Abstract
Background
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of advanced or recurrent non‐small cell lung cancer (NSCLC). They cause immune‐related adverse events (irAEs), but the underlying mechanisms and predictors remain to be fully elucidated. In this retrospective study, we investigated the association between pretreatment neutrophil‐to‐lymphocyte ratio (NLR) and the occurrence of irAEs.
Methods
The study involved 115 patients with NSCLC who started ICI‐only treatment in our hospital between January 2016 and April 2020.
Results
Forty‐five patients (39.1%) had irAEs, and pretreatment NLR was significantly lower in the irAEs group than in the non‐irAEs group (2.8 vs. 4.1; p = 0.036). The cutoff value of the NLR was 2.86 (area under curve, 0.62; sensitivity, 0.56; specificity, 0.71), and the incidence rate of irAEs was significantly higher in the NLR < 2.86 group than in the NLR ≥2.86 group (p = 0.004; odds ratio [OR]: 3.12; 95% confidence interval [CI]: 1.43–6.84). The multivariate analysis showed that the NLR was significantly associated with the occurrence of irAEs (p = 0.016; OR: 2.69; 95% CI: 1.21–6.01).
Conclusions
Low pretreatment NLR may be a predictive factor for the occurrence of irAEs. By focusing on the potential risk of irAEs in patients with a low pretreatment NLR, irAEs can be appropriately managed from an early period.
Keywords: immune checkpoint inhibitor, immune‐related adverse event, neutrophil‐to‐lymphocyte rate, non‐small cell lung cancer
Here, we report that low pretreatment NLR may be a predictive factor for the occurrence of irAEs in NSCLC patients. The cutoff value of the NLR was 2.86, and the univariate and multivariate analysis showed that the NLR was significantly associated with the occurrence of irAEs.
INTRODUCTION
Immune checkpoint inhibitors (ICIs) have paved a new era for the treatment of advanced or recurrent non‐small cell lung cancer (NSCLC). Nivolumab, pembrolizumab, atezolizumab, durvalumab, and ipilimumab have been approved for the treatment of NSCLC, and monotherapy and combination therapy have been established as a standard course of care. 1
ICIs exert antitumor effects by binding to inhibitory receptors expressed on T cells, or their ligands, and blocking the inhibitory signals of the immune system. 2 An increase in immune functions induced by ICIs may cause immune‐related adverse events (irAEs) due to the disruption of immune function homeostasis. 3 irAEs pose a risk of poor prognosis in severe cases, and ICI‐related mortality rate is in the range of 0.36%–1.23%. 4 Appropriate treatment from an early period is required when patients experience irAEs; while there have been some reports on the predictive factors of irAEs, 5 , 6 , 7 , 8 , 9 there are no established factors.
The neutrophil‐to‐lymphocyte ratio (NLR) is an index that reflects systemic inflammation. It can be easily calculated from the results of inexpensive routine blood tests; the prognostic value of NLR has been proven in cardiovascular diseases and infections. 10 , 11 Reportedly, the NLR can serve as a prognostic factor for solid tumors 12 , 13 , 14 and a predictor of clinical benefits of ICIs. 15 , 16 , 17 Furthermore, its relationship with irAEs has been investigated in recent years 8 , 9 ; however, there is still no unified view. In this study, we retrospectively investigated the association between NLR and the occurrence of irAEs in patients with advanced/recurrent NSCLC treated with ICIs.
METHODS
Patients
This retrospective study involved 115 patients with NSCLC who started treatment with ICIs between January 2016 and April 2020 in the National Hospital Organization Kyushu Medical Center. We excluded patients who had been treated with other ICIs before starting ICIs, patients who received ICIs in combination with chemotherapy, and patients whose peripheral blood neutrophils and lymphocytes were not measured within one week before the start of ICIs. We retrospectively surveyed the medical records of patients and investigated the irAEs. The NLR was calculated as follows: neutrophil count (/μl) / lymphocyte count (/μl). The Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 was used to assess irAEs. The observation period was from January 2016 to September 2020.
Ethical approval
This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and the ethical guidelines for Medical and Health Research Involving Human Subjects. This study was approved by the investigational review board of the ethics committee of Kyushu Medical Center (approval no. 20C200).
