Abstract
Background
Prevalence estimates of autistic traits in individuals with psychotic disorders (PD) vary greatly and it is unclear whether individuals with a familial risk (FR) for psychosis have an increased propensity to display autistic traits. Furthermore, it is unknown whether the presence of comorbid autism traits disproportionally affects the cognitive and behavioral aspects of social functioning in PD.
Methods
In total, 504 individuals with PD, 587 unaffected siblings with FR, and 337 typical comparison (TC) individuals (16–50 years) were included. Autistic and psychotic traits were measured with the Autism Spectrum Quotient (AQ) and the Community Assessment of Psychic Experiences (CAPE). Social cognition was assessed with the Picture Sequencing Task (PST) and social behavior with the Social Functioning Scale (SFS).
Results
For PD 6.5% scored above AQ clinical cut-off (⩾32), 1.0% for FR, and 1.2% for TC. After accounting for age, sex, and IQ, the PD group showed significantly more autistic traits and alterations in social behavior and cognition, while FR and TC only displayed marginal differences. Within the PD group autistic traits were a robust predictor of social behavior and there were no interactions with positive psychotic symptoms.
Conclusions
Levels of autistic traits are substantially elevated in PD and have a profoundly negative association with social functioning. In contrast, autistic traits above the clinical cut-off are not elevated in those with FR, and only marginally on a dimensional level. These findings warrant specific clinical guidelines for psychotic patients who present themselves with autistic comorbidity to help address their social needs.
Key words: autism, functional outcome, mentalizing, psychosis, schizophrenia, social cognition
Introduction
Since the concept of ‘autism’ was conceived by Bleuler in 1911 (Bleuler, 1950), it has been intertwined with the diagnosis of schizophrenia and evolved from a central feature of severe psychotic disorders (PD) to a separate spectrum of clinical disorders. The literature suggests that autism spectrum disorders (ASD) and PD still co-occur more frequently than one would expect based on the prevalence rates in the general population, which gravitate around 1% for both (Chisholm, Lin, Abu-Akel, & Wood, 2015). In addition, findings from longitudinal, registry-based studies convincingly suggest that individuals diagnosed with ASD are not only at an increased risk for non-affective PD such as schizophrenia, but also for affective PD, as observed in diagnosed mood disorders (Schalbroeck, Termorshuizen, Visser, Van Amelsvoort, & Selten, 2018; Selten, Lundberg, Rai, & Magnusson, 2015). Vice versa, the prevalence of ASD diagnoses in individuals with an established PD has been investigated less systematically and is mostly restricted to the observations in adult populations of modest sample size (Chisholm et al., 2015; Kincaid, Doris, Shannon, & Mulholland, 2017; Padgett, Miltsiou, & Tiffin, 2010). Findings from the largest patient sample to date indicate a substantially elevated prevalence (3.6%, N = 197) (Davidson, Greenwood, Stansfield, & Wright, 2014) of ASD diagnoses among PD individuals, but this analysis did not include the prevalence estimates of sub-clinical autism traits.
Recent efforts have therefore focused more on dimensional rather than categorical approaches, under the assumption that both conditions represent extremes on a continuum of symptomatic severity, and that even isolated or low-intensity traits may affect clinical outcomes. Such studies have provided further evidence that autistic and psychotic traits co-occur at an elevated behavioral level in clinical samples (Barneveld et al., 2011; De Crescenzo et al., 2019; Esterberg, Trotman, Brasfield, Compton, & Walker, 2008; Fagel et al., 2013; Kincaid et al., 2017; Ziermans, Swaab, Stockmann, de Bruin, & van Rijn, 2017). From a cognitive and functional perspective, it is also well established that both ASD and PD are characterized by (partially) overlapping impairments compared to typical healthy comparisons, particularly in the social domain (Martinez et al., 2017; Pinkham et al., 2019; Sasson et al., 2007, 2011, 2016; Velthorst et al., 2018). However, it is currently unclear whether the presence of comorbid autistic and psychotic traits has a detrimental effect on the outcome. A recent study in schizophrenia patients with a positive screening for ASD (28 out of 75) showed an increased duration of illness, more general psychopathology, and worse cognitive functioning (working memory and processing speed) compared to patients without ASD traits, yet similar levels of IQ, social cognition, and functioning (Barlati, Deste, Gregorelli, & Vita, 2019). In contrast, Vaskinn and Abu-Akel (2019) used a dimensional approach and concluded that the presence of more psychotic and autistic traits in schizophrenia patients (N = 81) was associated with relatively better functioning and mentalizing. The latter finding also provided clinical support for the (counterintuitive) diametrical model of autism and psychosis (Crespi & Badcock, 2008), which posits that both disorders have opposite genetic relations and that co-occurrence would diametrically modulate behavior toward normality.
Whether genetic markers associated with autism and psychosis are diametrical opposites remains open to debate. There is ample evidence, however, that etiological overlap between both conditions does exist, as further supported by recent studies focusing on polygenic risk for both autism and schizophrenia (Fromer et al., 2016; Pourcain et al., 2018; Velthorst et al., 2018), as well as the presence of rare copy-number variations in both populations (Kushima et al., 2018; Zarrei et al., 2019). In addition, given the highly heritable nature of both ASD and PD, it is reasonable to expect a higher co-occurrence of subclinical traits of both dimensions in unaffected relatives of either patient population. Indeed, twin data have suggested that psychiatric disorders in general are associated with continuously distributed genetic risks throughout the general population (Taylor et al., 2019). An important next step is to establish whether concurrent traits of autism and psychosis are also associated with social impairments in at-risk samples.
The primary aim of the current study was to establish the prevalence of autistic traits in a substantial group of PD individuals compared to their unaffected siblings with a familial risk for PD (FR) and a typical comparison (TC) group. It was expected that the PD group would show more autistic traits than the TC group, with a large effect size, and the FR group in an intermediate position. The second aim was to establish to what extent the levels of autism or psychotic traits were associated with social cognition and social functioning, using both categorical and dimensional approaches. It was expected that both psychotic and autism traits would show independent negative associations with social cognition and functioning across groups, but most profoundly within the PD group as we assumed they would display more variability in trait scores and therefore stronger associations. A third and final aim was to explore whether our data could provide any further support for the diametric hypothesis, which postulates that negative main effects of both autistic and positive psychotic traits on social functioning are modulated toward positivity if both are present.
Methods
The current cross-sectional study is part of a longitudinal cohort study, Genetic Risk and Outcome of Psychosis (GROUP). The main objective of GROUP is to elucidate etiological and pathogenetic factors influencing the onset and course of PD in patients, their unaffected family members, and non-related controls. For a full description of recruitment and assessment procedures, the reader is referred to a previous publication on this topic (Korver et al., 2012).
