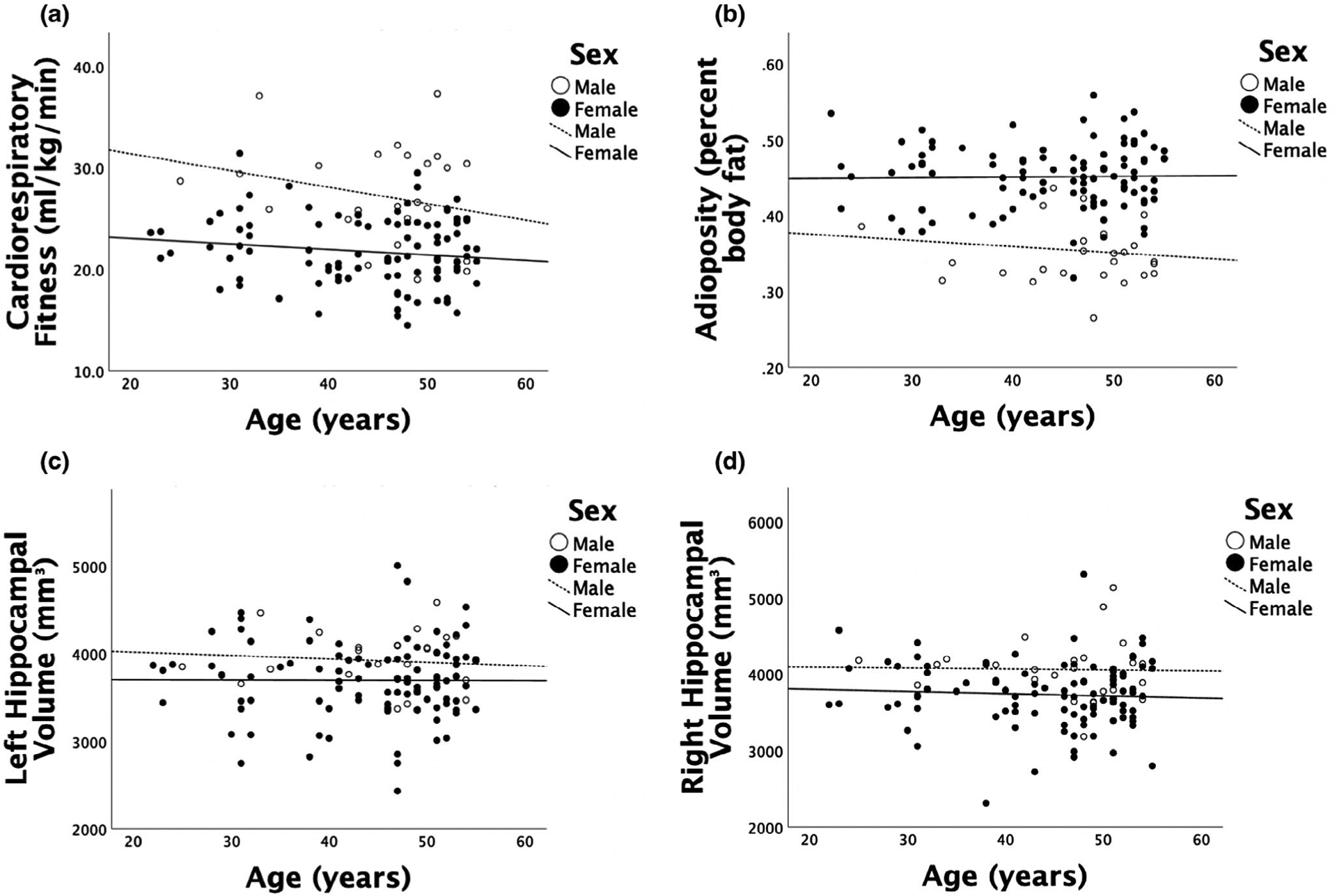
(a) Scatter plot of age by CRF as a function of sex. (b) Scatter plot of age by adiposity as a function of sex. (c) Scatter plot of age by left hippocampal volume as a function of sex. (d) Scatter plot of age by right hippocampal volume as a function of sex. Age was not significantly correlated with CRF, adiposity, or hippocampal volume (ranging between r = −.095, p = .291 and r = .003, p = .977)
3.2 |. CRF, but not adiposity, was associated with hippocampal volume
Hierarchical regression models were conducted for the right and left hippocampus separately, as depicted in Table 3.
TABLE 3.