Dosage of ICIs
Nivolumab was administered every two weeks at a dose of 3 mg/kg until August 31, 2018, and 240 mg/bodyweight after November 1, 2018. During the two months between September 2018 and October 2018, a dose of either 3 mg/kg or 240 mg/bodyweight was chosen for each patient. Pembrolizumab was administered at a dose of 200 mg/bodyweight every three weeks, and atezolizumab was administered at 1200 mg/bodyweight every three weeks.
Statistical analysis
In this study, patients were divided into two groups, namely, the irAEs and non‐irAEs groups. We used Fisher's exact test and the chi‐square test to compare the qualitative data between the groups, and Mann–Whitney U test to compare the quantitative data. Patients were dichotomized according to the cutoff values of NLR < 2.86 versus ≥2.86. The NLR cutoff value before the start of treatment was calculated using receiver operating characteristic (ROC) curve analysis. Multivariable logistic regression analysis was used to identify the predictive factors of irAEs.
Overall survival (OS) was defined as the period from the initiation of ICIs to patient death from any cause or to the last date of confirmation of survival based on the medical records. The OS was compared between the groups using the log‐rank test, and Holm method of the post‐hoc test was used to compare the OS among the four groups. Results with p < 0.05 were considered statistically significant. All statistical analyses were performed using EZR software version 1.53. 18
RESULTS
Patient characteristics
This study involved 115 patients with advanced NSCLC, except for one patient with stage I who was inoperable because of poor lung function and was treated with an ICI after the failure of stereotactic irradiation. Forty‐five patients (39.1%) experienced irAEs (Table 1), with four patients experiencing multiple irAEs. There were no significant differences between the groups in terms of age, sex, Eastern Cooperative Oncology Group performance status (ECOG‐PS), smoking status, histopathological diagnosis, driver gene alteration, tumor proportion score, tumor stage, type of ICI, previous chemotherapy, previous thoracic radiotherapy, taking systemic corticosteroids, white blood cell count, neutrophil count, and lymphocyte count. The median (range) pretreatment NLR was 2.8 (0.9–12.0) and 4.1 (0.8–10.7) in the irAEs and non‐irAEs groups, respectively, and the difference was significant (p = 0.036).
TABLE 1.
Patient characteristics of the non‐irAEs and irAEs groups
| Factor | Non‐irAEs (n = 70) n (%) | irAEs (n = 45) n (%) | p‐value | |
|---|---|---|---|---|
| Age (years) | Median (range) | 69 (44–85) | 68 (45–87) | 0.866 |
| Sex | Male | 52 (74.3) | 32 (71.1) | 0.531 |
| Female | 18 (25.7) | 14 (31.1) | ||
| ECOG‐PS | 0 | 34 (48.6) | 26 (57.8) | 0.429 |
| 1 | 28 (40.0) | 17 (37.8) | ||
| 2 | 8 (11.4) | 2 (4.4) | ||
| Smoking status | Never | 16 (22.9) | 16 (35.6) | 0.200 |
| Ever | 54 (77.1) | 29 (64.4) | ||
| Histological subtype | Adenocarcinoma | 49 (70.0) | 28 (62.2) | 0.526 |
| Squamous cell carcinoma | 18 (25.7) | 13 (28.9) | ||
| Others | 3 (4.3) | 4 (8.9) | ||
| EGFR mutation | Yes | 9 (12.9) | 7 (15.6) | 0.918 |
| No | 48 (68.6) | 30 (66.7) | ||
| Unknown | 13 (18.6) | 8 (17.8) | ||
| ALK rearrangement | Yes | 1 (1.4) | 1 (2.2) | 0.612 |
| No | 46 (65.7) | 26 (57.8) | ||
| Unknown | 23 (32.9) | 18 (40.0) | ||
| PD‐L1 (TPS) | < 1% | 9 (12.9) | 8 (17.8) | 0.523 |
| 1 ~ 49% | 21 (30.0) | 10 (22.2) | ||
| ≥ 50% | 17 (56.7) | 8 (17.8) | ||
| Unknown | 23 (76.7) | 19 (42.2) | ||
| Stage a | I | 0 (0.0) | 1 (1.9) | 0.351 |