Participants
Participant groups for this study consisted of patients diagnosed with schizophrenia or related PD, unaffected siblings with FR and TC individuals from the general population. Participants were recruited in 36 mental health care institutes in the Netherlands and Belgium. PD were identified through clinicians in the participating institutes by applying the following inclusion criteria: (1) age between 16 and 50 years, (2) meet DSM-IV-TR (American Psychiatric Association, 2000) criteria for a non-affective PD, (3) good command of the Dutch language, and (4) able and willing to provide informed consent. Siblings were not allowed to meet the criteria for a lifetime diagnosis of any PD at baseline. Healthy control participants had no lifetime diagnosis of a PD at baseline and no first-degree relative with a lifetime PD. For the purpose of the present study, we only included participants from the database (Data release 6.0) with available data on the Autism Spectrum Quotient (AQ) (Baron-Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001) measured at the 6-year follow-up assessment (T3). The study was approved by the Medical Ethics Committee of the University Medical Center Utrecht and subsequently by local review boards of each participating institute. All subjects gave written informed consent in accordance with the committee's guidelines.
Instruments
Global cognitive functioning
All participants were assessed with a cognitive task battery that included four tasks (Arithmetic, Block Design, Digit Symbol-Coding, and Information) of the Dutch version of the Wechsler Adult Intelligence Scale – Third Edition (WAIS-III; Wechsler, 1997), resulting in a total IQ estimate.
Psychotic traits
Psychotic traits were assessed with the Community Assessment of Psychic Experiences (CAPE; Stefanis et al., 2002). This self-report scale typically measures the lifetime prevalence of positive, negative, and depressive traits on both a frequency scale and a distress scale. Because the instrument was assessed as part of a follow-up study, individuals were instructed to answer the questions with regard to the last 3 years (since the previous assessment). Only the more commonly used frequency scores were included in this study.
Autistic traits
The AQ is a well-validated, 50-item self-report questionnaire that measures autism traits and is commonly used to screen for ASD diagnosis (Woodbury-Smith, Robinson, Wheelwright, & Baron-Cohen, 2005). AQ scores fall within the range of 0–50 and higher scores indicate a higher level of autism traits. In addition, AQ scores of ⩾32 indicate that an ASD diagnosis may be appropriate, and individuals with ASD are unlikely to score below a threshold of 26 (Baron-Cohen et al., 2001; Woodbury-Smith et al., 2005). The AQ consists of five subscales measuring the following domains: social skills, communication, imagination, attention switching, and attention to detail. The Dutch translation of the AQ was used for this study (Hoekstra, Bartels, Cath, & Boomsma, 2008).
Social functioning
Cognition. The Picture Sequencing Task (PST; Langdon and Coltheart, 1999) was assessed to measure mentalizing ability in participants. The PST consists of stories depicted in four illustrated (black and white) card picture sequences. Subjects are asked to turn over the cards placed in front of them and to place them in the correct order to show a logical sequence of events. The false-belief (PST-FB) stories depict a story character who, unaware of an event that occurred in a story, acted on his or her misinformation. These stories have previously been associated with underperformance in individuals with schizophrenia (Langdon, Coltheart, & Ward, 2006) and autism (Baron-Cohen, Leslie, & Frith, 1986). Total score ranges between 0 and 24.
Behavior. On a daily behavioral level, social functioning was assessed with the Social Functioning Scale (SFS; Birchwood, Smith, Cochrane, Wetton, and Copestake, 1990), a widely used self-report instrument to measure the areas of functioning essential for successful community maintenance. The total scaled score on the SFS was used as a measure of overall social functioning in the past 3 months. Higher scores on the SFS indicate higher levels of social functioning. It has a mean standardized score of 100, with a standard deviation of 15. It is commonly assessed in PD, and adults with ASD have an equally reduced score on this instrument compared to TC (Chan et al., 2019).
Statistical analysis
The χ2 and ANOVA tests were used for the comparisons of group characteristics, with Cramer's V and partial η2 as respective effect sizes, followed by Games–Howell or Hochberg's G2 post hoc tests, where applicable. To compare the prevalence of autism and psychotic traits (AQ and CAPE) across groups, a missing value analysis was conducted and missing items were imputed with the predicted mean matching method (Markov chain Monte Carlo; 50 iterations) to generate subscale and total scores for all individuals. Proportions above cut-off scores were compared with χ2 tests. Next, (M)ANCOVA was implemented by using general linear models with age, sex, and IQ entered as covariates and Bonferroni-corrected post hoc comparisons.
Next, we assessed the relative impact of autism traits on social functioning across groups by conducting two separate series of multilevel mixed linear models for both dependent variables. A two-level model with random intercepts was applied, i.e. measurements within families and within study sites. Age, IQ, and trait scores were centered around their respective means. In the initial models, group was entered as a factor. Significant analyses were continued for PD only, as this was the primary group of interest. The relative goodness of fit of the competing models was evaluated with the Akaike information criterion (AIC) and the Bayesian information criterion (BIC), where smaller values indicate a better fit. Finally, to test for a possible diametric effect between positive symptoms and autistic symptoms, the analyses were repeated with AQ Total, CAPE positive, and their interaction term included. Multilevel models were computed with the unstructured covariance setting and maximum likelihood estimation.
To improve reproducibility and reduce the chance of Type I error, a critical p value of <0.005 was applied for all ad hoc analyses (Benjamin et al., 2018) and p < 0.05 for post hoc and explorative analyses. Cohen's d, based on pooled variances and estimated marginal means, was calculated for the effect sizes of group differences and the standardized β coefficient was reported as an effect size for individual predictors in multilevel analyses.
Results
Demographics and clinical characteristics
Data were available for 504 PD, 572 FR, and 337 TC individuals (N = 1413). Demographic data and clinical characteristics are provided in Table 1. Groups differed significantly on sex, age, education, and IQ estimate (all p < 0.001). Post hoc testing showed that there were significantly more males in the PD group than in the FR (χ2 = 161.16, p < 0.001, V = 0.28) or TC group (χ2 = 129.89, p < 0.001, V = 0.30). Both the PD and FR groups had a lower mean age than the TC group (both p < 0.001, η2partial = 0.08 and 0.06) and education (post hoc, all p < 0.001, Vrange = 0.14–0.44) and IQ estimates differed for all group comparisons (post hoc, all p < 0.01, η2partial, range = 0.01–0.14) with PD showing the lowest average IQ score and TC the highest.
Table 1.
