Abstract
Since December 2020, four vaccines for SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) have been developed, and three have been approved for immediate use in the United States. Two are mRNA vaccines, and one uses a viral vector mechanism. Thrombotic complications have been reported after vaccine administration, which were primarily cerebral sinus thromboses after administration of the viral vector vaccines. To the best of our knowledge, we are the first to report venous thrombotic complications within days of administration of the mRNA-1273 (Moderna) vaccine. We present a series of three women who developed venous thromboembolism after RNA-1273 vaccination at a single healthcare system.
Keywords: COVID-19, Deep vein thrombosis, Pulmonary embolism, Venous thromboembolism
In response to the coronavirus disease 2019 (COVID-19) pandemic caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), four vaccines were rapidly developed worldwide. Two of these vaccines use mRNA coded to the spike protein antigen of SARS-CoV-2, and two use a viral vector for vaccination.1 Three of these vaccines have been approved in the United States, and since December 2020, the United States has vaccinated >60 million people. Although the vast majority of persons have experienced uncomplicated vaccinations, documentation of serious thrombotic complications after administration of COVID-19 vaccines has been growing.2 , 3 Furthermore, recent cases of cerebral venous sinus thrombosis in women after receiving the Ad26.COV2.S (Janssen Pharmaceuticals, Johnson & Johnson, Beerse, Belgium) vaccine have been reported,4 which caused the U.S. Food and Drug Administration to recommend a pause in its use in the United States on April 13, 2021.5
The mRNA-1273 (Moderna) vaccine (Moderna, Cambridge, Mass) was first approved in December 2020. Since then, its safety profile has been excellent, with most adverse events reported involving flu-like symptoms and muscle pain.6 Although cases of thrombotic complications have been documented after other vaccines, to the best of our knowledge, no cases of thrombotic complications after the mRNA-1273 vaccine have been documented. We present a series of three women who presented to a single hospital system with acute venous thromboembolism (VTE) shortly after vaccination with the mRNA-1273 vaccine.
Case report
Index patient
An otherwise healthy 25-year-old woman had presented to the emergency department 2 days after receiving the first of the mRNA-1273 vaccine series with acute-onset shortness of breath and dyspnea on exertion. She had been taking oral contraceptive pills (OCPs) for years before the present admission.
The clinical details are outlined in the Table . On presentation, she did not require supplemental oxygen. An echocardiogram was performed, which revealed mild to moderate right ventricular strain. Computed tomography angiography (CTA) with a dedicated pulmonary embolism (PE) protocol (CTA-PE) revealed bilateral segmental PE (Fig 1 ). Venous Doppler ultrasound scans showed no deep vein thrombosis (DVT). She was admitted to the intensive care unit, intravenous heparin was started, and she was monitored. Her symptoms improved with heparin administration. She was discharged on hospital day 3 after transitioning to apixaban.
Table.
Clinical details of each patient
| Variable | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Hemoglobin, g/dL | 14.8 | 16.9 | 12.7 |
| Platelet count, per mm3 | 315 | 162 | 144 |
| International normalized ratio | 1.0 | 1.2 | 1.1 |
| Troponin, ng/mL (normal <0.01 ng/mL) | 0.28 | 0.46 | NT |
| BNP, pg/mL | 99 | 667 | NT |
| D-dimer, μg/mL | NT | 10.61 | NT |
| Pulmonary embolism | Yes | Yes | No |
| RV TAPSE on echocardiogram | 1.6 | 1 | NT |
| RV/LV ratio | 1.8 | 2.4 | NT |
| Other thrombosis | No | DVT | DVT |
| Symptom onset after vaccination, days | 2 | 3 | 3 |
| Initial anticoagulation treatment | Heparin | Enoxaparin | Heparin |
| Discharge anticoagulation therapy | Apixaban | Rivaroxaban | Apixaban |
BNP, Brain natriuretic peptide; DVT, deep vein thrombosis; LV, left ventricle; NT, not tested; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion.
Fig 1.