| II | 0 (0.0) | 0 (0.0) | ||
| III | 4 (6.5) | 6 (11.3) | ||
| IV | 58 (93.5) | 46 (86.8) | ||
| ICIs | Nivolumab | 38 (54.3) | 27 (60.0) | 0.703 |
| Pembrolizumab | 16 (22.9) | 11 (24.4) | ||
| Atezolizumab | 16 (22.9) | 7 (15.6) | ||
| Number of previous chemotherapy | 0 | 10 (14.3) | 10 (22.2) | 0.426 |
| 1 or 2 | 47 (67.2) | 25 (55.6) | ||
| ≥ 3 | 13 (18.6) | 10 (22.2) | ||
| Previous thoracic radiotherapy | Yes | 12 (17.1) | 5 (11.1) | 0.431 |
| No | 58 (82.9) | 40 (88.9) | ||
| Corticosteroid use b | Yes | 9 (12.9) | 1 (2.2) | 0.086 |
| No | 61 (87.1) | 44 (97.8) | ||
| WBC (/μl) | Median (range) | 6600 (2900–20 600) | 6300 (2600–11 200) | 0.281 |
| Neut (/μl) | Median (range) | 4397 (1676–17 222) | 4315 (1027–8400) | 0.139 |
| Lym (/μl) | Median (range) | 1162 (520–4870) | 1280 (380–3254) | 0.451 |
| NLR | Median (range) | 4.1 (0.8–10.7) | 2.8 (0.9–12.0) | 0.036 |
Abbreviations: ECOG‐PS, Eastern Cooperative Oncology Group Performance Status; ICIs, immune checkpoint inhibitors; irAEs, immune‐related adverse events; Lym, lymphocyte; NLR, neutrophil‐to‐lymphocyte rate; PD‐L1, programmed death‐ligand 1; TPS, tumor proportion score; WBC, white blood cell; Neut, neutrophil.
Tumor Nodes Metastasis Classification.
Administration of corticosteroids at the initiation of ICIs.
Immune‐related adverse event
Table 2 shows the occurrence of irAEs and their severity. The most frequent irAEs was thyroid‐related events, followed by skin‐related events and interstitial lung diseases. Skin‐related events were rash and pruritus; none of the patients had vitiligo. The severity of thyroid‐related events was grade ≤ 2, and the treatment was interrupted in four patients, but treatment discontinuation was not required for any patient. The severity of interstitial lung disease was grade ≥ 3 in 5 patients, and treatment interruption and discontinuation were required for two and six patients, respectively. Other irAEs that required the discontinuation of treatment were colitis, encephalitis, hepatopathy, cardiac‐related events, thrombocytopenia, and hypoadrenocorticism. Colitis was considered to be immune‐related enteritis in all patients; all patients were treated with corticosteroids, except one patient, who was treated with infliximab. With respect to encephalitis, two patients presented decreased consciousness level of grade 3; with regard to cardiac‐related events, pericardial tamponade was observed in one patient and acute myocardial infarction in one patient. Two patients presented with immunogenic thrombocytopenia and one patient developed symptoms two months after ICI discontinuation.
TABLE 2.
Summary of irAEs
| irAEs | All grade n (%) | Grade, n (%) | ICI interruption n (%) | ICI discontinuation n (%) | ||
|---|---|---|---|---|---|---|
| 1 | 2 | ≥ 3 | ||||
| Thyroiditis / hypothyroidism | 16 (13.9) | 4 (25.0) | 12 (75.0) | ‐ | 4 (25.0) | ‐ |
| Skin‐related events | 14 (12.2) | 9 (64.3) | 5 (35.7) | ‐ | ‐ | ‐ |
| Interstitial lung disease | 8 (7.0) | 1 (12.5) | 2 (25.0) | 5 (62.5) | 2 (25.0) | 6 (75.0) |
| Colitis | 5 (4.3) | 1 (20.0) | 2 (40.0) | 2 (40.0) | ‐ | 3 (40.0) |
| Encephalitis | 2 (1.7) | ‐ | ‐ | 2 (100) | ‐ | 2 (100) |
| Cardiac‐related events | 2 (1.7) | ‐ | ‐ | 2 (100) | ‐ | 2 (100) |
| Thrombocytopenia | 2 (1.7) | ‐ | 1 (50.0) | 1(50.0) | ‐ | 1 (50.0) |
| Hypoadrenocorticism | 2 (1.7) | ‐ | ‐ | 2 (100) | 1 (50.0) | 1 (50.0) |
| Hepatopathy | 2 (1.7) | 1 (50.0) | ‐ | 1 (50.0) | ‐ | 1 (50.0) |
| Renal dysfunction | 1 (0.9) | 1 (100) | ‐ | ‐ | 1 (100) | ‐ |
Abbreviations: ICI, immune checkpoint inhibitors; irAEs, immune‐related adverse events.