Demographic and clinical characteristics of patients, siblings, and healthy controls in the GROUP study, mean scores (standard deviations) and absolute numbers (%)
| PD (504) | FR (572) | TC (337) | Statistic | p value | ES | Post hoc | |
|---|---|---|---|---|---|---|---|
| Sex (male) | 365 (72.4) | 254 (44.4) | 153 (42.4) | χ2 = 108.17 | <0.001 | V = 0.27 | PD > FR & TC |
| Age (years) | 33.4 (7.2) | 34.0 (7.9) | 38.5 (10.6) | F = 30.89 | <0.001 | η2p = 0.06 | PD & FR < TC |
| Age of onset (years)b | 22.4 (6.5) | – | – | ||||
| Illness duration (years) | 11.5 (4.3) | – | – | ||||
| Estimated IQb | 100.7 (17.9) | 111.5 (17.7) | 115.4 (17.1) | F = 83.15 | <0.001 | η2p = 0.11 | PD < FR < TC |
| Education | χ2 = 198.45 | <0.001 | V = 0.26 | PD < FR < TC | |||
| Low | 133 (26.4) | 52 (9.1) | 11 (3.3) | ||||
| Middle | 260 (51.6) | 227 (39.7) | 116 (34.4) | ||||
| High | 111 (22.0) | 293 (51.2) | 210 (62.3) | ||||
| CAPE scoresa,b | |||||||
| Positive | 29.3 (9.7) | 21.7 (2.5) | 21.5 (3.0) | F = 493.83 | <0.001 | η2p = 0.26 | PD > FR & TC |
| Negative | 26.2 (7.8) | 20.7 (5.7) | 19.7 (4.8) | F = 280.57 | <0.001 | η2p = 0.17 | PD > FR > TC |
| Depression | 15.1 (4.8) | 12.1 (3.3) | 11.8 (3.3) | F = 203.81 | <0.001 | η2p = 0.13 | PD > FR & TC |
| DSM-IV Diagnosisc | |||||||
| Schizophrenia | 310 (61.5) | – | – | ||||
| Schizophreniform d. | 36 (7.1) | – | – | ||||
| Schizoaffective d. | 67 (13.3) | – | – | ||||
| Psychotic d. | 79 (15.7) | – | – | ||||
| Delusional d. | 12 (2.4) | – | – | ||||
| In remission >6 monthsb,d | |||||||
| Yes/no/unknown | 198/274/29 | ||||||
| Antipsychotic medication | |||||||
| Yes/no/unknown | 345/4/155 | – | – |
PD, psychotic disorder group; FR, familial risk group; TC, typical comparison group; ES, effect size.
Games–Howell, p < 0.05.
Missing data: Age of onset – 1 PD, IQ – 1 FR, CAPE – 2 PD & 3 FR, PANSS – max. 20 PD.
Based on the Comprehensive Assessment of Symptoms and History (CASH; Andreasen, Flaum, and Arndt, 1992) and the Schedules for Clinical Assessment for Neuropsychiatry (SCAN 2.1; Wing et al., 1990) at baseline, reported for schizophrenia spectrum only.
Based on PANSS remission tool (Andreasen et al., 2005).
In the PD group, schizophrenia diagnoses were the most common primary DSM-IV diagnosis (61.5%), followed by psychotic (15.7%) and schizoaffective (13.7%) diagnoses. Psychotic traits on all three CAPE dimensions (Positive, Negative, and Depressive) were significantly more frequent in PD compared to FR and TC (all p < 0.001, η2partial, range = 0.12–0.23). In addition, FR reported significantly more negative traits than TC on average (p = 0.018, η2partial = 0.01), but no difference in positive (p = 0.561, η2partial = 0.00) or depressive traits (p = 0.542, η2partial = 0.00).
Autistic traits
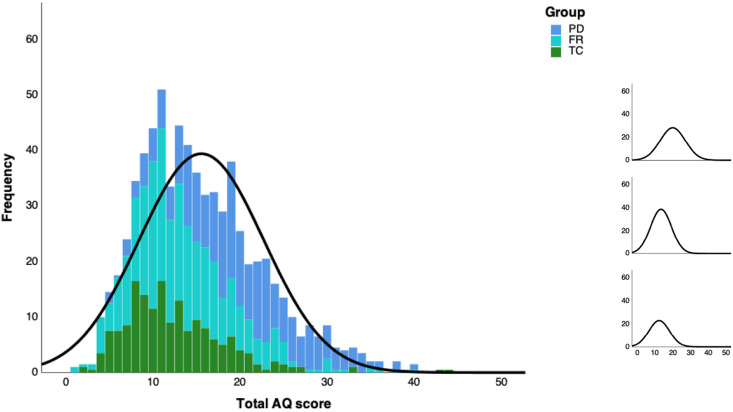
Missing value analysis indicated that for 54 individuals (3.8%; 31 PD, 17 FR, 6 TC) AQ data were incomplete, with a total of 0.1% of items missing. Due to the small proportion of missing values, a single imputed data set was generated. Total AQ score distributions are illustrated in Fig. 1 and reported by group and gender for all subscales in Table 2. Based on recommended and most stringent clinical cut-offs for Total AQ scores (Table 2), 6.5% of PD scored ⩾32 and 21.4% ⩾26, while lower proportions scored above these thresholds in the FR (1.0% and 2.8%) and TC (1.2% and 2.3%) groups, respectively. Proportions differed significantly across all groups for AQ ⩾ 32 (χ2 = 32.62, p < 0.001, V = 0.15) and AQ ⩾ 26 (χ2 = 135.19, p < 0.001, V = 0.31). Post hoc comparisons showed that PD differed from the other groups on both cut-offs (all p < 0.001, Vrange = 0.13–0.27), but FR did not differ from TC (p = 0.85, V = 0.01 and p = 0.70, V = 0.01, respectively).
Fig. 1.
Left: Histogram plot with the normal curve of Total AQ scores stacked across groups. Right: Normal curves per group: PD (top), FR (middle), and TC (bottom).
Table 2.
AQ data per group, mean scores (standard deviations) and number of participants exceeding standard AQ cut-off scores (%)
| PD | FR | TC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (504) | Male (365) | Female (139) | Total (572) | Male (254) | Female (318) | Total (337) | Male (143) | Female (194) | |
| AQ Total (0–50) | 20.04 (7.06) | 20.18 (6.81) | 19.68 (7.69) | 13.48 (5.89) | 14.42 (6.39) | 12.72 (5.36) | 12.43 (5.82) | 13.50 (5.76) | 11.64 (5.75) |
| Social skill | 3.57 (2.35) | 3.48 (2.31) | 3.80 (2.44) | 1.98 (1.96) | 2.06 (2.15) | 1.91 (1.79) | 1.75 (1.82) | 1.81 (1.92) | 1.70 (1.75) |
| Communication | 3.27 (1.96) | 3.28 (1.94) | 3.23 (2.02) | 1.97 (1.69) | 2.19 (1.87) | 1.80 (1.52) | 1.82 (1.64) | 2.22 (1.66) | 1.53 (1.57) |
| Imagination | 4.19 (2.00) | 4.45 (1.84) | 3.51 (2.23) | 3.11 (1.80) | 3.55 (1.81) | 2.76 (1.72) | 2.93 (1.77) | 3.21 (1.88) | 2.72 (1.66) |
| Attention to detail | 4.03 (2.20) | 4.00 (2.23) | 4.11 (2.10) | 3.33 (1.87) | 3.34 (1.91) | 3.32 (1.84) | 3.15 (1.93) | 3.14 (1.99) | 3.15 (1.88) |
| Attention switching | 4.99 (2.35) | 4.06 (2.31) | 5.03 (2.22) | 3.08 (1.97) | 3.28 (2.02) | 2.93 (1.92) | 2.79 (2.01) | 3.12 (2.14) | 2.54 (1.87) |
| AQ ⩾ 32 (%) | 33 (6.55) | 24 (6.58) | 9 (6.47) | 6 (1.05) | 5 (1.97) | 1 (0.31) | 4 (1.18) | 2 (1.40) | 2 (1.03) |
| AQ ⩾ 26 (%) | 108 (21.43) | 80 (21.92) | 28 (20.14) | 16 (2.80) | 10 (3.94) | 6 (1.89) | 8 (2.36) | 4 (2.80) | 4 (2.06) |
Next, mean comparisons showed that groups differed significantly on the overall level of autistic traits, covaried for age, sex, and IQ (F2,1406 = 129.28, p < 0.001, η2partial = 0.16). Covariates were significant as well, with positive effects for age (p = 0.004, η2partial = 0.01) and male sex (p < 0.001, η2partial = 0.01), and a negative effect for IQ (p < 0.001, η2partial = 0.01). Bonferroni-corrected post hoc comparisons indicated that PD reported significantly more autistic traits than FR (p < 0.001, d = 0.80) and TC (p < 0.001, d = 0.92). FR also reported more autistic traits than TC, albeit with a small effect size (p = 0.028, d = 0.18). On a subscale level MANCOVA analysis showed similar group differences on all subscales (all p < 0.001), except post hoc tests were not significant for mean differences between FR and TC.