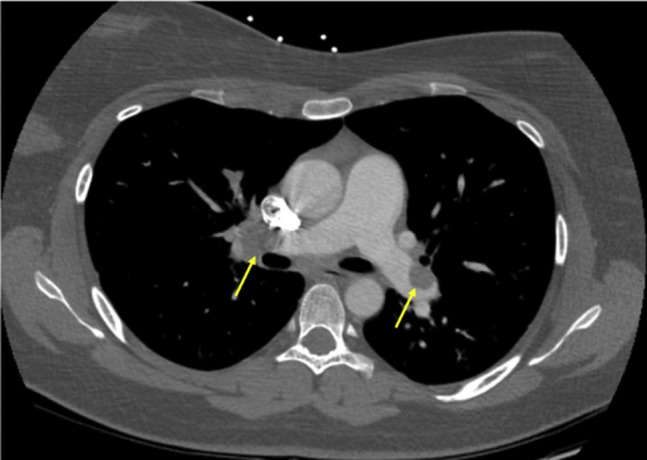
Computed tomography scan showing bilateral segmental pulmonary embolism (PE; arrows) in a 25-year-old woman.
Subsequent patients
A 77-year-old woman with a history of gastrointestinal bleeding had presented to the emergency department with a 4-day history of shortness of breath. She had received the first of the mRNA-1273 vaccine series 3 days before symptom onset. She had a remote history of breast cancer, which had been diagnosed and treated in 2009. She had been prescribed raloxifene for osteoporosis, which she stated she had been taking for years.
Her clinical details are also listed in the Table. She had required 6 L of supplemental oxygen on arrival to the hospital. The echocardiogram showed mild right ventricular strain. The CTA-PE was notable for bilateral segmental PE (Fig 2 ). Venous Doppler ultrasound scans revealed DVT of the right common femoral, femoral, and profunda veins. She was admitted to the intensive care unit and received twice-daily therapeutic enoxaparin (Lovenox). Overnight, her symptoms improved, and her oxygen requirement decreased to 3 L. She was discharged on hospital day 5 with a prescription for rivaroxaban.
Fig 2.
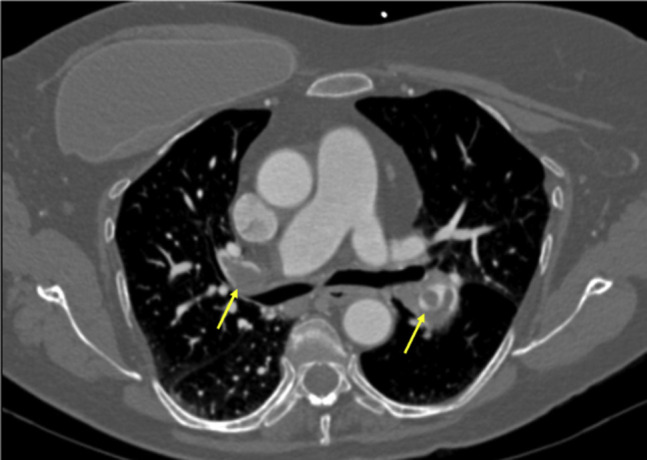
Computed tomography scan showing bilateral segmental pulmonary embolism (PE; arrows) in a 77-year-old woman.
An 84-year-old woman had presented with an 8-day history of left leg pain and swelling. Her symptoms had started 3 days after receiving the second injection of the mRNA-1273 vaccine series. The CTA-PE showed no PE but revealed thrombus in the common femoral vein (Fig 3 ). Venous duplex ultrasound confirmed left common femoral, popliteal, and peroneal DVT. She was admitted to a monitored floor and intravenous heparin was started. Her symptoms improved with the intravenous heparin, and she was ultimately discharged on hospital day 3 with a prescription for apixaban.
Fig 3.