Association between NLR and irAEs
The cutoff value of pretreatment NLR for the occurrence of irAEs was 2.86 (area under curve, 0.62; 95% confidence interval [CI]: 0.50–0.73; sensitivity, 0.56; specificity, 0.71; Figure 1). Among 115 patients treated with ICIs, 70 (60.9%) had an NLR of ≥2.86, and 45 (39.1%) had an NLR of <2.86, and the univariate analysis showed that the occurrence rate of irAEs was significantly higher in the NLR < 2.86 group than in the NLR ≥2.86 group (p = 0.004; Table 3). The multivariate analysis revealed that the NLR < 2.86 can be an independent predictive factor for the occurrence of irAEs (p = 0.016; odds ratio [OR]: 2.69; 95% Cl: 1.21–6.01; Table 3). There was no significant difference between the grade of irAEs and level of NLR (grade 1, 2 vs. > 3; p = 0.577, date not shown).
FIGURE 1.
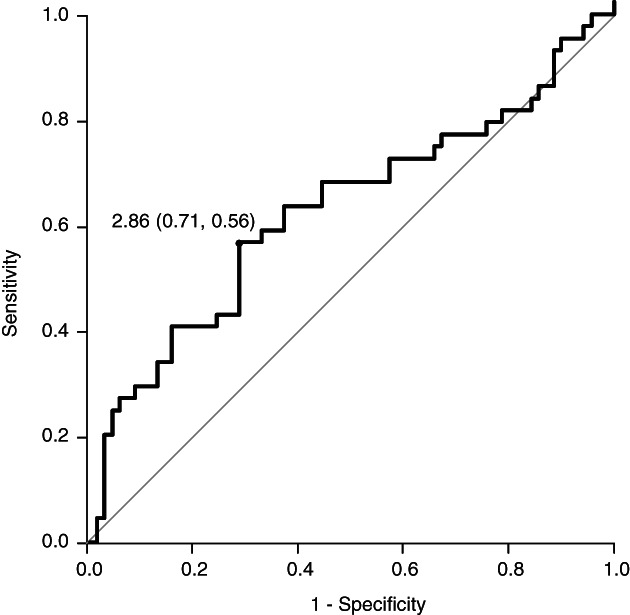
Receiver operating characteristic curve of pretreatment neutrophil‐to‐lymphocyte ratio (NLR) for the occurrence of immune‐related adverse events (irAEs). The cutoff value of pretreatment NLR for the occurrence of irAEs was 2.86 (area under curve, 0.62; 95% confidence interval [CI]: 0.50–0.73; sensitivity, 0.56; specificity, 0.71)
TABLE 3.
Univariate and multivariate logistic regression analyses of factors associated with irAEs
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| ORs | 95% CI | p‐value | ORs | 95% CI | p‐value | |
| Age | ||||||
| <65 vs. ≥65 | 0.81 | 0.37–1.8 | 0.611 | 0.796 | 0.34–1.85 | 0.596 |
| Sex | ||||||
| Male vs. female | 0.75 | 0.34–1.75 | 0.529 | 0.889 | 0.42–2.15 | 0.811 |
| ECOG‐PS | ||||||
| 0,1 vs. 2 | 2.80 | 0.56–13.70 | 0.211 | 2.01 | 0.36–11.10 | 0.422 |
| Corticosteroid use a | ||||||
| Yes vs. No | 0.154 | 0.15–1.23 | 0.081 | 0.227 | 0.03–1.96 | 0.178 |
| NLR | ||||||
| <2.86 vs. ≥2.86 | 3.12 | 1.43–6.84 | 0.004 | 2.69 | 1.21–6.01 | 0.016 |
Administration of corticosteroids at the initiation of ICIs.
Abbreviations: 95% Cl, 95% confidence interval; ECOG‐PS, Eastern Cooperative Oncology Group Performance Status; irAEs, immune‐related adverse events; NLR, neutrophil‐to‐lymphocyte rate; ORs, odds ratios.