Hierarchical regression models of CRF and adiposity with hippocampal volume
| Model | β | p value | R2 | Model | β | p value | R2 | ||
|---|---|---|---|---|---|---|---|---|---|
| Left hippocampal volume | Right hippocampal volume | ||||||||
| 1 | Sex | .132 | .154 | .301 | 1 | Sex | .028 | .763 | .318 |
| IV | .612 | <.001** | – | IV | .579 | <.001** | – | ||
| 2 | Sex | .228 | .024* | .330 | 2 | Sex | .050 | .617 | .319 |
| IV | .591 | <.001** | – | IV | .574 | <.001** | – | ||
| CRF | .203 | .023* | – | CRF | .049 | .587 | – | ||
| 3 | Sex | .221 | .062 | .330 | 3 | Sex | .070 | .553 | .320 |
| IV | .591 | <.001** | – | IV | .575 | <.001** | – | ||
| CRF | .207 | .031* | – | CRF | .038 | .694 | – | ||
| Adiposity | .013 | .910 | – | Adiposity | −.036 | .746 | – | ||
| Left anterior hippocampal volume | Right anterior hippocampal volume | ||||||||
| 1 | Sex | .082 | .370 | .318 | 1 | Sex | .009 | .918 | .336 |
| IV | .606 | <.001** | – | IV | .585 | <.001** | |||
| 2 | Sex | .164 | .100 | .340 | 2 | Sex | .032 | .751 | .338 |
| IV | .588 | <.001** | – | IV | .580 | <.001** | |||
| CRF | .175 | .049* | – | CRF | .047 | .591 | – | ||
| 3 | Sex | .177 | .133 | .340 | 3 | Sex | .064 | .583 | .339 |
| IV | .589 | <.001** | – | IV | .581 | <.001** | |||
| CRF | .168 | .077 | – | CRF | .030 | .754 | – | ||
| Adiposity | −.023 | .839 | – | Adiposity | −.059 | .593 | – | ||
| Left posterior hippocampal volume | Right posterior hippocampal volume | ||||||||
| 1 | Sex | .200 | .040* | .238 | 1 | Sex | .053 | .580 | .254 |
| IV | .572 | <.001** | – | IV | .532 | <.001** | |||
| 2 | Sex | .309 | .004** | .276 | 2 | Sex | .075 | .477 | .255 |
| IV | .548 | <.001** | – | IV | .527 | <.001** | |||
| CRF | .231 | .013* | – | CRF | .047 | .614 | – | ||
| 3 | Sex | .272 | .027* | .278 | 3 | Sex | .075 | .546 | .255 |
| IV | .547 | <.001** | – | IV | .527 | <.001** | |||
| CRF | .251 | .012* | – | CRF | .047 | .638 | – | ||
| Adiposity | .066 | .569 | – | Adiposity | <.001 | .999 | – | ||
Notes: Sex = male: 1, female: 2; IV = intracranial volume; CRF = cardiorespiratory fitness.
Statistical significance at the .05 level.
Statistical significance at the .01 level.
The regression analyses revealed that when all predictors were included in step three of the model, CRF was the only significant predictor of left hippocampal volume (β = .207, p = .031). In contrast, neither CRF (β = .038, p = .694) nor adiposity (β = −.036, p = .746) were significantly related to right hippocampal volume after controlling for sex and intracranial volume. Figures 2 and 3 depict scatter plots of CRF by left and right hippocampal volume, respectively.
FIGURE 2.
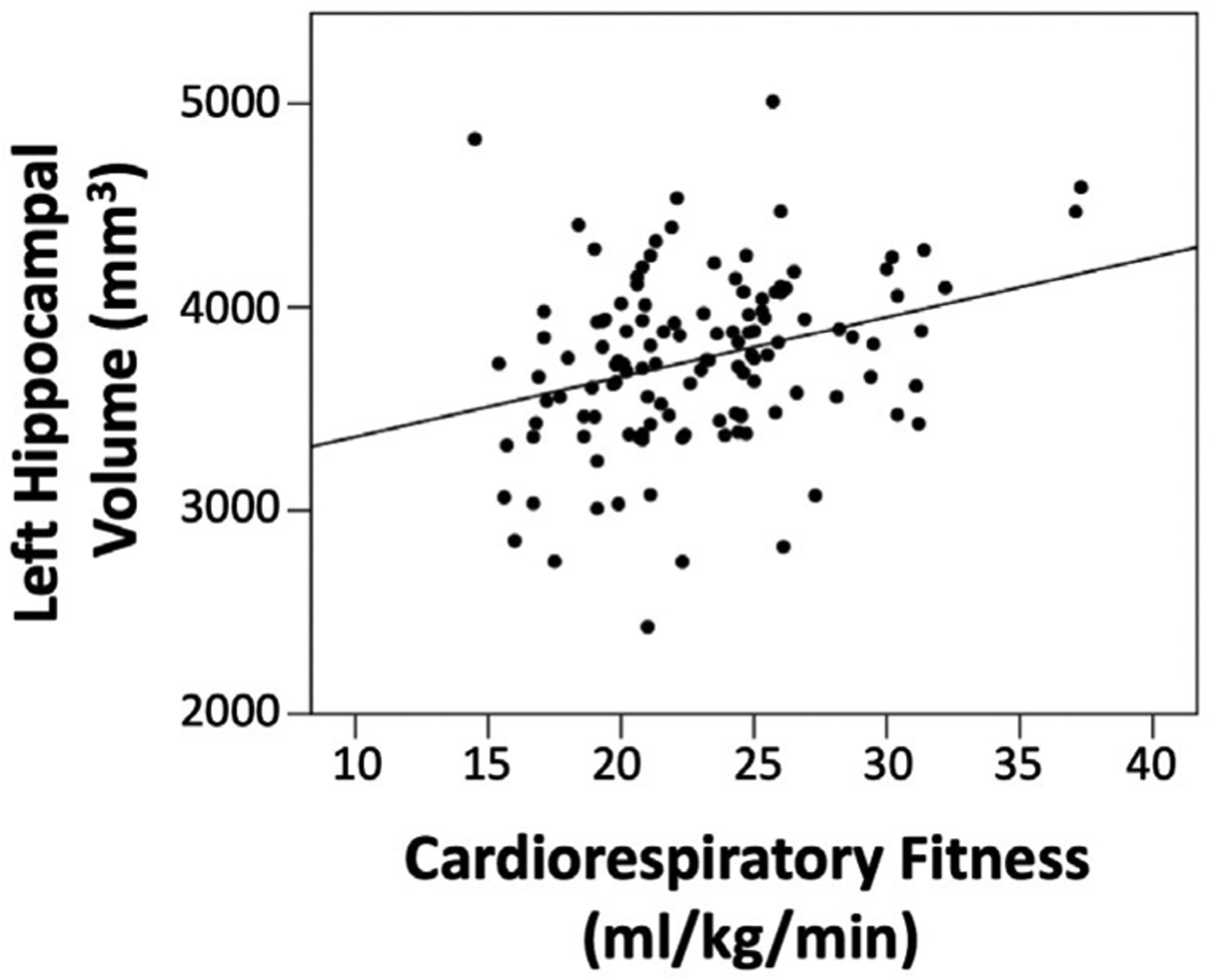
Scatter plot of CRF by left hippocampal volume. After accounting for sex, intracranial volume, and adiposity, the relationship between CRF and left hippocampal volume was significant (p = .031)
FIGURE 3.
