Abstract
Background
African American women (AAW) die more frequently from estrogen receptor (ER) positive breast cancer than European American women (EAW). We investigated the relationship between race, percent ER staining, treatment, and clinical outcomes.
Methods
Percent ER staining (weakly ER+: 1–10%, moderately ER+: 11–50%, strongly ER+: > 50%) was abstracted from pathology reports for 1573 women with ER+/HER2− invasive breast cancer treated at a single cancer center in Detroit, MI from 2010 to 2017. Clinical outcomes and tumor characteristics were obtained from the Metropolitan Detroit Cancer Surveillance System. Associations of ER levels with demographic and clinical characteristics were evaluated using logistic regression. Overall and breast cancer-specific (BCS) survival were evaluated using Cox proportional hazards models.
Results
AAW were more likely to have tumors with lower ER staining levels than EAW (weakly ER+: Odds ratio (OR) 2.19, p = 0.019; moderately ER+: OR 2.80, p = 0.005). Women with weakly compared to strongly ER+ tumors were less likely to receive endocrine therapy (ET) regardless of race (OR 0.79, p < 0.001). Mortality was predicted by both AA race (Overall hazard ratio (HR) = 1.72, p < 0.001; BCS HR 1.45, p = 0.08) and low (1–50%) ER (Overall HR 1.57, p = 0.083; BCS HR 2.11, p = 0.017) adjusting for clinic-pathologic characteristics. ET was associated with improved BCS survival in all women (1–50%: HR 0.11, p < 0.001; > 50%: HR 0.24, p < 0.001).
Conclusion
The biology of ER+/HER2− tumors varies by race, although this does not appear to account for racial differences in survival. Although ET substantially reduces mortality among women with weakly ER+ tumors, these women are less likely to be treated with ET and have poorer outcomes.
Keywords: Gene expression, Pathology, Clinical outcomes, Racial disparities
Introduction
Breast cancer incidence among African American women (AAW) in the USA has increased in recent years, with AAW now equally likely to be diagnosed with breast cancer compared to European American women (EAW) [1]. Disparities in breast cancer mortality, however, continue to widen, where AAW are 1.4 times as likely to die from breast cancer compared to EAW [2]. Despite the fact that women with estrogen receptor positive (ER+)/human epidermal growth factor receptor 2-negative (HER2−) breast cancer have the highest 5-year survival rates compared to other breast cancer subtypes [3], recent studies have shown that AAW are nearly twice as likely to die from ER+/HER2− disease than EAW even when accounting for clinical and tumor characteristics associated with poor prognosis [4–6]. It has been hypothesized that this disparity is partially driven by the reductions in treatment course because of toxicity or under-dosing, but there is also evidence for racial differences in tumor biology among ER+/HER2− breast tumors [7–9].
In 2010, the American Society of Clinical Oncology and the College of American Pathologists recommended that tumors should be classified as ER+ if they show as little as 1% ER staining of tumor cells by immunohistochemistry [10], and this threshold for ER positivity is the current standard for oncology practice in the USA [11]. While women with tumors across the ER+ spectrum benefit from endocrine therapy and have better survival compared to women with ER-negative tumors [12–14], there is evidence for heterogeneity in tumor biology among the ER+ group. Tumors with 1–10% ER staining show molecular properties more similar to triple negative breast cancers, a subtype that is twice as common among AAs compared to EAW [15], than to strongly ER+ tumors [16]. Specifically, there is an increased prevalence of the basal-like subtype among weakly ER+ tumors compared to strongly ER+ tumors, corresponding to reduced overall survival rates in the weakly compared to strongly ER+ groups [16]. We also recently showed that AAW are 75% more likely to have higher predicted risk of distant recurrence than EAW using the 21-gene recurrence score (RS) assay, OncotypeDx [17], which is used as a clinical decision-making tool to guide the recommendation for adjuvant chemotherapy [17–20] and is highly correlated with ER expression levels [21–25]. However, it is unclear whether these molecular differences by ER expression levels translate into differences in clinical outcomes.
Clinical decision-making with respect to treatment recommendations for women with ER+/HER2− breast cancer is also likely to be impacted by the percentage of tumor cells that stain positive for ER. This happens directly through the correlation between ER expression and the 21-gene RS assay, where low ER expression correlates with high RS and increased recommendations for chemotherapy [21–25]. However, only a relatively small proportion of women with ER+/HER2− breast cancer meet NCCN guidelines for and receive 21-gene RS testing, and utilizing ER protein expression levels when evaluating treatments and prognosis is more broadly applicable. There is evidence that women with weakly ER+ tumors are less likely to be prescribed adjuvant endocrine therapy compared to women with strongly ER+ breast cancer [26], despite the fact that evidence-based guidelines recommend endocrine therapy for all patients with ER+ breast cancer [27]. This is possibly due to clinicians’ perceptions that weakly ER+ tumors have low anticipated benefit from endocrine therapy relative to the side effects of such therapy and their impact on quality of life. Establishing whether ER staining levels influence receipt of endocrine therapy is important given that endocrine therapy impacts survival for all levels of ER staining. The main goal of this analysis was to evaluate the hypothesis that differences in ER expression partially explain racial disparities in survival among all women treated for ER+/HER2− breast cancer at a single cancer center in Detroit, MI. To do this, we first estimated racial differences in ER protein expression levels and then evaluated the impact of race and clinical characteristics on treatment and survival in our cohort.
