Abstract
Zearalenone (ZEN) is one of the most common contaminating mycotoxins and is mainly produced by Fusarium graminearum. ZEN and its metabolites can interfere with estrogen function and affect animals' reproductive ability. Pigs are most susceptible to ZEN, and ZEN is less harmful to poultry than to pigs. The exact mechanism for the difference in susceptibility remains unclear. In this review, we summarized some possible reasons for the relative insensitivity of poultry to ZEN, such as the lower total amount of α-zearalenol (α-ZOL) and the α-ZOL-to-β-ZOL ratio which reduce the toxicity of ZEN to poultry. The faster hepatic and enteric circulation, and excretion capacity in poultry can excrete more ZEN and its metabolites. There are other possible factors such as the transformation of intestinal microorganisms, differences in hydroxysteroid dehydrogenases' activity, high estrogen levels, and low estrogen receptors affinity which can also cause poultry to be relatively insensitive to ZEN. In this review, we summarized the hazards, pollution status, metabolic pathways, and some measures to mitigate ZEN's harmfulness. Specifically, we discussed the possible mechanisms of low reproductive toxicity by ZEN in poultry.
Keywords: Zearalenone, Poultry, Low sensitivity, Metabolism, Detoxification
1. Introduction
1.1. The toxicity of zearalenone to animals
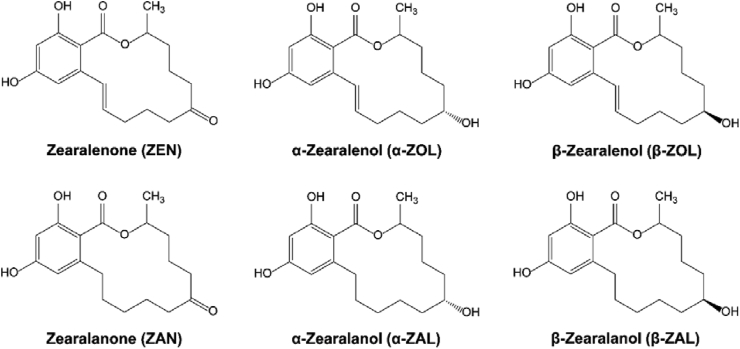
Zearalenone (ZEN) is mainly produced by Fusarium graminearum as a secondary metabolite with estrogenic properties. Pure ZEN is a white crystal, chemical name 6-(10-hydroxy-6-oxy-trans-1-undecenyl)-β-ryanolide (molecular formula C18H22O5, molecular weight 318.36, CAS 17924- 92-4), with a melting point from 161 to 163 °C, being insoluble in water and easily soluble in alkaline solution, ether, benzene, methanol, and ethanol. ZEN has thermal stability and is challenging to decompose during feed processing (crushing, squeezing, storage, or heating). ZEN has reproductive toxicity, liver and kidney toxicity, and immunotoxicity, among which the most typical is reproductive toxicity (Abid-Essefi et al., 2004; Zinedine et al., 2007). ZEN and its metabolites have a biological structure similar to 17-β estradiol (E2) and can bind to estrogen receptors (ER) in reproductive organs (Fig. 1 for some products). This can interfere with the estrogen levels of livestock and poultry and cause reproductive disorders such as miscarriage, stillbirth, and teratogenesis (Kouadio et al., 2005).
Fig. 1.
The chemical structure of zearalenone (ZEN) and its metabolites.
Zearalenone is highly toxic and widely contaminates corn, wheat, barley, sorghum, rice, and other grains. It has been reported that many agricultural products are seriously contaminated by ZEN, with an average concentration ranging from 5 to 50 mg/kg and a maximum concentration from 120 to 180 mg/kg (Zinedine et al., 2007). Ma et al. (2018) collected 1,569 samples (including 742 feed material samples and 827 compound feed samples) from different regions of China. The results showed that the ZEN contamination rate in the samples was 88%, and the highest contamination level was 729.2 μg/kg. Overall, 10.8% of the tested samples exceeded China's standard limit for ZEN in feed (150 to 500 μg/kg) (Ma et al., 2018). There can also be a combination of multiple mycotoxins and interactions between mycotoxins in feed and feed ingredients (Rai et al., 2020). ZEN enhances the damage of deoxynivalenol and aflatoxin B1 to broilers' and laying hens' production performance and immunity (Danicke et al., 2002). Additionally, damage to the liver and jejunum, and increased residues of deoxynivalenol and aflatoxin B1 in serum, liver, chest muscle, small intestine, and excreta have been described (Chang et al., 2020). ZEN is highly toxic and enhances other mycotoxins' harm to poultry during co-contamination, making ZEN-contaminated feed a severe threat to poultry health. In short, ZEN could reduce the nutritional value of feed and damage the growth and health of livestock and poultry, causing severe economic losses to the livestock industry (Binder, 2007; Wu, 2004).
1.2. The metabolic pathway of zearalenone in animals
Zearalenone has 2 main metabolic pathways in animals: 1) reduction reaction and 2) binding reaction.
When feeds containing ZEN were fed to animals, part of the toxin is excreted with urine and feces without being absorbed. The remaining ZEN is quickly absorbed by small intestinal epithelial cells into the blood circulation and then metabolized by the liver into various metabolites. ZEN is reduced to 2 isomers, α/β-zearalenol (α/β-ZOL), by 3α/β-hydroxysteroid dehydrogenase (3α/β-HSD). The structure of α/β-ZOL is similar to ZEN. The estrogen potency of α-ZOL is nearly 500 times stronger than that of ZEN, but that of β-ZOL is 16 times weaker than that of ZEN and is almost harmless (Zinedine et al., 2007). Therefore, the reaction to produce α-ZOL can be regarded as the toxicity enhancement pathway, and the reaction to produce β-ZOL can be considered as the detoxification pathway (Malekinejad et al., 2005a; Yang et al., 2017). In pigs and ruminants, a small amount of ZEN is also metabolized to zearalanone (ZAN), and part of α/β-ZOL can be further reduced to α/β-zearalanol (α/β-ZAL) (Erasmuson et al., 1995). After ZEN is metabolized in the liver, part of ZEN and its metabolites can be excreted into the intestines via bile and then return to the liver via the enterohepatic circulation, continuously circulating. Studies have shown that the half-life of ZEN in plasma is 87 h. Blocking the enterohepatic circulation by ligating the bile duct can shorten the half-life to 3 h (Biehl et al., 1993). Furthermore, milk is also one of the excretion pathways of ZEN and its metabolites. ZEN and its metabolites have been detected in the milk of cows and sows (Prelusky et al., 1990; Schoevers et al., 2012).
