Epstein-Barr virus (EBV) has etiological implications in the development of a wide range of lymphoproliferative lesions and malignant lymphomas of B-cell or T/NK-cell origin. Among peripheral T-cell lymphomas and diffuse large B-cell lymphomas, EBV-associated cases have a poorer prognosis compared to that of EBV-negative lymphomas. 1,2 Given that CD30, a member of the tumor necrosis factor receptor family, is frequently expressed in EBV-infected lymphoid cells, CD30 might represent an ideal target for the treatment of EBV-positive and CD30- positive lymphomas. Brentuximab vedotin is an antibody- drug conjugate comprising the chimeric IgG1 antibody cAC10 specific for human CD30 and the microtubule- disrupting agent monomethyl auristatin E via a protease-cleavable linker.
An open-label, multicenter, investigator-initiated phase II study was designed to evaluate the activity of brentuximab vedotin in patients with EBV-positive and CD30- positive non-Hodgkin lymphomas with various levels of CD30 in the relapsed or refractory setting (registered at Clinicaltrials.gov as NCT02388490). The optimal Simon two-stage design was used to test the null hypothesis of an objective response rate (ORR) of 20% against the alternative hypothesis of a 45% ORR. According to this design, ten patients were accrued in the first stage. Stage II was delayed until the completion of four or more cycles of study medication in the last subject in stage I. If more than three responders were observed, 15 additional patients would be accrued for a total of 25. This study design yields a type I error of 10% and a power of 90%. The study was approved by the institutional review board at each participating site and written informed consent was obtained in accordance with the Declaration of Helsinki.
A total of 25 patients with relapsed or refractory EBVpositive lymphomas also positive for CD30 (≥ 1% of tumor cells) were enrolled in this study between March 2016 and January 2018 at Seoul National University Hospital and Seoul National University Bundang Hospital. Positivity for EBV and CD30 expression was reviewed by experienced hematopathologists in each participating center and confirmed by a specialized hematopathologist (YKJ). All patients were administered 1.8 mg/kg brentuximab vedotin intravenously every 3 weeks for up to 16 cycles or until disease progression. Tumor measurements were assessed at screening, after completion of cycle 2, every 6 weeks until cycle 16, and at 4 to 8 weeks after the last dose of brentuximab vedotin. The patients’ baseline characteristics and diagnostic data are presented in Online Supplementary Table S1. The median age of the 20 men and 5 women was 67 years (range, 38-87) and most patients had EBV-positive mature T/NK-cell neoplasms (88%). Most patients were diagnosed at advanced stage (III or IV) and about a quarter of the patients were heavily pretreated; one patient had undergone a prior autologous stem cell transplant. Patients received a median of nine cycles of brentuximab vedotin (range, 1-16), and nine patients completed the planned 16 cycles of brentuximab vedotin administration. The most common reasons for discontinuation were progressive disease (n=14, 56%) and adverse events (n=2, 8%).
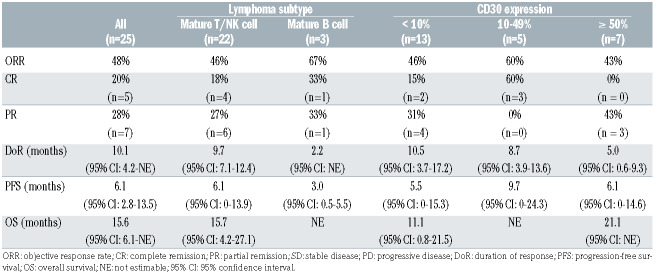
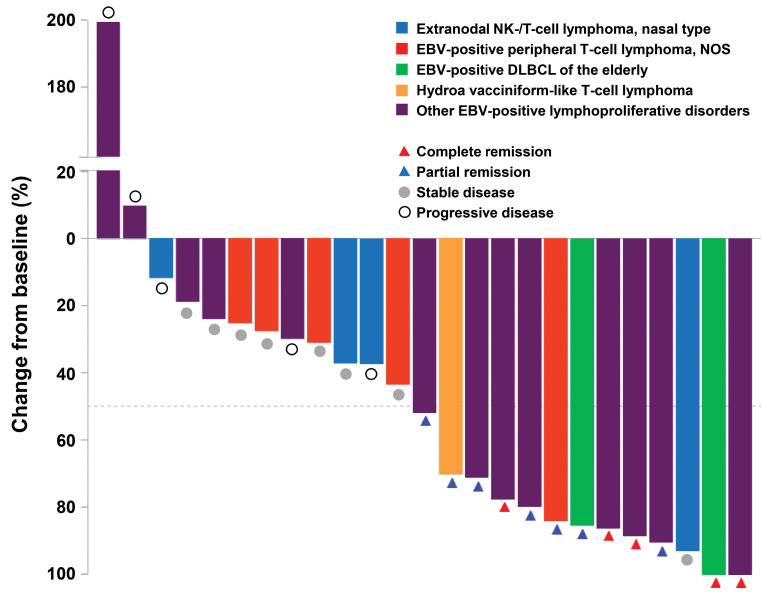
The primary endpoint was the ORR based on the revised Cheson criteria or modified Severity Weighted Assessment Tool (SWAT) criteria in the case of cutaneous lymphomas.3,4 The ORR in the intention-to-treat population of 25 patients was 48% (90% confidence interval [CI]: 31%-64%) (Table 1). The amount of tumor shrinkage of the target lesion from baseline for individual patients is shown in Figure 1. The correlation of CD30 expression level with the clinical response was assessed according to subgroup analysis. However, CD30 expression levels and ORR were not considerably different, although clinical responses were noted across all expression levels (Table 1).