Overall survival
The median OS was 20.6 (95% CI: 14.8–23.9) months in the NLR < 2.86 group and 8.3 (95% CI: 6.7–13.0) months in the NLR ≥2.86 group (p = 0.003; Figure 2). Among 70 patients with a pretreatment NLR of ≥2.86, 20 (28.6%) developed irAEs (NLR ≥2.86/irAEs), and 50 (71.4%) patients did not experience irAEs (NLR ≥2.86/non‐irAEs). Among 45 patients with a pretreatment NLR of <2.86, 25 (55.6%) developed irAEs (NLR < 2.86/irAEs), and 20 (44.4%) patients did not experience irAEs (NLR < 2.86/non‐irAEs). The median OS was 20.5 (95% CI: 7.4–29.0) months for NLR ≥2.86/irAEs, 6.9 (95% CI: 4.5–10.5) months for NLR ≥2.86/non‐irAEs, 23.0 months (95% CI: 17.8 months to not available) for NLR < 2.86/irAEs, and 15.5 (95% CI: 7.7–22.1) months for NLR < 2.86/non‐irAEs (p < 0.001; Figure 3).
FIGURE 2.
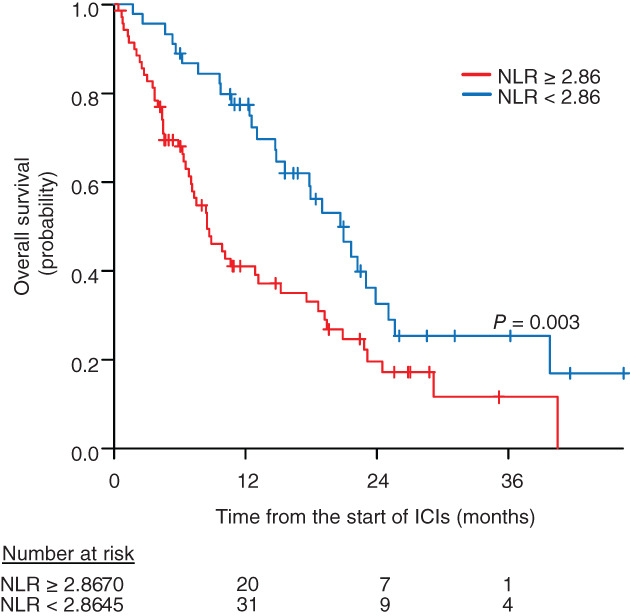
Overall survival (OS) according to pretreatment neutrophil‐to‐lymphocyte ratio (NLR). The median OS was 20.6 months (95% confidence interval [CI]: 14.8–23.9 months) in the NLR < 2.86 group and 8.3 months (95% CI: 6.3–12.7 months) in the NLR ≥2.86 group (p = 0.002)
FIGURE 3.
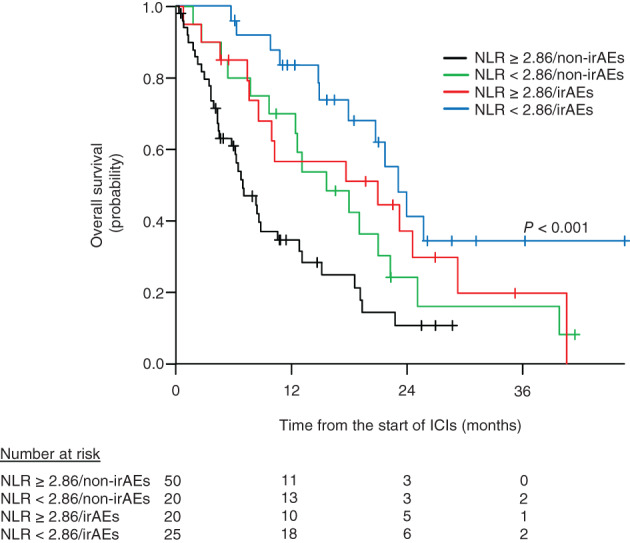
Overall survival (OS) according to pretreatment neutrophil‐to‐lymphocyte ratio (NLR) and immune‐related adverse events (irAEs). The median OS was 6.9 months (95% confidence interval [CI]: 4.5–10.5 months) for NLR ≥2.86/non‐irAEs, 15.5 months (95% CI: 7.6–22.1 months) for NLR < 2.86/non‐irAEs, 20.7 months (95% CI: 7.4–29.0 months) for NLR ≥2.86/irAEs, and 23.0 months (95% CI: 17.8 months to not available) for NLR < 2.86/irAEs. All, p < 0.001; NLR ≥2.86/non‐irAEs versus NLR < 2.86/irAEs, p < 0.001; NLR ≥2.86/non‐irAEs versus NLR < 2.86/non‐irAEs, p = 0.199; NLR ≥ 2.86/non‐irAEs versus NLR ≥ 2.86/irAEs, p = 0.082; NLR < 2.86/non‐irAEs versus NLR < 2.86/irAEs, p = 0.199; NLR < 2.86/non‐irAEs versus NLR ≥2.86/irAEs, p = 0.679; NLR ≥2.86/irAEs versus NLR < 2.86/irAEs, p = 0.199
Discussion
In this study, pretreatment NLR was significantly lower in the irAEs group than in the non‐irAEs group, with a cutoff value of 2.86. Additionally, the occurrence rate of irAEs was significantly higher in the NLR < 2.86 group than in the NLR ≥2.86 group. This result is in agreement with the results of Pavan et al. 8 and Eun et al. 9 who showed that a pretreatment NLR of <3 is a risk factor for the occurrence of irAEs. In these studies, the NLR cutoff value was set to 3. This value was not calculated as a risk factor for the development of irAEs, but was considered from previous studies reporting the association between NLR and prognosis of solid tumors and ICI treatment outcomes. 