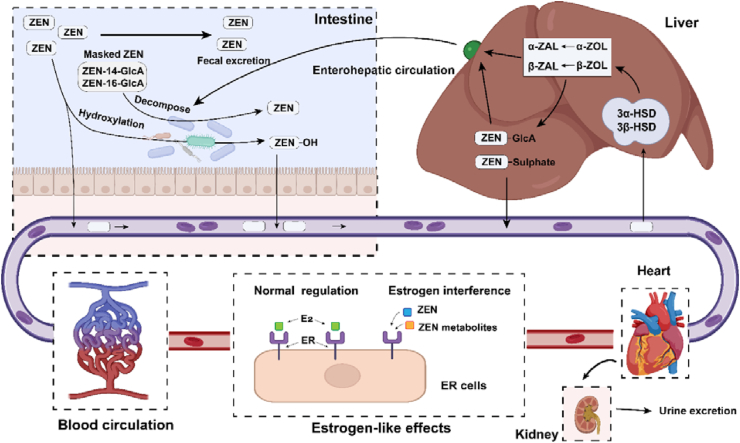
Zearalenone and its metabolites can be coupled with glucuronic acid and sulfate under the catalysis of uridine diphosphate glucuronidase transferase and sulfotransferase, respectively. Most of the produced conjugated compounds enter the bile and are excreted into the intestine, and then they are absorbed and metabolized by intestinal mucosal cells. These compounds then circulate through the portal vein blood to various body organs (Biehl et al., 1993), and the remaining glucuronide conjugates are excreted in urine and bile (Kiessling and Pettersson, 1978). Glucuronic acid chimeric compounds lack estrogen's biological activity, so the combination of ZEN and glucuronic acid is considered a detoxification reaction. The body clears ZEN and its metabolites through glucuronic acid acidification and enterohepatic circulation. Furthermore, it has been reported that ZEN can be hydroxylated into 8-OH-ZEN, 13-OH-ZEN, and 15-OH-ZEN in humans and rats (Drzymala et al., 2015). Their similar estrogen effects are less than ZEN, indicating that oxidation is also a detoxification pathway of ZEN (Bravin et al., 2009). Fig. 2 summarizes the metabolic process of ZEN in animals. A key part of developing an antidote to reduce the toxicity of ZEN is to clarify zearalenone metabolism in poultry.
Fig. 2.
The metabolic process of zearalenone (ZEN) in animals. Intestine: After intestinal absorption of ZEN, some part of ZEN is affected by intestinal microbes and is hydroxylated, and the remaining ZEN is excreted with feces. Liver: Some ZEN metabolism and detoxification products are excreted into the intestines through bile, entering the enterohepatic circulation. The metabolism and detoxification products are absorbed into the blood circulation, and part is excreted in urine and milk. ER cells: ZEN and its metabolites compete for the binding site of E2 and interfere with the normal function of estrogen. ZAL = zearalanol; ZOL = zearalenol; HSD = hydroxysteroid dehydrogenase; GlcA = glucuronic acid; E2 = 17-β estradiol; ER = estrogen receptor.
1.3. The different sensitivity of animals to zearalenone
There are apparent species differences in the sensitivity of animals to ZEN. The sensitivity hierarchy to ZEN is generally considered to be pig > rodent > poultry (Biehl et al., 1993). When the dietary ZEN content reaches 1 mg/kg, it can cause estrogen effects to sows in the first stage estrus (Malekinejad et al., 2006). Higher doses can even cause embryonic dysplasia and miscarriage in pregnant sows (Zhang et al., 2018). ZEN can also cause reproductive disorders such as testicular atrophy and decreased male hormone secretion in boars (Berger et al., 1981). Poultry have a relatively stronger tolerance of ZEN. Apparent reproductive toxicity has only been observed at 400 mg/kg ZEN in finishing broiler chickens and young turkeys (Allen et al., 1981a, Allen et al., 1981b), and reduced growth performance and achondroplasia at 2 mg/kg ZEN (Chen et al., 2019). The fact that pigs are more sensitive to ZEN and chickens are relatively insensitive raises a question: whether chickens have a different metabolic mechanism for ZEN than pigs, resulting in chickens’ relative tolerance of ZEN? Therefore, the purpose of this review is to discuss and summarize the latest data on ZEN toxicity to poultry and methods to mitigate ZEN toxicity. In addition, this review also focuses on possible mechanisms by which poultry are relatively insensitive to ZEN, such as the low absorption rate of ZEN and the rapid elimination of metabolites, the relatively high proportion of β-ZOL produced by the liver, and the metabolic effects of intestinal microorganisms on ZEN.
2. The toxicity of zearalenone to poultry
2.1. Reproductive toxicity
Zearalenone and its metabolites can compete with E2 to bind to ER to produce estrogen-like effects, which can interfere with the expression and function of estrogen in the gonads (Bovee et al., 2004; Ueno et al., 1983). At present, the contamination and harm caused by ZEN are concerning for feed and poultry production. Feeding laying hens with 10 mg/kg ZEN-contaminated feed resulted in the accumulation of large amounts of ZEN metabolites in the liver, egg yolk, and excrement (Dailey et al., 1980). Feeding adult female turkeys with 100 mg/kg ZEN feed resulted in a 20% reduction in egg production (Allen et al., 1983). Male turkeys fed 400 and 800 mg/kg ZEN had a reduced sperm percentage and fertilization rate, and it promoted precocity (Allen et al., 1981a, Allen et al., 1981b). Therefore, ZEN has low reproductive toxicity to poultry.
2.2. Other toxicity
Although ZEN has low reproductive toxicity to poultry, studies have found that ZEN is more toxic to other organs of poultry. As many tissues and cells such as the uterus, breast, liver, and immune cells also have ER and are regulated by estrogen (Hueza et al., 2014), the destruction of the estrogen balance can also damage their health. The classic nuclear receptors of estrogen are divided into ER-α and ER-β. Feeding 0.4 mg/kg of ZEN changed the serum levels of aspartate aminotransferase (AST) and serum alkaline phosphatase (ALP) in adult hens (Cheng et al., 2017) and increased serum levels of low-density lipoprotein (LDL) and cholesterol in growing layers (Wu et al., 2016). ZEN at 2 mg/kg can cause reduced growth performance and achondroplasia in broilers and increase liver weight (Chen et al., 2019), ZEN, and ZEN's metabolites residues in liver (Zhu et al., 2016). ZEN at 2 mg/kg also caused a decrease in total protein levels, albumin, and antioxidant enzymes in broilers' serum, and an increase in AST and Alanine aminotransferase (ALT) (Zhu et al., 2016). Ling et al. (2019) found that ZEN higher than 5 mg/kg increased the average feed-to-egg ratio of laying hens and induced severe inflammation (Ling et al., 2019). The addition of 7.9 mg/kg of ZEN to the broiler diet increased the activity of glutathione peroxidase (GSH-Px) in kidney tissue, increased the activity of γ-glutamyl transferase (GGT) in plasma, and oxidative stress occurred (Gresakova et al., 2012). Interestingly, studies have shown that estrogen is an important regulator of the immune system, and ER-α is expressed in many types of immune cells (Li et al., 2018). However, current research on immunotoxicity concerns mostly cell research, and there is a lack of research on ZEN-induced immunotoxicity in poultry. Therefore, we should pay attention to other types of damage caused by ZEN to poultry. Table 1 provides an introduction to research on the toxicity of ZEN to poultry.