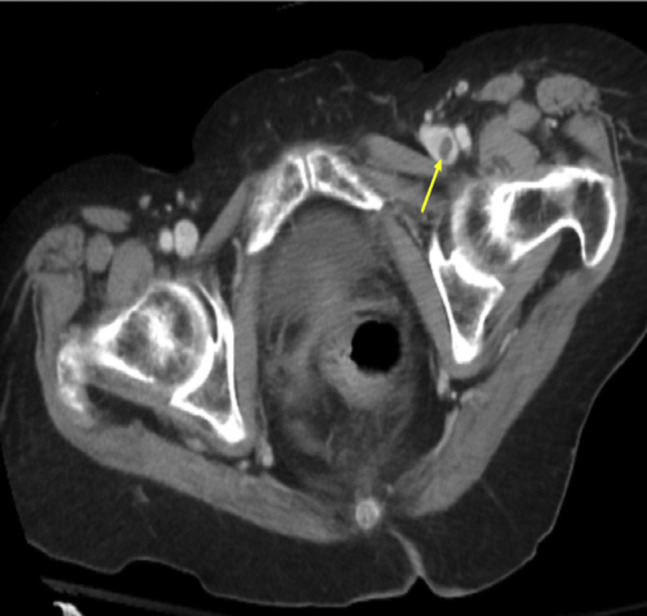
Computed tomography scan showing left common femoral venous thrombosis (arrow) in an 85-year-old woman.
In all three cases, the patients were active and lived independently. They denied a personal or family history of VTE, recent surgery, trauma, and any recent travel. Each patient had been tested for COVID-19 with negative results. The hypercoagulable panel performed for all three patients was negative for a genetic predisposition to VTE (hospital days 2-4). None of the three women has been seen in follow-up. All three patients involved in the present series provided written informed consent for their case details and images to be included in our report.
Discussion
We have presented three cases of acute venous thrombosis that developed after the patients had received the mRNA-1273 (Moderna) vaccine. None of the patients had been infected with COVID-19. All three were otherwise active and independent women with no personal or family history of thrombotic disorders.
Although the risk of thrombosis after infection with COVID-19 is well documented,7, 8, 9 the risk after receipt of a COVID-19 vaccine is not well known. Recognition has been increasing of a potential thrombotic risk after vaccination for COVID-19. Cerebral venous sinus thrombosis has been identified after both the Ad26.COV2.S and the ChAdOx1 nCov-19 vaccines.2 , 4 , 5 Patients with these complications have been found to develop a spontaneous immune thrombotic thrombocytopenia mediated by platelet-activating antibodies against PF4, similar to autoimmune heparin-induced thrombocytopenia.2 , 3 In March 2021, the use of the ChAdOx1 nCov-19 vaccine was temporarily halted in some European countries because of these safety concerns.10 Additionally, these cases led the Food and Drug Administration to recommend a temporary pause in the use of the Ad26.COV2.S vaccine in the United States.5 It is possible that a similar mechanism had occurred in the patients in the present case series.
Although most thrombotic complications after administration of the COVID-19 vaccines have been occurred primarily with the ChAdOx1 nCov-19 or Ad26.COV2.S vaccines, one case of a popliteal and peroneal DVT has been reported after the second dose of the BNT162b2 (Pfizer-BioNTech) vaccine (BioNTech, Mainz, Germany; Pfizer, New York, NY).11 However, that patient was found to be heterozygous for the factor V Leiden mutation. To date, the present series is the first to report VTE after the mRNA-1273 vaccine in three patients with no other apparent risk factors.
One of the patients in the present series had a remote history of breast cancer and had also been concomitantly prescribed raloxifene for osteoporosis. Raloxifene has been documented to result in a slightly elevated risk of VTE compared with those not taking raloxifene (0.27 vs 0.39; hazard ratio, 1.44).12 However, that patient in our series had endorsed chronic use of this medication without any prior adverse effects. Additionally, another patient in the present series had been taking OCPs at the time of her event. Those taking OCPs have a relative risk of venous thrombosis of 3.5 compared with nonusers.13 Similarly, this patient had endorsed chronic use of OCPs without prior adverse effects. Although it is impossible to determine the exact mechanism of venous thrombosis, the timing of onset is concerning for a potential connection with the COVID-19 vaccine itself. Additionally, it was not documented whether these patients had experienced malaise after the vaccine or mobility-limiting complications after vaccine administration. It is possible that these patients could have experienced a reduction in functional mobility that could have led to venous stasis and, thus, VTE.
