Abstract
Mesenchymal stem cells (MSCs) are pluripotent progenitor cells with the capabilities of self-renewing, differentiating into multiple lineages, and achieving trophic effects during tissue repair. MSCs can secrete extracellular vesicles (EVs) including exosomes and microvesicles, which mediate their trophic effects on other cells. Carrying a variety of intracellular molecules of MSCs including lipids, proteins, RNA (mRNA and non-coding RNA), and DNA, EVs deliver them into other cells to regulate tissue regeneration process. The therapeutic effects of MSC-derived EVs have been observed in a number of animal disease models. In this review, we focus on the current state and future directions of MSC-derived EVs in regenerative medicine.
Keywords: Extracellular vesicles, Exosomes, Mesenchymal stem cells, Regenerative medicine
INTRODUCTION
Extracellular vesicles (EVs) are heterogeneous small vesicles with a bilayer of phospholipids. EVs are secreted by almost all cell types and found in a variety of biological fluids (blood, urine, saliva, cerebrospinal fluid, breast milk, etc.). Once released into extracellular environment, they act on nearby recipient cells through paracrine regulation. In addition, they can also act on recipient cells far from the parental cells through systemic circulation.
As early as the 1960s, membrane vesicles in the extracellular space were observed, but their importance was unclear (Behnke, 1968). Since RNAs including microRNAs were observed in EVs (Ratajczak et al., 2006), EVs have attracted a great deal of renewed attention as mediators of intercellular communication (Valadi et al., 2007). According to the size, composition and origin, EVs can be classified into three types: (a) apoptotic bodies, (b) microvesicles/ ectosomes and (c) exosomes (Cocucci et al., 2009; Thery, 2011). Apoptotic bodies are released when plasma membrane blebbing occurs during apoptosis (Collino et al., 2015). These specialized EVs during cell death are not included in this review. The difference between the two remaining types of vesicles is mainly based on size: the range of exosomes is 30–150 nm, while the range of microvesicles is 100–1,000 nm. In addition, the origin of these two types of vesicles is also different: exosomes originate from endosomal membrane invagination, whereas microvesicles originate from the surface of cell membrane (Figure. 1).
Figure 1. Summary of biogenesis, biological components and therapeutic potential of MSC-derived EVs in a variety of biological events.
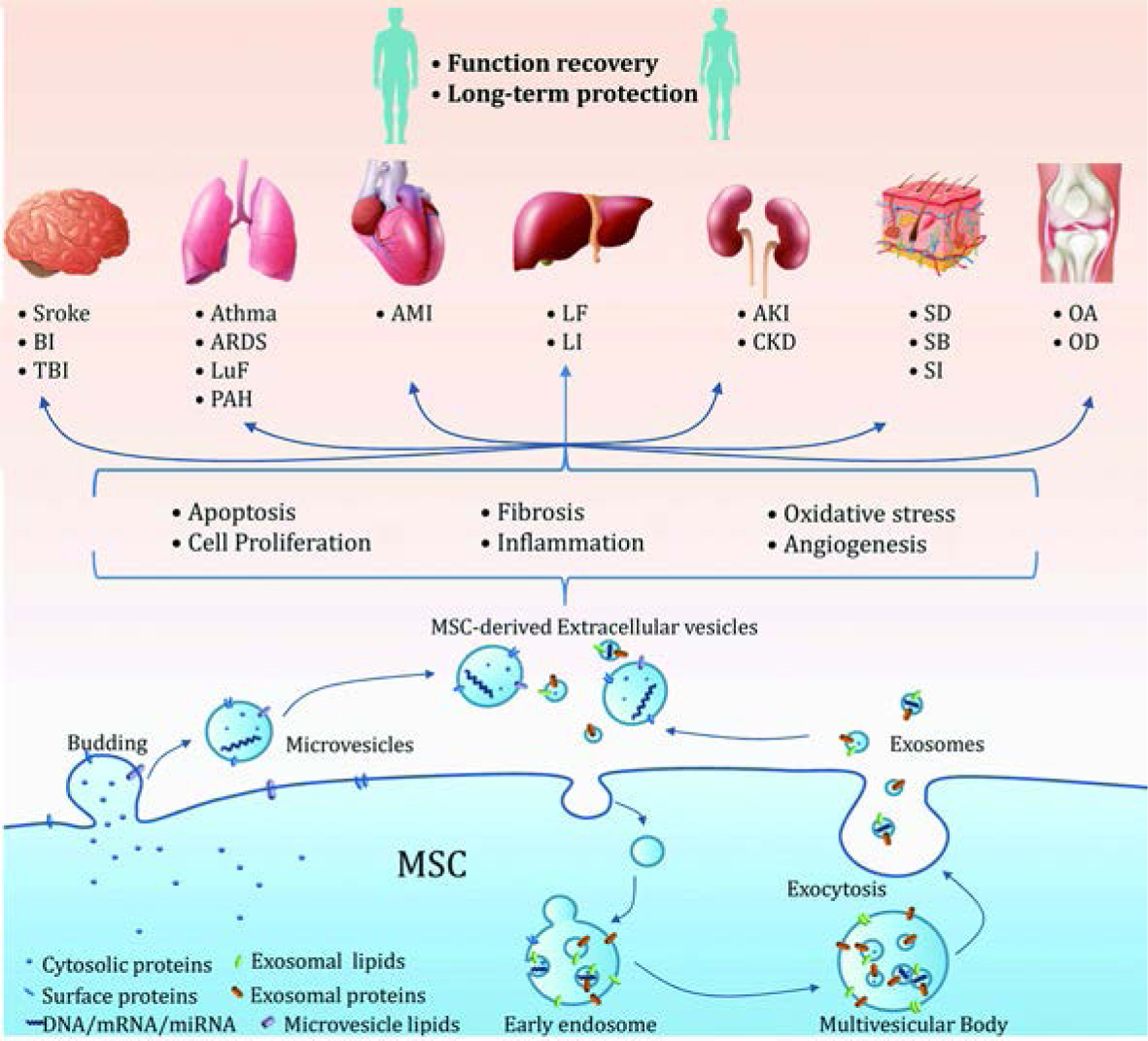
AD, adipose-derived; AKI, acute kidney injury; ALI, acute lung injury; AMI, acute myocardial ischemia; ARDS, acute respiratory distress syndrome; BI, brain injury; CKD, chronic kidney disease; MSC, mensenchymal stem cell; LI, liver injury; LF, liver fibrosis; LuF, lung fibrosis; OA, osteoarthritis; OD, osteochondral defects; PAH, pulmonary arterial hypertension; SB, skin burn; SD, skin defect, SI, skin incision; TBI, traumatic brain injury.
