Abstract
Background
Primary inflammatory breast cancer (IBC) is a rare and aggressive entity whose prognosis has been improved by multimodal therapy. However, 5-year overall survival (OS) remains poor. Given its low incidence, the prognosis of IBC at metastatic stage is poorly described.
Materials and methods
This study aimed to compare OS calculated from the diagnosis of metastatic disease between IBC patients and non-IBC patients in the Epidemiological Strategy and Medical Economics database (N = 16 702 patients). Secondary objectives included progression-free survival (PFS) after first-line metastatic treatment, identification of prognostic factors for OS and PFS, and evolution of survival during the study period.
Results
From 2008 to 2014, 7465 patients with metastatic breast cancer and known clinical status of their primary tumor (T) were identified (582 IBC and 6883 non-IBC). Compared with metastatic non-IBC, metastatic IBC was associated with less hormone receptor-positive (44% versus 65.6%), more human epidermal growth factor receptor 2-positive (30% versus 18.6%), and more triple-negative (25.9% versus 15.8%) cases, more frequent de novo M1 stage (53.3% versus 27.7%; P < 0.001), and shorter median disease-free interval (2.02 years versus 4.9 years; P < 0.001). With a median follow-up of 50.2 months, median OS was 28.4 months [95% confidence interval (CI) 24.1-33.8 months] versus 37.2 months (95% CI 36.1-38.5 months) in metastatic IBC and non-IBC cases, respectively (P < 0.0001, log-rank test). By multivariate analysis, OS was significantly shorter in the metastatic IBC group compared with the metastatic non-IBC group [hazard ratio = 1.27 (95% CI 1.1-1.4); P = 0.0001]. Survival of metastatic IBC patients improved over the study period: median OS was 24 months (95% CI 20-31.9 months), 29 months (95% CI 21.7-39.9 months), and 36 months (95% CI 27.9-not estimable months) if diagnosis of metastatic disease was carried out until 2010, between 2011 and 2012, and from 2013, respectively (P = 0.003).
Conclusion
IBC is independently associated with adverse outcome when compared with non-IBC in the metastatic setting.
Key words: metastatic breast cancer, inflammatory breast cancer, real-life study, prognostic factors, multimodal therapy
Highlights
-
•
IBC is a rare and aggressive form of breast cancer with poor prognosis.
-
•
OS was compared between IBC and non-IBC patients in a national French cohort of metastatic breast cancer.
-
•
IBC was correlated with more pejorative histologic characteristics.
-
•
Outcomes (OS and PFS) were significantly and independently worse in IBC than in non-IBC metastatic breast cancer.
Introduction
Primary inflammatory breast cancer (IBC) is a rare (5% of all cases) and aggressive form of breast cancer. IBC is classified as T4d in the American Joint Committee on Cancer (AJCC) Cancer Staging, eighth edition,1,2 and diagnosis is based on inflammatory clinical signs arising quickly and pathological confirmation of an invasive carcinoma. Survival of IBC patients was greatly improved by the introduction of a multimodal therapeutic strategy including neoadjuvant chemotherapy. However, the 5-year survival of non-metastatic stages still remains close to 50%-60%.2
Such a poor prognosis of IBC is due in a large part to its strong metastatic potential. Thus, patients with IBC are three times as likely as those with non-inflammatory breast cancer (non-IBC) to present with metastasis on diagnosis.3, 4, 5, 6, 7 In addition, several retrospective studies comparing non-metastatic IBC and locally advanced non-IBC have suggested a significantly worse outcome.8, 9, 10 Yet, in the neoadjuvant setting, our recent results suggest that IBC is not less sensitive to chemotherapy than non-IBC.11
Among stage IV disease, whether the outcome of IBC patients is worse than that of non-IBC patients is still under debate. An analysis of the Surveillance Epidemiology and End Results (SEER) registry found a reduced breast cancer-specific survival in stage IV IBC (n = 1085) compared to stage IV non-IBC (n = 13 280), but the limited number of available clinical data prevented specific multivariate analysis.12 A recent monocentric study from the MD Anderson Cancer Center involving 1504 patients with stage IV disease, including 206 IBC and 1298 non-IBC, was reported. With a median follow-up period of 4.7 years, patients with IBC had a shorter median overall survival (OS) than those with non-IBC, and IBC status was an independent poor prognosis factor.13 Yet, this study did not examine outcomes of metastatic IBC patients with metachronous disease. In addition, patients were enrolled over a large period of time (from 1987 to 2012), which may favor heterogeneity of diagnostic and therapeutic procedures. Thus, data remain limited comparing specific features and outcome of IBC at the metastatic stage.
The Epidemiological Strategy and Medical Economics (ESME) program is an academic initiative led by Unicancer, the French network of cancer centers, to centralize real-life data on metastatic breast cancer (MBC).14 Such a large clinically annotated cohort may be of interest in a rare disease such as IBC. The main objective of the present study was to describe the OS of metastatic IBC patients comparatively to metastatic non-IBC patients. Secondary objectives included description of the population in terms of clinical, pathological, and therapeutic features, the progression-free survival (PFS) after first-line metastatic treatment, specific prognostic factors, and evolution of survival outcome with time.