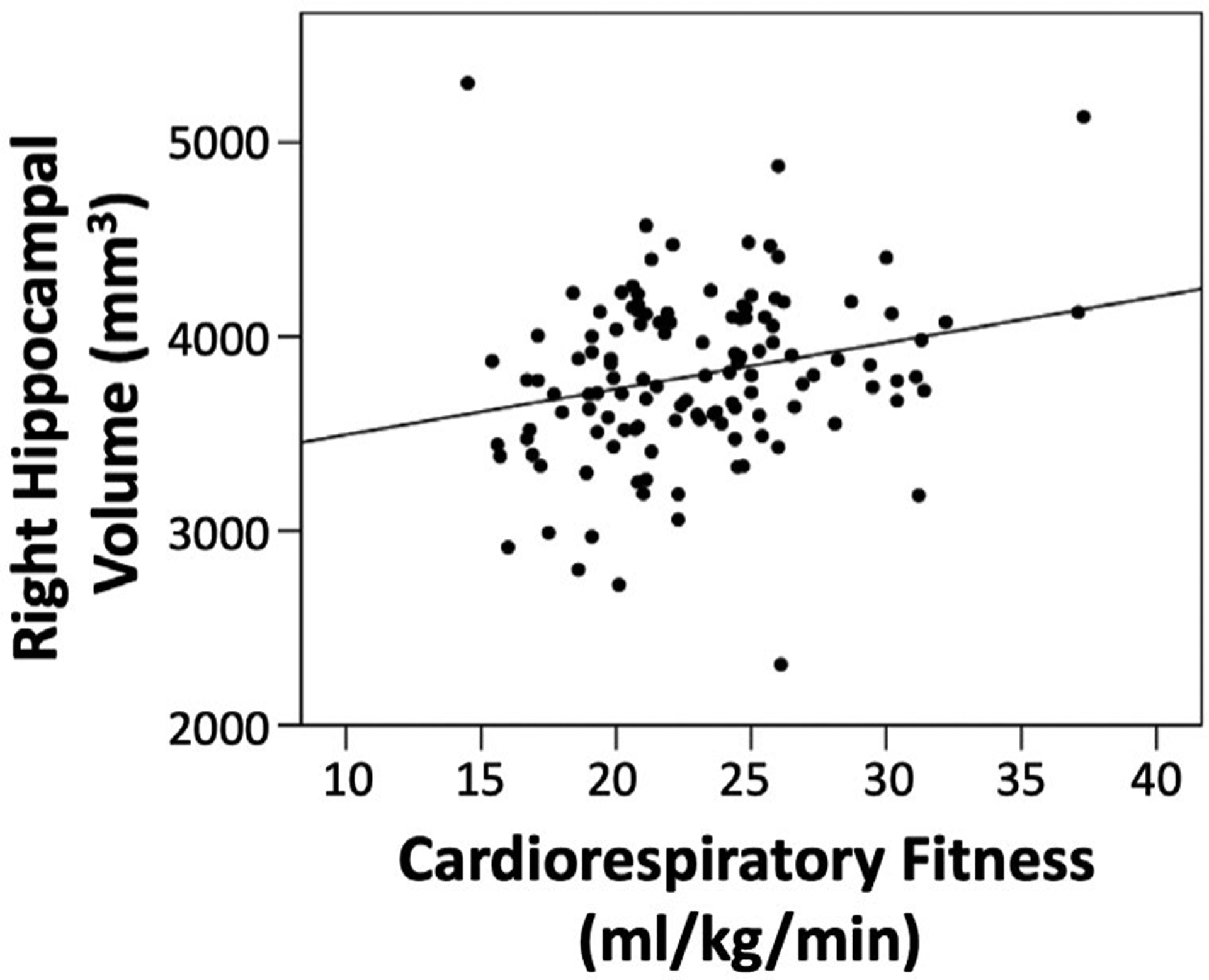
Scatter plot of CRF by right hippocampal volume. After accounting for sex, intracranial volume, and adiposity, the relationship between CRF and right hippocampal volume was not significant (p = .694)
When the predictor variables were entered in the reverse order, the associations remained unchanged such that the introduction of CRF into the model significantly improved prediction of left hippocampal volume above and beyond sex, intracranial volume, and adiposity (Fchange(1,119) = 4.761, p = .031). Furthermore, the interaction between adiposity and CRF on left (F(5,118) = 11.631, p = .910) and right (F(5,118) = 11.216, p = .533) hippocampal volume was not significant.
CRF was significantly associated with both anterior and posterior left hippocampal volume, as depicted in Table 3. Specifically, after controlling for sex and intracranial volume, higher CRF was significantly associated with greater left anterior (β = .175, p = .049) and left posterior (β = .231, p = .013) hippocampal volume. Adiposity was not significantly associated with either anterior or posterior hippocampal volumes (left anterior: β = −.023, p = .839; left posterior: β = .066, p = .569; right anterior: β = −.059, p = .593; right posterior: β < .001, p = .999). When all predictors were included in step three, CRF was significantly related to left posterior hippocampal volume (β = .251, p = .012) and marginally related to left anterior hippocampal volume (β = .168, p = .077).
3.3 |. Neither CRF nor adiposity were associated with thalamus or caudate nucleus volume
Consistent with prior research, CRF did not significantly explain thalamus (β = −.027, p = .731) or caudate nucleus (β = −.067, p = .462) volume after controlling for sex and intracranial volume, as shown in Table 4.