Impact of autistic traits on social functioning in PD
Group, Total AQ, and CAPE Positive were entered by default in the initial models. Linearity assumptions (p < 0.05; correlations in online Supplementary Tables) were checked next to determine which additional variables were entered into the models. In the initial models only main effects were included. Additional two-way interactions were also examined for potential model optimization.
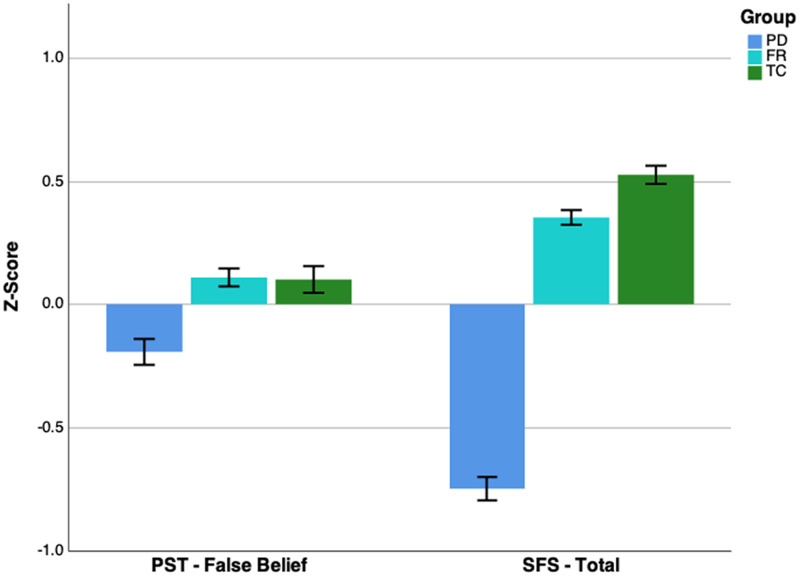
Cognition. PST-FB data were missing for 55 individuals (17 PD, 18 FR, 20 TC). Standardized group means are displayed in Fig. 2. In addition to Group, Total AQ, and CAPE Positive, the covariates age and IQ were also included. There was no significant group effect on PST-FB (p = 0.124), hence model comparison was continued for the total sample without Group as a factor. The best fit (see Table 3a) included: Total AQ (β = −0.09, p = 0.001), age (β = −0.20, p < 0.001), IQ (β = 0.28, p < 0.001), and Total AQ × age (β = −0.07, p = 0.002). CAPE Positive was not significant and no longer included in the final model.
Fig. 2.
Z-transformed group means for Picture Sequencing Test – False Belief (PST-FB; left) and the Social Functioning Scale (SFS; right). Error bars represent ±1 standard error.
Table 3.
Best-fitting multilevel models for social functioning
| B | s.e. | p | 95% CI | |
|---|---|---|---|---|
| (a) Dependent: picture sequencing task – false belief stories (total sample) | ||||
| Intercept | 19.676 | 0.116 | <0.001 | 19.450–19.903 |
| Total AQ | −0.051 | 0.015 | 0.001 | −0.080 to −0.021 |
| Age | −0.010 | 0.013 | <0.001 | −0.125 to −0.021 |
| IQ | 0.064 | 0.006 | <0.001 | 0.052–0.076 |
| Total AQ × age | −0.006 | 0.002 | 0.002 | −0.009 to −0.002 |
| Centered around the means: Total AQ = 15.57, age = 34.85, IQ = 108.59. | ||||
Centered around the means: Total AQ = 20.04, age = 33.38, IQ = 100.72, CAPE Negative = 26.25.
Behavior. SFS data were missing for 10 individuals (four PD, four FR, two TC). See Fig. 2 for standardized group means. All candidate predictors were included. There was a significant group effect on SFS (F = 45.70, p < 0.001). Bonferroni-corrected post hoc comparisons indicated that PD differed significantly from the other groups (p < 0.001), but FR did not differ from TC. Model comparison was then continued for the PD group only. The best model fit (see Table 3b) included fixed effects for age (β = −0.09, p = 0.019), sex (β = 0.21, p < 0.001), IQ (β = 0.19, p < 0.001), Total AQ (β = −0.37, p < 0.001), and CAPE Negative (β = −0.24, p < 0.001). Neither CAPE Positive and CAPE Depressive, nor any two-way interactions improved both AIC and BIC values.
Interaction autistic and positive symptoms. Adding the interaction term Total AQ × CAPE Positive did not yield significant predictors or model improvements for PST-FB or SFS. To allow for better statistical comparison with previous results from Vaskinn and Abu-Akel (2019), the analyses were repeated in generalized linear models for PD only, without region and family ID included in the models. Again, no significant interactions supporting a diametrical model were detected (see online Supplementary Tables).
Discussion
The current study provides robust evidence for a substantially increased presence of autistic traits in individuals diagnosed with PD compared to their unaffected siblings and TC individuals. This concerns both categorical and dimensional levels of autistic traits, which may arguably reflect separate etiologies (Abu-Akel, Allison, Baron-Cohen, & Heinke, 2019; Linscott & van Os, 2010). Concerning categorical classifications, an AQ cut-off of 32 is known to discriminate satisfactorily between ASD and TC individuals, and a cut-off of 26 shows reasonable sensitivity (Baron-Cohen et al., 2001; Woodbury-Smith et al., 2005). Therefore, our findings suggest that one in 15 individuals with PD falls above the clinical cut-off, and one in five above the sub-clinical threshold. For FR, the odds are, respectively, 6 and 7 times smaller than for PD, and comparable with TC. However, the predictive diagnostic accuracy of AQ thresholds in suspected ASD appears modest (Ashwood et al., 2016), and in our sample, only six (1.2%) PD individuals had a suspected diagnosis of ASD at intake. This raises the question whether elevated autistic traits in PD may reflect symptomatic overlap, in particular with negative psychotic traits. Based on the assessments used in this study, there is evident overlap between the social skill subscale of AQ (10 items; e.g. ‘I enjoy meeting new people’) and social withdrawal items of the CAPE (four items; e.g. ‘Do you ever feel you have no interest to be with other people’). Nevertheless, most items do not resemble each other as closely and correlations of AQ and CAPE in PD individuals were modest and comparable for all dimensions (0.32–0.36; online Supplementary Table). Therefore, symptom overlap can only provide a limited explanation for the increased presence of autistic traits in PD. Regardless, the absence of diagnoses in our sample further emphasizes that comorbid ASD is potentially overlooked or wrongfully dismissed by clinicians presented with PD cases.