Materials and methods
Study design and data source
We conducted a non-interventional, retrospective, comparative study based on the ESME-MBC database that is managed by R&D Unicancer. This database gathers individual data from all patients, male or female, ≥18 years, with MBC whose first metastatic disease was treated (either completely or partially) in one of the 18 French cancer centers participating in the ESME program. The resulting cohort represents a nation-wide, population-based registry. As previously described,14 these centralized data do not contain any personal data on patients. In compliance with the authorization delivered by the French Data Protection agency to R&D Unicancer (registration ID 1704113 and authorization N°DE-2013.-117, NCT03275311), only aggregated statistical reports were provided. Moreover, in compliance with the applicable European regulations, a complementary authorization was obtained on 14 October 2019 regarding the ESME Research Data Warehouse. Accordingly, no informed consent signature was required. The present study was approved by an independent ethics committee (Comité de Protection des Personnes Sud-Est II-2015-79). In this study, data collection and follow-up were conducted until the cut-off date of 15 January 2016.
Raw data were generated at the Unicancer large-scale facility. Derived data supporting the findings of this study are available from the corresponding author upon request.
Study population
Eligible patients were diagnosed for metastatic disease between 1 January 2008 and 31 December 2014 and had their initial AJCC T stage available in the database. According to AJCC TNM (tumor–node–metastasis) classification, patients were considered as IBC (T4d) or non-IBC [T0, Tis, Tis (ductal carcinoma in situ), Tis (lobular caricnoma in situ), Tis (Paget), T1, T1 mic, T1a, T1b, T1c, T2, T3, T4, T4a, T4b, T4c]. Diagnosis of IBC was based on clinical signs (redness, edema, ‘peau d'orange’) arising quickly and involving more than one-third of the breast, with or without an underlying palpable tumor with pathological confirmation of an invasive carcinoma. The metastatic disease was defined as de novo (M1) when the metastasis was diagnosed synchronously or ≤6 months after the diagnosis of primary tumor, and recurrent (M0) when the metastasis was diagnosed >6 months after the diagnosis of primary tumor. MBC treatment strategy could include surgery, radiotherapy, chemotherapy, targeted therapy, and endocrine therapy. Breast cancer was hormone receptor positive (HR+) if estrogen receptor or progesterone receptor expression was ≥10% (immunohistochemistry). Human epidermal growth factor receptor 2 (HER2) immunohistochemical (IHC) score 3+ or IHC score 2+ with a positive fluorescence in situ hybridization or chromogenic in situ hybridization classified the tumors as HER2+. Four subtypes were defined according to HR and HER2 statutes: HER2+/HR−, HER2+/HR+, HER2−/HR+, and HR−/HER2− [triple-negative (TN) breast cancer (TNBC)]. HR and HER2 status were evaluated on primary tissue when possible or on metastatic tissue when primary tissue was not available. Menopausal status was approximated according to age, with 52 years as a cut-off (pre-menopausal <52 and post-menopausal ≥52).
Statistical analysis
Descriptive statistics were used to summarize patients' initial characteristics at diagnosis of metastatic disease. They were compared between groups using chi-square or Fisher's exact test for categorical data, and Student's t-test or non-parametric Wilcoxon's test for continuous data; a P value <0.05 was considered statistically significant. The OS was defined as time (months) between diagnosis of metastatic disease and date of death (any cause) or censored to date of latest news. The PFS was defined as time between the starting date of first-line metastatic treatment and date of first disease progression or death, or censored to date of latest news or data cut-off (15 January 2016). Disease progression was defined as the appearance of a new metastatic site, progression of existing metastasis, or local or locoregional recurrence of the primary tumor. Survival curves for OS and PFS with associated log-rank tests were generated using the Kaplan–Meier method. The reverse Kaplan–Meier method was used to estimate the median follow-up duration, beginning at the date of diagnosis of metastatic disease. The Cox proportional hazards model was used to adjust on prognostic factors for the comparison of OS and PFS between IBC and non-IBC. We also used the Cox proportional hazards model to identify prognostic factors for OS and PFS in IBC patients. Pre-specified potential prognostic factors for survival investigated in univariate Cox proportional hazards model were: age at MBC diagnosis (<52 versus ≥52 years), molecular subtypes (HER2+/HR+, HER2+/HR−, HR+/HER2−, TNBC), disease-free interval (synchronous, metachronous ≤24 months or >24 months from primary tumor), number of metastatic sites [(0-3) versus >3], type of metastatic sites (non-visceral metastasis: bone, skin, metastatic lymph nodes; brain visceral metastasis: brain and meninges; non-brain visceral metastasis: liver, lung, other organ), circumstances of diagnosis (systematic exam or symptoms), recurrence (no recurrence, local recurrence, locoregional recurrence), first-line metastatic treatment (endocrine therapy, chemotherapy ± endocrine therapy), and previous adjuvant treatment for M0 disease (none, endocrine therapy, chemotherapy, or both). Variables significant at a 10% level were included in a backward selection procedure to keep factors significant at a 5% level in the final multivariate model. Hazard ratios (HRs) are presented with 95% confidence interval (CI). A logistic regression model was used to identify the risk factors for the presence of brain metastasis. Odds ratios (ORs) are presented with 95% CI. We used SAS software (Statistical Analysis Software, Cary, NC, version 9.4) for all statistical analyses.