TABLE 4.
Hierarchical regression models of CRF and adiposity with thalamus and caudate nucleus volume
| Model | β | p value | R2 | |
|---|---|---|---|---|
| Thalamus volume | ||||
| 1 | Sex | −.061 | .451 | .464 |
| IV | .645 | <.001** | – | |
| 2 | Sex | −.074 | .409 | .465 |
| IV | .648 | <.001** | – | |
| CRF | −.027 | .731 | – | |
| 3 | Sex | −.078 | .461 | .465 |
| IV | .648 | <.001** | – | |
| CRF | −.025 | .767 | – | |
| Adiposity | .007 | .945 | – | |
| Caudate nucleus volume | ||||
| 1 | Sex | .221 | .020* | .284 |
| IV | .627 | <.001** | ||
| 2 | Sex | .189 | .069 | .288 |
| IV | .634 | <.001** | ||
| CRF | −.067 | .462 | – | |
| 3 | Sex | .115 | .342 | .296 |
| IV | .631 | <.001** | ||
| CRF | −.027 | .783 | – | |
| Adiposity | .135 | .241 | – | |
Notes: Sex = male: 1, female: 2; IV = intracranial volume; CRF = cardiorespiratory fitness.
Statistical significance at the .05 level.
Statistical significance at the .01 level.
Furthermore, adiposity was not significantly associated with either thalamus (β = .007, p = .945) or caudate nucleus (β = .135, p = .241) volumes.
3.4 |. CRF was associated with the shape of the hippocampus, but not the shape of the thalamus or caudate nucleus
The selective association between CRF and the left hippocampus was further evaluated via a shape analysis. We found that CRF was significantly related to outward shape differences of the left hippocampus, but not the right. Significant associations were apparent along the surface of the subiculum and CA1 regions of the left hippocampus, as depicted in Figure 4.
FIGURE 4.

Mapping of significant left hippocampal shape differences related to CRF. Significant differences at each point are displayed using a significance-corrected 1-p value color scale, such that a value of .95 corresponds to p = .05. All analyses controlled for sex and adiposity. Significant outward shape differences were found along the surface of the subiculum and CA1 regions of the left hippocampus. Only the most significant sides of the nuclei are displayed
There was no evidence that CRF was associated with inward shape differences of either the right or the left hippocampus. Adiposity was not significantly associated with shape differences in the hippocampus. Furthermore, neither CRF nor adiposity were significantly associated with shape differences in the thalamus or caudate nucleus.
4 |. DISCUSSION
Prior research suggests that CRF plays a more important role in cardiovascular health than adiposity (Barry et al., 2014; Fogelholm, 2010; Hainer et al., 2009). Based on this literature, we predicted that CRF would explain significantly more individual variability in hippocampal structure than adiposity. Consistent with our expectations, higher CRF, but not adiposity, was significantly associated with greater left hippocampal volume, with outward shape differences occurring along the surface of the subiculum and CA1 regions of the left hippocampus. These results are consistent with prior literature demonstrating an association between higher CRF and greater preservation of the anterior and subiculum sections of the hippocampus (Erickson et al., 2011; Stillman et al., 2018; Varma et al., 2016). These results may have implications for patient populations, such as those with major depressive disorder, schizophrenia, and Alzheimer’s disease, since studies have revealed reduced hippocampal volume among these groups (Maller et al., 2012; Wang et al., 2003). For example, inward surface deformations along the left hippocampus in cognitively normal individuals has been shown to be an early predictor of Alzheimer’s disease (Csernansky et al., 2005). Our results may suggest that higher CRF might reduce the risk for hippocampal volume loss typical of major depressive disorder, schizophrenia, and Alzheimer’s disease, with measurable differences even as early as midlife. Yet, research in these populations is necessary for making definitive conclusions.
CRF is a physiological measure, but it is modifiable through engagement in regular aerobic exercise. Thus, studies of aerobic exercise might describe possible mechanisms for associations between CRF and hippocampal structure. Recent evidence suggests that inflammatory cytokines may be an important pathway. Habitual exercise creates an anti-inflammatory environment by inducing long-term changes in circulating cytokines (Petersen & Pedersen, 2005). It is thought that higher CRF as a result of exercise may contribute to the cytokine response. Specifically, higher CRF has been associated with lower circulating levels of cytokines known to have pro-inflammatory actions, such as interleukin (IL) -6, and greater circulating levels of anti-inflammatory cytokines, such as IL-10 (Kullo, Khaleghi, & Hensrud, 2007; Wedell-Neergaard et al., 2018). Animal research shows an association between increased IL-6 levels in the periphery and greater levels of cytokines in the hippocampus, where the highest levels of IL-6 and IL-6 receptors are detected (Gadient & Otten, 1994). Increased IL-6 levels in the hippocampus interferes with long-term potentiation, neurogenesis, and neural plasticity (Heyser, Masliah, Samimi, Campbell, & Gold, 1997). In middle-aged adults, higher peripheral levels of IL-6 were associated with reduced left hippocampal volume (Marsland, Gianaros, Abramowitch, Manuck, & Hariri, 2008). This relationship may be even more evident in populations with overweight/obesity, as higher BMI is associated with increased IL-6 levels (Khaodhiar, Ling, Blackburn, & Bistrian, 2004). Thus, higher CRF may be associated with reduced pro-inflammatory cytokines and elevated anti-inflammatory cytokines, thereby stimulating hippocampal plasticity. However, this study does not include a measurement of inflammation, allowing only speculation as to whether this could be one of the underlying mechanisms.