Methods
Study population
Using the Metropolitan Detroit Cancer Surveillance System (MDCSS) database, we identified 1652 women who (1) were diagnosed with and underwent surgery for ER+/HER2− invasive breast cancer from 2010 to 2017 at the Karmanos Cancer Institute (KCI) in Detroit, MI and (2) identified as AA or EA. 2010 is the first year for which HER2 status data were available in MDCSS. MDCSS is a founding member of the Surveillance, Epidemiology, and End Results (SEER) Program [28], and has been continuously collecting population-based cancer data since 1973. This study was granted concurrence of exemption by the Wayne State University Institutional Review Board (IRB).
Estrogen receptor staining levels
ER staining levels, measured as the percentage of cells staining positive for ER by immunohistochemistry, were abstracted from KCI pathology reports and entered into a de-identified database. ER staining was categorized as weakly ER+ (1–10%), moderately ER+ (11–50%), and strongly ER+ (> 50%). Two records where percent ER staining was reported as a range were excluded because the range fell within more than one ER category (> 1% and 30–60%). The threshold for strongly ER+ tumors was set at 50% by fitting a two-component mixture model of Weibull distributions to the percent ER variable using the “mixtools” R package (https://cran.r-project.org/). Percent ER staining was not reported for 77 women, and we restricted our dataset to include only those women with known ER percent staining (n = 1573).
Clinical and demographic variables
Clinical, treatment, and outcomes data were obtained via linkage with the MDCSS registry, including stage, grade, age at diagnosis, race/ethnicity, node status, histology, tumor size, 21-gene recurrence score (RS), ER and PR status, surgery type, systemic therapy type, radiation, vital status at last contact, cause of death, and date of last contact. Treatment data for KCI patients in MDCSS are abstracted directly from KC medical records by MDCSS staff. For those women who had treatment data recorded in MDCSS (n = 1614, 99.3%), first course of treatment was defined as receipt of therapy for a cancer diagnosis before disease progression or recurrence and included type of surgery (breast-conserving versus mastectomy), adjuvant chemotherapy, endocrine therapy, and radiation therapy.
Statistical analysis
Univariable associations between demographics, clinical characteristics, tumor characteristics, and race were examined using χ2 tests and Wilcoxon rank sum tests for categorical and continuous variables, respectively. Because 21-gene RS scores were not available for the majority of women in the cohort, this variable was not included in subsequent analyses. Multinomial logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI) for predictors of ER staining levels, where the reference outcome category was > 50% ER staining. Binomial logistic regression was used to evaluate associations between demographic/tumor characteristics and receiving endocrine therapy. Cox proportional hazards regression was used to estimate associations with overall survival and breast cancer-specific (BCS) survival. For all regression models, covariates were included based on a priori evidence for being related to the outcome and main exposure of interest. Adjusted survival curves were generated by applying the “survfit” function of the R package survival to the previously fitted Cox proportional hazards models, where we specified a new data frame consisting of the median values for each of the variables in the original Cox model (race, age at diagnosis, grade, node status, stage, chemotherapy) with indicators for hormone therapy status. All data were analyzed using R statistical software (https://cran.r-project.org/). All statistical tests were two-sided, with a p-value of < 0.05 considered to be statistically significant.
Results
Racial differences in ER+/HER2− tumor characteristics
Overall, weakly ER+ (1–10%), moderately ER+ (11–50%), and strongly ER+(> 50%) staining levels accounted for 3.2%, 2.7%, and 94.1% of tumors, respectively; however, this differed by race, with weakly and moderately ER+ staining accounting for 1% each of tumors in EAW and 4% and 3%, respectively, in AAW (p < 0.001). Low ER staining levels were associated with younger age at diagnosis (p < 0.001), AA race (p < 0.001), ductal histology (p = 0.008), higher grade (p < 0.001), and 21-gene RS high risk category (p < 0.001) (Table 1). AAW were more than twice as likely to have weakly ER+ tumors (OR 2.40, 95% CI 1.28–4.49, p = 6.4 × 10−3) and moderately ER+ tumors (OR 2.79, 95% CI 1.40–5.58, p = 3.7 × 10−3) than EAW. AAW were also more likely to be diagnosed at an older age (p = 0.002), had higher 21-gene RS scores among the 38% of women who received the test (p = 0.016), and were more likely to have tumors with higher grade (p = 0.013) and larger size (p = 0.001) (Table S1). Race remained significantly associated with ER staining level (1–10%: adjusted OR 2.19, p = 0.019; 11–50%: adjusted OR 2.80, p = 0.005) after adjustment for age, tumor size, grade, and stage (Table 2). Further adjustment for histology did not appreciably change the effect estimates, and histology was not associated with ER staining levels in the multivariable model.
Table 1.