Results
Patients' characteristics and treatments
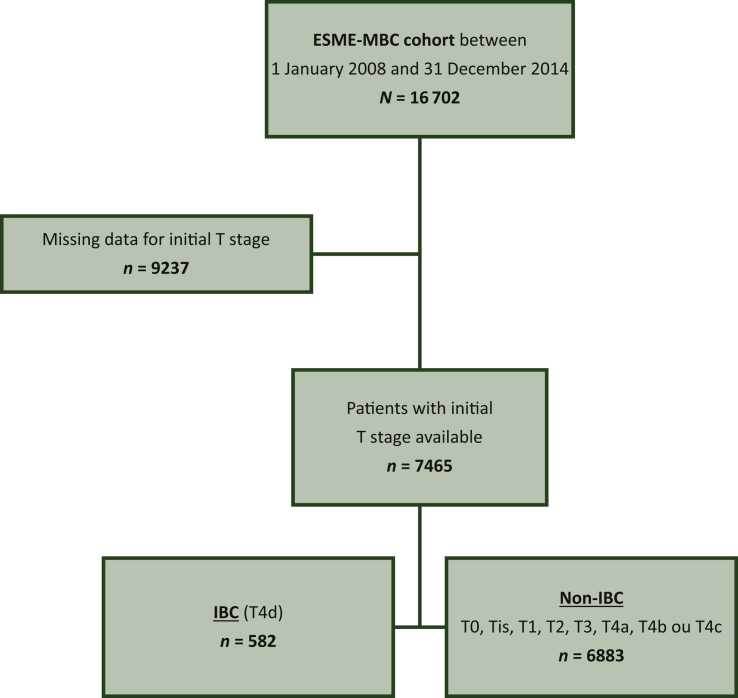
Among the 16 702 patients identified in the ESME-MBC database from January 2008 to December 2014, 7465 had diagnosis of MBC and known clinical status of their primary tumor (T), including 582 IBC (T4d) and 6883 non-IBC (Figure 1).
Figure 1.
Flow chart.
ESME, Epidemiological Strategy and Medical Economics; IBC, inflammatory breast cancer; MBC, metastatic breast cancer.
Patients’ characteristics at initial diagnosis of breast cancer are shown in Table 1. Almost all IBC and non-IBC patients were female. At diagnosis of primary tumor, the median age was not different between IBC and non-IBC patients. Lobular pathological type was less frequent (6.9% versus 14.1%; P < 0.001). Regarding the molecular subtypes of primary tumor and compared to non-IBC, metastatic IBC was significantly associated with less HR+/HER2− tumors (44% versus 65.6%), more HER2+ (30% versus 18.6%), and more TNBC (25.9% versus 15.8%) (P < 0.001). Of note, HR−/HER2+ tumors were more frequent in IBC (18% versus 7%), while HR+/HER2+ had a similar incidence between IBC and non-IBC patients. Regarding treatments of primary tumor in patients with initial M0 stage (272 IBC and 4978 non-IBC), IBC patients received more (neo)adjuvant chemotherapy with or without endocrine therapy (95.2% versus 75.3%) and less endocrine therapy alone (3.3% versus 17.2%) than non-IBC patients (P < 0.001).
Table 1.
Patients and tumor characteristics at initial diagnosis of breast cancer in the whole population
| Non-IBC n (%) |
IBC n (%) |
All n (%) |
P value | |
|---|---|---|---|---|
| n = 6883 | n = 582 | N = 7465 | ||
| Sex | ||||
| Male | 67 (1.0) | 3 (0.5) | 70 (0.9) | 0.3 |
| Female | 6816 (99.0) | 579 (99.5) | 7395 (99.1) | |
| Age at initial diagnosis (years) | ||||
| Median (min; max) | 54 (22; 96) | 55.0 (22; 91) | 54.0 (22; 96) | 0.5 |
| Menopausal status at initial diagnosisa | ||||
| No | 715 (23.5) | 82 (29.3) | 797 (24.0) | — |
| Yes | 2276 (74.8) | 196 (70.0) | 2472 (74.4) | |
| NA (men) | 51 (1.7) | 2 (0.7) | 53 (1.6) | |
| Missing data | 3841 | 302 | 4143 | |
| Histologic type | ||||
| Ductal | 4760 (82.5) | 439 (89.6) | 5199 (83.1) | <0.001 |
| Lobular | 814 (14.1) | 34 (6.9) | 848 (13.5) | |
| Mixed | 82 (1.4) | 3 (0.6) | 85 (1.4) | |
| Other | 113 (2.0) | 14 (2.9) | 127 (2.0) | |
| Missing data | 1114 | 92 | 1206 | |
| Subtypesb | ||||
| HR+ HER2+ | 736 (11.6) | 63 (11.2) | 799 (11.6) | <0.001 |
| HR+ HER2− | 4153 (65.6) | 248 (44.0) | 4401 (63.9) | |
| HR− HER2+ | 442 (7.0) | 106 (18.8) | 548 (8.0) | |
| HR− HER2− | 998 (15.8) | 146 (25.9) | 1144 (16.6) | |
| Missing data | 554 | 19 | 573 | |
| Adjuvant treatment, only for M0 | n = 4978 | n= 272 | N = 5250 | |
|---|---|---|---|---|
| Adjuvant systemic treatment | ||||
| Chemotherapy | 1244 (25.1) | 135 (50.2) | 1379 (26.4) | <0.001 |
| Chemotherapy + endocrine therapy | 2493 (50.2) | 121 (45.0) | 2614 (50.0) | |
| Endocrine therapy | 854 (17.2) | 9 (3.3) | 863 (16.5) | |
| Nothing | 372 (7.5) | 4 (1.5) | 376 (7.2) | |
| Missing data | 15 | 3 | 18 | |
| Adjuvant radiotherapy | ||||
| No | 492 (9.9) | 31 (11.4) | 523 (10.0) | 0.4 |
| Yes | 4483 (90.1) | 241 (88.6) | 4724 (90.0) | |
HR, hormonal receptor; HER2, human epidermal growth factor receptor 2; IBC, inflammatory breast cancer; M0, no metastasis at diagnosis and until 6 months after diagnosis; M1, de novo metastatic disease.