Table 1.
The toxicity of zearalenone (ZEN) to poultry.
| Item | ZEN Concentration | Symptoms | Reference |
|---|---|---|---|
| Laying hens | 0.4 mg/kg | increased relative weight of oviduct and ovary, degeneration and atrophy of the ovarian tissues | Chen et al. (2017) |
| 0.4 mg/kg | increased activity of GSH-Px and T-SOD | Wu et al. (2016) | |
| 5 to 200 mg/kg | interfered with the secretion of sex hormones β-endorphin, LH, and progesterone. Renal edema and nephremia, increased content of urea and creatinine | Ling et al. (2019) | |
| 7.9 mg/kg | increased activity of GSH-Px and GGT, increased the levels of MDA | Gresakova et al. (2012) | |
| Broilers | 0.85 mg/kg | decreased the concentrations of triglyceride and globulin, increased ALT activities | Wang (2016) |
| 2 mg/kg | increased relative weight of liver, ALT and AST activities | Chen et al. (2019) | |
| 2 mg/kg | increased the levels of MDA, decreased the concentrations of SOD and GSH-Px | Zhu et al. (2016) | |
| 800 mg/kg | reduced weight of comb and testes | Allen et al. (1981) | |
| Turkey | 400 mg/kg | increased development of dewlaps and caruncles, and exhibited considerable strutting behavior | Allen et al. (1981) |
| Chicken lymphocytes | 0.1, 1 μg/mL | increased activity of acetyl cholinesterase, increased the levels of MDA | Lautert et al. (2014) |
| 5 μg/mL | increased ROS generation, induced ER stress, and triggered apoptosis | Xiao et al. (2019a) | |
| 1.6 to 25 μg/mL | pH, calmodulin concentrations of supernatants, and intracellular Na+/K+-ATPase and Ca2+-ATPase activities decreased | Wang et al. (2012a) | |
| 6.25 and 25 μg/mL | IL-2 levels increased; IL-6 levels were critically suppressed | Wang et al. (2012b) |
GSH-Px = glutathione peroxidase; T-SOD = total superoxide dismutase; LH = luteinizing hormone; GGT = gamma glutamyl transferase; MDA = malondialdehyde; ALT = alanine aminotransferase; AST = acute suppurative thyroiditis; SOD = superoxide dismutase; ROS = reactive oxygen species; ER = estrogen receptor; IL = interleukin.
3. Possible mechanisms of low reproductive toxicity of zearalenone in poultry
Early studies reported that 50 to 200 mg/kg of ZEN in diets affected 30-week-old laying hens and turkeys, and although some physiological and biochemical indicators were affected, it did not affect reproductive function (Allen et al., 1981a, Allen et al., 1981b; Chi et al., 1980). Only at 400 and 800 mg/kg ZEN, it increased dewlaps, caruncles, and considerable strutting behavior exhibited (Allen et al., 1981a, Allen et al., 1981b; Chi et al., 1980). Conversely, 1.1 mg/kg of ZEN can cause reproductive system diseases in pigs, including redness and swelling of the vulva, rectal and vaginal bleeding, enlarged uterus, and ovarian atrophy (Jiang et al., 2011). Why are pigs more sensitive to ZEN whereas chickens are relatively insensitive? The current widely held view is that pigs metabolize ZEN more into α-ZOL, which has a strong estrogen effect, and chickens produce more β-ZOL (Devreese et al., 2015). Furthermore, this review also discusses and summarizes the possible reasons why other poultry are relatively insensitive to ZEN, such as the activity of 3-HSD, excretion of feces, enterohepatic circulation, and influence of microorganisms on ZEN hydroxylation and masked toxins.
3.1. The levels of α-ZOL and β-ZOL and the ratio of α-ZOL to β-ZOL
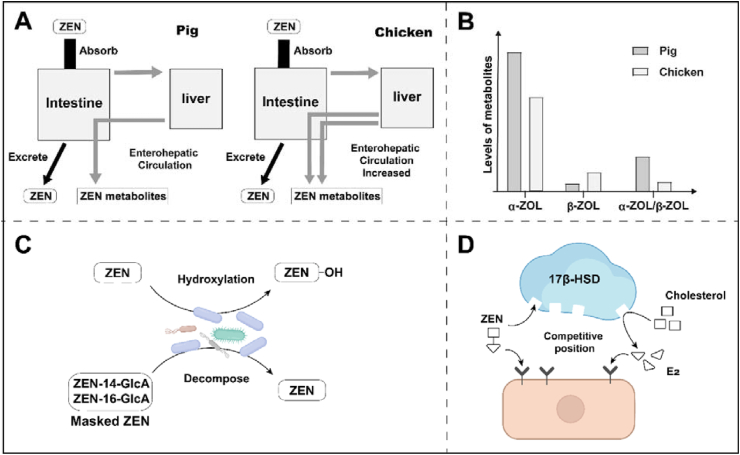
Zearalenone produces different metabolites in different species. Zearalenone is mainly metabolized to α-ZOL in humans, pigs, and mice (Malekinejad et al., 2005b; Yang et al., 2007) whereas it is mainly metabolized to β-ZOL in poultry and ruminants (Pillay et al., 2002; Videmann et al., 2008). If sorted, the ratio of α-ZOL to β-ZOL is 1 to 2 for quails, about 2 for geese, about 3 to 5 for ducks and chickens, and more than 10 for rats (Kolf-Clauw et al., 2008). The traditional concept suggests that the different ratios of α-ZOL to β-ZOL produced by ZEN metabolism cause differences in the sensitivity of pigs and chickens. It is worth noting that most of these conclusions are based on in vitro experiments using microsomal metabolism. It has been reported that the production of chicken α-ZOL and β-ZOL in vivo experiments is similar, which is significantly different from the in vitro results reported at the same time (Yang et al., 2017). Danicke et al. (2002) detected that the production of α-ZOL and β-ZOL in liver and bile were roughly similar. These studies indicate that the in vivo animal experiment results and in vitro microsome or cell experiments may be inconsistent. Recent studies have shown that α-ZOL appears to be the major metabolite of ZEN in broilers. After broilers were gavaged with ZEN at 1.2 mg/kg BW, the contents of α-ZOL in the liver, kidney, and small intestine were 105.2 ± 43.8 and 30.9 ± 13.7, 77.99 ± 23.1, respectively, and the contents of β-ZOL were 36.6 ± 9.1, 626.1 ± 135.6 and 198.3 ± 60.2 μg/kg, respectively (Buranatragool et al., 2015). Contrary to Pillay et al. (2002) and Videmann et al. (2008), Kolf-Clauw et al. (2008) have found that poultry mainly metabolize ZEN into α-ZOL, and the degree of metabolization is lower than that of rats (Kolf-Clauw et al., 2008). This suggests that the ratio of α-ZOL to β-ZOL may not be the only reason for chickens’ low sensitivity. The sensitivity of animals to ZEN may also have a special relationship with the production level of α-ZOL (Fig. 3A).