In addition to inflammation, CRF and adiposity might influence the hippocampus via other cellular and molecular pathways. Obesity impairs insulin signaling and decreases mitochondrial efficiency, which reduces metabolic function (Stranahan, 2015). These cellular and molecular effects evoke a series of signaling cascades that are deleterious to the structural integrity of the brain (Stranahan, 2015). In contrast, higher CRF is associated with greater peripheral insulin sensitivity and mitochondrial oxidative capacity, two cellular factors thought to mediate metabolic flexibility (Lalia et al., 2016). In parallel, elevated metabolic flexibility has been found during exercise within the context of obesity (Goodpaster & Sparks, 2017). These cellular and molecular effects increase neural plasticity and promote the structural integrity of the brain (Colcombe et al., 2004). Thus, opposing alterations in energy metabolism appear to be a potential mechanism regulating plasticity at the neural level. The significant association between higher CRF and hippocampal structure in our sedentary sample suggests that these cellular and molecular mechanisms may be at play even at low levels of habitual activity.
Secondary analyses revealed regional specificity of the associations, such that CRF was associated with hippocampal structure but not thalamus or caudate nucleus structure. This is consistent with previous research demonstrating an increase in volume of the hippocampus, but not the thalamus or the caudate nucleus in the context of an exercise intervention of older adults (Erickson et al., 2011). Cross-sectional studies have also found that the volume of the hippocampus, but not the caudate nucleus or thalamus, are sensitive to CRF (Erickson et al., 2011; Ortega et al., 2019; Stillman et al., 2018). Reasons for the regional specificity of these associations are generally unknown, but these results suggest that the hippocampus is uniquely sensitive to individual variability in CRF. Regional specificity of the associations might be explained by the particular cellular and molecular milieu of the hippocampus that is highly sensitive to environmental enrichment and stimulation.
Although our results replicate previous research showing an association between CRF and hippocampal morphology, there was no indication of a significant association between the hippocampus and adiposity. Most previous research linking adiposity to hippocampal volume has used BMI measures rather than measures assessing body composition (DXA), and has found greater BMI to be associated with decreased hippocampal volume (Raji et al., 2010; Taki et al., 2008). However, these prior studies did not measure or account for CRF levels, limiting the ability to make inferences about the independent association of adiposity with hippocampal volume. We found CRF and adiposity to have 33.9% shared variance (r = −.582), suggesting that previous studies may have been partially detecting the effect of CRF rather than that of adiposity. Given that our sample consisted of relatively sedentary adults, it is possible that adiposity may be differently associated with the hippocampus among habitually active adults. Nevertheless, we found that objectively measured adiposity was unrelated to the volume and shape of the hippocampus even without including CRF in the model. In an exploratory analysis, we also failed to find an association of hippocampal volume with BMI or fat mass from the DXA assessment (p > .10). It is also possible that the effect sizes for associations of BMI or body composition with hippocampal volume are much smaller than that of CRF due to CRF counterbalancing the detrimental effects of adiposity. Finally, we cannot conclude that BMI or body composition would not have different effects at different age ranges or at different adiposity levels.
Our results were specific to the left hippocampus; the explanation for these laterality differences is unclear. Previous studies examining the relationship between CRF and hippocampal volume have often aggregated results across hemispheres, limiting the ability to detect, or report laterality differences (Boots et al., 2015; Jonasson et al., 2016; Maass et al., 2015; Morris et al., 2017; Szabo et al., 2011; Varma et al., 2016). However, one study that did examine laterality differences found higher CRF in children with overweight/obesity to be associated with greater hippocampal volume in the left hemisphere (Esteban-Cornejo et al., 2017). Furthermore, a few exercise interventions have examined hemispheric differences and reported greater associations with the left hippocampus, which is consistent with our results (Erickson et al., 2011; Firth et al., 2018; Niemann et al., 2014). One potential factor contributing to the emergence of such a pattern may be asymmetry in neuronal connections (Shipton et al., 2014). Specifically, rodent studies have found that the ability to induce long-term potentiation differs across the left and right hippocampus (Kohl et al., 2011). In particular, the left CA3 axons are better able to induce long-term potentiation than the right CA3 axons, generating differences in the size of CA1 pyramidal cell synapses between hemispheres (Kohl et al., 2011; Shinohara et al., 2008). Therefore, although exercise increases neuronal survival, synaptic development, and plasticity, the fundamental characteristics of axonal projections to the left and right hippocampus may be influencing their amenability for growth. However, this is only speculative, highlighting the need for future research to examine laterality effects in the context of plausible mechanisms.
The results of the current study should be interpreted in the context of several limitations. As with all cross-sectional studies, it is not possible to draw causal relationships from these findings. However, our results are consistent with those of longitudinal studies and randomized controlled trials that have found exercise-related changes in hippocampal volume (Erickson et al., 2010, 2011; Niemann et al., 2014). In addition, this study does not include a measurement of mechanisms, allowing only speculation as to the underlying causes of the observed associations. Furthermore, the particular imaging sequence does not allow us to reliably quantify the amount and shape of the ammonic subfields of the hippocampus. Thus, we are unable to make inferences regarding the morphology of the inner subfields of the hippocampus, such as the dentate gyrus. Additionally, measures of memory were not collected, limiting our ability to examine the impact these results have on associated memory function. Moreover, although VO2submax and DXA are highly reliable and valid measures, there are other measures of CRF and adiposity that may capture different components of these constructs (e.g., hydrostatic weighing). Furthermore, CRF is a multifactorial physiological construct that is influenced by a variety of factors, including exercise habits and genetics (Newson & Kemps, 2006). It will be important for future studies to determine whether the importance of CRF can be explained by genetic factors or habitual participation in aerobic activity.