Menopausal status determined by sex and age (cut-off of 52 years).
Subtype phenotypes determined on primary tumor or, if not available, on metastatic tissue.
Patients’ characteristics at diagnosis of metastasis are shown in Table 2. Median age was significantly younger (56 versus 60 years; P < 0.001) and more patients were considered as pre-menopausal (37.5% versus 29.1%; P < 0.001) in the IBC group. Moreover, we observed more frequent de novo (M1 stage at diagnosis) metastatic disease (53.3% versus 27.7%; P < 0.001) and shorter median disease-free interval (2.02 years versus 4.9 years; P < 0.001) in IBC patients. The median number of metastatic sites was similar between both groups. Lung (25.5% versus 17.7%; P < 0.001) and bone (58.1% versus 46.9%; P < 0.001) metastases were more frequent in non-IBC, whereas lymph node (35.6% versus 26.8%; P < 0.001), brain (11.2% versus 7.3%; P < 0.001), and skin metastases (16.3% versus 9.8%; P < 0.001) were more frequent in IBC. The distribution of metastatic involvement was significantly different in M0 patients between the two groups: brain metastases (19.9% versus 8.8%) and non-visceral metastases (43% versus 39.5%) were more frequent, and non-brain visceral metastases were less frequent (37.1% versus 51.7%) in IBC than in non-IBC patients (P < 0.001). On the contrary, this distribution of metastatic sites was similar between IBC and non-IBC for M1 patients (P = 0.7). Of note, the higher frequency of HR− HER2+ and TN subtypes in IBC versus non-IBC was observed in both M0 and M1 groups. There were more HR− HER2+ and less TN in M1 than in M0 patients and it was slightly more pronounced in IBC than in non-IBC (Supplementary Table S1A, available at https://doi.org/10.1016/j.esmoop.2021.100220). Thus, the different distribution of metastatic sites between IBC and non-IBC observed in the M0 group only was unlikely to be essentially explained by a different repartition in subtypes. To examine whether IBC was independently associated with brain metastases, we carried out a logistic regression analysis including the initial stage (M0 or M1), subtypes, and IBC status. We found that IBC patients have a higher risk of brain metastases even after adjustment on all these factors [OR = 1.7 (95% CI 1.23-2.21); P = 0.0008] (Supplementary Table S1B, available at https://doi.org/10.1016/j.esmoop.2021.100220)
Table 2.
Patients and tumor characteristics at metastasis diagnosis in the whole population
| Non-IBC |
IBC |
All |
P value | |
|---|---|---|---|---|
| n = 6883 | n = 582 | N = 7465 | ||
| Age at metastasis diagnosis (years) | ||||
| Median (min; max) | 60.0 (22; 97) | 56.0 (22; 91) | 60.0 (22; 97) | <0.001 |
| Menopausal status at metastasis diagnosis,an (%) | ||||
| No | 2004 (29.1) | 218 (37.5) | 2222 (29.8) | <0.001 |
| Yes | 4812 (69.9) | 361 (62.0) | 5173 (69.3) | |
| NA (men) | 67 (1.0) | 3 (0.5) | 70 (0.9) | |
| Metastatic status at diagnosis, n (%) | ||||
| M0 | 4978 (72.3) | 272 (46.7) | 5250 (70.3) | <0.001 |
| De novo (M1) | 1905 (27.7) | 310 (53.3) | 2215 (29.7) | |
| Metastatic sites, n (%) | ||||
| Visceral disease | 4018 (58.4) | 326 (56.0) | 4344 (58.2) | 0.3 |
| Bone | 4001 (58.1) | 273 (46.9) | 4274 (57.3) | <0.001 |
| Brain | 499 (7.3) | 65 (11.2) | 564 (7.6) | <0.001 |
| Lung | 1754 (25.5) | 103 (17.7) | 1857 (24.9) | <0.001 |
| Lymph node | 1843 (26.8) | 207 (35.6) | 2050 (27.5) | <0.001 |
| Pleura | 739 (10.7) | 55 (9.5) | 794 (10.6) | 0.3 |
| Skin | 677 (9.8) | 95 (16.3) | 772 (10.3) | <0.001 |
| Liver | 1898 (27.6) | 159 (27.3) | 2057 (27.6) | 0.9 |
| Visceral involvement for M0, n (%) | ||||
| n | 4978 | 272 | 5250 | |
| Brain visceral metastasis | 440 (8.8) | 54 (19.9) | 494 (9.4) | <0.001 |
| Non-brain visceral metastasis | 2574 (51.7) | 101 (37.1) | 2675 (51.0) | |
| Non-visceral metastasis | 1964 (39.5) | 117 (43.0) | 2081 (39.6) | |
| Visceral involvement for M1, n (%) | ||||
| n | 1905 | 310 | 2215 | |
| Brain visceral metastasis | 59 (3.1) | 11 (3.5) | 70 (3.2) | 0.7 |
| Non-brain visceral metastasis | 945 (49.6) | 160 (51.6) | 1105 (49.9) | |
| Non-visceral metastasis | 901 (47.3%) | 139 (44.8%) | 1040 (47.0) | |
| Number of metastatic sites | ||||
| Median (min; max) | 1.0 (1; 8) | 1.0 (1; 6) | 1.0 (1; 8) | 0.4 |
| Delay between initial diagnosis and metastases onset (year) only for M0 | ||||
| n | 4978 | 272 | 5250 | |
| Median (min; max) | 4.90 (0.50; 47.94) | 2.02 (0.50; 31.41) | 4.68 (0.50; 47.94) | <0.001 |
| Diagnosis of metastatic relapse, n (%) | ||||
| Systematic examination | 3446 (52.9) | 356 (63.3) | 3802 (53.7) | <0.001 |
| Symptom | 3072 (47.1) | 206 (36.7) | 3278 (46.3) | |
| Missing data | 365 | 20 | 385 | |
| Local or locoregional relapse, n (%) | ||||
| None | 6183 (89.9) | 537 (92.3) | 6720 (90.1) | 0.1 |
| Local relapse | 176 (2.6) | 8 (1.4) | 184 (2.5) | |
| Locoregional relapse | 516 (7.5) | 37 (6.4) | 553 (7.4) | |
| Missing data | 8 | 0 | 8 | |
| First-line treatment, n (%) | ||||
| Chemotherapy ± endocrine therapy ± target therapy | 4413 (66.8) | 477 (86.4) | 4890 (68.3) | <0.001 |
| Endocrine therapy ± target therapy | 2198 (33.2) | 75 (13.6) | 2273 (31.7) | |
IBC, inflammatory breast cancer; M0, no metastasis at diagnosis and until 6 months after diagnosis; M1, de novo metastatic disease; NA, not applicable.