With mass vaccination campaigns and new vaccines arriving on the market, incentive exists to identify and quantify the incidence of thrombotic events after COVID-19 vaccinations. Li et al8 analyzed a multinational network of administrative claims data to characterize and estimate adverse events occurring after COVID-19 vaccination. PE was estimated to be as high as 427 events per 100,000 person-years in patients aged >85 years. Moving forward, it will continue to be imperative, not only to accurately quantify thrombotic complications of vaccines in large databases, but also to identify the clinical details in reports such as ours to better understand the clinical presentation and physiology of these patients. A possibility exists that the present case series represents a normal incidence of VTE among a population that is experiencing a mass vaccination campaign and that the vaccination itself was a confounding variable. However, the timing of the VTE events suggest a possible relationship that is important to document. It is imperative to obtain the COVID-10 vaccination history from patients with symptoms of VTE with no other obvious predisposing factors. Furthermore, given the rapid and widespread vaccination campaigns, reports of adverse events occurring after administration of the new COVID-19 vaccines are essential to the proper care and education of these patients.
Conclusions
We have reported three cases of VTE occurring after vaccination with the mRNA-1273 vaccine at a single healthcare system. Although vaccination for COVID-19 is essential in the pandemic, we should continue to be vigilant for any potential adverse effects.
Footnotes
This research was supported in part by the National Heart, Lung, and Blood Institute, United States (grant 5T32HL0098036 to E.A.A.). The University of Pittsburgh, United States holds a Physician-Scientist Institutional Award from the Burroughs Wellcome Fund (to E.A.A.).
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Centers for Disease Control and Prevention COVID-19 Vaccines. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html Available at: [PubMed]
- 2.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castelli G.P., Pognani C., Sozzi C., Franchini M., Vivona L. Cerebral venous sinus thrombosis associated with thrombocytopenia post-vaccination for COVID-19. Crit Care. 2021;25:137. doi: 10.1186/s13054-021-03572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks P., Schuchat A. Joint CDC and FDA Statement on Johnson & Johnson COVID-19 Vaccine. https://www.fda.gov/news-events/press-announcements/joint-cdc-and-fda-statement-johnson-johnson-covid-19-vaccine Available at:
- 6.Centers for Disease Control and Prevention Moderna COVID-19 Vaccine Overview and Safety. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Moderna.html Available at:
- 7.Sjöström A., Wersäll J., Warnqvist A., Farm M., Magnusson M., Oldner A., et al. Platelet count rose while D-dimer levels dropped as deaths and thrombosis declined, an observational study on anticoagulation shift in COVID-19 [e-pub ahead of print] https://doi.org/10.1055/a-1477-3829 Thromb Haemost. [DOI] [PubMed]
- 8.Li X., Ostropolets A., Makadia R., Shaoibi A., Rao G., Sena A.G., et al. Characterizing the incidence of adverse events of special interest for COVID-19 vaccines across eight countries: a multinational network cohort study [e-pub ahead of print] https://doi.org/10.1101/2021.03.25.21254315 medRxiv. [DOI] [PMC free article] [PubMed]
- 9.Erben Y., Franco-Mesa C., Gloviczki P., Stone W., Quinones-Hinojosa A., Meltzer A.J., et al. Deep venous thrombosis and pulmonary embolism among hospitalized coronavirus disease 2019 (COVID-19) positive patients predict higher mortality, prolonged intensive care unit and hospital stays in a multi-site healthcare system [e-pub ahead of print] https://doi.org/10.1016/j.jvsv.2021.03.009 J Vasc Surg Venous Lymphat Disord. [DOI] [PMC free article] [PubMed]
- 10.Oldenburg J., Klamroth R., Langer F., Albisetti M., von Auer C., Ay C., et al. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseologie. 2021;41:184–189. doi: 10.1055/a-1469-7481. [DOI] [PubMed] [Google Scholar]
- 11.Carli G., Nichele I., Ruggeri M., Barra S., Tosetto A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern Emerg Med. 2021;16:803–804. doi: 10.1007/s11739-021-02685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosca L., Grady D., Barrett-Connor E., Collins P., Wenger N., Abramson B.L., et al. Effect of raloxifene on stroke and venous thromboembolism according to subgroups in postmenopausal women at increased risk of coronary heart disease. Stroke. 2009;40:147–155. doi: 10.1161/STROKEAHA.108.518621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stegeman B.H., de Bastos M., Rosendaal F.R., van Hylckama Vlieg A., Helmerhorst F.M., Stijnen T., et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;347:f5298. doi: 10.1136/bmj.f5298. [DOI] [PMC free article] [PubMed] [Google Scholar]


