Abstract
T lymphoma cells may constitutively express PD-1 and PD-L1. The relative role of PD-1 and PD-L1 in T lymphoma is incompletely understood. We report here that PD-1+ PDL-1+ human T lymphoma cells exhibit constitutive hyperactivation of the TCR signaling and do not respond to PD-L1-mediated suppression in vitro. Knocking out PD-1 or PD-L1 has no effects on T lymphoma cell apoptosis and proliferation in vitro, but significantly increased tumor-bearing mouse survival. Our findings determine that the constitutively active TCR signaling pathway maintain T lymphoma cell growth in vitro and that both PD-1 and PD-L1 promote T lymphoma growth in vivo.
Keywords: PD-1, PD-L1, T lymphoma, T cell receptor
Introduction
PD-1 is up-regulated by the TCR signaling pathway during T cell activation [1]. Programmed cell death 1 ligand 1 (PD-L1) is the physiological ligand of PD-1. PD-L1 is expressed on various types of cells, including antigen-presenting cells and tumor cells [2]. The normal function of PD-1 is to terminate T cell function after an immune response to prevent autoimmunity [3]. Binding of PD-1 by PD-L1 activates the PD-1 receptor which recruits and phosphorylates SHP2. Once phosphorylated, SHP2 is activated and then de-phosphorylates ZAP70 and CD28, resulting in inhibition of T cell activation [1, 4]. Therefore, the TCR, PD-1 receptor and its physiological ligand PD-L1 work in concert to ensure a controlled immune response that eliminates infected cells while preventing autoimmunity. Tumor cells escape from host cancer immune surveillance by expressing PD-L1 [5]. Based on this phenomenon, blocking antibodies that neutralize the PD-L1 and PD-1 interaction between T cells and tumor cells have been developed and approved for human cancer immunotherapy [6].
T lymphoma cells originate from T cells that express PD-1 and thus can be considered as deregulated T cells [7-9]. In a rare case, it was reported that PD-1 inhibitor immunotherapy causes clonal proliferation of abnormal T-cell clone leading to T-cell lymphoma [10]. Clinically, blockade of PD-L1 binding to PD-1 with a PD-1 or PD-L1 monoclonal antibody has shown a remarkable overall response rate in patients with relapsed or refractory lymphoma, leading to the approval of PD-1 blockade monoclonal antibodies for human lymphoma immunotherapy[6]. However, PD-1 is activated by TCR signaling [1] and may acts as a suppressor of T cell lymphomas under certain cellular conditions [7], blocking PD-1 may promotes T lymphoma growth, which may offset PD-1 blockade immunotherapy efficacy. We therefore aimed at determining the relative roles of PD-1 and PD-L1 in T lymphoma growth and pathogenesis. Our data indicate that PD-1 suppressive function is impaired in T cell lymphoma in vitro, but contrary to reported function of PD-1 as a T lymphoma suppressor, Both PD-1 and PD-L1 promote PD-1+PD-L1+ T lymphoma growth in syngeneic immune competent mice in vivo.
Materials and Methods
Cells:
Human T lymphoma cell line HPB-ALL and mouse lymphoma cell line EL4 were obtained from American Type Culture Collection (ATCC, Manassas, VA). 293FT cell line was obtained from ThermoFisher Scientific (Grand Island, NY). Normal human peripheral blood mononuclear cells (PBMC) were obtained from consented healthy donors in Augusta Shepard Community bank. All studies with human samples were approved by Augusta University Institutional Review Board.
Mice:
C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME). All mice used in this study were female and between 2–3 months old. All studies with mice were approved by Augusta University Institutional Animal Care and Use Committee (Protocol #: 2008-0162). EL4 tumor cells were injected intraperitoneally into C57BL/6 mice (2x105 cells/mouse). Mice were monitored for survival.
T cell purification:
CD3 positive T cells were purified from human PBMCs using the MojoSort Human CD3 T Cell Isolation Kit (Biolegend, San Diego, CA) according to the manufacturer’s protocol.
T cell stimulation:
For stimulation of T cells with anti-CD3 and anti-CD28 antibodies, tissue culture plates were coated with anti-CD3 (Clone OKT3, Biolegend, 8 μg/mL) and anti-CD28 (clone 28.2, Biolegend, 10 μg/mL) antibodies at 4°C overnight. Purified T cells were cultured in the antibody-coated plates. For stimulation with PMA (Phorbol 12-Myristate 13-Acetate, 25 ng/mL) and Ionomycin, purified T cells were cultured in uncoated plates. PMA (Sigma, St Luis, MO, 25 ng/ml) and Ionomycin (Sigma, 1 μM) were added directly to the culture media.