MSCs secrete various factors extensively to alter microenvironment following tissue damage. These factors affect regenerative processes including cell migration, proliferation, differentiation, and matrix synthesis (Meirelles Lda et al., 2009). In addition, these trophic factors suppress the local immune system, inhibit fibrosis and apoptosis, enhance angiogenesis, and stimulate mitosis and differentiation of reparative cells (Caplan, 2007). Most of the initial efforts were made to find bioactive therapeutic factors secreted by the MSC including cytokines, chemokines and growth factors (Vizoso et al., 2017). Although there are many promising candidates, none could adequately explain the trophic mediator role of MSCs. Since the demonstration that microvesicles secreted by MSCs could prevent acute renal tubule epithelium injury (Bruno et al., 2009) , more and more evidence have emerged regarding the role of MSC derived EVs as bioactive therapeutic factors during tissue regeneration (Akyurekli et al., 2015). Exosomes derived from different types of stem cells have been demonstrated to facilitate tissue repair in the skin (Zhang et al., 2015c), limbs (Hu et al., 2015), heart (Lai et al., 2010), joint (Zhu et al., 2017) and other tissues.
In this review, we focus on the current state of MSC-derived EVs including exosomes and microvesicles in regenerative medicine. We conducted search of the following key words in the Web of Science database: extracellular vesicles, microvesicles, exosomes and stem cells.
General Characteristics of EVs
Biogenesis
The assembly of both exosomes and microvesicles involves the accumulation of components in the small membrane domains, which sprout and fall off the membrane (Figure 1). Nevertheless, the initial processes leading to the generation of exosomes and microvesicles are largely different.
Exosomes are formed by reverse germination, and thus contain cytosol (Thery et al., 2002). The biogenesis of the exosomes begins with the formation of early endosomes from endocytoses of the plasma membrane with its cargos. The early endosomes become multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs). They are either degraded by lysosomes or released as exosomes (Alenquer and Amorim, 2015).
In contrast, the initial process leading to microvesicles assembly is largely different from exosomes assembly. During microvesicle assembly, cargos are trafficked to the plasma membrane regions named cholesterol-rich microdomains, which are full of ceramide and lipid rafts. These membrane regions with their cargos are then protruded or budded to the outside of the cells to produce microvesicles (Nawaz et al., 2014).
Isolation Methods
There are numerous techniques to isolate EVs, although they have not been standardized. The original EVs isolation and purification method was differential centrifugation based on the size of the EVs. Thery et al. introduced a detailed protocol of this method (Thery et al., 2006). However, EVs vary in size, origin and molecular compositions, and different EVs populations overlap considerably in their size and phenotype. According to a large survey of current worldwide practices for the isolation of EVs (Gardiner et al., 2016) , ultracentrifuge method is still the most commonly used method (81%), followed by density gradient centrifugation (20%), filter (18%), size exclusion chromatography (15%), and precipitation (14%). Ninety-one percent of respondents used two or more methods. Up to now, no consensus on the “gold standard” method of EVs isolation and purification has been reached.