On a dimensional level, group differences in autism traits are equally striking for PD and in line with previous findings in smaller samples (Lugnegard, Hallerbäck, & Gillberg, 2015; Sasamoto et al., 2011). Additionally, individuals with FR reported marginally more autism traits than TC when the potential effects of age, sex, and IQ were accounted for. However, this group difference dissipated on a subscale level. Importantly, our sibling group was unaffected in terms of schizophrenia spectrum diagnoses, but not completely devoid of other comorbid conditions, in particular mood disorders (n = 111, including 74 remissions). Explorative post hoc analyses indicated that those who met the criteria for any DSM-IV disorder (n = 126; M = 15.48) scored significantly higher on AQ than those not meeting any criteria (n = 446, M = 12.91, p < 0.001). In addition, the few siblings who did acquire a PD after baseline were reassigned to the patient group. This likely led to a slight underestimation of psychotic and autistic traits in the sibling group than would be expected based on family history alone (Sullivan et al., 2012). As such, we can ascertain that only unaffected siblings of individuals with PD are relatively resistant to developing autistic traits.
As expected, the results provide strong evidence for a profoundly negative relation of autism traits with social functioning in PD. Comparable data are surprisingly scarce or only available for modest and mostly pediatric samples, with three notable exceptions that all used the PANSS Autism Severity Score (PAUSS; Kästner et al., 2015) to measure autism symptoms in adult PD. Barlati et al. (2019) showed that psychosocial functioning did not differ between schizophrenia patients (N = 75) with and without autism, after patients were thoroughly screened for ASD. However, global measures of functioning were used (Honos, GAF) and the prediction of autism severity scales was dichotomized, making it difficult to establish any potential linear dosage effects on a dimensional scale. Vaskinn and Abu-Akel (2019) did report a negative linear relation between autistic symptoms and functioning in PD (N = 81) by using both GAF and SFS as outcome measures, but also found positive two-way interaction effects with positive symptoms that were suggestive of diametric effects on functioning (discussed further below). Finally, Harvey et al. also found a small but significant effect of autistic symptoms on real-world functioning in PD (N = 171) (Harvey et al., 2019). Our results add to these findings a more robust outcome and direct comparisons with a related and unrelated group of individuals without PD.
Although it is known that cognitive and negative symptoms are better predictors of functional outcome (Malla & Payne, 2005; Stouten, Veling, Laan, van der Helm, & van der Gaag, 2017), it was still somewhat unexpected that there were no significant relations between positive psychotic traits and social functioning. The positive traits were also relatively low in our PD sample, perhaps due to treatment effects, which may help explain the absence of associations with functioning measures. To improve our understanding of the dynamics between co-occurring autistic traits, psychotic traits, and functioning in PD, it would therefore be of great interest to study the strength of relations on a subdomain level and determine centrality measures within a network approach (van Rooijen et al., 2018; Wigman et al., 2015; Wüsten et al., 2018).
Social cognitive functioning was also negatively associated with autistic traits in line with previous findings (Harvey et al., 2019; Vaskinn & Abu-Akel, 2019) but, contrary to expectations, the effect was small and comparable across groups. Likewise, the correlation between social cognition and social functioning was surprisingly small. We used a classic mentalizing (false belief) paradigm, which is typically performed less accurately by individuals with PD (Langdon et al., 2006), though not always (Pepper et al., 2018). Group differences in mentalizing were obscured by relatively strong effects of IQ and age in our study. However, these differences may be meaningful in their own right and covarying for them may not always be necessary or preferable in some instances (Miller & Chapman, 2001). The influence of other factors affecting outcome may also help explain why mentalizing performance may not be sensitive enough to reveal strong relations with the functional outcome (Ohmuro et al., 2016). Notwithstanding, it is safe to conclude from our results that autistic traits are negatively associated with mentalizing abilities in general, hence also for PD. Possibly, the effect would have been stronger if other, lower-order social cognition tasks (i.e. emotion recognition) were used, as these appear to be more clearly impaired in both PD and ASD (Eack et al., 2013; Pepper et al., 2018; Sasson, Pinkham, Weittenhiller, Faso, & Simpson, 2016). Future studies may benefit in general from broader and more ecologically valid assessments of social cognition.
A final aim of this study was to explore the presence of diametric effects of autistic and positive psychotic traits on social functioning. Contrary to recent exciting findings in non-clinical (Abu-Akel, Wood, Hansen, & Apperly, 2015, 2017b, 2017a, 2018a) and clinical (Abu-Akel et al., 2018b; Vaskinn & Abu-Akel, 2019) samples in the literature, our results do not provide further support for the diametric hypothesis. Although there are some differences in samples and methods across studies, the type, quality, and amount of data available to address this question in the GROUP cohort would have been sufficient to detect even small interaction effects if a diametric relation had been present. It is unclear how to interpret these opposing findings and we propose future studies address the diametric hypothesis by cross-validation in different samples with identical methods.
Several limitations should be considered when interpreting our findings. First, despite their psychometric strengths and common use, self-report questionnaires such as AQ-50 and SFS have notable shortcomings. For example, the AQ-50 is not equivalent to an objective assessment of autistic traits, potentially affected by subgroup bias (Agelink van Rentergem, Lever, & Geurts, 2019) and has a debatable factor structure (Lundqvist & Lindner, 2017). Additional observations, interviews, or informant reports are required to confirm the presence of traits and/or a clinical diagnosis of ASD in PD. Concurrently, it would help establish convergent validity of ASD assessments in PD and reduce potential bias introduced by mono-method associations (i.e. based on self-report). The SFS was constructed specifically to tap those areas of functioning that are crucial to individuals with schizophrenia and related disorders and generally has good psychometric properties, but its use may be suboptimal for assessing social functioning in individuals with ASD (Chan et al., 2019) and some domains may have low ecological validity (Schneider et al., 2017). Second, data were assessed approximately 6 years after baseline. Consequently, a relatively large portion of PD individuals (approximately 40%) fulfilled PANSS remission criteria. It is up to speculation if and how this affected the prevalence rates and relations with social functioning. Interestingly, when assessed post hoc, PD individuals in remission had significantly lower AQ scores than those not in remission, which could indicate that the impact of autistic traits extends beyond social functioning. Third, the data were cross-sectional and can therefore only establish concurrent relations and not whether autism traits are an actual determinant of poor outcome. Only follow-up data can and should address this in the future.