Menopausal status determined by sex and age (cut-off of 52 years).
Consistently with more de novo metastatic disease (M1 stage), the diagnosis of metastases was more frequently based on systematic imaging work-up (63.3% versus 52.9%) than on symptoms in IBC than in non-IBC. Regarding the first-line systemic treatment for metastatic disease, IBC patients were treated more frequently with chemotherapy ± endocrine therapy than non-IBC patients (86.4% versus 66.8%) and less frequently with endocrine therapy ± targeted therapy (13.6% versus 33.2%). Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100220, displays the systemic treatments received for metastatic disease in the whole population. Regarding anti-HER2 drugs received during systemic treatment for metastatic disease, most of HER2+ patients received trastuzumab at least once during the course of the metastatic disease: percentage of patients who received trastuzumab (16.2% for non-IBC and 29.9% for IBC) correspond approximately to HER2+ population (18.6% for non-IBC and 30% for IBC). A minority of patients received anti-HER2 treatment of second generation in both IBC and non-IBC groups.
Overall survival and progression-free survival under first-line treatment in all patients
With a median follow-up of 50.2 months (95% CI 0-104 months) in the whole population, 4307 deaths were reported, and the median OS was 36.4 months (95% CI 35.5-37.9 months). With a similar follow-up between both groups, the median OS was 28.4 months (95% CI 24.1-33.8 months) versus 37.2 months (95% CI 36.1-38.5 months) in IBC and non-IBC cases, respectively (P < 0.0001) (Figure 2A). The 4-year OS was 31% (95% CI 27% to 36%) in IBC and 41% (95% CI 39% to 42%) in non-IBC. In univariate analysis for OS in the whole population, the HR for death was 1.26 (95% CI 1.13-1.41) in IBC patients versus non-IBC patients (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100220). In a multivariate Cox model including all other variables associated with OS by univariate analysis (Figure 2B), IBC remained independently associated with shorter OS [Figure 2B, HR = 1.27 (95% CI 1.12-1.43); P = 0.0001].
Figure 2.
Overall survival (OS) by IBC status (A) and multivariate Cox analyses for OS (B) in the whole population.
CI, confidence interval; CT, chemotherapy; ET, endocrine therapy; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IBC, inflammatory breast cancer.
Among the whole population, 7163 patients received first-line treatment (68.3% by chemotherapy and/or endocrine therapy and/or target therapy; 31.7% by endocrine therapy and/or target therapy). During the follow-up, 6232 disease progressions or deaths were reported. The median PFS was 7.2 months (95% CI 6.6-8.3 months) versus 9.5 months (95% CI 9.1-9.8 months) in IBC and non-IBC cases, respectively (P = 0.01; Supplementary Figure S1A, available at https://doi.org/10.1016/j.esmoop.2021.100220). In univariate analysis for PFS, the HR for disease progression or death was 1.12 (95% CI 1.02-1.23) in IBC patients versus non-IBC patients (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2021.100220). In a multivariate Cox model (Supplementary Figure S1B, available at https://doi.org/10.1016/j.esmoop.2021.100220), IBC remained associated with shorter PFS [HR = 1.15 (95% CI 1.04-1.27); P = 0.007], suggesting independent unfavorable prognostic value.
Specific prognostic factors for survival in IBC patients
We carried out prognostic analyses for OS and first-line PFS specifically in the group of IBC patients (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2021.100220). Four factors were independently associated with OS in multivariate analysis: disease-free interval, nature and number of metastatic sites, and IHC subtypes (Table 3). IBC patients with no synchronous metastatic disease [<2 years versus de novo: HR = 3.0 (95% CI 2.3-4.0); >2 years versus de novo: HR = 1.5 (95% CI 1.15-1.98); P < 0.0001], with brain metastases and non-brain visceral metastases [HR = 2.64 (95% CI 1.84-3.79) and HR = 2.15 (95% CI 1.68-2.74), respectively; P < 0.0001], with more than three metastases sites [HR = 1.52 (95% CI 1.04-2.23); P = 0.03], and with HER2− subtypes, including TN [HR+/HER2−: HR = 1.51 (1.01-2.25); HR−/HER2−: HR = 3.10 (95% CI 2.05-4.70); RH−/HER2+: HR = 0.98 (0.62-1.53); P < 0.0001], were associated with shorter OS. Regarding the PFS under first-line treatment, the same prognostic factors were identified (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2021.100220).
Table 3.