Fig. 3.
Metabolic characteristics and estrogenic effects of zearalenone (ZEN). (A) Compared with pigs, chickens excrete more ZEN and metabolites of ZEN through bile. (B) The main metabolite of ZEN is α-ZOL in both chickens and pigs. Compared with pigs, chickens produce less α-ZOL, more β-ZOL, and a smaller ratio of α-ZOL to β-ZOL. (C) Intestinal microbes can hydroxylate ZEN or release ZEN after metabolizing masked ZEN. (D) ZEN can compete with E2 and cholesterol for substrate sites, affecting estrogen function and synthesis. ZEN = zearalenone; ZOL = zearalenol; HSD = hydroxysteroid dehydrogenase; GlcA = glucuronic acid; E2 = 17-β estradiol.
3.2. Hydroxylation of zearalenone
Based on toxicokinetic information, the bioavailability of ZEN in pigs is as high as 80% to 85%, while it is less than 10% in poultry and mice (Mendez-Catala et al., 2020; Yang et al., 2017). The hydroxylation of ZEN is considered a detoxification process. Pigs metabolize ZEN into α-ZOL rather than hydroxylation. The lower degree of ZEN hydroxylation may partly explain the higher sensitivity of pigs to ZEN. In vitro microsomal experiments have shown that, compared with pigs and humans, ZEN is more likely to be hydroxylated by chicken microsomes to produce 4-OH-ZEN, 5-OH-ZEN, and 9-OH-ZEN, but in vivo experiments did not show this. Hydroxylated metabolites were found in chicken feces (Yang et al., 2017). In vivo experiments have further proved that reduction, hydroxylation, glucuronidation, and glucuronidation are the primary metabolic pathways in rats, but reduction and sulfation dominate in chickens (Yang et al., 2017).
It should be noted that for the hydroxylated metabolites of ZEN, in vitro and in vivo results differ. For example, 8-OH-ZEN, 13-OH-ZEN, and 15-OH-ZEN are the primary of ZEN metabolites in rat liver microsomes, but the 4-OH-ZEN and 5-OH- ZEN are the primary of ZEN metabolites in rat's liver (Yang et al., 2017). The microbial flora of the rat's intestinal tract may be affected by the hydroxylation process of ZEN. In some studies, after intravenous injection of ZEN in broilers and laying hens, α-ZOL and β-ZOL were equally detected in the serum, and after oral administration, the biotransformation of β-ZOL was increased (Devreese et al., 2015). This indicates that the mode of administration can affect ZEN's metabolic pathway, which is mutually corroborated by the results of Devreese et al. (2015). In conclusion, ZEN's hydroxylation in the liver and intestinal microflora may be a reason for the difference in insensitivity.
3.3. Fecal excretion and enterohepatic circulation
The adverse effects of zearalenone are partly determined by the processes of elimination, and fecal and biliary excretion are important processes affecting the fate of ZEN. It has been reported that after exposure to ZEN, broilers began to excrete large amounts of ZEN metabolized α-ZOL and β-ZOL through feces at 9 h (Buranatragool et al., 2015). In turkeys fed with 800 mg/kg of ZEN, the top 3 samples with ZEN and α-ZOL content were the fecal (182 ± 33 and 644 ± 86 μg/g), liver (276 ± 54 and 2,715 ± 590 ng/g), and kidney (122 ± 25 and 477 ± 53 ng/g). Only trace amounts of β-ZOL were detected in the plasma, excreta, and tissues (Olsen et al., 1986). ZEN being excreted largely by α-ZOL of chickens explains the different sensitivity between animals from another perspective (Minervini and Dell'Aquila, 2008).
As a component of the enterohepatic circulation, bile also plays an important role in the excretion of ZEN and its metabolites by poultry (Danicke et al., 2004). Notably, one study showed that the average of total ZEN toxin level in chicken liver is 3.25 μg/kg, while the average toxin level in bile is as high as 165.0 μg/L (Danicke et al., 2002). The total ZEN toxin level was 52.7 to 123.8 μg/L in pig bile and 3.2 to 22 μg/kg in liver, respectively. In other words, the level of ZEN in pig bile only 4.3 to 38.7 times that of the liver (Schneweis et al., 2005), which is lower than the excretion capacity of chickens. The excretion capacity of feces and bile of chickens may be stronger than that of pigs, so ZEN and its metabolites can be excreted faster, shortening the time for harmful substances to affect chickens adversely (Fig. 3B).
3.4. Masked toxins
In addition to ZEN's metabolism by the liver, it is interesting that microorganisms are also involved in the metabolism of other forms of ZEN (Boevre et al., 2015). ZEN's modification (extractable conjugated binding form) and masked (non-extractable binding form) are not easily detected by conventional analytical procedures (Knutsen et al., 2017). The gut microbes can metabolize modified ZEN and then release ZEN (Gratz et al., 2017). Plants can metabolize ZEN into more polar derivatives, such as ZEN-14-sulfate, ZEN-14-glucoside, and ZEN-16-glucoside (Fig. 3C) (Catteuw et al., 2019). These substances can be converted back to ZEN in the gastrointestinal tract of pigs and restore toxicity (Binder et al., 2017). From the previous summary, we can see that intestinal microbes are involved in ZEN's hydroxylation and the conversion of masked toxins. Studies have used an in vitro model system to evaluate the contribution of intestinal microbial metabolism to the activation and detoxification of ZEN. It was found that the activity of intestinal microbes was equivalent to 36% of liver activity, and this may also be one of the important factors in the sensitivity of different animals to ZEN (Mendez-Catala et al., 2020). However, this system has only been established in mammals. The toxicological model of intestinal microbial metabolism of ZEN in poultry, the effects of modified and masked forms of intestinal microbial metabolism of ZEN, and the metabolites need to be further studied. The modified and masked ZEN forms need to be considered for risk assessment of poultry to ZEN in the future (Liu and Applegate, 2020).
3.5. Other factors
Hydroxysteroid dehydrogenase (HSD) catalyze ZEN's biotransformation and play an important role in regulating the homeostasis of hormones in advance of receptor levels. The abundance of nicotinamide adenine dinucleotide phosphate (NADPH) in porcine liver microsomes can promote the reduction of 3α-HSD and inhibit the reducibility of 3β-HSD, thereby promoting ZEN conversion to α-ZOL (Malekinejad et al., 2005a; Penning et al., 2004). In addition, the ability of pigs to metabolize ZEN into α-ZOL than β-ZOL is greater than that of poultry, which may also be an important factor in the sensitivity of pigs to ZEN (Frizzell et al., 2015). Studies have suggested that the enzyme types and activities of 3α-HSD and 3β-HSD in poultry may be different from those in mammals, and this has been proven by in vitro experiments (Kolf-Clauw et al., 2008). There may be many types of ZEN reductase in poultry (Zinedine et al., 2007). These studies suggest that differences in HSD may be an important factor in the relative insensitivity of poultry.