Secondary objectives of the study were to evaluate the safety profile, progression-free survival, duration of response, and overall survival. The duration of response was assessed in 12 patients who had objective responses and the median duration was 10 months (95% CI: 4.2-21.0). The response continued even after the completion of the planned 16 cycles of brentuximab vedotin administration in three of these 12 patients (25%) at the time of data cut-off. The median duration of follow-up was 20 months (range, 1.7-30.4 months). For the intention-to-treat population, the median progression-free survival and overall survival were 6·2 months (95% CI: 2.9-13.6) and 15.7 months (95% CI: 6.1-not reached), respectively (Online Supplementary Figure S1A, B). As of January 2, 2019, 13 patients had died of disease progression. Pretreatment serum samples were collected from the whole blood of all patients. The level of soluble CD30 (sCD30) was determined using an enzyme-linked immunosorbent assay. The median value of sCD30 was 99.03 ng/mL (range 2.67-2155.78 ng/mL), and the ORR in the groups with high sCD30 (≥ 99.03 ng/mL) and low sCD30 (<99.03 ng/mL) were not significantly different (46% vs. 50%; P=0.85). In addition, the sCD30 level was not significantly associated with either progression-free survival or overall survival (Online Supplementary Figure S1C, D).
Table 1.
Clinical outcomes of brentuximab vedotin treatment according to lymphoma subtype and CD30 expression.
Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4.03. Treatmentrelated adverse events are summarized in Online Supplementary Table S2. All patients except for one had adverse events of any grade, among which 45% were considered treatment-related by the investigators. Nine patients (36%) had 15 serious adverse events during the study. The most common treatment-related adverse events were peripheral neuropathy (48%), neutropenia (44%), thrombocytopenia (20%), and rash (16%). The most common treatment-related adverse events of grade 3/4 were neutropenia (20%), thrombocytopenia (12%), and anemia (8%). Overall, the grade 3/4 adverse events were manageable with the standard guidelines and patients recovered completely.
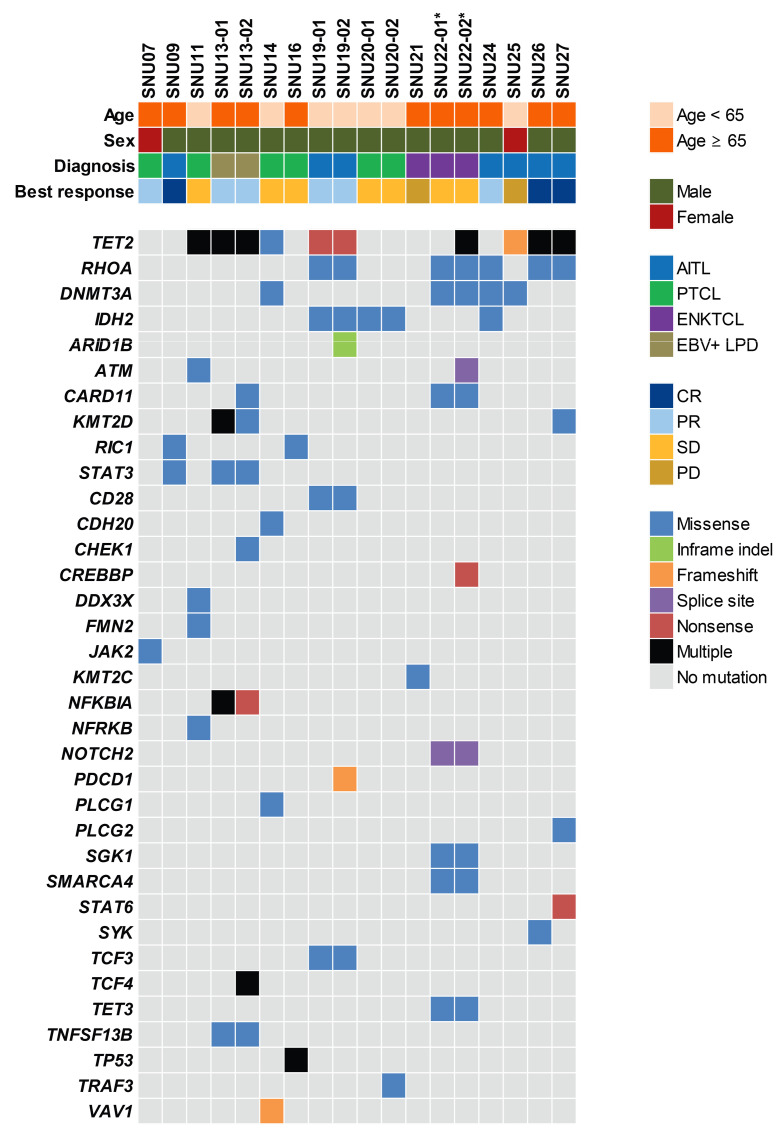
To explore the potential molecular biomarkers and resistance mechanisms, DNA obtained from formalinfixed paraffin-embedded tumor tissues was deeply sequenced using a customized multigene panel test comprising 120 genes. The mutational profiles of 18 tumors in 14 patients are summarized in Figure 2. The samples of tumor tissue were taken pretreatment from ten patients and both before and after treatment with brentuximab vedotin from four patients. A majority of patients (n=8, 57%) carried TET2 mutations and five patients (36%) carried RHOA mutations. In addition, DMNT3A and IHD2 mutations were detected in four and three patients, respectively. Among the four patients with genomic profiles from paired pre- and post-treatment samples, a novel TRAF3D483E mutation (variant allele frequency, 5.2%) was only identified in resistant tumors of patient SNU20. In addition, CARD11V1073M (variant allele frequency, 3.18%) mutations were acquired at disease progression in patient SNU13.