There are also a number of limitations in terms of our sample. First, our inclusion of sedentary individuals with overweight or obesity limits our ability to examine whether differences in hippocampal morphology are apparent in the lower––normal range of adiposity or among habitually active adults. Second, the small proportion of men in this study limits our ability to examine sex differences, which moderates the association between CRF and cognition (Barha, Davis, Falck, Nagamatsu, & Liu-Ambrose, 2017). Yet, our large sample allows us to characterize these associations in midlife. This is especially important, as previous studies are mostly comprised of older adults despite the 56% increase in risk for mortality associated with midlife obesity (Janssen & Bacon, 2008).
Despite these limitations, we used sensitive measures of body composition and CRF, as well as advanced neuroimaging techniques, to examine the importance of fitness over body fat in the context of hippocampal structure. For the first time, we extend the fitness versus body fat debate to brain health and demonstrate that higher CRF, but not adiposity, is associated with greater volume and outward shape differences along the surface of the subiculum and CA1 regions of the left hippocampus. These results have public health implications, highlighting the importance of exercise interventions that promote increases in CRF within the context of obesity to reduce risk for decreased hippocampal volume typical of many different disorders. However, given that the observed results only account for a small portion of the variance in hippocampal volume, many other genetic and environmental factors are likely influencing hippocampal morphology. Future research would benefit from examining these results among a diverse age range to ascertain whether the importance of CRF and adiposity shifts across the life span.
Funding information
This research was supported by grants awarded from the National Institutes of Health to SLA (T32GM081760), KIE (R01 DK095172), JMJ (R01-HL103646), and University of Pittsburgh Clinical and Translational Science Institute (CTSI) (UL1 TR001857). Irene Esteban-Cornejo was supported by a grant from the Alicia Koplowitz Foundation
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Abe T, Thiebaud RS, Loenneke JP, & Young KC (2015). Prediction and validation of DXA-derived appendicular lean soft tissue mass by ultrasound in older adults. Age (Dordrecht, Netherlands), 37(6), 114. 10.1007/s11357-015-9853-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Andělová M, Pfister A, Mueller-Lenke N, Traud S, Reinhardt J, … Sprenger T (2015). Subcortical brain segmentation of two dimensional T1-weighted data sets with FMRIB’s Integrated Registration and Segmentation Tool (FIRST). Neuroimage. Clinical, 7, 43–52. 10.1016/j.nicl.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Davis JC, Falck RS, Nagamatsu LS, & Liu-Ambrose T (2017). Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Frontiers in Neuroendocrinology, 46, 71–85. 10.1016/j.yfrne.2017.04.002 [DOI] [PubMed] [Google Scholar]
- Barry VW, Baruth M, Beets MW, Durstine JL, Liu J, & Blair SN (2014). Fitness vs. fatness on all-cause mortality: A meta-analysis. Progress in Cardiovascular Diseases, 56(4), 382–390. 10.1016/j.pcad.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Boots EA, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, … Okonkwo OC (2015). Cardiorespiratory fitness is associated with brain structure, cognition, and mood in a middle-aged cohort at risk for Alzheimer’s disease. Brain Imaging and Behavior, 9(3), 639–649. 10.1007/s11682-014-9325-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, VanPatter M, … Kramer AF (2010). A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Research, 1358, 172–183. 10.1016/j.brainres.2010.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Eadie BD, Kannangara TS, Robillard JM, Shin J, & Titterness AK (2008). Exercising our brains: How physical activity impacts synaptic plasticity in the dentate gyrus. Neuromolecular Medicine, 10(2), 47–58. 10.1007/s12017-008-8033-2 [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, … Elavsky S (2004). Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America, 101(9), 3316–3321. 10.1073/pnas.0400266101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, & Berchtold NC (2002). Exercise: A behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences, 25(6), 295–301. 10.1016/s0166-2236(02)02143-4 [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, & Christie LA (2007). Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends in Neurosciences, 30(9), 464–472. 10.1016/j.tins.2007.06.011 [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Swank J, Miller JP, Gado M, McKeel D, … Morris JC (2005). Preclinical detection of Alzheimer’s disease: Hippocampal shape and volume predict dementia onset in the elderly. Neuroimage, 25(3), 783–792. 10.1016/j.neuroimage.2004.12.036 [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, … Kramer AF (2009). Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus, 19(10), 1030–1039. 10.1002/hipo.20547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, … Kuller LH (2010). Physical activity predicts gray matter volume in late adulthood: The cardiovascular health study. Neurology, 75(16), 1415–1422. 10.1212/WNL.0b013e3181f88359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, … Kramer AF (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences, 108(7), 3017–3022. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban-Cornejo I, Cadenas-Sanchez C, Contreras-Rodriguez O, Verdejo-Roman J, Mora-Gonzalez J, Migueles JH, … Ortega FB (2017). A whole brain volumetric approach in overweight/obese children: Examining the association with different physical fitness components and academic performance. The ActiveBrains Project. Neuroimage, 159, 346–354. 10.1016/j.neuroimage.2017.08.011 [DOI] [PubMed] [Google Scholar]