Size and Morphology
In whole-mount samples under electron microscope (EM), exosomes display cup-shaped appearance. However, this feature, produced by the fixation and contrast procedure that leads to exosome membrane shrinking, may be an artifact of exosomes. The true appearance of exosomes is a round shape, which is observed under cryo-EM and verified (Conde-Vancells et al., 2008). The diameter of the exosomes obtained by high-speed centrifugation is 30–150 nm while that of microvesicles is 100–1000 nm. These observations are consistent across different studies (Baietti et al., 2012).
Physical Features
EVs can be separated by density gradients. Obtained by ultracentrifugation, exosomes densities range from 1.15 to 1.19 g/ml whereas microvesicles range from 1.12 to 1.20 g/ml in sucrose (Robbins and Morelli, 2014). Such a range of densities indicates the heterogeneity of vesicles. Thus, various subpopulations exist in the preparations and separate analysis of these subpopulations is needed.
MSCs in regenerative medicine
Biochemical Components of MSC-derived EVs
MSC-derived EVs contain a large number of biochemical components including proteins, lipids and nucleic acids. Several databases were built to facilitate transmission of these data. Exocarta contained the database of mammalian exosomes (Mathivanan et al., 2012) , which has been incorporated into Vesiclepedia (http://microvesicles.org), a more comprehensive database. Another database (http://evpedia.info) contain information of non-mammal EVs (Kim et al., 2013).
Proteins
There are three groups of proteins, namely common proteins, enzymes and signaling molecules, according to their specific functions in MSC-derived EVs. Approximately 2,000 proteins in MSC-derived exosome come from MSC membranes, Golgi, nuclei and cytoplasm, and occasionally from ER or mitochondria (Choi et al., 2013). In MSC-derived exosomes, the common membrane proteins include CD9, CD63, CD82, CD81, HSP70, MHC-I, MCH-II while cell structure and motility proteins include tubulin, myosin and actin. MSC-derived exosomes contain five important enzymes that play a key role in the glycolysis process (Lai et al., 2011). Signaling molecules such as chemokines, cytokines, interleukins and growth factors were also found in MSC-derived exosomes (Lai et al., 2010; Formiga et al., 2014). It is worth noting that the exosomes protein composition reflects the physiological and pathological status of host cells, which can change in response to stress signals in the microenvironment (Salomon et al., 2013).
Lipids
Lipids are involved in EVs biogenesis. An important protein ESCRT can interact with lipids and enzymes related to lipid metabolism during EVs biogenesis (Wang et al., 2005). Moreover, Hsc-70 specifically binds to phosphatidylserine in the endosomal membrane, followed by ESCRT-III binding to polyglycerol phospholipids (monoglycerides) phosphate (Mobius et al., 2003). In addition, production of exosomes also needs the participation of cholesterol, oxysterols, ceramides and lipid transporters (Record et al., 2011). Many bioactive lipids, as well as lipid metabolism-related enzymes, are transported by exosomes (Subra et al., 2010). Once ingested by the target cell, the bioactive lipids of exosomes are delivered, which can affect the signaling pathways and lipid homeostasis of target cells (Howcroft et al., 2011).
Nucleic acids
MSC-derived EVs contain mRNAs of various sizes and small RNAs including microRNAs. However, the levels of ribosomal 18 S and 28 S RNA are low or undetected. RNAs are not randomly included in exosomes. Different sequences are either secreted preferentially or retained in cells (Ratajczak et al., 2006; Valadi et al., 2007). Exosomes are enriched with miRNAs, most of which are in the form of pre-miRNAs that are inactive before they are transformed into mature miRNAs (Chen et al., 2010). Exosomal miRNAs are secreted in a strict regulatory process, which is affected by the source and development stage of host cells. A number of miRNAs have been identified in MSC-derived exosomes, which can function in recipient cells (Halkein et al., 2013; Bang et al., 2014; Wang et al., 2014b). Thus, exosomal miRNAs may play an important role in mediating intercellular communication. The type and amount of miRNAs encapsulated into exosome are affected by pathophysiological stress stimuli and the state of the microenvironment (Jelonek et al., 2016).
Therapeutic potential of MSC-derived EVs
The effect of MSC-derived EVs on treatment of diseases in various organs has been studied. They are summarized as follows.
Brain disease
Potential regenerative effect of MSC-EVs has been observed for treatment of neurological and neurodegenerative diseases. In a rat stroke model, intravenous administration of MSC-EVs enhanced neurite remodeling, neurogenesis, and angiogenesis, and improved functional recovery (Xin et al., 2013). MSC-EVs have also been tested in traumatic brain injury (TBI) models. By promoting endogenous neurogenesis and angiogenesis, MSC-EV improved recovery of brain function after TBI (Zhang et al., 2015d). Mechanistically, MSC-EV acts by improving the recovery of the impairment of both spatial learning and pattern separation (Kim et al., 2016), and by inhibiting neuro-inflammation (Zhang et al., 2015d) (Zhang et al., 2015d; Kim et al., 2016).