19 , 20 , 21 , 22 , 23 We focused on irAEs and calculated the cutoff value of NLR, and the value was close to 3. Pavan et al. 8 reported the findings of a study in Europe and Eun et al. 9 investigated multiple cancer types. It has been reported that the types of irAEs and their occurrence rates differ depending on race and cancer type. 24 , 25 Our findings demonstrated the association between low pretreatment NLR and high occurrence rates of irAEs in Japanese patients with NSCLC. As far as we know, this is the first study to have demonstrated the significance of NLR in predicting irAEs in Japanese patients with NSCLC. On the basis of these results, we speculate that the NLR may be an important factor for predicting irAEs, regardless of race and cancer type.
We showed that OS of patients with a pretreatment NLR of <2.86 was significantly longer than that of patients with a pretreatment NLR of ≥2.86. This indicates that patients with a pretreatment NRL of <2.86 are at a high risk of developing irAEs, but good clinical outcomes can be expected. In addition, the OS of patients with NLR < 2.86/irAEs was significantly prolonged compared with that of patients with NLR ≥2.86/non‐irAEs. Although no significant difference was observed, the survival curve suggested that the OS of patients in the NLR < 2.86/irAEs group may be longer than that of patients in the NLR < 2.86/non‐irAEs group. This difference may be because a lower NLR has been associated with a better outcome of ICI treatment, 16 , 17 and the development of irAEs has been found to be related to survival benefits in patients with NSCLC. 26 , 27 , 28
Lymphocytes, mainly T cells, are involved in the antitumor immune response and suppress the growth of tumor cells. 29 In contrast, neutrophils promote tumor development by acting on tumor cells and the tumor microenvironment, 30 and peripheral neutrophil counts have been reported to correlate with intertumoral neutrophil counts. 31 Furthermore, the increase in peripheral neutrophils adversely affects the cytotoxic activity of lymphocytes and may attenuate the antitumor response. 30 The main mechanism of the occurrence of irAEs is considered to be the damage to self‐tissues due to the activation of lymphocytes that react with self‐antigens following the administration of ICIs. 32 Low NLRs reflect low peripheral neutrophil counts and high lymphocyte counts, indicating the maintenance of antitumor response of the immune system as well as the increased risk of the presence of lymphocytes that inappropriately react to self‐antigens. Therefore, we consider that a low pretreatment NLR leads to the improvement of positive outcomes of ICI treatment and the increased risk of irAEs.
Biomarkers for irAEs have been extensively investigated. To date, such biomarkers including HLA genotypes, soluble serum proteins, and gut microbiota have been reported to predict irAEs. 33 , 34 , 35 However, evaluation of these biomarkers is associated with clinical challenges, such as accessibility, complexity, and high cost. Compared with these biomarkers, NLR is easy to measure and inexpensive. The NLR could be a clinically cost‐effective and useful predictive factor for irAEs.