Other factors may affect the sensitivity of poultry to ZEN. The level of estrogen in the blood of poultry is naturally 1.1 to 3.0 times higher than that of pigs, which may help poultry adapt and resist the interference of ZEN on estrogen at low concentrations and reduce sensitivity to ZEN (Liu and Applegate, 2020). Cell tests have shown that ZEN is a complete activator of ER-α receptors but only a partial activator of ER-β (Kuiper et al., 1998). In addition, it has been reported that the relative affinities of α-ZOL to the ER of pig uterus, rat uterus, and chicken fallopian tube cytoplasm are 1.00, 0.68, and 0.40, respectively (Fitzpatrick et al., 1989). This shows that the affinity of chicken ER to α-ZOL is lower than that of pigs, which also contributes to tolerance of ZEN. Moreover, ZEN has an estrogen-like effect and a substrate that competes with anabolic hormone-related enzymes, such as 17α/β-HSD (Fig. 3D), may affect the synthesis and metabolism of steroid hormones, and then cause the body disorders of hormone secretion (Zheng et al., 2019).
4. Detoxification measures for zearalenone
Although ZEN has a low reproductive toxicity to poultry, it can still damage the liver and kidneys, affect blood lipid levels, and induce oxidative stress in the body. Therefore, ZEN contamination in feed still needs to be detoxified. At present, physical adsorbents, exogenous substances, or biological methods are predominantly used to eliminate or reduce the toxicity of ZEN. The physical adsorbent mainly adsorbs ZEN through physical means to prevent its absorption in the animal intestine. This includes hydrated sodium calcium aluminosilicates, activated carbon, montmorillonite clays, clays such as kaolinite, and yeast cell walls (Kogan and Kocher, 2007). Selenium also has a protective effect on ZEN-induced cytotoxicity (Xiao et al., 2019, Xiao et al., 2019). Plant extracts generally speed up the elimination of ZEN from the body or reduce the binding to target organs by improving the metabolic function or stress state of the animal. Gao et al. (2018) found that silymarin can reduce ZEN-induced hepatotoxicity and reproductive toxicity in rats. Ben et al. (2015) found that crocin can significantly alleviate oxidative stress caused by ZEN in the liver and kidneys of mice. Boeira et al. (2014) also reported that lycopene has an excellent protective effect on hematology, reproduction, and pathological damage in mice caused by high-dose ZEN treatment.
In addition to physical adsorbents and detoxification mitigators, the reduction of ZEN by microorganisms has also received greater attention in recent years. Molnar et al. (2004) found that Trichosporon mycotoxinivorans can detoxify 1 mg/kg ZEN into substances without estrogenic effects within 24 h. The Pseudomonas putida can effectively degrade ZEN into low- or non-toxic substances (Altalhi and El-Deeb, 2009). It has also been reported that Aspergillus niger strain FS10 can reduce the content of ZEN by destroying the ring structure of ZEN (Sun et al., 2014). However, only a few ZEN-degrading bacteria have a determined mechanism of detoxification, and most ZEN-degrading bacteria work through unexplained detoxification mechanisms. For example, a non-pathogenic Rhodococcus pyridinivorans K408 strain proved to have a degradation efficiency of 87.2% for ZEN. This strain does not produce any metabolites with estrogenic effects, but the degradation products are unknown (Kriszt et al., 2012).
In summary, physical adsorbents currently remain the most widely used detoxification method, and plant extracts are often used in combination because of their comprehensive sources and easy availability. Presently, microbial feed detoxification agents have gradually become a central issue in detoxification agents’ research, but more research and breakthroughs are still needed. In the future, it will be necessary to explore the mechanism of toxicity of ZEN to poultry and develop suitable detoxifiers based on its metabolic characteristics to reduce the harm of ZEN (Zinedine et al., 2007).
5. Conclusion
Zearalenone is one of the main contaminating mycotoxins in feed. The metabolic pathway of ZEN is mainly a reduction reaction and binding reaction in animals. Chickens have a high tolerance to ZEN, but ZEN can also damage poultry's physiological functions at low doses and cause reproductive toxicity in poultry at high doses. Based on previous studies, we believe that the possible reasons for poultry's relative insensitivity to ZEN include the following: 1) Although α-ZOL is also the main metabolite of ZEN in poultry, the amount of α-ZOL and the ratio of α-ZOL to β-ZOL in poultry are still lower than in mammals; 2) Due to the stronger enterohepatic circulation and poultry's excretion capacity, ZEN and its metabolites can be excreted quickly and in large quantities; 3) In addition, the intestinal microbes of poultry further assist some ZEN being converted to low-toxicity products such as hydroxylate ZEN, and the intestinal microbes of poultry may have lower utilization of masked toxins in the feed; 4) Some other factors, such as the difference in the activity of chicken and pig HSD, the high level of natural estrogen in chickens, and the low affinity of ER for α-ZOL, are caused by the same factors. In the future, we should clarify ZEN's toxicity mechanism in poultry, supplement and improve the limit standards of ZEN and laws in the feed and food industries, and develop effective detoxification measures to reduce the losses caused by ZEN to the poultry industry.
Author contributions
Kuntan Wu: writing – reviewing, editing, and visualization. Chenxi Ren: writing – original draft preparation, conceptualization. Yangfan Gong: data curation. Xin Gao: investigation, software. Shahid Ali Rajput: methodology. Desheng Qi and Shuai Wang: supervision.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This research was funded by the National Key Research and Development Program of China (Project no. 2016YFD0501207).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Desheng Qi, Email: qds@mail.hzau.edu.cn.
Shuai Wang, Email: wangshuai@mail.hzau.edu.cn.