Cell proliferation assays:
Purified T cells were labeled with CFSE tracking dye (Invitrogen, CA) according to the manufacturer’s instructions. The labeled cells were cultured in anti-CD3 and anti-CD28-coated plates as described above. For PD-L1 protein inhibition of T cells and T lymphoma cells, tissue culture plates were coated with anti-CD3 (0.8 μg/ml) + anti-CD28 (1 μg/ml), anti-CD3+ anti-CD28+ IgG (82 μg/ml), or anti-CD3+anti-CD28+PD-L1 recombinant protein (R&D System, 82 μg/ml) at 4°C overnight. Purified T cells and HPB-ALL lymphoma cells were then cultured in the coated plates for 3 days. Cells were collected and analyzed by flow cytometry for CFSE intensity. Cell proliferation index was calculated as previously described [11].
Generation of PD-1 and PD-L1 knock out cells:
293FT cells were co-transfected with pCMV-VSV-G (Addgene #8454), psPAX2 (Addgene #12260) and lentiCRISPRv2 vector containing specific sgRNA-coding sequence (Genscript Inc., Piscataway, NJ) using Lipofectamine 2000 (Life Technologies, Carlsbad, CA). Scramble sgRNA sequence is 5’-CTCGTATCTTTTCCCACGGC-3’. The Pdcd1 sgRNA sequence is 5’-CGGAGGATCTTATGCTGAAC-3’. The Cd274 sgRNA sequence is 5’-CCAAAGGACTTGTAACGTGG-3. After forty-eight hours, lentiviral particles were harvested and used to transduce EL4 cells. Successful transductions were enriched by selection with puromycin (5 μg/ml, Santa Cruz Biotechnology, Dallas, TX) and flow sorting for cells lacking the marker of interest.
Apoptosis analysis:
Cells (1x105 cells/well in 96-well plate) were cultured for 24h, harvested, and stained Annexin V (Biolegend, San Diego, CA) in 4°C for 30 min, followed by staining with Propidium Iodide (PI, 1 μg/100μl) for 10 min. Cells were then analyzed by flow cytometry. Annexin V+PI+ cells and Annexin V+ cells were quantified using Flowjo program (BD Biosciences, San Diego, CA).
Cell cycle analysis:
EL4 cells (1x105 cells/well in 24-well plate) were allowed to grow logarithmically for 36 hours. Cells were harvested, fixed in 70% ethanol, washed, and treated with RNase A (100 μg/ml). The cells were then stained in 400 μl PI (50mg/ml) for 30 min and analyzed by flow cytometry. DNA content was calculated by analyzing PI intensity using Flowjo (BD Biosciences, San Diego, CA) to determine cell cycle.
Flow cytometry:
Fluorescent dye-conjugated antibodies that are specific for CD3, CD4, CD8, PD-1, and PD-L1, respectively were obtained from BioLegend. Cells were stained with the antibodies either alone or in combinations and analyzed by flow cytometry.
Western blotting analysis:
Cells were lysed in total lysis buffer (20mM Hepes, pH 7.4, 100mM NaCl, 10% glycerol, and 1% Triton X-100). Total protein lysates were separated by a 4-20% SDS-polyacrylamide gel electrophoresis and blotted to nylon membranes. The membrane was hybridized with antibodies that are specific for pZAP70, ZAP70 (Abcam, Cambridge, MA), pAKT (S473), pAKT (T308), and AKT (Cell Signaling Tech, Danvers, MA).
Statistical analysis:
The student t test was used to determine the difference between two treatments. Mouse survival differences were calculated by Logrank.
Results
The T cell receptor signaling pathway is constitutively active in T human lymphoma
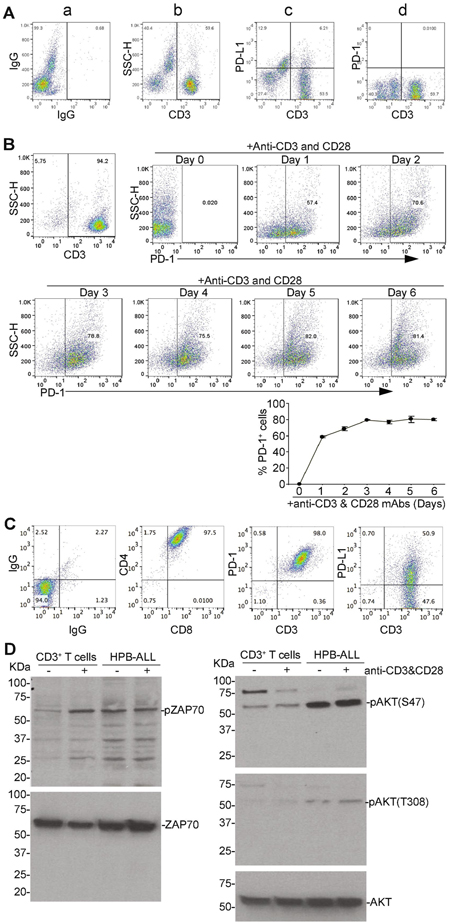
Human T cells were used as a non-neoplastic control cells for human T lymphoma cells. To determine PD-1 expression profiles in human T cells, purified CD3+ human T cells were analyzed for PD-1 and PD-L1 expression levels. A small portion of CD3+ T cells are PD-L1+ and almost all CD3+ T cells are PD-1− before stimulation (Fig. 1A). Stimulation with anti-CD3 and anti-CD28 antibodies rapidly increased PD-1+ cells. The percentage of PD-1+ cells plateaued about 3 days after stimulation (Fig. 1B).