There were some limitations to the study, including its retrospective nature, single‐institutional design, and limited sample size. Large‐scale, multi‐institutional, retrospective, and prospective studies on the relationship between the NLR and the occurrence of irAEs are warranted. Additionally, we cannot deny that the association between a low NLR and higher incidence of irAEs may be caused by the longer OS in a low NLR group; longer OS usually means exposure to more doses of ICIs. The relationship between the duration of exposure or the cumulative dose of ICIs and irAEs has not been clarified, and this should be investigated in the future.
In conclusion, a low NLR may be a predictive factor for the onset of irAEs. The NLR can be measured in an inexpensive and easy manner through routine clinical practice. By focusing on the potential risk of irAEs in patients with a low pretreatment NLR, irAE management can be appropriately managed from an early period. We consider that this could maximize the benefits of ICI treatment.
CONFLICT OF INTEREST
All authors declare no potential conflict of interest.
Fujimoto A, Toyokawa G, Koutake Y, et al. Association between pretreatment neutrophil‐to‐lymphocyte ratio and immune‐related adverse events due to immune checkpoint inhibitors in patients with non‐small cell lung cancer. Thorac Cancer. 2021;12:2198–2204. 10.1111/1759-7714.14063
REFERENCES
- 1. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre‐Finn C, et al. Metastatic non‐small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2018;29:iv192–237. [DOI] [PubMed] [Google Scholar]
- 2. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Postow MA. Managing immune checkpoint‐blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015;35:76–83. [DOI] [PubMed] [Google Scholar]
- 4. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta‐analysis. JAMA Oncol. 2018;4:1721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Fujimura T, Yamamoto Y, et al. Fluctuations in routine blood count might signal severe immune‐related adverse events in melanoma patients treated with nivolumab. J Dermatol Sci. 2017;88:225–31. [DOI] [PubMed] [Google Scholar]
- 6. Nakamura Y, Tanaka R, Maruyama H, Ishitsuka Y, Okiyama N, Watanabe R, et al. Correlation between blood cell count and outcome of melanoma patients treated with anti‐PD‐1 antibodies. Jpn J Clin Oncol. 2019;49:431–7. [DOI] [PubMed] [Google Scholar]
- 7. Diehl A, Yarchoan M, Hopkins A. Relationships between lymphocyte counts and treatment‐related toxicities and clinical responses in patients with solid tumors treated with PD‐1 checkpoint inhibitors. Oncotarget. 2017;8:11426–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pavan A, Calvetti L, Maso AD, Attili I, Del Bianco P, Pasello G, et al. Peripheral blood markers identify risk of immune‐related toxicity in advanced non‐small cell lung cancer treated with immune‐checkpoint inhibitors. Oncologist. 2019;24:1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eun Y, Kim IY, Sun JM, Lee J, Cha H‐S, Koh E‐M, et al. Risk factors for immune‐related adverse events associated with anti‐PD‐1 pembrolizumab. Sci Rep. 2019;19:14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Azab B, Chainani V, Shah N, McGinn JT. Neutrophil‐lymphocyte ratio as a predictor of major adverse cardiac events among diabetic population: a 4‐year follow‐up study. Angiology. 2013;6:456–65. [DOI] [PubMed] [Google Scholar]
- 11. Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil‐to‐lymphocyte ratio in sepsis: a meta‐analysis. Am J Emerg Med. 2020;38:641–7. [DOI] [PubMed] [Google Scholar]
- 12. Dirican N, Dirican A, Anar C, Atalay S, Ozturk O, Bircan A, et al. A new inflammatory prognostic index, based on C‐reactive protein, the neutrophil to lymphocyte ratio and serum albumin is useful for predicting prognosis in non‐small cell lung cancer cases. Asian Pac J Cancer Prev. 2016;17:5101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bowen RC, Little NAB, Harmer JR, Ma J, Mirabelli LG, Roller KD, et al. Neutrophil‐to‐lymphocyte ratio as prognostic indicator in gastrointestinal cancers: a systematic review and meta‐analysis. Oncotarget. 2017;19:32171–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mano Y, Shirabe K, Yamashita Y, Harimoto N, Tsujita E, Takeishi K, et al. Preoperative neutrophil‐to‐lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258:301–5. [DOI] [PubMed] [Google Scholar]