References
- Abid-Essefi S., Ouanes Z., Hassen W., Baudrimont I., Creppy E., Bacha H. Cytotoxicity, inhibition of DNA and protein syntheses and oxidative damage in cultured cells exposed to zearalenone. Toxicol Vitro. 2004;18(4):467–474. doi: 10.1016/j.tiv.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Allen N.K., Mirocha C.J., Aakhus-Allen S., Bitgood J.J., Weaver G., Bates F. Effect of dietary zearalenone on reproduction of chickens. Poultry Sci. 1981;60(6):1165–1174. doi: 10.3382/ps.0601165. [DOI] [PubMed] [Google Scholar]
- Allen N.K., Mirocha C.J., Weaver G., Aakhus-Allen S., Bates F. Effects of dietary zearalenone on finishing broiler chickens and young Turkey poults. Poultry Sci. 1981;60(1):124–131. doi: 10.3382/ps.0600124. [DOI] [PubMed] [Google Scholar]
- Allen N.K., Peguri A., Mirocha C.J., Newman J.A. Effects of fusarium cultures, T-2 toxin, and zearalenone on reproduction of Turkey females. Poultry Sci. 1983;62(2):282–289. doi: 10.3382/ps.0620282. [DOI] [PubMed] [Google Scholar]
- Altalhi A.D., El-Deeb B. Localization of zearalenone detoxification gene(s) in pZEA-1 plasmid of Pseudomonas putida ZEA-1 and expressed in Escherichia coli. J Hazard Mater. 2009;161(2–3):1166–1172. doi: 10.1016/j.jhazmat.2008.04.068. [DOI] [PubMed] [Google Scholar]
- Ben S.I., Boussabbeh M., Helali S., Abid-Essefi S., Bacha H. Protective effect of Crocin against zearalenone-induced oxidative stress in liver and kidney of Balb/c mice. Environ Sci Pollut Res Int. 2015;22(23):19069–19076. doi: 10.1007/s11356-015-5086-2. [DOI] [PubMed] [Google Scholar]
- Berger T., Esbenshade K.L., Diekman M.A., Hoagland T., Tuite J. Influence of prepubertal consumption of zearalenone on sexual development of boars. J Anim Sci. 1981;53(6):1559–1564. doi: 10.2527/jas1982.5361559x. [DOI] [PubMed] [Google Scholar]
- Biehl M.L., Prelusky D.B., Koritz G.D., Hartin K.E., Buck W.B., Trenholm H.L. Biliary excretion and enterohepatic cycling of zearalenone in immature pigs. Toxicol Appl Pharmacol. 1993;121(1):152–159. doi: 10.1006/taap.1993.1140. [DOI] [PubMed] [Google Scholar]
- Binder E.M. Managing the risk of mycotoxins in modern feed production. Anim Feed Sci Technol. 2007;133(1):149–166. [Google Scholar]
- Binder S.B., Schwartz-Zimmermann H.E., Varga E., Bichl G., Michlmayr H., Adam G. Metabolism of zearalenone and its major modified forms in pigs. Toxins. 2017;9(2) doi: 10.3390/toxins9020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeira S.P., Filho C.B., Del'Fabbro L., Roman S.S., Royes L.F., Fighera M.R. Lycopene treatment prevents hematological, reproductive and histopathological damage induced by acute zearalenone administration in male Swiss mice. Exp Toxicol Pathol. 2014;66(4):179–185. doi: 10.1016/j.etp.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Boevre M.D., Graniczkowska K., Saeger S.D. Metabolism of modified mycotoxins studied through in vitro and in vivo models: an overview. Toxicol Lett. 2015;233(1):24–28. doi: 10.1016/j.toxlet.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Bovee T.F., Helsdingen R.J., Rietjens I.M., Keijer J., Hoogenboom R.L. Rapid yeast estrogen bioassays stably expressing human estrogen receptors alpha and beta, and green fluorescent protein: a comparison of different compounds with both receptor types. J Steroid Biochem Mol Biol. 2004;91(3):99–109. doi: 10.1016/j.jsbmb.2004.03.118. [DOI] [PubMed] [Google Scholar]
- Bravin F., Duca R.C., Balaguer P., Delaforge M. In vitro cytochrome p450 formation of a mono-hydroxylated metabolite of zearalenone exhibiting estrogenic activities: possible occurrence of this metabolite in vivo. Int J Mol Sci. 2009;10(4):1824–1837. doi: 10.3390/ijms10041824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buranatragool K., Poapolathep S., Isariyodom S., Imsilp K., Klangkaew N., Poapolathep A. Dispositions and tissue residue of zearalenone and its metabolites alpha-zearalenol and beta-zearalenol in broilers. Toxicol Rep. 2015;2:351–356. doi: 10.1016/j.toxrep.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catteuw A., Broekaert N., De Baere S., Lauwers M., Gasthuys E., Huybrechts B. Insights into in vivo absolute oral bioavailability, biotransformation, and toxicokinetics of zearalenone, alpha-zearalenol, beta-zearalenol, zearalenone-14-glucoside, and zearalenone-14-sulfate in pigs. J Agric Food Chem. 2019;67(12):3448–3458. doi: 10.1021/acs.jafc.8b05838. [DOI] [PubMed] [Google Scholar]
- Chang J., Wang T., Wang P., Yin Q., Liu C., Zhu Q. Compound probiotics alleviating aflatoxin B1 and zearalenone toxic effects on broiler production performance and gut microbiota. Ecotoxicol Environ Saf. 2020;194:110420. doi: 10.1016/j.ecoenv.2020.110420. [DOI] [PubMed] [Google Scholar]
- Chen P., Liu T., Jiang S., Yang Z., Huang L., Liu F. Effects of purified zearalenone on selected immunological and histopathologic measurements of spleen in post-weanling gilts. Anim Nutr. 2017;3(3):212–218. doi: 10.1016/j.aninu.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Cheng Y., Wen C., Wang W., Kang Y., Wang A. The protective effects of modified palygorskite on the broilers fed a purified zearalenone-contaminated diet. Poultry Sci. 2019;98(9):3802–3810. doi: 10.3382/ps/pez085. [DOI] [PubMed] [Google Scholar]
- Cheng Q., Jiang S.Z., Li S.Q., Wang Y.X., Zhang C.Y., Yang W.R. Effects of low-dose zearalenone-contaminated diets with or without montmorillonite clay adsorbent on nutrient metabolic rates, serum enzyme activities, and genital organs of growing-laying hens. J Appl Poultry Res. 2017;26(3):367–375. [Google Scholar]
- Chi M.S., Mirocha C.J., Weaver G.A., Kurtz H.J. Effect of zearalenone on female White Leghorn chickens. Appl Environ Microbiol. 1980;39(5):1026–1030. doi: 10.1128/aem.39.5.1026-1030.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey R.E., Reese R.E., Brouwer E.A. Metabolism of [14C] zearalenone in laying hens. J Agric Food Chem. 1980;28(2):286–291. doi: 10.1021/jf60228a008. [DOI] [PubMed] [Google Scholar]
- Danicke S., Ueberschar K.H., Halle I., Matthes S., Valenta H., Flachowsky G. Effect of addition of a detoxifying agent to laying hen diets containing uncontaminated or Fusarium toxin-contaminated maize on performance of hens and on carryover of zearalenone. Poultry Sci. 2002;81(11):1671–1680. doi: 10.1093/ps/81.11.1671. [DOI] [PubMed] [Google Scholar]