Multivariate Cox analyses for OS and PFS in IBC
| Hazard ratio (95% CI) |
P value |
Hazard ratio (95% CI) |
P value |
|
|---|---|---|---|---|
| OS | PFS | |||
| Disease-free interval | <0.0001 | |||
| De novo | <0.0001 | |||
| 6 months-2 years | 3.00 (2.27-3.96) | 2.51 (1.97-3.21) | ||
| >2 years | 1.51 (1.15-1.98) | 1.34 (1.06-1.70) | ||
| Visceral involvement | ||||
| Non-visceral metastasis | <0.0001 | <0.0001 | ||
| Brain visceral metastasis | 2.64 (1.84-3.79) | 1.69 (1.20-2.39) | ||
| Non-brain visceral metastasis | 2.15 (1.68-2.74) | 1.60 (1.31-1.96) | ||
| Number of metastatic sites | ||||
| 0-3 | 0.03 | 0.03 | ||
| >3 | 1.52 (1.04-2.23) | 1.47 (1.04-2.06) | ||
| IHC subtype | ||||
| HR+ HER2+ | <0.0001 | <0.0001 | ||
| HR+ HER2− | 1.51 (1.01-2.25) | 1.05 (0.76-1.44) | ||
| HR− HER2+ | 0.98 (0.62-1.53) | 0.85 (0.59-1.23) | ||
| HR− HER2− | 3.10 (2.05-4.70) | 1.62 (1.14-2.30) | ||
CI, confidence interval; HR, hormonal receptor; HER2, human epidermal growth factor receptor 2; IBC, inflammatory breast cancer; IHC, immunohistochemical; OS, overall survival; PFS, progression-free survival.
Evolution of survival over time in IBC patients
During the study period (2008-2014), OS and PFS improved over time in IBC patients. Median OS was 24 months (95% CI 20-31.9 months), 29 months (95% CI 21.7-39.9 months), and 36 months (95% CI 27.9-not estimable months), when the diagnosis of metastatic disease was carried out until 2010, between 2011 and 2012, and from 2013, respectively (P = 0.003) (Supplementary Figure S2A, available at https://doi.org/10.1016/j.esmoop.2021.100220). The same time effect was observed for PFS with median values equal to 6.5 months (95% CI 5.1-7.3), 8.3 months (95% CI 6.4-10.3), and 8.3 months (95% CI 6.6-10.9), for each period, respectively (P = 0.03; Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2021.100220). However, a separate analysis by subtype revealed that a significant improvement in OS and PFS over time (P = 0.0007 and P = 0.01, respectively) was solely demonstrated in HER2+ IBC patients (Supplementary Figure S2B, available at https://doi.org/10.1016/j.esmoop.2021.100220), but not in HER2−/HR+ (Supplementary Figure S2C, available at https://doi.org/10.1016/j.esmoop.2021.100220) or TNBC (Supplementary Figure S2D, available at https://doi.org/10.1016/j.esmoop.2021.100220) cases.
Discussion
The present study sheds light on important clinical features of IBC treated in the metastatic setting. Firstly, as already described at the non-metastatic stage, metastatic IBC patients were younger and lobular histology was uncommon. Secondly, the distribution of IBC subtypes was also consistent with that observed in non-metastatic disease: IBC tumors commonly lacked HR expression and had HER2 amplification3,15 and as expected, we observed more TNBC and HER2+ subtypes in IBC (25.9% and 30%, respectively) than in non-IBC (15.8% and 18.6%, respectively) patients. Importantly, only HER2+/HR− were overrepresented in metastatic IBC, while HER2+/HR+ had a similar prevalence in both IBC and non-IBC patients. This observation confirms a specific and subtle interplay between HR and HER2 in IBC. Thirdly, consistent with the higher metastatic ability of IBC, more IBC than non-IBC patients had de novo metastatic disease and, for metachronous disease, the disease-free interval was shorter in IBC patients. Lung and bone metastases were less frequent, while skin and lymph node locations were more frequent in IBC patients, in concordance with the known tropisms of IBC. Of note, while the distribution of metastatic sites was similar in de novo metastatic disease for IBC and non-IBC patients, it was not the case in recurrent disease in which brain metastases were more common and non-brain visceral metastases were less frequent in IBC patients. This may be related to differences in systemic treatments administered at the initial stage, as indicated by the larger prevalence of (neo)adjuvant chemotherapy in IBC patients from this subgroup, consistent with the recent demonstration that previous treatments may dramatically alter genomic makeup and the resulting clinical features and outcomes.16
A main result of our study was the independent poor prognosis value of IBC phenotype, on both PFS after first-line treatment and OS. A previous study, enrolling de novo metastatic patients (stage IV) only and conducted at a single center in a large and relatively earlier period of time (1990-2008), also revealed that IBC phenotype independently conferred a poor prognosis in the metastatic setting.13 Thus, to our knowledge, the present series is the largest reported to date examining the prognostic impact of IBC phenotype, focusing on a modern era (2008-2014), and the first one including patients with both de novo and metachronous disease, the latter representing nearly half of IBC patients with metastatic disease in our series. The reasons behind the poorer outcome in IBC patients even when considered at a metastatic stage are unclear. Yet, this observation supports the hypothesis of an intrinsic distinct biology of the disease associated with higher metastatic propensity than non-IBC, lethality, and therapeutic resistance.