Associations between ER staining level and demographic, tumor, and clinical characteristics of 1573 women with ER+/HER2− invasive breast cancer
| 1–10% |
11–50% |
> 50% |
p-value* | ||||
|---|---|---|---|---|---|---|---|
|
N = 50 |
N = 43 |
N = 1480 |
|||||
| n | % | n | % | n | % | ||
| Demographics | |||||||
| Race | < 0.001 | ||||||
| White | 14 | 29% | 11 | 26% | 722 | 49% | |
| African American | 35 | 71% | 32 | 74% | 753 | 51% | |
| Age at diagnosis (years) | < 0.001 | ||||||
| Median, IQR | 53 | 22 | 52 | 24.5 | 60 | 17.5 | |
| Clinical/tumor characteristics | |||||||
| Histology | 0.008 | ||||||
| Ductal | 41 | 83% | 35 | 81% | 974 | 66% | |
| Ductal + Lobular | 1 | 2% | 0 | 0% | 120 | 8% | |
| Lobular | 0 | 0% | 4 | 9% | 209 | 14% | |
| Other | 7 | 14% | 4 | 9% | 170 | 11% | |
| Grade | < 0.001 | ||||||
| 1 | 0 | 0% | 7 | 16% | 333 | 23% | |
| 2 | 8 | 16% | 9 | 20% | 795 | 55% | |
| 3 | 40 | 83% | 27 | 62% | 299 | 20% | |
| Stage | 0.48 | ||||||
| Local | 19 | 38% | 20 | 46% | 677 | 45% | |
| Regional | 13 | 26% | 14 | 32% | 415 | 28% | |
| Distant | 4 | 8% | 3 | 6% | 61 | 4% | |
| Unknown | 14 | 28% | 6 | 13% | 327 | 22% | |
| Node involvement | 0.23 | ||||||
| No | 27 | 55% | 28 | 65% | 769 | 52% | |
| Yes | 22 | 44% | 15 | 34% | 706 | 47% | |
| 21-gene RS risk categorya | < 0.001 | ||||||
| Low | 1 | 25% | 3 | 18% | 332 | 58% | |
| Intermediate | 1 | 25% | 2 | 12% | 182 | 31% | |
| High | 2 | 50% | 11 | 68% | 56 | 9% | |
| Tumor size (mm) | 0.15 | ||||||
| Median, IQR | 31.11 | 23.58 | 28.48 | 14.66 | 24.92 | 21.50 | |
| Treatment-related factors | |||||||
| Received 21-gene RS testing | < 0.001 | ||||||
| No | 46 | 92% | 27 | 63% | 910 | 61% | |
| Yes | 4 | 8% | 16 | 37% | 570 | 39% | |
| Received chemotherapy | < 0.001 | ||||||
| No | 12 | 24% | 15 | 34% | 933 | 63% | |
| Yes | 37 | 75% | 28 | 65% | 541 | 36% | |
| Received endocrine therapy | < 0.001 | ||||||
| No | 16 | 32% | 4 | 9% | 159 | 10% | |
| Yes | 33 | 67% | 39 | 90% | 1315 | 89% | |
| Underwent surgery | 0.027 | ||||||
| No | 40 | 80% | 39 | 90% | 1349 | 91% | |
| Yes | 10 | 20% | 4 | 9% | 131 | 8% | |
| Survival characteristics | |||||||
| Vital status | 0.028 | ||||||
| Alive | 35 | 71% | 37 | 86% | 1258 | 85% | |
| Dead | 14 | 28% | 6 | 13% | 217 | 14% | |
| Months of active follow-up | 0.31 | ||||||
| Median, IQRa | 36 | 26 | 43 | 27 | 41 | 24 | |
Among 592 women who received OncotypeDx testing
IQR Interquartile range
p-values: χ2 tests for categorical variables, Wilcoxon rank sum tests for continuous variables, log-rank test for follow-up time
Table 2.
Associations between race and ER staining level in a multivariable model adjusted for age, tumor size, grade, and stage among 1,573 women with ER+/HER2− invasive breast cancer
| Predictors in multivariable model | 1–10% vs. > 50% |
11–50% < vs. > 50% |
||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Race (AAW vs. EAW) | 2.19 (1.14–4.23) | 0.019 | 2.80 (1.37–5.71) | 0.005 |
| Age at diagnosis (years) | 0.98 (0.96–1.00) | 0.078 | 0.95 (0.93–0.98) | < 0.001 |
| Tumor size (mm) | 1.00 (0.99–1.01) | 0.96 | 1.00 (0.98–1.01) | 0.92 |
| Grade (1 vs 3) | –a | –a | 0.23 (0.09–0.59) | 0.002 |
| Grade (2 vs 3) | 0.08 (0.04–0.17) | < 0.001 | 0.13 (0.06–0.28) | < 0.001 |
| Stage (Regional vs. Local) | 0.52 (0.23–1.21) | 0.13 | 0.69 (0.31–1.51) | 0.35 |
| Stage (Distant vs. Local) | 1.49 (0.43–5.12) | 0.53 | 1.23 (0.32–4.77) | 0.77 |
| Stage (Unknown vs. Local) | 1.01 (0.47–2.16) | 0.98 | 0.41 (0.15–1.14) | 0.089 |
No tumors were grade 1 with 1–10% ER staining
We next evaluated whether racial differences in tumor characteristics persisted when accounting for ER staining level (Table S2). Tumor size was only significantly associated with race among the > 50% ER staining group (AA: 20 mm, EA: 18 mm p = 0.048). In contrast to the overall analysis, we identified a marginally significant association between race and node involvement among weakly ER+ tumors, where AAW were more likely to be node positive than EAW (54% vs. 21%, respectively; p = 0.057). Similarly, while not statistically significant, AAW with moderately ER+ tumors were more likely to be node positive than EAW. This association remained marginally significant when adjusting for tumor size with AAW fourfold more likely to be node positive than EAW (adjusted OR 3.94, 95% CI 0.92–16.87, p = 0.065). While not statistically significant, AAW with moderately ER+ tumors were approximately twofold more likely to be node positive than EAW adjusting for tumor size (OR 2.09, p = 0.42). No difference in node status was seen among strongly ER+ tumors, and no other differences by ER staining level were observed for associations between race and tumor characteristics.
ER staining levels, race, and treatment
We next evaluated the impact of ER staining levels on whether a woman received endocrine therapy and if this differed for AAW compared to EAW, noting that there is potential for therapy misclassification due to unknown therapy information (Tables 1, 3). Adjusted for age at diagnosis, tumor size, node involvement, grade, stage, and receiving chemotherapy, women with weakly ER+ tumors were 20% less likely to receive endocrine therapy compared to those with strongly ER+ tumors (OR 0.79, 95% CI 0.72–0.86, p < 0.001) (Table 3). The association between weakly ER+ status and receiving endocrine therapy did not differ for AAW compared to EAW (AA: OR 0.77, white OR 0.83, p-interaction = 0.33).