- Danicke S., Ueberschar K.H., Valenta H., Matthes S., Matthaus K., Halle I. Effects of graded levels of Fusarium-toxin-contaminated wheat in Pekin duck diets on performance, health and metabolism of deoxynivalenol and zearalenone. Br Poultry Sci. 2004;45(2):264–272. doi: 10.1080/00071660410001715876. [DOI] [PubMed] [Google Scholar]
- Devreese M., Antonissen G., Broekaert N., De Baere S., Vanhaecke L., De Backer P. Comparative toxicokinetics, absolute oral bioavailability, and biotransformation of zearalenone in different poultry species. J Agric Food Chem. 2015;63(20):5092–5098. doi: 10.1021/acs.jafc.5b01608. [DOI] [PubMed] [Google Scholar]
- Drzymala S.S., Binder J., Brodehl A., Penkert M., Rosowski M., Garbe L.A. Estrogenicity of novel phase I and phase II metabolites of zearalenone and cis-zearalenone. Toxicon. 2015;105:10–12. doi: 10.1016/j.toxicon.2015.08.027. [DOI] [PubMed] [Google Scholar]
- Erasmuson A.F., Scahill B.G., West D.M. Natural zeranol (alpha.-zearalanol) in the urine of pasture-fed animals. J Agric Food Chem. 1995;42(12):2721–2725. [Google Scholar]
- Fitzpatrick D.W., Picken C.A., Murphy L.C., Buhr M.M. Measurement of the relative binding affinity of zearalenone, alpha-zearalenol and beta-zearalenol for uterine and oviduct estrogen receptors in swine, rats and chickens: an indicator of estrogenic potencies. Comp Biochem Physiol C Comp Pharmacol Toxicol. 1989;94(2):691–694. doi: 10.1016/0742-8413(89)90133-3. [DOI] [PubMed] [Google Scholar]
- Frizzell C., Uhlig S., Miles C.O., Verhaegen S., Elliott C.T., Eriksen G.S. Biotransformation of zearalenone and zearalenols to their major glucuronide metabolites reduces estrogenic activity. Toxicol Vitro. 2015;29(3):575–581. doi: 10.1016/j.tiv.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Gao X., Xiao Z.H., Liu M., Zhang N.Y., Khalil M.M., Gu C.Q. Dietary silymarin supplementation alleviates zearalenone-induced hepatotoxicity and reproductive toxicity in rats. J Nutr. 2018;148(8):1209–1216. doi: 10.1093/jn/nxy114. [DOI] [PubMed] [Google Scholar]
- Gratz S.W., Dinesh R., Yoshinari T., Holtrop G., Richardson A.J., Duncan G. Masked trichothecene and zearalenone mycotoxins withstand digestion and absorption in the upper GI tract but are efficiently hydrolyzed by human gut microbiota in vitro. Mol Nutr Food Res. 2017;61(4) doi: 10.1002/mnfr.201600680. [DOI] [PubMed] [Google Scholar]
- Gresakova L., Borutova R., Faix S., Placha I., Cobanova K., Kosikova B. Effect of lignin on oxidative stress in chickens fed a diet contaminated with zearalenone. Acta Vet Hung. 2012;60(1):103–114. doi: 10.1556/AVet.2012.009. [DOI] [PubMed] [Google Scholar]
- Hueza I.M., Raspantini P.C., Raspantini L.E., Latorre A.O., Gorniak S.L. Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins. 2014;6(3):1080–1095. doi: 10.3390/toxins6031080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S.Z., Yang Z.B., Yang W.R., Gao J., Liu F.X., Broomhead J. Effects of purified zearalenone on growth performance, organ size, serum metabolites, and oxidative stress in postweaning gilts. J Anim Sci. 2011;89(10):3008–3015. doi: 10.2527/jas.2010-3658. [DOI] [PubMed] [Google Scholar]
- Kiessling K.H., Pettersson H. Metabolism of zearalenone in rat liver. Acta Pharmacol Toxicol. 1978;43(4):285–290. doi: 10.1111/j.1600-0773.1978.tb02267.x. [DOI] [PubMed] [Google Scholar]
- Knutsen H.K., Alexander J., Barregard L., Bignami M., Bruschweiler B., Ceccatelli S. Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J. 2017;15(7) doi: 10.2903/j.efsa.2017.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan G., Kocher A. Role of yeast cell wall polysaccharides in pig nutrition and health protection. Livest Sci. 2007;109(1–3):161–165. [Google Scholar]
- Kolf-Clauw M., Ayouni F., Tardieu D., Guerre P. Variations in zearalenone activation in avian food species. Food Chem Toxicol. 2008;46(5):1467–1473. doi: 10.1016/j.fct.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Kouadio J.H., Mobio T.A., Baudrimont I., Moukha S., Dano S.D., Creppy E.E. Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology. 2005;213(1–2):56–65. doi: 10.1016/j.tox.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Kriszt R., Krifaton C., Szoboszlay S., Cserhati M., Kriszt B., Kukolya J. A new zearalenone biodegradation strategy using non-pathogenic Rhodococcus pyridinivorans K408 strain. PloS One. 2012;7(9) doi: 10.1371/journal.pone.0043608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper G.G., Shughrue P.J., Merchenthaler I., Gustafsson J.A. The estrogen receptor beta subtype: a novel mediator of estrogen action in neuroendocrine systems. Front Neuroendocrinol. 1998;19(4):253–286. doi: 10.1006/frne.1998.0170. [DOI] [PubMed] [Google Scholar]
- Lautert C., Ferreiro L., Wolkmer P., Paim F., Da S.C., Jaques J. Individual in vitro effects of ochratoxin A, deoxynivalenol and zearalenone on oxidative stress and acetylcholinesterase in lymphocytes of broiler chickens. SpringerPlus. 2014;1(3):506. doi: 10.1186/2193-1801-3-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zheng R., Gu Y., Tao S., Ren Z., Wang Y. Effects of zearalenone on apoptosis of cultured chicken spleen lymphocytes in vitro. Chinese Journal of Animal Nutrition. 2018;(8):3285–3292. [Google Scholar]
- Ling A., Guo J., Guo W., Yang J., Zhao Z. Effects of zearalenone on performance, Blood index and reproductive hormone levels of laying hens. Shanghai Journal of Agriculture. 2019;35(4):100–106. [Google Scholar]
- Liu J., Applegate T. Zearalenone (ZEN) in livestock and poultry: dose, toxicokinetics, toxicity and estrogenicity. Toxins. 2020;12(6) doi: 10.3390/toxins12060377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R., Zhang L., Liu M., Su Y.T., Xie W.M., Zhang N.Y. Individual and combined occurrence of mycotoxins in feed ingredients and complete feeds in China. Toxins. 2018;10(3) doi: 10.3390/toxins10030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekinejad H., Maas-Bakker R.F., Fink-Gremmels J. Bioactivation of zearalenone by porcine hepatic biotransformation. Vet Res. 2005;36(5–6):799–810. doi: 10.1051/vetres:2005034. [DOI] [PubMed] [Google Scholar]
- Malekinejad H., Maas-Bakker R.F., Fink-Gremmels J. Enzyme kinetics of zearalenone biotransformation: pH and cofactor effects. Arch Toxicol. 2005;79(10):547–553. doi: 10.1007/s00204-005-0664-6. [DOI] [PubMed] [Google Scholar]