In conclusion, this report set out to establish the prevalence of (self-reported) autistic traits in an unprecedented sample size of individuals with PD and their unaffected siblings compared to typical comparisons. Our results confirm that the prevalence is overwhelmingly higher in PD, both categorically and dimensionally, an only marginally higher in FR on a dimensional level. The higher prevalence in PD is accompanied by a strong negative relation with social functioning on a behavioral level and to a lesser extent on a cognitive level. This indicates a need for better transdiagnostic clinical guidelines. For example, it is generally accepted that psychotic decompensation can occur due to a maladaptive stress response. For individuals on the autism spectrum, there are often clear indicators which situations trigger a severe stress response, e.g. sensory overload or unexpected, life-changing events. Therefore, when they present themselves with prodromal symptoms, these individuals can benefit from supervised daily schedules and access to a stimulus-free room to help prevent the onset/relapse of psychosis and potentially marginalize the need for antipsychotic treatment. These and other alternative treatment hypotheses need to be substantiated with additional scientific evidence (e.g. from clinical trials), but are worthy of consideration. Taken together, our findings suggest that autistic comorbidity in PD represents a very relevant clinical issue that needs to be addressed in order to improve treatment outcome.
Acknowledgements
We are grateful for the generosity of time and effort by the patients, their families, and healthy subjects. Furthermore, thanks to all research personnel involved in the GROUP project, in particular: Joyce van Baaren, Erwin Veermans, Ger Driessen, Truda Driesen, Erna van ‘t Hag.
Financial support
This work was supported by the Brain & Behaviour Research Foundation (T.Z., NARSAD Young Investigator grant, number 25500). The infrastructure for the GROUP study is funded through the Geestkracht program of the Dutch Health Research Council (Zon-Mw, grant number 10-000-1001), and matching funds from participating pharmaceutical companies (Lundbeck, AstraZeneca, Eli Lilly, Janssen Cilag) and universities and mental health care organizations.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291720000458.
click here to view supplementary material
Conflict of interest
None.
References
- Abu-Akel, A., Allison, C., Baron-Cohen, S., & Heinke, D. (2019). The distribution of autistic traits across the autism spectrum: Evidence for discontinuous dimensional subpopulations underlying the autism continuum. Molecular Autism, 10, 1752–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Akel, A., Apperly, I., Spaniol, M. M., Geng, J. J., & Mevorach, C. (2018a). Diametric effects of autism tendencies and psychosis proneness on attention control irrespective of task demands. Scientific Reports, 8, 8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Akel, A. M., Apperly, I. A., Wood, S. J., & Hansen, P. C. (2017b). Autism and psychosis expressions diametrically modulate the right temporoparietal junction. Social Neuroscience, 12, 506–518. [DOI] [PubMed] [Google Scholar]
- Abu-Akel, A., Apperly, I. A., Wood, S. J., Hansen, P. C., & Mevorach, C. (2017a). Autism tendencies and psychosis proneness interactively modulate saliency cost. Schizophrenia Bulletin, 43, 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Akel, A., Testa, R. R., Jones, H. P., Ross, N., Skafidas, E., Tonge, B., & Pantelis, C. (2018b). Attentional set-shifting and social abilities in children with schizotypal and comorbid autism spectrum disorders. Australian and New Zealand Journal of Psychiatry, 52, 68–77. [DOI] [PubMed] [Google Scholar]
- Abu-Akel, A. M., Wood, S. J., Hansen, P. C., & Apperly, I. A. (2015). Perspective-taking abilities in the balance between autism tendencies and psychosis proneness. Proceedings of the Royal Society B: Biological Sciences, 282, 20150563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agelink van Rentergem, J. A., Lever, A. G., & Geurts, H. M. (2019). Negatively phrased items of the Autism Spectrum Quotient function differently for groups with and without autism. Autism, 23, 1752–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2000). Diagnostic and statistical manual of mental Disorders (4th ed). Washington, DC: DSM-IV-TR. [Google Scholar]
- Andreasen, N. C., Carpenter, W. T., Kane, J. M., Lasser, R. A., Marder, S. R., & Weinberger, D. R. (2005). Remission in schizophrenia: Proposed criteria and rationale for consensus. American Journal of Psychiatry, 162, 441–449. [DOI] [PubMed] [Google Scholar]
- Andreasen, N. C., Flaum, M., & Arndt, S. (1992). The Comprehensive Assessment of Symptoms and History (CASH): An instrument for assessing diagnosis and psychopathology. Archives of General Psychiatry, 49, 615–623. [DOI] [PubMed] [Google Scholar]
- Ashwood, K. L., Gillan, N., Horder, J., Hayward, H., Woodhouse, E., McEwen, F. S., … Murphy, D. G. (2016). Predicting the diagnosis of autism in adults using the Autism-Spectrum Quotient (AQ) questionnaire. Psychological Medicine, 46, 2595–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlati, S., Deste, G., Gregorelli, M., & Vita, A. (2019). Autistic traits in a sample of adult patients with schizophrenia: Prevalence and correlates. Psychological Medicine, 49, 140–148. [DOI] [PubMed] [Google Scholar]
- Barneveld, P. S., Pieterse, J., de Sonneville, L., van Rijn, S., Lahuis, B., van Engeland, H., & Swaab, H. (2011). Overlap of autistic and schizotypal traits in adolescents with autism spectrum disorders. Schizophrenia Research, 126, 231–236. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen, S., Leslie, A. M., & Frith, U. (1986). Mechanical, behavioural and intentional understanding of picture stories in autistic children. British Journal of Developmental Psychology, 4, 113–125. [Google Scholar]
- Baron-Cohen, S., Wheelwright, S., Skinner, R., Martin, J., & Clubley, E. (2001). The Autism-Spectrum Quotient (AQ): Evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31, 5–17. [DOI] [PubMed] [Google Scholar]
- Benjamin, D. J., Berger, J. O., Johannesson, M., Nosek, B. A., Wagenmakers, E. J., Berk, R., … Johnson, V. E. (2018). Redefine statistical significance. Nature Human Behaviour, 2, 6–10. [DOI] [PubMed] [Google Scholar]
- Birchwood, M., Smith, J., Cochrane, R., Wetton, S., & Copestake, S. (1990). The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. British Journal of Psychiatry, 157, 853–859. [DOI] [PubMed] [Google Scholar]
- Bleuler, E. (1950). Dementia praecox or the group of schizophrenias. Oxford, England: International Universities Press. [Google Scholar]