- Firth J, Stubbs B, Vancampfort D, Schuch F, Lagopoulos J, Rosenbaum S, & Ward PB (2018). Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage, 166, 230–238. 10.1016/j.neuroimage.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Flegal KM, Kit BK, Orpana H, & Graubard BI (2013). Association of all-cause mortality with overweight and obesity using standard body mass index categories. Journal of the American Medical Association, 309(1), 71–82. 10.1001/jama.2012.113905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelholm M (2010). Physical activity, fitness and fatness: Relations to mortality, morbidity and disease risk factors. A systematic review. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity, 11(3), 202–221. 10.1111/j.1467-789X.2009.00653.x [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Fox NC, Jack CR, Scheltens P, & Thompson PM (2010). The clinical use of structural MRI in Alzheimer disease. Nature Reviews Neurology, 6(2), 67–77. 10.1038/nrneurol.2009.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadient RA, & Otten U (1994). Expression of interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) mRNAs in rat brain during post-natal development. Brain Research, 637(1–2), 10–14. 10.1016/0006-8993(94)91211-4 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, & Sparks LM (2017). Metabolic flexibility in health and disease. Cell Metabolism, 25(5), 1027–1036. 10.1016/j.cmet.2017.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainer V, Toplak H, & Stich V (2009). Fat or fit: What is more important? Diabetes Care, 32(Suppl 2), S392–397. 10.2337/dc09-S346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CM, Carroll MD, Fryar CD, & Ogden CL (2017). Prevalence of obesity among adults and youth: United States, 2015–2016. National Center for Health Statistics Data Brief, 288, 1–8. [PubMed] [Google Scholar]
- Heckers S, & Konradi C (2002). Hippocampal neurons in schizophrenia. Journal of Neural Transmission (Vienna, Austria: 1996), 109(5–6), 891–905. 10.1007/s007020200073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, & Nagel BJ (2012). Aerobic fitness relates to learning on a virtual Morris water task and hippocampal volume in adolescents. Behavioural Brain Research, 233(2), 517–525. 10.1016/j.bbr.2012.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Masliah E, Samimi A, Campbell IL, & Gold LH (1997). Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proceedings of the National Academy of Sciences of the United States of America, 94(4), 1500–1505. 10.1073/pnas.94.4.1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, & Hatfield BD (2017). Run for your life! Childhood physical activity effects on brain and cognition. Kinesiology Review, 6(1), 12–21. 10.1123/kr.2016-0034 [DOI] [Google Scholar]
- Janssen I, & Bacon E (2008). Effect of current and midlife obesity status on mortality risk in the elderly. Obesity (Silver Spring, Md.), 16(11), 2504–2509. 10.1038/oby.2008.400 [DOI] [PubMed] [Google Scholar]
- Jonasson LS, Nyberg L, Kramer AF, Lundquist A, Riklund K, & Boraxbekk CJ (2016). Aerobic exercise intervention, cognitive performance, and brain structure: Results from the physical influences on brain in aging (PHIBRA) study. Frontiers in Aging Neuroscience, 8, 336. 10.3389/fnagi.2016.00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AP, Shea JL, & Sun G (2009). Comparison of the classification of obesity by BMI vs. Dual-energy X-ray absorptiometry in the Newfoundland population. Obesity (Silver Spring, Md.), 17(11), 2094–2099. 10.1038/oby.2009.101 [DOI] [PubMed] [Google Scholar]
- Khaodhiar L, Ling PR, Blackburn GL, & Bistrian BR (2004). Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. Journal of Parenteral and Enteral Nutrition, 28(6), 410–415. 10.1177/0148607104028006410 [DOI] [PubMed] [Google Scholar]
- Kohl MM, Shipton OA, Deacon RM, Rawlins JNP, Deisseroth K, & Paulsen O (2011). Hemisphere-specific optogenetic stimulation reveals left-right asymmetry of hippocampal plasticity. Nature Neuroscience, 14(11), 1413–1415. 10.1038/nn.2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullo IJ, Khaleghi M, & Hensrud DD (2007). Markers of inflammation are inversely associated with VO2 max in asymptomatic men. Journal of Applied Physiology (Bethesda, Md.: 1985), 102(4), 1374–1379. 10.1152/japplphysiol.01028.2006 [DOI] [PubMed] [Google Scholar]
- Lalia AZ, Dasari S, Johnson ML, Robinson MM, Konopka AR, Distelmaier K, … Lanza IR (2016). Predictors of whole-body insulin sensitivity across ages and adiposity in adult humans. The Journal of Clinical Endocrinology and Metabolism, 101(2), 626–634. 10.1210/jc.2015-2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Ge T, Leng Y, Pan Z, Fan J, Yang W, & Cui R (2017). The role of neural plasticity in depression: From hippocampus to prefrontal cortex. Neural Plasticity, 2017, 6871089. 10.1155/2017/6871089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Düzel S, Goerke M, Becke A, Sobieray U, Neumann K, … Düzel E (2015). Vascular hippocampal plasticity after aerobic exercise in older adults. Molecular Psychiatry, 20(5), 585–593. 10.1038/mp.2014.114 [DOI] [PubMed] [Google Scholar]
- Maller JJ, Daskalakis ZJ, Thomson RHS, Daigle M, Barr MS, & Fitzgerald PB (2012). Hippocampal volumetrics in treatment-resistant depression and schizophrenia: The devil’s in detail. Hippocampus, 22(1), 9–16. 10.1002/hipo.20873 [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, & Hariri AR (2008). Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biological Psychiatry, 64(6), 484–490. 10.1016/j.biopsych.2008.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley PA, & Beavers KM (2014). Contribution of cardiorespiratory fitness to the obesity paradox. Progress in Cardiovascular Diseases, 56(4), 434–440. 10.1016/j.pcad.2013.09.006 [DOI] [PubMed] [Google Scholar]
- Miller AA, & Spencer SJ (2014). Obesity and neuroinflammation: A pathway to cognitive impairment. Brain, Behavior, and Immunity, 42, 10–21. 10.1016/j.bbi.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Morris JK, Vidoni ED, Johnson DK, Van Sciver A, Mahnken JD, Honea RA, … Burns JM (2017). Aerobic exercise for Alzheimer’s disease: A randomized controlled pilot trial. Public Library of Science One, 12(2), e0170547. 10.1371/journal.pone.0170547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gómez-Pinilla F, Choi J, & Cotman C (1995). Exercise and brain neurotrophins. Nature, 373(6510), 109. 10.1038/373109a0 [DOI] [PubMed] [Google Scholar]