The impact of MSC-EVs on Alzheimer’s disease was also investigated. EVs from human adipose tissue-derived mesenchymal stem cells (ADSCs) decreased the levels of both secreted and intracellular β-amyloid peptide in the neuroblastoma cells (Katsuda et al., 2013). This effect might be achieved by neprilysin in the ADSCs, an important enzyme during degradation of β-amyloid peptide (Katsuda et al., 2013).
Lung diseases
In allergic asthma mice model, ADSCs and their EVs relieved inflammation, reduced eosinophil counts, and regulated airway rebuilding (de Castro et al., 2017). In silicotic mice model, MSCs-derived EVs decreased pulmonary fibrosis through inflammation remission and collagen deposition reduction in lung parenchyma (Choi et al., 2014b). In pulmonary arterial hypertension (PAH) mice model, MSC-exosomes were found to reverse PAH through increasing the levels of anti-inflammatory, anti-proliferative miRNAs (Chen et al., 2014).
Cardiac injury
In mouse myocardial ischemia/reperfusion injury model, exosomes from human embryonic stem cell (ESC) remarkably reduced the size of myocardial infarct (Lai et al., 2010; Arslan et al., 2013). In acute myocardial infarction (AMI) rat model, EVs from hypoxic human BM-MSCs via intramyocardial injection markedly enhanced blood flow recovery and reduced infarct size (Bian et al., 2014). Among human BM-MSCs, ADSCs, and endometrium-derived MSCs (EnMSCs), EnMSCs were most effective in infact size reduction, cardiac function restoration, and angiogenesis in ischemic zone (Wang et al., 2017).
Liver injury
In a mouse liver fibrosis model, administering exosomes from human umbilical cord MSCs improved liver fibrosis and restored liver function by inhibiting the epithelial–mesenchymal transition of hepatocytes and collagen production (Li et al., 2013b). By transferring miR-122 into hepatic stem cells (HSCs), exosomes from ADSCs regulate collagen maturation and cell proliferation of HSCs through inhibiting the target genes of miR-122 (Li et al., 2013a).
Kidney injury
In ischemia-reperfusion injury (IRI)-induced acute kidney injury (AKI) mouse model, MSC-EVs protected mouse from AKI and restored kidney function by inhibiting apoptosis and stimulating tubular epithelial cell proliferation (Gatti et al., 2011; Wang et al., 2014a; Zhang et al., 2016b). Interestingly, these reno-protective effects were also found in the EVs isolated from kidney resident MSCs (Choi et al., 2014a).
In AKI mouse models induced by drug three cisplatin (Bruno et al., 2012) or glycerol (Bruno et al., 2017) , systemic administration of MSC-EVs ameliorated tubular injury and restored renal function. Such EVs induced renoprotection was abolished if the internal mRNAs of EVs were degraded. It suggested that these EVs-derived mRNAs play an important role in renal protection.
Skin disease
In a full-thickness skin defect rat model, subcutaneous administration of exosomes derived from human induced pluripotent stem cells (iPSCs) accelerated re-epithelialization, reduced scar widths, and promoted collagen maturity and angiogenesis (Zhang et al., 2015c). ADSCs-derived exosomes also protected skin by enhancing cell proliferation and increasing collagen content in a dose-dependent manner after ingestion by skin fibroblasts (Hu et al., 2016).
In a second-degree burn rat model, subcutaneous injection of exosomes from human umbilical cord-derived mesenchymal stem cells (hUCMSC) accelerated re-epithelialization and increased type I collagen deposition (Zhang et al., 2015a; Zhang et al., 2015b). Mechanistically, these exosomes enhanced skin repair by accelerating the Wnt/beta-catenin signaling pathway and by inhibiting the YAP signaling pathway (Zhang et al., 2016a).
Joint disorder
MSC-derived EVs enhanced cartilage repair and inhibited cartilage degeneration related to osteoarthritis (OA) (Cosenza et al., 2017) (Zhang et al., 2016c; Zhang et al., 2018b) (Tao et al., 2017). In collagenase-induced OA mice model, single intra-articular administration of exosomes or microvesicles from murine BM-MSC inhibited chondrocyte apoptosis and macrophage activation (Cosenza et al., 2017). In femoral groove osteochondral defect rat model, weekly intra-articular injection of human embryonic stem cells-derived exosomes increased cell proliferation and matrix synthesis, enhanced the regenerative immune phenotype, and repaired cartilage defects (Zhang et al., 2016c; Zhang et al., 2018b). In a trauma-induced OA rat model, exosomes from synovial MSCs overexpressing chondroprotective miR-140-5p facilitated cartilage regeneration and inhibited OA pathogenesis (Tao et al., 2017).