- Chan, E. H. C., Kong, S. D. X., Park, S. H., Song, Y. J. C., Demetriou, E. A., Pepper, K. L., … Guastella, A. J. (2019). Validation of the social functioning scale: Comparison and evaluation in early psychosis, autism spectrum disorder and social anxiety disorder. Psychiatry Research, 276, 45–55. [DOI] [PubMed] [Google Scholar]
- Chisholm, K., Lin, A., Abu-Akel, A., & Wood, S. J. (2015). The association between autism and schizophrenia spectrum disorders: A review of eight alternate models of co-occurrence. Neuroscience and Biobehavioral Reviews, 55, 173–183. [DOI] [PubMed] [Google Scholar]
- Crespi, B., & Badcock, C. (2008). Psychosis and autism as diametrical disorders of the social brain. Behavioral and Brain Sciences, 31, 241–261. [DOI] [PubMed] [Google Scholar]
- Davidson, C., Greenwood, N., Stansfield, A., & Wright, S. (2014). Prevalence of Asperger syndrome among patients of an early intervention in psychosis team. Early Intervention in Psychiatry, 8, 138–146. [DOI] [PubMed] [Google Scholar]
- De Crescenzo, F., Postorino, V., Siracusano, M., Riccioni, A., Armando, M., Curatolo, P., & Mazzone, L. (2019). Autistic symptoms in schizophrenia spectrum disorders: A systematic review and meta-analysis. Frontiers in Psychiatry, 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack, S. M., Bahorik, A. L., McKnight, S. A. F., Hogarty, S. S., Greenwald, D. P., Newhill, C. E., … Minshew, N. J. (2013). Commonalities in social and non-social cognitive impairments in adults with autism spectrum disorder and schizophrenia. Schizophrenia Research, 148, 24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterberg, M. L., Trotman, H. D., Brasfield, J. L., Compton, M. T., & Walker, E. F. (2008). Childhood and current autistic features in adolescents with schizotypal personality disorder. Schizophrenia Research, 104, 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagel, S. S. A. A., Swaab, H., De Sonneville, L. M. J., Van Rijn, S., Pieterse, J. K., Scheepers, F., & Van Engeland, H. (2013). Development of schizotypal symptoms following psychiatric disorders in childhood or adolescence. European Child and Adolescent Psychiatry, 22, 683–692. [DOI] [PubMed] [Google Scholar]
- Fromer, M., Roussos, P., Sieberts, S. K., Johnson, J. S., Kavanagh, D. H., Perumal, T. M., … Sklar, P. (2016). Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nature Neuroscience, 11, 1442–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey, P. D., Deckler, E., Jones, M. T., Jarskog, L. F., Penn, D. L., & Pinkham, A. E. (2019). Autism symptoms, depression, and active social avoidance in schizophrenia: Association with self-reports and informant assessments of everyday functioning. Journal of Psychiatric Research, 115, 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra, R. A., Bartels, M., Cath, D. C., & Boomsma, D. I. (2008). Factor structure, reliability and criterion validity of the autism-spectrum quotient (AQ): A study in Dutch population and patient groups. Journal of Autism and Developmental Disorders, 38, 1555–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kästner, A., Begemann, M., Michel, T. M., Everts, S., Stepniak, B., Bach, C., … Ehrenreich, H. (2015). Autism beyond diagnostic categories: Characterization of autistic phenotypes in schizophrenia. BMC Psychiatry, 15, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid, D. L., Doris, M., Shannon, C., & Mulholland, C. (2017). What is the prevalence of autism spectrum disorder and ASD traits in psychosis? A systematic review. Psychiatry Research, 250, 99–105. [DOI] [PubMed] [Google Scholar]
- Korver, N., Quee, P. J., Boos, H. B. M., Simons, C. J. P., De Haan, L., Kahn, R. S., & Linszen, D. H. (2012). Genetic Risk and Outcome of Psychosis (GROUP), a multi site longitudinal cohort study focused on gene-environment interaction: Objectives, sample characteristics, recruitment and assessment methods. International Journal of Methods in Psychiatric Research, 21, 205–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushima, I., Aleksic, B., Nakatochi, M., Shimamura, T., Okada, T., Uno, Y., … Arioka, Y. (2018). Comparative analyses of copy-number variation in autism spectrum disorder and schizophrenia reveal etiological overlap and biological insights. Cell Reports, 24, 2838–2856. [DOI] [PubMed] [Google Scholar]
- Langdon, R., & Coltheart, M. (1999). Mentalising, schizotypy, and schizophrenia. Cognition, 71, 43–71. [DOI] [PubMed] [Google Scholar]
- Langdon, R., Coltheart, M., & Ward, P. B. (2006). Empathetic perspective-taking is impaired in schizophrenia: Evidence from a study of emotion attribution and theory of mind. Cognitive Neuropsychiatry, 11, 133–155. [DOI] [PubMed] [Google Scholar]
- Linscott, R. J., & van Os, J. (2010). Systematic reviews of categorical versus continuum models in psychosis: Evidence for discontinuous subpopulations underlying a psychometric continuum. Implications for DSM-V, DSM-VI, and DSM-VII. Annual Review of Clinical Psychology, 6, 391–419. [DOI] [PubMed] [Google Scholar]
- Lugnegard, T., Hallerbäck, M. U., & Gillberg, C. (2015). Asperger syndrome and schizophrenia: Overlap of self-reported autistic traits using the Autism-spectrum Quotient (AQ). Nordic Journal of Psychiatry, 69, 268–274. [DOI] [PubMed] [Google Scholar]
- Lundqvist, L. O., & Lindner, H. (2017). Is the Autism-Spectrum Quotient a valid measure of traits associated with the autism spectrum? A Rasch validation in adults with and without autism spectrum disorders. Journal of Autism and Developmental Disorders, 47, 2080–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malla, A., & Payne, J. (2005). First-episode psychosis: Psychopathology, quality of life, and functional outcome. Schizophrenia Bulletin, 31, 650–671. [DOI] [PubMed] [Google Scholar]
- Martinez, G., Alexandre, C., Mam-Lam-Fook, C., Bendjemaa, N., Gaillard, R., Garel, P., … Krebs, M. O. (2017). Phenotypic continuum between autism and schizophrenia: Evidence from the Movie for the Assessment of Social Cognition (MASC). Schizophrenia Research, 185, 161–166. [DOI] [PubMed] [Google Scholar]
- Miller, G. A., & Chapman, J. P. (2001). Misunderstanding analysis of covariance. Journal of Abnormal Psychology, 110, 40–48. [DOI] [PubMed] [Google Scholar]
- Ohmuro, N., Katsura, M., Obara, C., Kikuchi, T., Sakuma, A., Iizuka, K., … Matsmoto, K. (2016). Deficits of cognitive theory of mind and its relationship with functioning in individuals with an at-risk mental state and first-episode psychosis. Psychiatry Research, 243, 318–325. [DOI] [PubMed] [Google Scholar]
- Padgett, F. E., Miltsiou, E., & Tiffin, P. A. (2010). The co-occurrence of nonaffective psychosis and the pervasive developmental disorders: A systematic review. Journal of Intellectual and Developmental Disability, 35, 187–198. [DOI] [PubMed] [Google Scholar]