Figure 1. The TCR signaling pathway is hyperactive in the PD-1+PD-L1+ human T lymphoma cells in vitro.
A. Human peripheral blood mononuclear cells (PBMC) were stained with IgG siotype control, and CD3−, PD-L1−, and PD-1-specific antibodies and analyzed by flow cytometry. Shown are representative plots of IgG (a), CD3+ T cells (b), CD3+ PD-L1+ (c), and CD3+ PD-1+ (d) cells. B. CD3+ T cells were purified from human PBMC as shown in A (top left panel) and stimulated with anti-CD3 and CD28 antibodies. Cells were collected at the indicated time points and stained with PD-1-specific antibodies. PD-1+ cells were quantified by analysis with flow cytometry. Shown are representative dot plots of PD-1 expression in purified CD3+ T cells (top right and middle panels). The PD-1+ cells were quantified and prewnted at the bottom panel. Shown are one representative experiments of three independent experiments. C. Human T lymphoma cell line HPB-ALL were stained with either IgG or CD4−, CD8−, CD3−, PD-1, and PD-L1-specific antibodies and analyzed by flow cytometry. Shown are representative plots of the phenotypes. D. Purified human CD3+ T cells and HPB-ALL cells were stimulated with anti-CD3 and anti-CD28 antibodies and analyzed by Western blotting for antibodies that are specific for phosphorylated ZAP70 (pZAP70) and AKTs [pAKT(S473), pAKT(T308)]. Total ZAP70 and AKT are used as normalization control.
T lymphoma cells express PD-L1 [3]. We analyzed T cell markers and PD-L1 and PD-1 expression levels on T lymphoma HPB-ALL cells. HPB-ALL cells are a patient-derived T-ALL cell line that exhibits a CD4+CD8+ immature T cell phenotype (Fig. 1C). In standard cell culture conditions, all of HPB-ALL cells are PD-1+ and approximately 50% of HPB-ALL cells are PD-L1+ (Fig. 1C). Because PD-1 expression is a hallmark of activated T cells, the above finding that all HPB-ALL cells are PD-1+ cells suggest that HPB-ALL lymphoma cells may mimic a constitutively active human T cell population. To test this hypothesis, we performed Western blotting analysis of total proteins from unstimulated and anti-CD3 + anti-CD28 antibody-stimulated HPB-ALL. Purified human CD3+ T cells were included as normal T cell controls. ZAP70 and AKT phosphorylation are considered as indicators of T cell receptor signaling pathway activation, which are the initial steps of T cell activation after TCR engagement [12]. As expected, stimulation of normal human T cells increased ZAP70 phosphorylation (pZAP70). Phosphorylation level of AKT at position serine 473 [pAKT (S473)] is increased after stimulation in the normal T cells. AKT phosphorylation in threonine 308 [pAKT(T308)] is weak in both unstimulated and stimulated normal human T cells (Fig. 1D). In contrast, the levels of pZAP70, pAKT (S473) and pAKT (T308) are all high in unstimulated and stimulated HPB-ALL cells (Fig. 1D). These findings indicate that human T lymphoma are constitutively activated T cells.
The PD-1 repressive signaling pathway is impaired in T lymphoma cells
PD-1 is a repressive receptor of T cells. The principal ligand for PD-1 is PD-L1 [3]. When PD-L1 binds to PD-1 on the T cell surface, T cell division is repressed [4]. Our above findings indicate that both PD-1 and PD-L1 are expressed on human T lymphoma surface (Fig. 1C). Therefore, PD-L1 expressed on T lymphoma cells surface may bind to PD-1 on another T lymphoma cell in trans to inhibit that T lymphoma cell growth. However, PD-L1+PD-1+ T lymphoma cells grow rapidly as a cell line. We therefore hypothesized that the PD-1 signaling pathway on human T lymphoma cells is impaired in vitro. To test this hypothesis, we coated tissue culture plates with anti-CD3+CD28, anti-CD3+CD28+IgG, and anti-CD3+CD28+PD-L1 protein. Purified human CD3+ T cells and human T lymphoma HPB-ALL cells were then cultured in the tissue culture plates coated as described above. As expected, normal T cells proliferate well in the control plates coated with anti-CD3 and anti-CD28 antibodies without PD-L1 protein (Fig. 2A: a1 & a2). PD-L1 protein significantly inhibited normal T cell proliferation (Fig. 2Aa3 & B). In contrast, the proliferation rates of human T lymphoma cells were not altered by PD-L1 protein (Fig. 2Ab3 & C).