Future direction: realizing tangible patient benefits
As described above, many studies have shown that MSC-derived EVs were effective for regenerating injured or even degenerated tissues or organs, thereby restoring their functions (Figure 1). Although multiple regenerative mechanisms could be involved potentially, some common features have emerged including anti-apoptosis, inhibiting inflammation, and enhancing cell proliferation. These are the inherent properties of young and healthy tissue resident MSCs (Caplan, 2017). Through transmitting these trophic effects of MSCs, MSC-derived EVs enhance tissue regeneration.
Despite these enormous progresses, the field of regenerative therapy using MSC-derived EVs is still in its early stage. No clinical trials have been published so far (http://www.clinicaltrials.gov/, accessed on May 2018). To date, there is only one interventional clinical trial of EV therapy, which demonstrated that immunotherapy of colorectal cancer using ascites-derived exosomes was safe and well tolerated. Although it was not a clinical trial using MSC-derived EV, it still instills the confidence about the future of clinical therapies using EVs.
A number of challenges need to be overcome to achieve the successful clinical translation of MSC-derived EV therapy. First, better treatment efficiency should be accomplished. Considering that MSC-derived EVs contain various bioactive cargos, such as mRNAs, microRNAs and proteins, treatment efficiency may be improved by enhancing the active ingredient within EVs. MSCs and their EVs can be modified through overexpressing or knocking down a target gene to improve therapeutic efficacy. For example, cartilage regenerative effect is improved by overexpressing chondroprotective miR-140–5p in rat synovial MSCs-derived exosomes in a trauma-induced OA rat model (Tao et al., 2017). Second, target delivery is essential to reduce off-target side effect and toxicity. This can be accomplished by local delivery to target tissues such as subcutaneous injection to the skin and intra-articular injection to the joint. Alternatively, the surface molecules of the EVs can be tailored for binding to the specific receptors on recipient cells. This can be accomplished through modifying the membrane molecules such as peptides, lipids or carbohydrates using cell engineering. For example, a brain-targeting peptide enables systemic exosomes delivery of siRNA to mouse brain (Alvarez-Erviti et al., 2011). Several subsequent studies confirmed the feasibility of this approach (Vader et al., 2016). Third, sustainability and longer half-life of EVs in tissues should be accomplished in vivo. Because of EVs small sizes in the nanometer to micrometer range, they are often cleared rapidly through blood vessels and lymphatic systems. Thus, it is crucial to understand the tissue transport and draining systems for EVs in vivo so as to develop more effective clinical treatment that evade clearance. In addition, the half-life of EVs can be improved by controlled and sustained release through a carrier. For instance, when MSC-derived exosomes were loaded with chitosan hydrogel, the stability and retention of exosomes were enhanced and the therapeutic effects for hindlimb ischemia were further augmented (Zhang et al., 2018a).
Last but not the least, EVs can be multi-functionalized to serve as not only therapeutic agents but also vehicles for drug delivery. For example, a novel carrier-in-carrier system was developed using MSC-derived EVs loaded with silk/curcumin nanoparticles (Perteghella et al., 2017). This drug delivery system combined beneficial effects of both regenerative cell therapies and pharmaceutical nanomedicine. Another example is the exosome-liposome hybrid nanoparticles, which could encapsulate large cargos more efficiently than exosomes alone (Lin et al., 2016). These hybrid nanoparticles have been used to deliver CRISPR/Cas9 system through endocytosis into MSCs and subsequent release of the encapsulated system within MSCs (Lin et al., 2018).
Based on these principles, we propose the following strategy for developing clinical application of MSC-derived EVs (Figure 2). First, tissue donor should be selected and examined for MSC-derived EVs production. The donor can be autologous or allogeneic, and stem cell may be obtained from different tissues. Safety of the cell sources should be ensured by comprehensive cell and molecular analysis. Second, MSCs modification by bioengineering may be considered to improve therapeutic efficacy of MSC-derived EVs. For instance, the abundance of a specific gene product in the MSC-derived EVs can be changed through gene editing using CRISPR/Cas9 in MSCs. Third, MSC-derived EVs are isolated and maybe modified. Standardized protocol for EV isolation is preferred. Therapeutic effects of EVs may be further improved by modifying EVs, for example, by encapsulating miRNA or siRNA in EVs. Fourth, the route of MSC-derived EVs administration should be selected. The EVs delivery routes should be determined according to the tissue target of the specific disease. They can be systemic (intravenous, inhale and oral) or local (topical, or intra-articular injection). Lastly, safety and potential side effects should be monitored. Although positive regenerative effects of MSC-derived EVs were observed in a number of studies, few of the studies evaluated the safety of the MSC-derived EVs. Safety will be the key to the success of employing MSC-derived EVs as a clinical therapy in the clinic.
Figure 2. A general strategy for clinical application of MSC-derived EVs.