Table 3.
Overall and race-specific associations between ER staining levels and receipt of endocrine therapy in multivariable models adjusted for age, tumor size, node involvement, grade, stage, and chemotherapy among women with ER+/HER2− invasive breast cancer
| Predictors in multivariable modela | Overall (n = 1573) |
AAW (n = 855) |
EAW (n = 762) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Race (AAW vs EAW) | 0.99 | 0.96–1.03 | 0.68 | – | – | – | – | – | – |
| ER level (1–10% vs. > 50%) | 0.79 | 0.72–0.86 | < 0.001 | 0.77 | 0.68–0.86 | < 0.001 | 0.83 | 0.71–0.98 | 0.027 |
| ER level (11–50% vs. > 50%) | 0.97 | 0.88–1.07 | 0.51 | 0.97 | 0.87–1.09 | 0.65 | 0.95 | 0.79–1.14 | 0.89 |
Adjusted for age at diagnosis, tumor size, node involvement, grade, stage, and chemotherapy
ER staining levels, race, and survival
We next evaluated the impact of race and ER staining levels on overall and BCS mortality. Given the small sample sizes and numbers of deaths in the weakly and moderately ER+ groups, we combined the 1–10% and 11–50% ER staining groups (1–50% n = 90, events n = 16). We adjusted for tumor characteristics as well as treatment to be able to evaluate the effect of endocrine therapy on mortality. AAW were significantly more likely to die from any cause (hazard ratio (HR) = 1.75, 95% CI 1.32–3.32, p < 0.001) and marginally significantly more likely to die from breast cancer (HR 1.48, 95% CI 0.99–2.21, p = 0.054) compared to EAW when adjusting for tumor characteristics and treatment (Table 4). When ER staining level was added to the models, the association between race and overall and BCS mortality did not change appreciably (overall HR 1.72, BCS HR 1.45). Having a weakly ER+tumor, however, was itself significantly associated with BCS mortality and marginally significantly associated with overall mortality (overall HR 1.57, p = 0.083; BCS HR 2.11, p = 0.017). Endocrine therapy was significantly associated with substantial reductions in overall and BCS mortality (Full model overall HR 0.35, p < 0.001; Full model BCS HR 0.23, p < 0.001). Given that endocrine therapy was one of the strongest predictors of mortality in our cohort, we performed a sensitivity analysis to evaluate the effects of race on mortality only among women who received endocrine therapy (Table S3). Race remained significantly associated with overall mortality when adjusting for ER staining level (HR 1.72, p = 0.001). While we had limited power for the breast cancer-specific mortality analyses due to a reduction in the number of breast cancer deaths, we observed a 22% increase in death in AAW compared to EAW, although this was not statistically significant.
Table 4.
Associations between race and mortality before and after adjusting for ER staining levels in multivariable models among 1573 women with ER+/HER2− invasive breast cancer
| Overall mortalitya |
Breast cancer-specific mortalityb |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1c | Model 1 + adjustment for % ER | Model 2d | Model 2 + adjustment for % ER | |||||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Race (AAW vs. EAW) | 1.75 | 1.32–3.32 | < 0.001 | 1.72 | 1.29–2.30 | < 0.001 | 1.48 | 0.99–2.21 | 0.054 | 1.45 | 0.96–2.20 | 0.080 |
| Age at diagnosis (years) | 1.03 | 1.01–1.04 | < 0.001 | 1.03 | 1.02–1.04 | < 0.001 | 1.01 | 0.99–1.02 | 0.43 | 1.01 | 0.99–1.02 | 0.20 |
| Grade (2 vs 1) | 1.75 | 1.13–2.72 | 0.012 | 1.70 | 1.10–2.64 | 0.018 | 4.26 | 1.53–11.8 | 0.54 | 3.85 | 1.38–10.8 | 0.0010 |
| Grade (3 vs 1) | 2.29 | 1.44–3.67 | < 0.001 | 2.07 | 1.28–3.34 | 0.003 | 6.54 | 2.32–18.5 | < 0.001 | 5.36 | 1.88–15.3 | 0.0017 |
| Node involvement (yes vs no) | 2.23 | 1.41–3.54 | < 0.001 | 2.74 | 1.70–4.42 | < 0.001 | 1.43 | 0.66–3.10 | 0.36 | 2.22 | 0.95–5.17 | 0.061 |
| Stage (regional vs. local) | 0.89 | 0.55–1.45 | 0.65 | 0.78 | 0.47–1.28 | < 0.001 | 1.71 | 0.75–3.91 | 0.20 | 1.35 | 0.56–3.25 | 0.50 |
| Stage (distant vs. local) | 9.21 | 5.40–15.7 | < 0.001 | 8.59 | 5.01–14.7 | 0.32 | 31.8 | 13.4–72.5 | < 0.001 | 27.5 | 11.4–66.3 | < 0.001 |
| Stage (unknown vs. local) | 2.66 | 1.37–5.16 | 0.004 | 2.77 | 1.38–5.56 | 0.004 | 3.61 | 1.18–11.0 | 0.024 | 4.38 | 1.35–14.2 | 0.014 |
| ET (yes vs. no) | 0.30 | 0.22–0.41 | < 0.001 | 0.35 | 0.25–0.49 | < 0.001 | 0.18 | 0.12–0.28 | < 0.001 | 0.23 | 0.14–0.38 | < 0.001 |