Figure 2. The PD-1 signaling pathway is impaired in human T lymphoma cells in vitro.
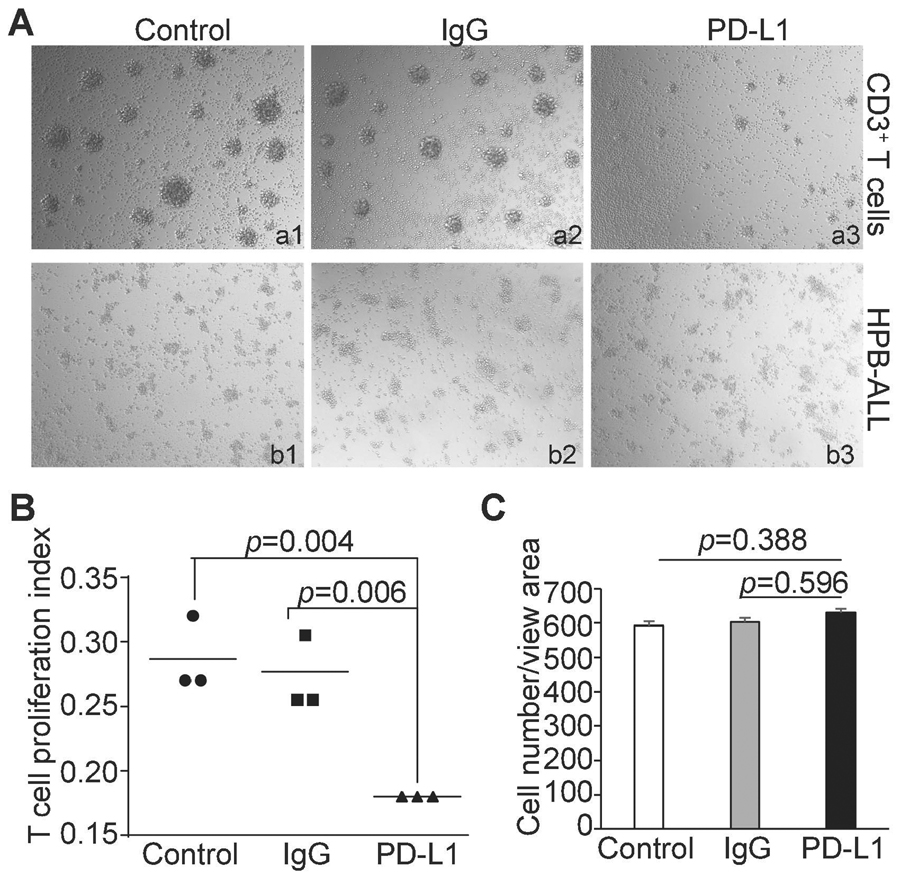
A. CD3+ T (a1-a3) were purified from human PBMC. The purified human CD3+ T cells and HPB-ALL (b1-b3) cells were labeled with CFSE tracking dye and cultured in control (a1 & b1), IgG-(a2 & b2), and PD-L1 (a3 & b3) protein-coated plates for 3 days. Shown are morphologies of cells under the three culture conditions. B. Purified CD3+ human T cells as shown in a1-a3 were analyzed by flow cytometry for CFSE intensity. T cell division index is defined as the average of cell division times as determined by the CFSE intensity as previousely described. C. HPB-ALL cells as shown in b1-b3 were quantified by cell counts.