AD, adipose-derived; BM, bone marrow; CP, chorionic plate; ESC, embryonic stem cells; EV, extracellular vesicles; iPSC, induced pluripotent stem cells; MSCs, mensenchymal stem cells; UC, umbilical cord.
CONCLUSIONS
MSC-derived EVs showed favorable regenerative therapeutic effects under various disease conditions in preclinical models, although their composition and function are not yet clearly understood. Research in this field is still in its early stage and no clinical trial has been published so far. MSC-derived EVs are envisaged as a promising therapy in regenerative medicine, because they not only mediate the trophic effects of MSC but also provide a simpler alternative to the current cell-based therapeutics. Therapeutic efficacy of MSCs-derived EVs can be further improved through modification by bio- and genetic engineering, drug encapsulation, and nanomaterial science. There are still many obstacles to overcome before clinical use, including standardized isolation and purification, large-scale production at pharmaceutical grade, and safety.
ACKNOWLEDGEMENTS
This study was supported by a scholarship from the China Scholarship Council to Q.Y., a grant (No. 81371987) from the National Natural Science Foundation of China to Y.Z., and by P20GM104937 and P30GM122732 from the NIGMS/NIH to Q.C.
Grant information:
Grant sponsor: NIGMS/NIH; Grant number: P20GM104937, P30GM122732
Grant sponsor: National Natural Science Foundation of China; Grant number: 81371987
Footnotes
COMPETING INTERESTS
No competing interests.
LITERATURE CITED
- Akyurekli C, Le Y, Richardson RB, Fergusson D, Tay J, Allan DS. 2015. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem cell reviews 11:150–160. [DOI] [PubMed] [Google Scholar]
- Alenquer M, Amorim MJ. 2015. Exosome Biogenesis, Regulation, and Function in Viral Infection. Viruses 7:5066–5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. 2011. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature biotechnology 29:341–345. [DOI] [PubMed] [Google Scholar]
- Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. 2013. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem cell research 10:301–312. [DOI] [PubMed] [Google Scholar]
- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G. 2012. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nature cell biology 14:677–685. [DOI] [PubMed] [Google Scholar]
- Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T. 2014. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. The Journal of clinical investigation 124:2136–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke O 1968. An electron microscope study of the megacaryocyte of the rat bone marrow. I. The development of the demarcation membrane system and the platelet surface coat. Journal of ultrastructure research 24:412–433. [DOI] [PubMed] [Google Scholar]
- Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. 2014. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. Journal of molecular medicine (Berlin, Germany) 92:387–397. [DOI] [PubMed] [Google Scholar]
- Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. 2012. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PloS one 7:e33115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. 2009. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. Journal of the American Society of Nephrology : JASN 20:1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno S, Tapparo M, Collino F, Chiabotto G, Deregibus MC, Soares Lindoso R, Neri F, Kholia S, Giunti S, Wen S, Quesenberry P, Camussi G. 2017. Renal Regenerative Potential of Different Extracellular Vesicle Populations Derived from Bone Marrow Mesenchymal Stromal Cells. Tissue engineering Part A 23:1262–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. 2007. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. Journal of cellular physiology 213:341–347. [DOI] [PubMed] [Google Scholar]
- Chen JY, An R, Liu ZJ, Wang JJ, Chen SZ, Hong MM, Liu JH, Xiao MY, Chen YF. 2014. Therapeutic effects of mesenchymal stem cell-derived microvesicles on pulmonary arterial hypertension in rats. Acta pharmacologica Sinica 35:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. 2010. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic acids research 38:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Kim DK, Kim YK, Gho YS. 2013. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics 13:1554–1571. [DOI] [PubMed] [Google Scholar]
- Choi HY, Moon SJ, Ratliff BB, Ahn SH, Jung A, Lee M, Lee S, Lim BJ, Kim BS, Plotkin MD, Ha SK, Park HC. 2014a. Microparticles from kidney-derived mesenchymal stem cells act as carriers of proangiogenic signals and contribute to recovery from acute kidney injury. PloS one 9:e87853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M, Ban T, Rhim T. 2014b. Therapeutic use of stem cell transplantation for cell replacement or cytoprotective effect of microvesicle released from mesenchymal stem cell. Molecules and cells 37:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci E, Racchetti G, Meldolesi J. 2009. Shedding microvesicles: artefacts no more. Trends in cell biology 19:43–51. [DOI] [PubMed] [Google Scholar]