- Newson RS, & Kemps EB (2006). Cardiorespiratory fitness as a predictor of successful cognitive ageing. Journal of Clinical and Experimental Neuropsychology, 28(6), 949–967. 10.1080/13803390591004356 [DOI] [PubMed] [Google Scholar]
- Niemann C, Godde B, & Voelcker-Rehage C (2014). Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Frontiers in Aging Neuroscience, 6, 170. 10.3389/fnagi.2014.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega FB, Campos D, Cadenas-Sanchez C, Altmäe S, Martínez-Zaldívar C, Martín-Matillas M, … Campoy C (2019). Physical fitness and shapes of subcortical brain structures in children. The British Journal of Nutrition, 122(s1), S49–S58. 10.1017/S0007114516001239 [DOI] [PubMed] [Google Scholar]
- Pajonk F-G, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, … Falkai P (2010). Hippocampal plasticity in response to exercise in schizophrenia. Archives of General Psychiatry, 67(2), 133–143. 10.1001/archgenpsychiatry.2009.193 [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, & Jenkinson M (2011). A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage, 56(3), 907–922. 10.1016/j.neuroimage.2011.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AMW, & Pedersen BK (2005). The anti-inflammatory effect of exercise. Journal of Applied Physiology (Bethesda, Md.: 1985), 98(4), 1154–1162. 10.1152/japplphysiol.00164.2004 [DOI] [PubMed] [Google Scholar]
- Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, … Thompson PM (2010). Brain structure and obesity. Human Brain Mapping, 31(3), 353–364. 10.1002/hbm.20870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, & Acker JD (2005). Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex (New York, N.Y.: 1991), 15(11), 1676–1689. 10.1093/cercor/bhi044 [DOI] [PubMed] [Google Scholar]
- Rogers RJ, Schelbert EB, Lang W, Fridman Y, Yuan N, & Jakicic JM (2020). Association of fitness and body fatness with left ventricular mass: The heart health study. Obesity Science & Practice, 6(1), 19–27. 10.1002/osp4.380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Bailey KR, Collazo-Clavell ML, & Lopez-Jimenez F (2008). Accuracy of body mass index to diagnose obesity in the US adult population. International Journal of Obesity (2005), 32(6), 959–966. 10.1038/ijo.2008.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara Y, Hirase H, Watanabe M, Itakura M, Takahashi M, & Shigemoto R (2008). Left-right asymmetry of the hippocampal synapses with differential subunit allocation of glutamate receptors. Proceedings of the National Academy of Sciences of the United States of America, 105(49), 19498–19503. 10.1073/pnas.0807461105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton OA, El-Gaby M, Apergis-Schoute J, Deisseroth K, Bannerman DM, Paulsen O, & Kohl MM (2014). Left–right dissociation of hippocampal memory processes in mice. Proceedings of the National Academy of Sciences, 111(42), 15238–15243. 10.1073/pnas.1405648111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicotte NL, Kern KC, Giesser BS, Arshanapalli A, Schultz A, Montag M, … Bookheimer SY (2008). Regional hippocampal atrophy in multiple sclerosis. Brain: A Journal of Neurology, 131(Pt 4), 1134–1141. 10.1093/brain/awn030 [DOI] [PubMed] [Google Scholar]
- Spielman LJ, Little JP, & Klegeris A (2014). Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration. Journal of Neuroimmunology, 273(1–2), 8–21. 10.1016/j.jneuroim.2014.06.004 [DOI] [PubMed] [Google Scholar]
- Stillman CM, Uyar F, Huang H, Grove GA, Watt JC, Wollam ME, & Erickson KI (2018). Cardiorespiratory fitness is associated with enhanced hippocampal functional connectivity in healthy young adults. Hippocampus, 28(3), 239–247. 10.1002/hipo.22827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM (2015). Models and mechanisms for hippocampal dysfunction in obesity and diabetes. Neuroscience, 309, 125–139. 10.1016/j.neuroscience.2015.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo AN, McAuley E, Erickson KI, Voss M, Prakash RS, Mailey EL, … Kramer AF (2011). Cardiorespiratory fitness, hippocampal volume, and frequency of forgetting in older adults. Neuropsychology, 25(5), 545–553. 10.1037/a0022733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K, … Fukuda H (2008). Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring, Md.), 16(1), 119–124. 10.1038/oby.2007.4 [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, & Gage FH (2005). Exercise enhances learning and hippocampal neurogenesis in aged mice. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(38), 8680–8685. 10.1523/JNEUROSCI.1731-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma VR, Tang X, & Carlson MC (2016). Hippocampal sub-regional shape and physical activity in older adults. Hippocampus, 26(8), 1051–1060. 10.1002/hipo.22586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, & Gomez-Pinilla F (2004). Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. The European Journal of Neuroscience, 20(10), 2580–2590. 10.1111/j.1460-9568.2004.03720.x [DOI] [PubMed] [Google Scholar]
- Wang L, Swank JS, Glick IE, Gado MH, Miller MI, Morris JC, & Csernansky JG (2003). Changes in hippocampal volume and shape across time distinguish dementia of the Alzheimer type from healthy aging. Neuroimage, 20(2), 667–682. 10.1016/S1053-8119(03)00361-6 [DOI] [PubMed] [Google Scholar]
- Wedell-Neergaard AS, Krogh-Madsen R, Petersen GL, Hansen ÅM, Pedersen BK, Lund R, & Bruunsgaard H (2018). Cardiorespiratory fitness and the metabolic syndrome: Roles of inflammation and abdominal obesity. Public Library of Science One, 13(3), e0194991. 10.1371/journal.pone.0194991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise CM, Thiyyagura P, Reiman EM, Chen K, & Krakoff J (2013). Fat-free body mass but not fat mass is associated with reduced gray matter volume of cortical brain regions implicated in autonomic and homeostatic regulation. Neuroimage, 64, 712–721. 10.1016/j.neuroimage.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]