The TCR signaling pathway negatively regulates PD-L1 expression in T lymphoma
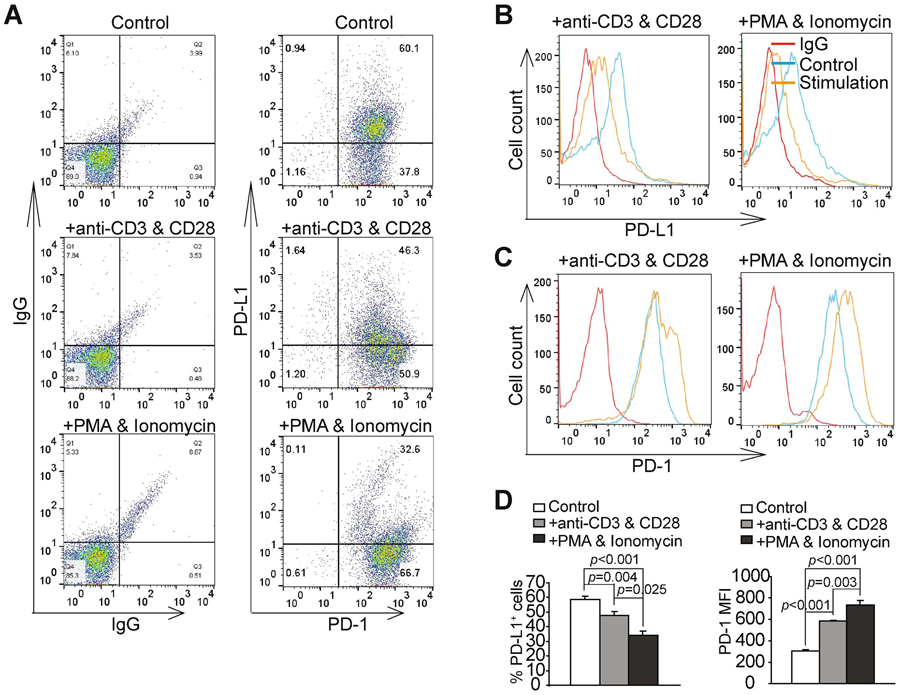
PD-1 expression in human T cells is coupled to and regulated by the TCR signaling pathway (Fig. 1). Given that PD-1 is highly expressed on human T lymphoma HPB-ALL cells (Fig. 1C), We then sought to determine whether PD-1 could be further increased by activating the TCR signaling pathway in human T lymphoma cells. Two methods were used to activate the TCR signaling pathway: stimulation with anti-CD3 and CD28 antibodies, or by stimulation with PMA and ionomycin. Analysis of PD-1 protein levels indicates that stimulation with either anti-CD3 and CD28 or PMA and ionomycin both significantly increased PD-1 protein levels on HPB-ALL cell surface (Fig. 3A & B). Stimulation with PMA and ionomycin caused a greater increase in PD-1 protein levels than stimulation with anti-CD3 and anti-CD28 antibodies (Fig. 3D). Surprisingly, stimulation of HPB-ALL cells with anti-CD3 and anti-CD28 or PMA and ionomycin both significantly decreased PD-L1+ cells (Fig. 3A & C). These findings indicate that the TCR signaling pathway activates PD-1, but decreases PD-L1 expression in T lymphoma cells, thereby may play a contrasting role in regulating PD-1 and PD-L1 expression in human T lymphoma growth.
Figure 3. The T cell receptor signaling pathway negatively regulates PD-L1 expression in human T lymphoma.
A. HPB-ALL cells were stimulated with IgG (control), anti-CD3 and anti-CD28, or PMA and ionomycin for three days. Cells were then stained IgG (as negative staining controls), PD-1− and PD-L1-specific antibodies, and analyzed by flow cytometry. Shown are representative plots of IgG, PD-1 and PD-L1 staining. B. PD-L1 MFIs of control and stimulated cells were determined. Shown are representative images of overlay of PD-L1 MFIs of the three cell groups. C. PD-1 MFIs of control and stimulated cells were determined. Shown are representative images of overlay of PD-L1 MFIs of the three cell groups. D. Quantification of PD-L1+ and PD-1+ cells of the three treatment groups. The percentages of PD-L1+ and PD-1+ cells in the control and stimulated cells (n=3) were quantified and analyzed by student t test for statistical significance.