- Malekinejad H., Maas-Bakker R., Fink-Gremmels J. Species differences in the hepatic biotransformation of zearalenone. Vet J. 2006;172(1):96–102. doi: 10.1016/j.tvjl.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Mendez-Catala D.M., Spenkelink A., Rietjens I., Beekmann K. An in vitromodel to quantify interspecies differences in kinetics for intestinal microbial bioactivation and detoxification of zearalenone. Toxicol Rep. 2020;7:938–946. doi: 10.1016/j.toxrep.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minervini F., Dell'Aquila M.E. Zearalenone and reproductive function in farm animals. Int J Mol Sci. 2008;9(12):2570–2584. doi: 10.3390/ijms9122570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar O., Schatzmayr G., Fuchs E., Prillinger H. Trichosporon mycotoxinivorans sp. nov., a new yeast species useful in biological detoxification of various mycotoxins. Syst Appl Microbiol. 2004;27(6):661–671. doi: 10.1078/0723202042369947. [DOI] [PubMed] [Google Scholar]
- Olsen M., Mirocha C.J., Abbas H.K., Johansson B. Metabolism of high concentrations of dietary zearalenone by young male Turkey poults. Poultry Sci. 1986;65(10):1905–1910. doi: 10.3382/ps.0651905. [DOI] [PubMed] [Google Scholar]
- Penning T.M., Jin Y., Steckelbroeck S., Lanisnik R.T., Lewis M. Structure-function of human 3 alpha-hydroxysteroid dehydrogenases: genes and proteins. Mol Cell Endocrinol. 2004;215(1–2):63–72. doi: 10.1016/j.mce.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Pillay D., Chuturgoon A.A., Nevines E., Manickum T., Deppe W., Dutton M.F. The quantitative analysis of zearalenone and its derivatives in plasma of patients with breast and cervical cancer. Clin Chem Lab Med. 2002;40(9):946–951. doi: 10.1515/CCLM.2002.166. [DOI] [PubMed] [Google Scholar]
- Prelusky D.B., Scott P.M., Trenholm H.L., Lawrence G.A. Minimal transmission of zearalenone to milk of dairy cows. J Environ Sci Health B. 1990;25(1):87–103. doi: 10.1080/03601239009372678. [DOI] [PubMed] [Google Scholar]
- Rai A., Das M., Tripathi A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit Rev Food Sci Nutr. 2020;60(16):2710–2729. doi: 10.1080/10408398.2019.1655388. [DOI] [PubMed] [Google Scholar]
- Schneweis I., Meyer K., Ritzmann M., Hoffmann P., Dempfle L., Bauer J. Influence of organically or conventionally produced wheat on health, performance and mycotoxin residues in tissues and bile of growing pigs. Arch Anim Nutr. 2005;59(3):155–163. doi: 10.1080/17450390500147594. [DOI] [PubMed] [Google Scholar]
- Schoevers E.J., Santos R.R., Colenbrander B., Fink-Gremmels J., Roelen B.A. Transgenerational toxicity of Zearalenone in pigs. Reprod Toxicol. 2012;34(1):110–119. doi: 10.1016/j.reprotox.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Sun X., He X., Xue K., Li Y., Xu D., Qian H. Biological detoxification of zearalenone by Aspergillus Niger strain FS10. Food Chem Toxicol. 2014;72:76–82. doi: 10.1016/j.fct.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Ueno Y., Tashiro F., Kobayashi T. Species differences in zearalenone-reductase activity. Food Chem Toxicol. 1983;21(2):167–173. doi: 10.1016/0278-6915(83)90232-6. [DOI] [PubMed] [Google Scholar]
- Videmann B., Mazallon M., Tep J., Lecoeur S. Metabolism and transfer of the mycotoxin zearalenone in human intestinal Caco-2 cells. Food Chem Toxicol. 2008;46(10):3279–3286. doi: 10.1016/j.fct.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Wang X. 2016. Study on the detoxification effect of mycotoxin detoxifier BZ on zearalenone. [Google Scholar]
- Wang Y.C., Deng J.L., Xu S.W., Peng X., Zuo Z.C., Cui H.M. Effects of zearalenone on calcium homeostasis of splenic lymphocytes of chickens in vitro. Poultry Sci. 2012;91(8):1956–1963. doi: 10.3382/ps.2011-02128. [DOI] [PubMed] [Google Scholar]
- Wang Y.C., Deng J.L., Xu S.W., Peng X., Zuo Z.C., Cui H.M. Effects of zearalenone on IL-2, IL-6, and IFN-gamma mRNA levels in the splenic lymphocytes of chickens. Sci World J. 2012;2012:567327. doi: 10.1100/2012/567327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F. Mycotoxin risk assessment for the purpose of setting international regulatory standards. Environ Sci Technol. 2004;38(15):4049–4055. doi: 10.1021/es035353n. [DOI] [PubMed] [Google Scholar]
- Wu Y., Yang W., Yang Z., Jiang S., Zhang G., Jiang X. Effects of low dose zearalenone and adsorbent on growth performance,serum biochemical and antioxidant indices of growing-laying hens. Chinese Journal of Animal Nutrition. 2016;28(4):1137–1144. [Google Scholar]
- Xiao Y., Xu S., Zhao S., Liu K., Lu Z., Hou Z. Protective effects of selenium against zearalenone-induced apoptosis in chicken spleen lymphocyte via an endoplasmic reticulum stress signaling pathway. Cell Stress Chaperones. 2019;24(1):77–89. doi: 10.1007/s12192-018-0943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Xu S., Zhao S., Liu K., Lu Z., Hou Z. Protective effects of selenium against zearalenone-induced apoptosis in chicken spleen lymphocyte via an endoplasmic reticulum stress signaling pathway. Cell Stress Chaperones. 2019;24(1):77–89. doi: 10.1007/s12192-018-0943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zhang Y., Wang Y., Cui S. Toxic effects of zearalenone and alpha-zearalenol on the regulation of steroidogenesis and testosterone production in mouse Leydig cells. Toxicol Vitro. 2007;21(4):558–565. doi: 10.1016/j.tiv.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Yang S., Zhang H., Sun F., De Ruyck K., Zhang J., Jin Y. Metabolic profile of zearalenone in liver microsomes from different species and its in vivo metabolism in rats and chickens using ultra high-pressure liquid chromatography-quadrupole/time-of-flight mass spectrometry. J Agric Food Chem. 2017;65(51):11292–11303. doi: 10.1021/acs.jafc.7b04663. [DOI] [PubMed] [Google Scholar]
- Zhang G.L., Feng Y.L., Song J.L., Zhou X.S. Zearalenone: a mycotoxin with different toxic effect in domestic and laboratory animals' granulosa cells. Front Genet. 2018;9:667. doi: 10.3389/fgene.2018.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W., Feng N., Wang Y., Noll L., Xu S., Liu X. Effects of zearalenone and its derivatives on the synthesis and secretion of mammalian sex steroid hormones: a review. Food Chem Toxicol. 2019;126:262–276. doi: 10.1016/j.fct.2019.02.031. [DOI] [PubMed] [Google Scholar]
- Zhu B., Zhang L., Yang W., Cheng Y., Wen C., Zhou Y. Effect of zearalenone on serum parameters, hepatic oxidative damage and residue of zearalenone in broilers. Anim Husb Vet Med. 2016;48(6):10–14. [Google Scholar]
- Zinedine A., Soriano J.M., Molto J.C., Manes J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: an oestrogenic mycotoxin. Food Chem Toxicol. 2007;45(1):1–18. doi: 10.1016/j.fct.2006.07.030. [DOI] [PubMed] [Google Scholar]