| Chemo (yes vs. no) | 0.84 | 0.61–1.16 | 0.29 | 0.85 | 0.61–1.20 | 0.36 | 1.02 | 0.66–1.59 | 0.92 | 1.00 | 0.62–1.62 | 0.99 |
| ER (1–50% vs. > 50%) | 1.57 | 0.94–2.58 | 0.083 | 2.11 | 1.14–3.87 | 0.017 | ||||||
Total number of deaths = 228
Total number of deaths due to breast cancer = 110
Model 1 evaluates the effect of race on overall mortality adjusting for age, grade, node involvement, stage, endocrine therapy, and chemotherapy
Model 2 evaluates the effect of race on breast cancer-specific mortality adjusting for age, grade, node involvement, stage, endocrine therapy, and chemotherapy
To directly evaluate the effect of endocrine therapy among women with low versus high ER staining levels, we next stratified our survival analyses by ER staining level (Fig. 1, Table S4). Endocrine therapy was significantly associated with reduced overall and BCS mortality for both the 1–50% ER staining group (Overall HR 0.17, p = 0.001; BCS HR 0.11, p < 0.001) and the > 50% ER staining group (Overall HR 0.41, p < 0.001; > 50% BCS HR 0.24, p < 0.001) (Table S4). There was a marginally significant interaction between ER staining level and endocrine therapy for overall survival (p = 0.074), where the effect estimate for endocrine therapy was strongest for the lower ER staining group. Importantly, while survival for women in the 1–50% ER group who did not receive endocrine therapy was poor, survival for women who did receive endocrine therapy was comparable for women in the 1–50% and the > 50% ER group (Fig. 1). Interestingly, the effect of race on overall survival appeared to vary by ER staining level, although these results should be interpreted with caution given the small sample sizes. AAW with 1–50% ER staining were threefold more likely to die from their breast cancer compared to EAW (HR 3.11, p = 0.067), in contrast to AAW with > 50% staining who were only 70% more likely to die from their breast cancer compared to EAW (HR 1.70, p < 0.001). There was no significant interaction between ER staining level and race for either overall mortality (p = 0.53) or BCS mortality (p = 0.37). While not statistically significant, mortality was also higher for AAW compared to EAW for those with 1–50% ER staining (HR 2.03, p = 0.26) and > 50% staining (HR 1.36, p = 0.17).
Fig. 1.
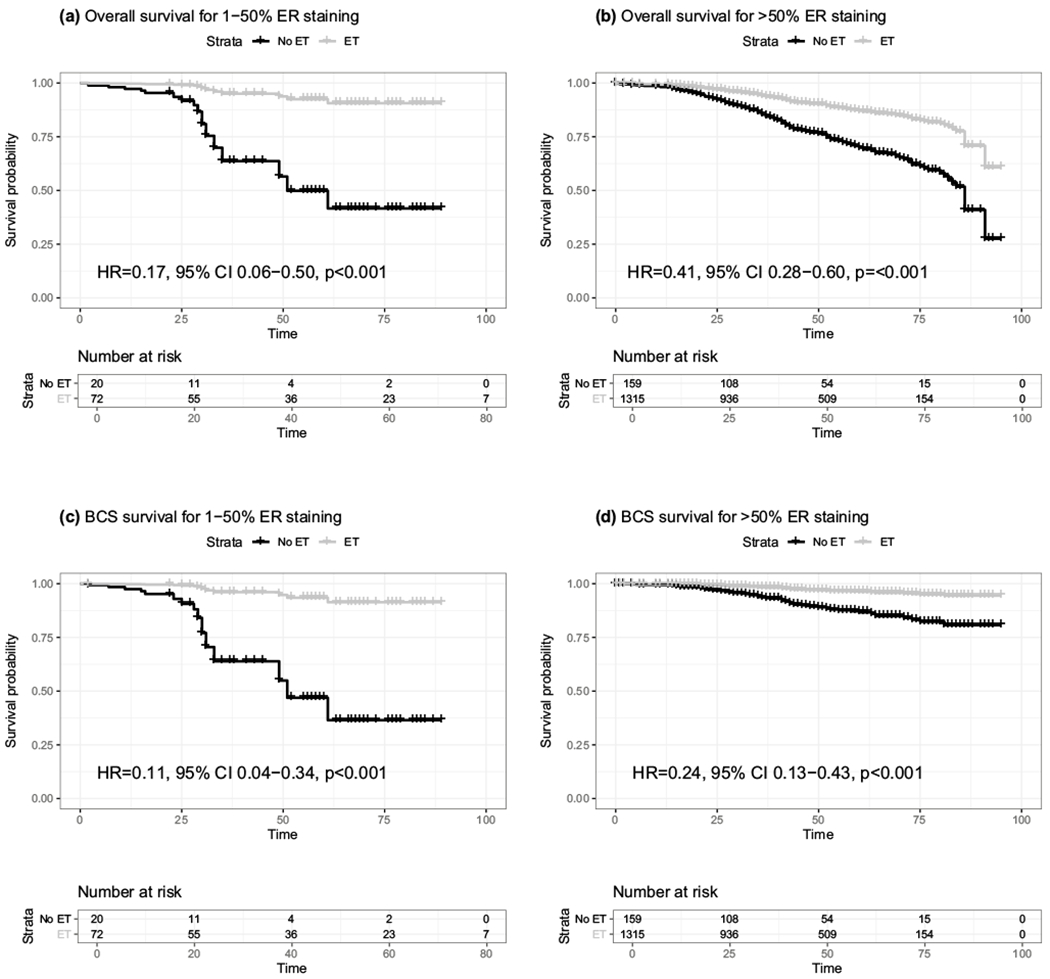
Overall and breast cancer-specific mortality curves by receipt of endocrine therapy and ER staining level. Survival curves adjusted for race, age at diagnosis, grade, node status, stage, and chemotherapy are shown for a overall survival for 1–50% ER staining, b overall survival for > 50% ER staining, c breast cancer-specific (BCS) survival for 1–50% ER staining, and d BCS survival for > 50% ER staining. Black lines represent survival curves among women who did not receive endocrine therapy and grey lines represent survival curves among women who did receive endocrine therapy. Censored events are indicated by vertical dashes along the survival curves
Discussion
This is the first report to show that AAW are more likely to have weakly ER+ breast cancer compared to EAW. We also provide additional evidence for differences in tumor biology by ER staining level. Specifically, lower levels of ER staining were associated with higher grade, primarily ductal histology, and higher predicted risk of recurrence. This is consistent with previous reports showing that weakly ER+ breast cancers are more likely to be classified as basal-like [16, 29]. It follows that the ER level-race association could be reflecting a higher proportion of basal-like tumors in AAW compared to EAW [30], but future molecular tumor-based studies are needed to confirm this. AAW with weakly ER+ breast cancer were also more than four times as likely to have node-positive tumors and have threefold higher risk of death compared to EAW. These two phenomena are likely related as nodal status is one of the best predictors of metastasis and survival [31, 32]. Interestingly, it appears that the proportion of AAW with node involvement is relatively constant across ER staining levels, in contrast to EAW, where the proportion of women with node involvement decreases for lower ER staining categories. This suggests that there may be racial differences in tumor biology beyond ER staining level alone.