Both PD-1 and PD-L1 promotes T lymphoma growth in vivo
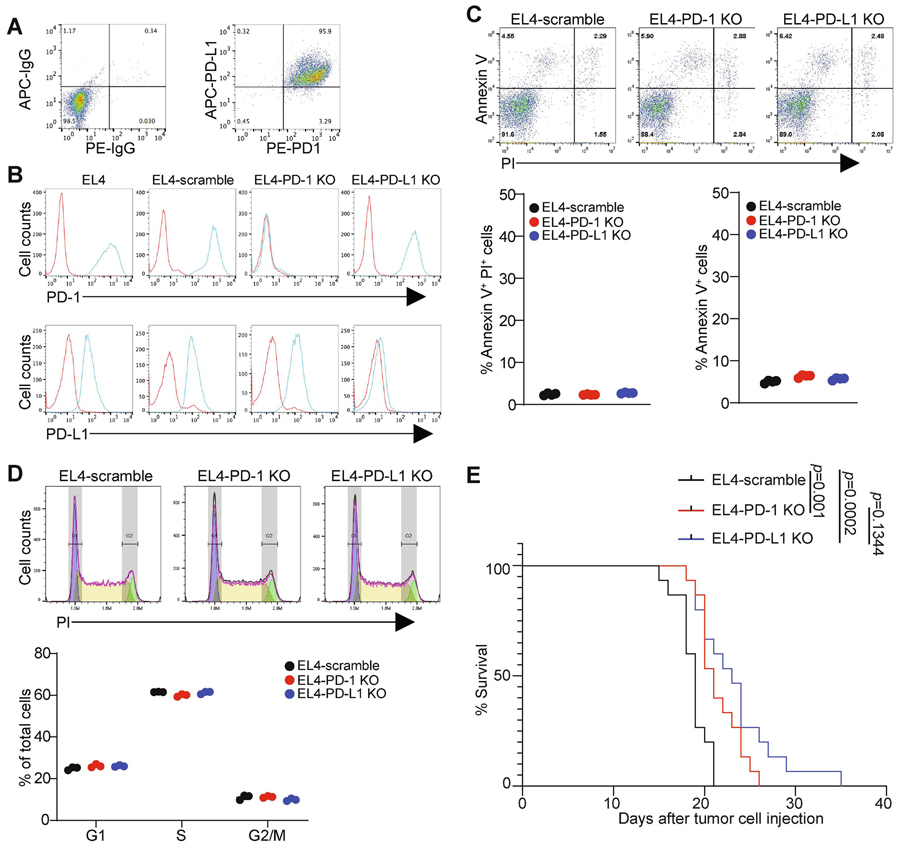
To determine the functions of PD-1 and PD-L1 in T lymphoma development in immune competent host, we made use of the murine T lymphoma cell line EL4. Similar to the human T lymphoma cell line HPB-ALL, EL4 cells constituitively express both PD-1 and PD-L1 (Fig. 4A). CRISPR-based KO approach specifically led to loss of PD-1 and PD-L1, respectively in EL4 cells (Fig. 4B). Knocking out PD-1 or PD-L1 had no significant effects on tumor cell apoptosis (Fig. 4C) or cell cycle in vitro (Fig. 4D). Next, the tumor cells were injected to immune competent syngeneic mice and monitored for survival. Surprisingly, although no growth suppression effect of PD-1 on human T lymphoma cells was observed in vitro, knocking out PD-1 in T lymphoma cells significantly increased tumor-bearing mouse survival. Knocking out PD-L1 also significantly increased the survival time of the tumor-bearing mice (Fig. 5). Taken together, our findings determined that both PD-1 and PD-L1 promote T lymphoma growth in vivo.
Figure 4. Function of PD-1 and PD-L1 in T lymphoma cell growth in vitro.
A. EL4 cells were stained with IgG isotype controls or anti-PD-1 and anti-PD-L1 antibodies and analyzed by flow cytometry. Shown is the representative dot plots of the cellular phenotypes. B. The indicated EL4 cells were stained with anti-PD-1 and anti-PD-L1 mAbs and analyzed by flow cytometry. C. The indicated cells were stained for propidium Iodide (PI) and Annexin V and analyzed by flow cytometry. Shown are representative dot plots. Apoptotic cell death (Annexin V+PI+) and apoptosis (Annexin V+) were quantified. Each circle represents each measurement. Results are representatives of two independent experiments. D. The indicated cells were allowed to grow logarithmically for 36 h. Cells were then fixed and stained by PI and analyzed by flow cytometry for cell cycle. Shown are representative images of cell cycle analysis output for the indicated cells (left panel). The cell cycle phases as shown at the left were quantified. Each circle represents each measurement. Results are representatives of two independent experiments. E. The indicated cells were injected intraperitoneally into C57BL/6 mice (n=15 for each cell lines) Mouse survival was recorded. P value was calculated by logrank.