- 15. Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil‐to‐lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta‐analysis. Onco Targets Ther. 2018;11:955–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y, Zhang Z, Hu Y, Yan X, Song Q, Wang G, et al. Pre‐treatment neutrophil‐to‐lymphocyte ratio (NLR) may predict the outcomes of advanced non‐small‐cell lung cancer (NSCLC) patients treated with immune checkpoint inhibitors (ICIs). Front Oncol. 2020;10:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, et al. Pretreatment neutrophil‐to‐lymphocyte ratio as a marker of outcomes in nivolumab‐treated patients with advanced non‐small‐cell lung cancer. Lung Cancer. 2017;106:1–7. [DOI] [PubMed] [Google Scholar]
- 18. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakaya A, Kurata T, Yoshioka H, Takeyasu Y, Niki M, Kibata K, et al. Neutrophil‐to‐lymphocyte ratio as an early marker of outcomes in patients with advanced non‐small‐cell lung cancer treated with nivolumab. Int J Clin Oncol. 2018;23:634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ozyurek BA, Ozdemirel TS, Ozden SB, Erdogan Y, Kaplan B, Kaplan T. Prognostic value of the neutrophil to lymphocyte ratio (NLR) in lung cancer cases. Asian Pac J Cancer Prev. 2017;18:1417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non‐small cell lung cancer. JAMA Oncol. 2018;4:351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Russo A, Franchina T, Ricciardi GRR, Battaglia A, Scimone A, Berenato R, et al. Baseline neutrophilia, derived neutrophil‐to‐lymphocyte ratio (dNLR), platelet‐to‐lymphocyte ratio (PLR), and outcome in non small cell lung cancer (NSCLC) treated with Nivolumab or docetaxel. J Cell Physiol. 2018;233:6386–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferrucci PF, Ascierto PA, Pigozzo J, del Vecchio M, Maio M, Antonini Cappellini GC, et al. Baseline neutrophils and derived neutrophil‐to‐lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2016;27:732–8. [DOI] [PubMed] [Google Scholar]
- 24. Peravali M, Gomes‐Lima C, Tefera E, Baker M, Sherchan M, Farid S, et al. Racial disparities in immune‐related adverse events (irAE) of immune checkpoint inhibitors (ICPi), and association with survival based on clinical and biochemical responses. J Clin Oncol. 2020;38:7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khoja L, Day D, Chen TWW, Sui LL, Hansen AR. Tumour and class‐specific patterns of immune‐related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28:2377–85. [DOI] [PubMed] [Google Scholar]
- 26. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune‐related adverse events with nivolumab efficacy in non‐small‐cell lung cancer. JAMA Oncol. 2018;4:374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cortellini A, Chiari R, Ricciuti B, Metro G, Perrone F, Tiseo M, et al. Correlations between the immune‐related adverse events spectrum and efficacy of anti‐PD1 immunotherapy in NSCLC patients. Clin Lung Cancer. 2019;4:237–47. [DOI] [PubMed] [Google Scholar]
- 28. Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, et al. Correlation between immune‐related adverse events and efficacy in non‐small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71–4. [DOI] [PubMed] [Google Scholar]
- 29. Chen SD, Mellman I. Oncology meets immunology: the cancer‐immunity cycle. Immunity. 2013;39:1–10. [DOI] [PubMed] [Google Scholar]
- 30. Treffers LW, Hiemstra IH, Kuijpers TW, Berg TK, Matlung HL. Neutrophils in cancer. Immunol Rev. 2016;273:312–28. [DOI] [PubMed] [Google Scholar]
- 31. Moses K, Brandau S. Human neutrophils: their role in cancer and relation to myeloid‐derived suppressor cells. Semin Immunol. 2016;28:187–96. [DOI] [PubMed] [Google Scholar]
- 32. Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2017;33:2092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, et al. Collateral damage, insulin‐dependent diabetes induced with checkpoint inhibitors. Diabetes. 2018;67:1471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fujimura T, Sato Y, Tanita K, Kambayashi Y, Otsuka A, Fujisawa Y, et al. Serum levels of soluble CD163 and CXCL5 may be predictive markers for immune‐related adverse events in patients with advanced melanoma treated with nivolumab: a pilot study. Oncotarget. 2018;9:15542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint‐blockade‐induced colitis. Nat Commun. 2016;7:10391. [DOI] [PMC free article] [PubMed] [Google Scholar]