Given that the observed ~ 70% increase in mortality among AAW compared to EAW was not substantially impacted when adjusting for ER staining level, these data do not support our original hypothesis that differences in ER expression levels partially explain racial disparities in survival. There was, however, a strong relationship between both race and ER staining level and survival. The literature on the relationship between quantitative ER staining and survival is sparse, although there is evidence that ten-year survival is substantially worse for weakly ER+ tumors than strongly ER+ tumors in a dose-dependent manner [33, 34]. A study of more than 1200 AAW and EAW showed overall that only women with strongly ER+ tumors (≥ 40%) had a survival advantage compared to ER− tumors in a dose–response manner, where women with the highest level of ER staining had the best survival [35]. This remained true among EAW, but for AAW, any level of ER staining, from 1 to 100%, was consistently associated with a 40% reduction in breast cancer-specific mortality. A separate study identified ER staining intensity as a significant prognostic factor, though only among non-Hispanic white women and percent ER staining was not associated with survival [36]. A set of genes co-expressed with ESR1 has also been significantly inversely associated with distant relapse and survival in both endocrine- and chemo-endocrine-treated cohorts, specifically using the genomic sensitivity to ET (SET) index [37]. Here we showed that while low ER staining appears to be associated with worse overall and breast cancer-specific survival, this association does not account for the worse survival among AAW with ER+/HER2− breast cancer. Indeed, there may be an interaction between race and ER staining levels, although this should be investigated in future studies to both increase the weakly ER+ sample size and to validate this initial finding. Further, the differences in survival that we observed for AAW and EAW could be reflecting differing rates of compliance with endocrine therapy between the two groups, which could not be evaluated in this study. Taken together, these data strongly suggest that not only AAW are disproportionately affected by this low ER+ subtype, but also clinical outcomes among this group are worse for AAW than EAW.
Women with weakly ER+ tumors were also 20% less likely to receive endocrine therapy, and importantly, our data suggest that women in the 1–50% ER staining group who receive endocrine therapy have survival comparable to women in the strongly ER+ group who receive endocrine therapy. These data are consistent with the literature suggesting that oncologists are less likely to prescribe endocrine therapy to women with weakly ER+ tumors due to a perception of low benefit in this group. Importantly, we show here that AAW are not less likely to receive endocrine therapy than EAW when accounting for ER staining level. Evidence-based guidelines recommend endocrine therapy for all patients with ER+ breast cancer regardless of ER/PR staining level, with the stipulation that physicians might wish to discuss the risks and benefits of endocrine therapy with patients in the 1–10% category [27]. These recommendations are based largely on evidence from a few studies showing that response to tamoxifen was observed among patients with as little as 1% ER tumor staining [13, 14]. In contrast to these recommendations, we showed that women with weakly ER+ breast cancer are less likely to be prescribed endocrine therapy, which is consistent with the limited literature on this topic [26] and may be in part due to inadequate evidence regarding the degree of benefit associated with adjuvant endocrine therapy for women specifically with weakly ER+ cancers [38]. Importantly, we provide compelling evidence that endocrine therapy is beneficial among weakly and moderately ER+ breast cancer as well as strongly ER+ cancers. While we did not observe racial differences in the receipt of endocrine therapy, it is important to note that AAW would be less likely to receive beneficial endocrine therapy because they are disproportionately affected by weakly ER+ breast cancer.
A major strength of our study is the use of data from the population-based MDCSS registry because invasive breast cancer cases are pathologically verified, follow-up data are high quality and highly complete, and standardized data entry is continuously monitored for accuracy. However, our analyses were limited to the Karmanos Cancer Institute because we required medical record review, and this hospital-based cohort may not be generalizable to the US population. Future studies that incorporate data from the larger MDCSS catchment area would increase generalizability. A second limitation is that data on chemotherapy or endocrine therapy is collected primarily at hospitals, radiation facilities, and laboratories, rather than physician offices where patients may undergo these treatments.