Discussion
T lymphoma originates from T cells and thus resembles activated T cells in TCR signaling and PD-1 expression [8, 9]. However, T lymphoma cells are no longer normal T cells, but tumor cells [13]. First, unlike normal resting T cells, T lymphoma cells proliferate constitutively without stimulation [14]. Secondly, we found that T lymphoma cells express higher levels of PD-1 and hyperactive TCR signaling as determined by pZAP70 and pAKT level. T lymphoma cells are thus hyperactive T cells. It is known that PD-1 can function as a tumor suppressor in T lymphoma cells in a mouse tumor model [7]. It is therefore striking that T lymphoma cells can express both PD-1 and PD-L1 and yet still proliferate rapidly. As PD-L1 can bind to PD-1 to suppress T cell proliferation, PD-L1 on one T-lymphoma cell should be able to bind to PD-1 on another T lymphoma cell to suppress T lymphoma proliferation [7, 15]. However, this does not happen as we observed that PD-1+PD-L1+ human T lymphoma HPB-ALL cells and PD-1+ PD-L1+ EL4 cells still proliferate rapidly in vitro. Therefore, it is likely that the PD-1 signaling pathway is impaired in PD-1+ PD-L1+ T lymphoma cells in vitro. This notion is supported by our observation that human T lymphoma cells maintain their proliferation rate in PD-L1 protein-coated culture plates while PD-L1 suppressed normal human T cell proliferation in vitro. The mechanism underlying PD-1 dysfunction in vitro in T lymphoma cells also requires further study. However, knocking out PD-1 significantly increased survival of tumor-bearing mice, indicating the PD-1 promotes T lymphoma growth in vivo. It is unknown whether PD-1 also acts as a tumor suppressor in human patients with T lymphoma, but it has been reported that melanoma cell-intrinsic PD-1 promotes tumorigenesis [16]. Similarly, hepatocellular carcinoma cell lines and clinical tissues also contain subpopulations that express PD-1, and HCC cell-intrinsic PD-1 promotes tumor progression [17]. Our finding thus extends PD-1 function as a tumor promoter from solid tumors to hematopoietic T lymphoma.
The U.S. Food and Drug Administration has recently approved an anti-PD-1 antibody immunotherapy (Opdivo) for the treatment of relapsed or progressive Hodgkin B cell lymphoma [18]. This treatment is based on the interruption of PD-1 and PD-L1 by Opdivo to block PD-L1 immunosuppressive function of the B cell lymphoma to boost the host T cell anti-tumor function [19]. However, anti-PD-1 cancer immunotherapy is currently not effective enough for the treatment of human patients with T cell lymphoma. Furthermore, the role of PD-1 as a tumor suppressor in mouse T lymphoma [7] suggests that use of PD-1 inhibitor may potentially cause clonal proliferation of abnormal T cell clone, leading to T-cell lymphoma [10]. It was recently reported the incidence of T cell lymphoma post-immune checkpoint inhibitor immunotherapy (pembrolizumab, nivolumab and ipilimumab) was approximately 0.02%, but the mortality is 17%. The relative risk probability T cell lymphoma risl compared with other drugs in pharmacovigilance database was increased at 1.91. [10]. Our data indicate that both PD-1 and PD-L1 function as tumor promoters in PD1+PD-L1+ T cell lymphoma. It is possible that the high level of PD-1 on the T lymphoma cells may deplete or reduce the anti-PD-1 antibodies available for T cell-expressed PD-1. Although blocking tumor cell PD-1 may suppress PD-1-mediated tumor growth promotion, but at the same time may not effectively block T cell PD-1. Therefore, a PD-L1-selective inhibitor or anti-PD-L1 antibody may be more effective in human T cell lymphoma immunotherapy. We also observed that activating the TCR signaling pathway effectively downregulate PD-L1 expression in T lymphoma cells. If PD-L1 were downregulated, its immune-suppressive function would be decreased. Therefore, a combined therapy of T cell activation therapy with anti-PD-L1 blockade immunotherapy may be another effective approach to suppress human T lymphoma, which requires further study.
Highlights.
Human T lymphoma cells express both PD-1 and PD-L1
TCR signaling activates PD-1 but represses PD-L1 expression in T lymphoma
PD-L1 promotes T lymphoma growth in vivo
PD-1 signaling pathways is impaired in vitro but promotes tumor growth in vivo
Acknowledgments:
We thank Ms. Jennifer Parks and Susan Dewes at Augusta Shepeard Community Blood Bank for providing blood samples from heathy donors. We also thank Dr. Jeanene Pihkala at Georgia Cancer Center Flow Cytometry Core Facility for assistance in cell sorting.
Funding:
This research was funded by National Institutes of Health, R01 CA133085, R01CA227433 (to K.L), and F30CA236436 (to J.D.K).
Footnotes
Institutional Review Board Statement: The animal study was conducted according to the approved protocol by Augusta University Institutional Animal Care and Use Committee (Protocol # 2008-0162).