Another important data generated by our study was the specific identification of prognostic factors within the population of metastatic IBC patients. Whereas disease-free interval, visceral involvement, and the number of metastatic sites were identified as independently associated with survival, as already described in non-IBC patients, a provocative result was that HER2+ subtypes displayed the best outcomes, without significant differences between HR−/HER2+ and HR+/HER2+. Conversely, TN, but also luminal HR+/HER2−, subtypes, were associated with poor OS. A similar result was found for PFS, except that HR+/HER2− and HR−/HER2+ had similar PFS as HR+/HER2+ subtypes. While the worst outcome of TN subtype was also pointed out in a recent IBC-specific Dutch study examining the prognostic impact of molecular subtypes in metastatic IBC patients,17 a better outcome for HER2+ compared to HR+/HER2− subtypes was not observed. However, in the latter study, only de novo metastatic IBC was considered and 25%-31% of HER2+ patients did not receive anti-HER2 treatment, while almost all patients with HER2+ IBC from our series received at least trastuzumab. A recent analysis from the overall ESME database also reported the same HER2+ subtype-associated survival advantage, suggesting that in IBC as in non-IBC patients, anti-HER2 treatments had a major impact on the natural history of the disease.18
We also found a significant increase in OS and PFS over time in metastatic IBC patients. However, it was almost exclusively restricted to the HER2+ subtypes. Yet, due to the considered period, only a marginal part of this population received first-line pertuzumab–trastuzumab combination and second-line trastuzumab–emtansine, both being associated with major survival gains, rendering plausible an even more striking progress in the more recent period. By contrast, there was no significant improvement with time for the HER2− subtypes. Thus, as in metastatic non-IBC, therapeutic innovations are eagerly awaited in the non-HER2+ subtypes of IBC.19 Of note, in the absence of IBC-specific data, it remains uncertain how the recent integration of CDK4/6 inhibitors to the therapeutic management of HR+/HER2− metastatic IBC will impact outcomes.20, 21, 22, 23 Similarly, other therapeutics with potential for improving OS in TN subtypes, such as immune checkpoint inhibitors, have not been specifically examined in metastatic IBC.24
As noted earlier, patients with recurrent disease had a particularly poor prognosis, which makes it critical to improve results in the ‘early’ IBC setting. This may rely upon the large use of pertuzumab in the neoadjuvant setting for HER2+ IBC, as well as the post-neoadjuvant trastuzumab–emtansine-based rescue in patients with residual disease, both being associated with significant reduction in disease relapse.25, 26, 27 Similarly, the incorporation of pembrolizumab immune checkpoint inhibitor in the neoadjuvant setting for TN subtypes may improve outcome for IBC patients as recently demonstrated in the general population of TNBC.28 The ongoing PELICAN study conducted in France specifically addresses this issue in a randomized phase II clinical trial enrolling HER2− non-metastatic IBC patients receiving neoadjuvant chemotherapy (NCT03515798).
A limitation of our work was that more than half of the initial population in the ESME database was excluded because of unknown clinical T status. However, we have compared patient characteristics between those with known and with unknown T stage and found that these populations were largely comparable (data not shown). In addition, the ultimate number of IBC patients (n = 582) in this study remains highly significant in such a rare disease. Indeed, to our knowledge, this study is the largest one comparing outcomes in metastatic IBC and non-IBC patients. This large cohort includes patients mostly treated in a real-life setting, avoiding over-selection of patients enrolled in clinical trials. Additional strengths of our study rely on the multicentric design, involving 18 academic centers across France, the relatively recent period of study (2008-2014) compared to other studies,12,13 the quality of data collected by expert centers, and the use of a consensual clinical definition of IBC.
Conclusion
In this large, national, and multicentric study, IBC was an independent factor associated with adverse outcome in the metastatic setting. Real-life databases are powerful tools to investigate clinical outcomes of rare diseases such as IBC. Further translational and clinical research, ideally specifically dedicated to IBC, is mandatory to improve our understanding of disease and the prognosis in this so-devastating disease.
Acknowledgements
We thank the 18 French comprehensive cancer centers for providing the data and each ESME local coordinator for managing the project at the local level. Moreover, we thank the ESME Scientific Group and Strategic Committee for their ongoing support.
Funding
The ESME-MBC database receives financial support from an industrial consortium (Roche, Pfizer, AstraZeneca, MSD, Eisai, and Daiichi-Sankyo) (no grant number). Data collection, analysis, and publication are managed entirely by Unicancer independently of the industrial consortium.
Disclosure
MC declares the following: Advisory board: AstraZeneca, Novartis, Abbvie, Sanofi, Pfizer, Sandoz, ACCORD, and Lilly GT1 group; consultant: Pierre Fabre Oncology, Sanofi, Novartis, and Servier; speaker bureau: Novartis; travel: Pfizer, Novartis, Roche, and AstraZeneca. BP declares the following: Consulting/advisor: Puma Biotechnology, Novartis, Myriad Genetics, and Pierre Fabre; personal fees: Novartis, AstraZeneca, MSD Oncology, and Pfizer; research funding: Daiichi, Puma Biotechnology, Novartis, Merus, Pfizer, and AstraZeneca. AG reports non-financial support from AstraZeneca, Roche, Pfizer, and Novartis. TdLMR reports grants, personal fees, and non-financial support from Pfizer; grants and non-financial support from Novartis and MSD; personal fees and non-financial support from AstraZeneca, Roche Genentech, and TESARO-GSK; and personal fees from CLOVIS ONCOLOGY and MYLAN, outside the submitted work. The remaining authors have declared no conflicts of interest.