The physiological impact of hormone activity in the tumor may be determined by the strength of the signaling within the receptor-positive cells, which in turn may be determined by the expression level of the receptor and as well a host of downstream effectors such as hormone receptor co-regulators. Strong hormone signaling in even a small fraction of the tumor cells could produce paracrine effects profoundly affecting tumor growth. Further studies of the molecular characteristics of weakly ER+ tumors, specifically with respect to hormone signaling and dependence, will be important in understanding the mechanisms of hormone sensitivity in these tumors.
Supplementary Material
Funding
This work was supported, in part, by the Epidemiology Research Core and the Biobanking and Correlative Sciences Core and National Institution of Health (NIH) Center Grant P30CA022453 to the Karmanos Cancer Institute at Wayne State University for conduct of the study. This work was also supported in part by contract (Grant No. HHSN261201300011I).
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10549-020-05607-4) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A (2016) Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin 66(1):31–42. 10.3322/caac.21320 [DOI] [PubMed] [Google Scholar]
- 2.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, Boscoe FP, Cronin KA, Lake A, Noone AM, Henley SJ, Eheman CR, Anderson RN, Penberthy L (2015) Annual Report to the Nation on the Status of Cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst 107(6):djv048. 10.1093/jnci/djv048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO, Blomqvist C, Heikkila P, Heikkinen T, Nevanlinna H, Akslen LA, Begin LR, Foulkes WD, Couch FJ, Wang X, Cafourek V, Olson JE, Baglietto L, Giles GG, Severi G, McLean CA, Southey MC, Rakha E, Green AR, Ellis IO, Sherman ME, Lissowska J, Anderson WF, Cox A, Cross SS, Reed MW, Provenzano E, Dawson SJ, Dunning AM, Humphreys M, Easton DF, Garcia-Closas M, Caldas C, Pharoah PD, Huntsman D (2010) Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med 7(5):e1000279. 10.1371/journal.pmed.1000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma H, Lu Y, Malone KE, Marchbanks PA, Deapen DM, Spirtas R, Burkman RT, Strom BL, McDonald JA, Folger SG, Simon MS, Sullivan-Halley J, Press MF, Bernstein L (2013) Mortality risk of black women and white women with invasive breast cancer by hormone receptors, HER2, and p53 status. BMC Cancer 13:225. 10.1186/1471-2407-13-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, Dressler LG, Geradts J, Millikan RC (2010) Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res 16(24):6100–6110. 10.1158/1078-0432.CCR-10-1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, Theriault RL, Blayney DW, Niland JC, Winer EP, Weeks JC, Partridge AH (2015) Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol 33(20):2254–2261. 10.1200/JCO.2014.57.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Arcy M, Fleming J, Robinson WR, Kirk EL, Perou CM, Troester MA (2015) Race-associated biological differences among Luminal A breast tumors. Breast Cancer Res Treat 152(2):437–448. 10.1007/s10549-015-3474-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huo D, Hu H, Rhie SK, Gamazon ER, Cherniack AD, Liu J, Yoshimatsu TF, Pitt JJ, Hoadley KA, Troester M, Ru Y, Lichtenberg T, Sturtz LA, Shelley CS, Benz CC, Mills GB, Laird PW, Shriver CD, Perou CM, Olopade OI (2017) Comparison of breast cancer molecular features and survival by African and European Ancestry in The Cancer Genome Atlas. JAMA Oncol. 10.1001/jamaoncol.2017.0595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holowatyj AN, Cote ML, Ruterbusch JJ, Ghanem K, Schwartz AG, Vigneau FD, Gorski DH, Purrington KS (2018) Racial differences in 21-gene recurrence scores among patients with hormone receptor-positive. Node-Negat Breast Cancer J Clin Oncol 36(7):652–658. 10.1200/JCO.2017.74.5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S (2010) American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract 6(4):195–197. 10.1200/JOP.777003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FCG, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer (Unabridged Version). Arch Pathol Lab Med 134(7):e48–e72. 10.1043/1543-2165-134.7.e48 [DOI] [PubMed] [Google Scholar]
- 12.Harvey JM, Clark GM, Osborne CK, Allred DC (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17(5):1474–1481. 10.1200/JCO.1999.17.5.1474 [DOI] [PubMed] [Google Scholar]
- 13.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795. 10.1200/JCO.2009.25.6529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ, Panel M (2009) Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol 20(8):1319–1329. 10.1093/annonc/mdp322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, Smith LV, Labbok MH, Geradts J, Bensen JT, Jackson S, Nyante S, Livasy C, Carey L, Earp HS, Perou CM (2008) Epidemiology of basal-like breast cancer. Breast Cancer Res Treat 109(1):123–139. 10.1007/s10549-007-9632-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwamoto T, Booser D, Valero V, Murray JL, Koenig K, Esteva FJ, Ueno NT, Zhang J, Shi W, Qi Y, Matsuoka J, Yang EJ, Hortobagyi GN, Hatzis C, Symmans WF, Pusztai L (2012) Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J Clin Oncol 30(7):729–734. 10.1200/JCO.2011.36.2574 [DOI] [PubMed] [Google Scholar]
- 17.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826. 10.1056/NEJMoa041588 [DOI] [PubMed] [Google Scholar]
- 18.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE Jr, Wickerham DL, Wolmark N (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24(23):3726–3734. 10.1200/JCO.2005.04.7985 [DOI] [PubMed] [Google Scholar]