Conflicts of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Oestreich KJ, Yoon H, Ahmed R, Boss JM, NFATc1 regulates PD-1 expression upon T cell activation, J Immunol, 181 (2008) 4832–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Goto T, Nishida T, Takagi E, Miyao K, Koyama D, Sakemura R, Hanajiri R, Watanabe K, Imahashi N, Terakura S, Murata M, Kiyoi H, Programmed Death-Ligand 1 on Antigen-presenting Cells Facilitates the Induction of Antigen-specific Cytotoxic T Lymphocytes: Application to Adoptive T-Cell Immunotherapy, J Immunother, 39 (2016) 306–315. [DOI] [PubMed] [Google Scholar]
- [3].Keir ME, Butte MJ, Freeman GJ, Sharpe AH, PD-1 and its ligands in tolerance and immunity, Annu Rev Immunol, 26 (2008) 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I, Vale RD, T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition, Science, 355 (2017) 1428–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Goodman A, Patel SP, Kurzrock R, PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas, Nat Rev Clin Oncol, 14 (2017) 203–220. [DOI] [PubMed] [Google Scholar]
- [6].Barta SK, Zain J, MacFarlane A.W.t., Smith SM, Ruan J, Fung HC, Tan CR, Yang Y, Alpaugh RK, Dulaimi E, Ross EA, Campbell KS, Khan N, Siddharta R, Fowler NH, Fisher RI, Oki Y, Phase II Study of the PD-1 Inhibitor Pembrolizumab for the Treatment of Relapsed or Refractory Mature T-cell Lymphoma, Clin Lymphoma Myeloma Leuk, 19 (2019) 356–364 e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wartewig T, Kurgyis Z, Keppler S, Pechloff K, Hameister E, Ollinger R, Maresch R, Buch T, Steiger K, Winter C, Rad R, Ruland J, PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis, Nature, 552 (2017) 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Trinquand A, Dos Santos NR, Tran Quang C, Rocchetti F, Zaniboni B, Belhocine M, Da Costa de Jesus C, Lhermitte L, Tesio M, Dussiot M, Cosset FL, Verhoeyen E, Pflumio F, Ifrah N, Dombret H, Spicuglia S, Chatenoud L, Gross DA, Hermine O, Macintyre E, Ghysdael J, Asnafi V, Triggering the TCR Developmental Checkpoint Activates a Therapeutically Targetable Tumor Suppressive Pathway in T-cell Leukemia, Cancer Discov, 6 (2016) 972–985. [DOI] [PubMed] [Google Scholar]
- [9].Wilcox RA, A three-signal model of T-cell lymphoma pathogenesis, Am J Hematol, 91 (2016)113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Anand K, Ensor J, Pingali SR, Hwu P, Duvic M, Chiang S, Miranda R, Zu Y, Iyer S, T-cell lymphoma secondary to checkpoint inhibitor therapy, J Immunother Cancer, 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Klement JD, Paschall AV, Redd PS, Ibrahim ML, Lu C, Yang D, Celis E, Abrams SI, Ozato K, Liu K, An osteopontin/CD44 immune checkpoint controls CD8+ T cell activation and tumor immune evasion, J Clin Invest, 128 (2018) 5549–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Smith-Garvin JE, Koretzky GA, Jordan MS, T cell activation, Annu Rev Immunol, 27 (2009) 591–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].O'Connor OA, Horwitz S, Masszi T, Van Hoof A, Brown P, Doorduijn J, Hess G, Jurczak W, Knoblauch P, Chawla S, Bhat G, Choi MR, Walewski J, Savage K, Foss F, Allen LF, Shustov A, Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study, J Clin Oncol, 33 (2015) 2492–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Graziano SL, Lehr BM, Merl SA, Ehrlich GD, Moore JL, Hallinan EJ, Hubbell C, Davey FR, Vournakis J, Poiesz BJ, Quantitative assay of human T-cell leukemia/lymphoma virus transformation, Cancer Res, 47 (1987) 2468–2473. [PubMed] [Google Scholar]
- [15].Kantekure K, Yang Y, Raghunath P, Schaffer A, Woetmann A, Zhang Q, Odum N, Wasik M, Expression patterns of the immunosuppressive proteins PD-1/CD279 and PD-L1/CD274 at different stages of cutaneous T-cell lymphoma/mycosis fungoides, Am J Dermatopathol, 34 (2012) 126–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kleffel S, Posch C, Barthel SR, Mueller H, Schlapbach C, Guenova E, Elco CP, Lee N, Juneja VR, Zhan Q, Lian CG, Thomi R, Hoetzenecker W, Cozzio A, Dummer R, Mihm MC Jr., Flaherty KT, Frank MH, Murphy GF, Sharpe AH, Kupper TS, Schatton T, Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth, Cell, 162 (2015) 1242–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li H, Li X, Liu S, Guo L, Zhang B, Zhang J, Ye Q, Programmed cell death-1 (PD-1) checkpoint blockade in combination with a mammalian target of rapamycin inhibitor restrains hepatocellular carcinoma growth induced by hepatoma cell-intrinsic PD-1, Hepatology, 66 (2017) 1920–1933. [DOI] [PubMed] [Google Scholar]
- [18].Kasamon YL, de Claro RA, Wang Y, Shen YL, Farrell AT, Pazdur R, FDA Approval Summary: Nivolumab for the Treatment of Relapsed or Progressive Classical Hodgkin Lymphoma, Oncologist, 22 (2017) 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P, PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma, N Engl J Med, 372 (2015) 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]