Supplementary data
References
- 1.Giuliano A.E., Connolly J.L., Edge S.B. Breast cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:290–303. doi: 10.3322/caac.21393. [DOI] [PubMed] [Google Scholar]
- 2.Dawood S., Merajver S.D., Viens P. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22:515–523. doi: 10.1093/annonc/mdq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson F.M., Bondy M., Yang W. Inflammatory breast cancer: the disease, the biology, the treatment. CA Cancer J Clin. 2010;60:351–375. doi: 10.3322/caac.20082. [DOI] [PubMed] [Google Scholar]
- 4.Wingo P.A., Jamison P.M., Young J.L., Gargiullo P. Population-based statistics for women diagnosed with inflammatory breast cancer (United States) Cancer Causes Control. 2004;15:321–328. doi: 10.1023/B:CACO.0000024222.61114.18. [DOI] [PubMed] [Google Scholar]
- 5.Curcio L.D., Rupp E., Williams W.L. Beyond palliative mastectomy in inflammatory breast cancer–a reassessment of margin status. Ann Surg Oncol. 1999;6:249–254. doi: 10.1007/s10434-999-0249-3. [DOI] [PubMed] [Google Scholar]
- 6.O'Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10(suppl 3):20–29. doi: 10.1634/theoncologist.10-90003-20. [DOI] [PubMed] [Google Scholar]
- 7.Miller K.D., Sledge G.W. The role of chemotherapy for metastatic breast cancer. Hematol Oncol Clin North Am. 1999;13:415–434. doi: 10.1016/s0889-8588(05)70063-0. [DOI] [PubMed] [Google Scholar]
- 8.Cristofanilli M., Valero V., Buzdar A.U. Inflammatory breast cancer (IBC) and patterns of recurrence: understanding the biology of a unique disease. Cancer. 2007;110:1436–1444. doi: 10.1002/cncr.22927. [DOI] [PubMed] [Google Scholar]
- 9.Dawood S., Ueno N.T., Valero V. Identifying factors that impact survival among women with inflammatory breast cancer. Ann Oncol. 2012;23:870–875. doi: 10.1093/annonc/mdr319. [DOI] [PubMed] [Google Scholar]
- 10.Low J.A., Berman A.W., Steinberg S.M. Long-term follow-up for locally advanced and inflammatory breast cancer patients treated with multimodality therapy. J Clin Oncol. 2004;22:4067–4074. doi: 10.1200/JCO.2004.04.068. [DOI] [PubMed] [Google Scholar]
- 11.Monneur A., Goncalves A., Gilabert M. Similar response profile to neoadjuvant chemotherapy, but different survival, in inflammatory versus locally advanced breast cancers. Oncotarget. 2017;8:66019–66032. doi: 10.18632/oncotarget.19732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlichting J.A., Soliman A.S., Schairer C., Schottenfeld D., Merajver S.D. Inflammatory and non-inflammatory breast cancer survival by socioeconomic position in the Surveillance, Epidemiology, and End Results database, 1990-2008. Breast Cancer Res Treat. 2012;134:1257–1268. doi: 10.1007/s10549-012-2133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fouad T.M., Kogawa T., Liu D.D. Overall survival differences between patients with inflammatory and noninflammatory breast cancer presenting with distant metastasis at diagnosis. Breast Cancer Res Treat. 2015;152:407–416. doi: 10.1007/s10549-015-3436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pérol D., Robain M., Arveux P. The ongoing French metastatic breast cancer (MBC) cohort: the example-based methodology of the Epidemiological Strategy and Medical Economics (ESME) BMJ Open. 2019;9:e023568. doi: 10.1136/bmjopen-2018-023568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Laere S.J., Van den Eynden G.G., Van der Auwera I. Identification of cell-of-origin breast tumor subtypes in inflammatory breast cancer by gene expression profiling. Breast Cancer Res Treat. 2006;95:243–255. doi: 10.1007/s10549-005-9015-9. [DOI] [PubMed] [Google Scholar]
- 16.Hu Z., Li Z., Ma Z., Curtis C. Multi-cancer analysis of clonality and the timing of systemic spread in paired primary tumors and metastases. Nat Genet. 2020;52:701–708. doi: 10.1038/s41588-020-0628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Uden D.J.P., van Maaren M.C., Strobbe L.J.A. Metastatic behavior and overall survival according to breast cancer subtypes in stage IV inflammatory breast cancer. Breast Cancer Res. 2019;21:113. doi: 10.1186/s13058-019-1201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deluche E., Antoine A., Bachelot T. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008-2016. Eur J Cancer Oxf Engl. 2020;129:60–70. doi: 10.1016/j.ejca.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Gobbini E., Ezzalfani M., Dieras V. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer. 2018;96:17–24. doi: 10.1016/j.ejca.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Verma S., Bartlett C.H., Schnell P. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III Study (PALOMA-3) Oncologist. 2016;21:1165–1175. doi: 10.1634/theoncologist.2016-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hortobagyi G.N., Stemmer S.M., Burris H.A. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 22.Goetz M.P., Toi M., Campone M. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 23.Petrelli F., Ghidini A., Pedersini R. Comparative efficacy of palbociclib, ribociclib and abemaciclib for ER+ metastatic breast cancer: an adjusted indirect analysis of randomized controlled trials. Breast Cancer Res Treat. 2019;174:597–604. doi: 10.1007/s10549-019-05133-y. [DOI] [PubMed] [Google Scholar]
- 24.Schmid P., Adams S., Rugo H.S. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 25.Gianni L., Pienkowski T., Im Y.H. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 26.von Minckwitz G., Procter M., de Azambuja E. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Eng J Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Minckwitz G., Huang C.S., Mano M.S. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 28.Schmid P., Chui S.Y., Emens L.A. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. Reply. N Engl J Med. 2019;380:987–988. doi: 10.1056/NEJMc1900150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.