- Pepper, K. L., Demetriou, E. A., Park, S. H., Song, Y. C., Hickie, I. B., Cacciotti-Saija, C., … Guastella, A. J. (2018). Autism, early psychosis, and social anxiety disorder: Understanding the role of social cognition and its relationship to disability in young adults with disorders characterized by social impairments. Translational Psychiatry, 8, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham, A. E., Morrison, K. E., Penn, D. L., Harvey, P. D., Kelsven, S., Ludwig, K., & Sasson, N. J. (2019). Comprehensive comparison of social cognitive performance in autism spectrum disorder and schizophrenia. Psychological Medicine, 1–9. [DOI] [PubMed] [Google Scholar]
- Pourcain, B. S., Robinson, E. B., Anttila, V., Sullivan, B. B., Maller, J., Golding, J., … Davey Smith, G. (2018). ASD and schizophrenia show distinct developmental profiles in common genetic overlap with population-based social communication difficulties. Molecular Psychiatry, 23, 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasamoto, A., Miyata, J., Hirao, K., Fujiwara, H., Kawada, R., Fujimoto, S., … Murai, T. (2011). Social impairment in schizophrenia revealed by Autism-Spectrum Quotient correlated with gray matter reduction. Social Neuroscience, 6, 548–558. [DOI] [PubMed] [Google Scholar]
- Sasson, N. J., Pinkham, A. E., Carpenter, K. L. H., & Belger, A. (2011). The benefit of directly comparing autism and schizophrenia for revealing mechanisms of social cognitive impairment. Journal of Neurodevelopmental Disorders, 3, 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson, N. J., Pinkham, A. E., Weittenhiller, L. P., Faso, D. J., & Simpson, C. (2016). Context effects on facial affect recognition in schizophrenia and autism: Behavioral and eye-tracking evidence. Schizophrenia Bulletin, 42, 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson, N., Tsuchiya, N., Hurley, R., Couture, S. M., Penn, D. L., Adolphs, R., & Piven, J. (2007). Orienting to social stimuli differentiates social cognitive impairment in autism and schizophrenia. Neuropsychologia, 45, 2580–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalbroeck, R., Termorshuizen, F., Visser, E., Van Amelsvoort, T., & Selten, J. P. (2018). Risk of non-affective psychotic disorder or bipolar disorder in autism spectrum disorder: A longitudinal register-based study in the Netherlands. Psychological Medicine, 49, 2543–2550. [DOI] [PubMed] [Google Scholar]
- Schneider, M., Reininghaus, U., van Nierop, M., Janssens, M., Myin-Germeys, I., & Investigators GROUP (2017). Does the Social Functioning Scale reflect real-life social functioning? An experience sampling study in patients with a non-affective psychotic disorder and healthy control individuals. Psychological Medicine, 47, 2777–2786. [DOI] [PubMed] [Google Scholar]
- Selten, J. P., Lundberg, M., Rai, D., & Magnusson, C. (2015). Risks for nonaffective psychotic disorder and bipolar disorder in young people with autism spectrum disorder: A population-based study. JAMA Psychiatry, 72, 483–489. [DOI] [PubMed] [Google Scholar]
- Stefanis, N. C., Hanssen, M., Smirnis, N. K., Avramopoulos, D. A., Evdokimidis, I. K., Stefanis, C. N., … Van Os, J. (2002). Evidence that three dimensions of psychosis have a distribution in the general population. Psychological Medicine, 32, 347–358. [DOI] [PubMed] [Google Scholar]
- Stouten, L. H., Veling, W., Laan, W., van der Helm, M., & van der Gaag, M. (2017). Psychosocial functioning in first-episode psychosis and associations with neurocognition, social cognition, psychotic and affective symptoms. Early Intervention in Psychiatry, 11, 23–36. [DOI] [PubMed] [Google Scholar]
- Sullivan, P. F., Magnusson, C., Reichenberg, A., Boman, M., Dalman, C., Davidson, M., … Lichtenstein, P. (2012). Family history of schizophrenia and bipolar disorder as risk factors for autism. Archives of General Psychiatry, 69, 1099–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, M. J., Martin, J., Lu, Y., Brikell, I., Lundström, S., Larsson, H., & Lichtenstein, P. (2019). Association of genetic risk factors for psychiatric disorders and traits of these disorders in a Swedish population twin sample. JAMA Psychiatry, 76, 280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooijen, G., Isvoranu, A. M., Kruijt, O. H., van Borkulo, C. D., Meijer, C. J., Wigman, J. T. W., … Bartels-Velthuis, A. A. (2018). A state-independent network of depressive, negative and positive symptoms in male patients with schizophrenia spectrum disorders. Schizophrenia Research, 193, 232–239. [DOI] [PubMed] [Google Scholar]
- Vaskinn, A., & Abu-Akel, A. (2019). The interactive effect of autism and psychosis severity on theory of mind and functioning in schizophrenia. Neuropsychology, 33, 195–202. [DOI] [PubMed] [Google Scholar]
- Velthorst, E., Froudist-Walsh, S., Stahl, E., Ruderfer, D., Ivanov, I., Buxbaum, J., … Martin, J. (2018). Genetic risk for schizophrenia and autism, social impairment and developmental pathways to psychosis. Translational Psychiatry, 8, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (1997). Wechsler adult intelligence scale – third edition (WAIS-III). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wigman, J. T. W., Van Os, J., Borsboom, D., Wardenaar, K. J., Epskamp, S., Klippel, A., … Wichers, M. (2015). Exploring the underlying structure of mental disorders: Cross-diagnostic differences and similarities from a network perspective using both a top-down and a bottom-up approach. Psychological Medicine, 45, 2375–2387. [DOI] [PubMed] [Google Scholar]
- Wing, J. K., Babor, T., Brugha, T., Burke, J., Cooper, J. E., Giel, R., … Sartorius, N. (1990). Schedules for clinical assessment in neuropsychiatry. Archives of General Psychiatry, 47, 589–593. [DOI] [PubMed] [Google Scholar]
- Woodbury-Smith, M. R., Robinson, J., Wheelwright, S., & Baron-Cohen, S. (2005). Screening adults for Asperger Syndrome using the AQ: A preliminary study of its diagnostic validity in clinical practice. Journal of Autism and Developmental Disorders, 35, 331–335. [DOI] [PubMed] [Google Scholar]
- Wüsten, C., Schlier, B., Jaya, E. S., Fonseca-Pedrero, E., Peters, E., Verdoux, H., … Lincoln, T. M. (2018). Psychotic experiences and related distress: A cross-national comparison and network analysis based on 7141 participants from 13 countries. Schizophrenia Bulletin, 44, 1185–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrei, M., Burton, C. L., Engchuan, W., Young, E. J., Higginbotham, E. J., MacDonald, J. R., … Scherer, S. W. (2019). A large data resource of genomic copy number variation across neurodevelopmental disorders. NPJ Genomic Medicine, 4, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziermans, T., Swaab, H., Stockmann, A., de Bruin, E., & van Rijn, S. (2017). Formal thought disorder and executive functioning in children and adolescents with autism spectrum disorder: Old leads and new avenues. Journal of Autism and Developmental Disorders, 47, 1756–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291720000458.
click here to view supplementary material