- 19.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Perez EA, Olson JA Jr, Zujewski J, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin P, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Atkins JN, Berenberg JL, Sledge GW (2015) Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 373(21):2005–2014. 10.1056/NEJMoa1510764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mamounas EP, Tang G, Fisher B, Paik S, Shak S, Costantino JP, Watson D, Geyer CE Jr, Wickerham DL, Wolmark N (2010) Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol 28(10):1677–1683. 10.1200/JCO.2009.23.7610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoury T, Huang X, Chen X, Wang D, Liu S, Opyrchal M (2016) Comprehensive histologic scoring to maximize the predictability of pathology-generated equation of breast cancer oncotype DX Recurrence Score. Appl Immunohistochem Mol Morphol 24(10):703–711. 10.1097/PAI.0000000000000248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eaton AA, Pesce CE, Murphy JO, Stempel MM, Patil SM, Brogi E, Hudis CA, El-Tamer M (2017) Estimating the OncotypeDX score: validation of an inexpensive estimation tool. Breast Cancer Res Treat 161(3):435–441. 10.1007/s10549-016-4069-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milburn M, Rosman M, Mylander C, Tafra L (2013) Is oncotype DX recurrence score (RS) of prognostic value once HER2-positive and low-ER expression patients are removed? Breast J 19(4):357–364. 10.1111/tbj.12126 [DOI] [PubMed] [Google Scholar]
- 24.Allison KH, Kandalaft PL, Sitlani CM, Dintzis SM, Gown AM (2012) Routine pathologic parameters can predict Oncotype DX recurrence scores in subsets of ER positive patients: who does not always need testing? Breast Cancer Res Treat 131(2):413–424. 10.1007/s10549-011-1416-3 [DOI] [PubMed] [Google Scholar]
- 25.Gage MM, Rosman M, Mylander WC, Giblin E, Kim HS, Cope L, Umbricht C, Wolff AC, Tafra L (2015) A validated model for identifying patients unlikely to benefit from the 21-gene recurrence score assay. Clin Breast Cancer 15(6):467–472. 10.1016/j.clbc.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CM, Jaswal J, Vandenberg T, Tuck A, Brackstone M (2013) Weakly hormone receptor-positive breast cancer and use of adjuvant hormonal therapy. Curr Oncol 20(6):e612–613. 10.3747/co.20.1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livaudais JC, Hershman DL, Habel L, Kushi L, Gomez SL, Li CI, Neugut AI, Fehrenbacher L, Thompson B, Coronado GD (2012) Racial/ethnic differences in initiation of adjuvant hormonal therapy among women with hormone receptor-positive breast cancer. Breast Cancer Res Treat 131(2):607–617. 10.1007/s10549-011-1762-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howlader NNA, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) SEER Cancer Statistics Review, 1975–2013. National Cancer Institute, Bethesda, MD, https://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 29.Badve S, Dabbs DJ, Schnitt SJ, Baehner FL, Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani SR, Palacios J, Rakha EA, Richardson AL, Schmitt FC, Tan PH, Tse GM, Weigelt B, Ellis IO, Reis-Filho JS (2011) Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol 24(2):157–167. 10.1038/modpathol.2010.200 [DOI] [PubMed] [Google Scholar]
- 30.Troester MA, Sun X, Allott EH, Geradts J, Cohen SM, Tse CK, Kirk EL, Thorne LB, Mathews M, Li Y, Hu Z, Robinson WR, Hoadley KA, Olopade OI, Reeder-Hayes KE, Earp HS, Olshan AF, Carey LA, Perou CM (2018) Racial differences in PAM50 subtypes in the Carolina Breast Cancer Study. J Natl Cancer Inst. 10.1093/jnci/djx135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buonomo OC, Caredda E, Portarena I, Vanni G, Orlandi A, Bagni C, Petrella G, Palombi L, Orsaria P (2017) New insights into the metastatic behavior after breast cancer surgery, according to well-established clinicopathological variables and molecular subtypes. PLoS ONE 12(9):e0184680. 10.1371/journal.pone.0184680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lale Atahan I, Yildiz F, Ozyigit G, Sari S, Gurkaynak M, Selek U, Hayran M (2008) Percent positive axillary lymph node metastasis predicts survival in patients with non-metastatic breast cancer. Acta Oncol 47(2):232–238. 10.1080/02841860701678761 [DOI] [PubMed] [Google Scholar]
- 33.Symmans WF, Hatzis C, Sotiriou C, Andre F, Peintinger F, Regitnig P, Daxenbichler G, Desmedt C, Domont J, Marth C (2010) Genomic index of sensitivity to endocrine therapy for breast cancer. J Clin Oncol 28(27):4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z (2009) Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 27(8):1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma H, Lu Y, Marchbanks PA, Folger SG, Strom BL, McDonald JA, Simon MS, Weiss LK, Malone KE, Burkman RT, Sullivan-Halley J, Deapen DM, Press MF, Bernstein L (2013) Quantitative measures of estrogen receptor expression in relation to breast cancer-specific mortality risk among white women and black women. Breast Cancer Res 15(5):R90. 10.1186/bcr3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill DA, Barry M, Wiggins C, Nibbe A, Royce M, Prossnitz E, Lomo L (2017) Estrogen receptor quantitative measures and breast cancer survival. Breast Cancer Res Treat 166(3):855–864. 10.007/s10549-017-4439-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Symmans WF, Hatzis C, Sotiriou C, Andre F, Peintinger F, Regitnig P, Daxenbichler G, Desmedt C, Domont J, Marth C, Delaloge S, Bauernhofer T, Valero V, Booser DJ, Hortobagyi GN, Pusztai L (2010) Genomic index of sensitivity to endocrine therapy for breast cancer. J Clin Oncol 28(27):4111–4119. 10.1200/JCO.2010.28.4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan DA, Refalo NA, Cheung KL (2011) Strength of ER-positivity in relation to survival in ER-positive breast cancer treated by adjuvant tamoxifen as sole systemic therapy. Breast 20(3):215–219. 10.1016/j.breast.2010.11.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