- Collino F, Bruno S, Incarnato D, Dettori D, Neri F, Provero P, Pomatto M, Oliviero S, Tetta C, Quesenberry PJ, Camussi G. 2015. AKI Recovery Induced by Mesenchymal Stromal Cell-Derived Extracellular Vesicles Carrying MicroRNAs. Journal of the American Society of Nephrology : JASN 26:2349–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Vancells J, Rodriguez-Suarez E, Embade N, Gil D, Matthiesen R, Valle M, Elortza F, Lu SC, Mato JM, Falcon-Perez JM. 2008. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. Journal of proteome research 7:5157–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noel D. 2017. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Scientific reports 7:16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro LL, Xisto DG, Kitoko JZ, Cruz FF, Olsen PC, Redondo PAG, Ferreira TPT, Weiss DJ, Martins MA, Morales MM, Rocco PRM. 2017. Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem cell research & therapy 8:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formiga FR, Pelacho B, Garbayo E, Imbuluzqueta I, Diaz-Herraez P, Abizanda G, Gavira JJ, Simon-Yarza T, Albiasu E, Tamayo E, Prosper F, Blanco-Prieto MJ. 2014. Controlled delivery of fibroblast growth factor-1 and neuregulin-1 from biodegradable microparticles promotes cardiac repair in a rat myocardial infarction model through activation of endogenous regeneration. Journal of controlled release : official journal of the Controlled Release Society 173:132–139. [DOI] [PubMed] [Google Scholar]
- Gardiner C, Di Vizio D, Sahoo S, Thery C, Witwer KW, Wauben M, Hill AF. 2016. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. Journal of extracellular vesicles 5:32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, Camussi G. 2011. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 26:1474–1483. [DOI] [PubMed] [Google Scholar]
- Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, Castermans K, Malvaux L, Lambert V, Thiry M, Sliwa K, Noel A, Martial JA, Hilfiker-Kleiner D, Struman I. 2013. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. The Journal of clinical investigation 123:2143–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howcroft TK, Zhang HG, Dhodapkar M, Mohla S. 2011. Vesicle transfer and cell fusion: Emerging concepts of cell-cell communication in the tumor microenvironment. Cancer biology & therapy 12:159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu GW, Li Q, Niu X, Hu B, Liu J, Zhou SM, Guo SC, Lang HL, Zhang CQ, Wang Y, Deng ZF. 2015. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem cell research & therapy 6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, Huang F, Zhang H, Chen L. 2016. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Scientific reports 6:32993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelonek K, Widlak P, Pietrowska M. 2016. The Influence of Ionizing Radiation on Exosome Composition, Secretion and Intercellular Communication. Protein and peptide letters 23:656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, Takeshita F, Sakai Y, Kuroda M, Ochiya T. 2013. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Scientific reports 3:1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK, Kang B, Kim OY, Choi DS, Lee J, Kim SR, Go G, Yoon YJ, Kim JH, Jang SC, Park KS, Choi EJ, Kim KP, Desiderio DM, Kim YK, Lotvall J, Hwang D, Gho YS. 2013. EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. Journal of extracellular vesicles 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ. 2016. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proceedings of the National Academy of Sciences of the United States of America 113:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. 2010. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem cell research 4:214–222. [DOI] [PubMed] [Google Scholar]
- Lai RC, Chen TS, Lim SK. 2011. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regenerative medicine 6:481–492. [DOI] [PubMed] [Google Scholar]
- Li J, Ghazwani M, Zhang Y, Lu J, Li J, Fan J, Gandhi CR, Li S. 2013a. miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression. Journal of hepatology 58:522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, Xu W. 2013b. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem cells and development 22:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KC, Yip HK, Shao PL, Wu SC, Chen KH, Chen YT, Yang CC, Sun CK, Kao GS, Chen SY, Chai HT, Chang CL, Chen CH, Lee MS. 2016. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. International journal of cardiology 216:173–185. [DOI] [PubMed] [Google Scholar]
- Lin Y, Wu J, Gu W, Huang Y, Tong Z, Huang L, Tan J. 2018. Exosome-Liposome Hybrid Nanoparticles Deliver CRISPR/Cas9 System in MSCs. Advanced science (Weinheim, Baden-Wurttemberg, Germany) 5:1700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. 2012. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic acids research 40:D1241–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. 2009. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine & growth factor reviews 20:419–427. [DOI] [PubMed] [Google Scholar]
- Mobius W, van Donselaar E, Ohno-Iwashita Y, Shimada Y, Heijnen HF, Slot JW, Geuze HJ. 2003. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic (Copenhagen, Denmark) 4:222–231. [DOI] [PubMed] [Google Scholar]
- Nawaz M, Camussi G, Valadi H, Nazarenko I, Ekstrom K, Wang X, Principe S, Shah N, Ashraf NM, Fatima F, Neder L, Kislinger T. 2014. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nature reviews Urology 11:688–701. [DOI] [PubMed] [Google Scholar]
- Perteghella S, Crivelli B, Catenacci L, Sorrenti M, Bruni G, Necchi V, Vigani B, Sorlini M, Torre ML, Chlapanidas T. 2017. Stem cell-extracellular vesicles as drug delivery systems: New frontiers for silk/curcumin nanoparticles. International journal of pharmaceutics 520:86–97. [DOI] [PubMed] [Google Scholar]
- Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. 2006. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20:847–856. [DOI] [PubMed] [Google Scholar]
- Record M, Amara S, Subra C, Jiang G, Prestwich GD, Ferrato F, Carriere F. 2011. Bis (monoacylglycero) phosphate interfacial properties and lipolysis by pancreatic lipase-related protein 2, an enzyme present in THP-1 human monocytes. Biochimica et biophysica acta 1811:419–430. [DOI] [PubMed] [Google Scholar]
- Robbins PD, Morelli AE. 2014. Regulation of immune responses by extracellular vesicles. Nature reviews Immunology 14:195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon C, Ryan J, Sobrevia L, Kobayashi M, Ashman K, Mitchell M, Rice GE. 2013. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PloS one 8:e68451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subra C, Grand D, Laulagnier K, Stella A, Lambeau G, Paillasse M, De Medina P, Monsarrat B, Perret B, Silvente-Poirot S, Poirot M, Record M. 2010. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. Journal of lipid research 51:2105–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. 2017. Exosomes derived from miR-140–5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 7:180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C 2011. Exosomes: secreted vesicles and intercellular communications. F1000 biology reports 3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Amigorena S, Raposo G, Clayton A. 2006. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current protocols in cell biology Chapter 3:Unit 3 22. [DOI] [PubMed] [Google Scholar]
- Thery C, Zitvogel L, Amigorena S. 2002. Exosomes: composition, biogenesis and function. Nature reviews Immunology 2:569–579. [DOI] [PubMed] [Google Scholar]
- Vader P, Mol EA, Pasterkamp G, Schiffelers RM. 2016. Extracellular vesicles for drug delivery. Advanced drug delivery reviews 106:148–156. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology 9:654–659. [DOI] [PubMed] [Google Scholar]
- Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. 2017. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Jiang Z, Webster KA, Chen J, Hu H, Zhou Y, Zhao J, Wang L, Wang Y, Zhong Z, Ni C, Li Q, Xiang C, Zhang L, Wu R, Zhu W, Yu H, Hu X, Wang J. 2017. Enhanced Cardioprotection by Human Endometrium Mesenchymal Stem Cells Driven by Exosomal MicroRNA-21. Stem cells translational medicine 6:209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhang Y, Li H, Chieu HK, Munn AL, Yang H. 2005. AAA ATPases regulate membrane association of yeast oxysterol binding proteins and sterol metabolism. The EMBO journal 24:2989–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Lin M, Li L, Li L, Qi G, Rong R, Xu M, Zhu T. 2014a. Bone marrow mesenchymal stem cell-derived exosome protects kidney against ischemia reperfusion injury in rats. Zhonghua yi xue za zhi 94:3298–3303. [PubMed] [Google Scholar]
- Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan GC. 2014b. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. Journal of molecular and cellular cardiology 74:139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. 2013. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 33:1711–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Shi Y, Gong A, Pan Z, Shi H, Yang H, Fu H, Yan Y, Zhang X, Wang M, Zhu W, Qian H, Xu W. 2016a. HucMSC Exosome-Delivered 14–3-3zeta Orchestrates Self-Control of the Wnt Response via Modulation of YAP During Cutaneous Regeneration. Stem cells (Dayton, Ohio) 34:2485–2500. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Shi H, Wu L, Zhu W, Qian H, Xu W. 2015a. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem cells (Dayton, Ohio) 33:2158–2168. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wu X, Zhang X, Sun Y, Yan Y, Shi H, Zhu Y, Wu L, Pan Z, Zhu W, Qian H, Xu W. 2015b. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/beta-catenin pathway. Stem cells translational medicine 4:513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Zou X, Huang Y, Wang F, Miao S, Liu G, Chen M, Zhu Y. 2016b. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect Against Acute Kidney Injury Through Anti-Oxidation by Enhancing Nrf2/ARE Activation in Rats. Kidney & blood pressure research 41:119–128. [DOI] [PubMed] [Google Scholar]
- Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, Xie Z, Zhang C, Wang Y. 2015c. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. Journal of translational medicine 13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Zhao X, Chen X, Wei Y, Du W, Wang Y, Liu L, Zhao W, Han Z, Kong D, Zhao Q, Guo Z, Han Z, Liu N, Ma F, Li Z. 2018a. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS applied materials & interfaces. [DOI] [PubMed] [Google Scholar]
- Zhang S, Chu WC, Lai RC, Lim SK, Hui JH, Toh WS. 2016c. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis and cartilage 24:2135–2140. [DOI] [PubMed] [Google Scholar]
- Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. 2018b. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 156:16–27. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y. 2015d. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. Journal of neurosurgery 122:856–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Wang Y, Zhao B, Niu X, Hu B, Li Q, Zhang J, Ding J, Chen Y, Wang Y. 2017. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem cell research & therapy 8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]