In this phase II study, the clinical activity of brentuximab vedotin in relapsed or refractory EBV-positive and CD30-positive non-Hodgkin lymphomas was significant and durable with an ORR of 48% and a duration of response of 10.1 months, and thus met the primary endpoint. In addition, brentuximab vedotin was well tolerated with manageable toxicities in these heavily pretreated patients with advanced diseases.
Several studies with brentuximab vedotin alone or in combination with chemotherapy have shown its remarkable success in Hodgkin lymphoma and systemic anaplastic large cell lymphoma with high CD30 expression in both the frontline as well as refractory settings.5-8 These previous studies provided the scientific rationale for considering that brentuximab vedotin might be active against other lymphomas expressing CD30 at varying rates (0-100%). So far, brentuximab vedotin has shown promising results in relapsed or refractory diffuse large B-cell lymphoma, T-cell lymphomas, and mycosis fungoides/ Sézary syndrome.9-12 The ORR of our study was similar to that of a previous study in relapsed and refractory T-cell lymphomas.10 However, considering the poor prognostic group of EBV-positive lymphomas in our study, the survival outcomes were encouraging and support the use of brentuximab vedotin for CD30-positive T- and B-cell lymphomas listed in National Comprehensive Cancer Network guidelines.
Figure 1.
Response to brentuximab vedotin. Waterfall plot of maximum percent change in tumor size of target lesions from baseline. NK: natural killer; NOS: not otherwise specified; EBV: Epstein-Barr virus; DLBCL: diffuse large B-cell lymphoma.
Although the nuclear factor kappa B (NF-kB) and mitogen- activated protein kinase pathways are known to be involved in CD30-mediated signaling,13 pleiotropic effects were observed in vitro after stimulation with CD30L or the monoclonal antibody Ki-1.14 This suggests that the CD30 signaling pathway might be differentially regulated depending on the cell type or tumor microenvironment. This would partly explain why other lymphomas, albeit with high CD30 expression, are not as responsive as Hodgkin lymphoma and systemic anaplastic large cell lymphoma, as evident from recent studies, thereby showing no clear relation between CD30 expression and clinical response.9-11 Conversely, even if CD30 expression was very low, tumor response was observed. Furthermore, tumor heterogeneity or unrevealed mechanisms of resistance may also contribute to the irrelevance of CD30 expression and efficacy of brentuximab vedotin. A recent study demonstrated that A20 negatively regulates the NF-kB pathway, and upregulation of NF-kB activity mediates resistance to brentuximab vedotin in Hodgkin lymphoma cell lines.15 In this study, targeted sequencing of paired tumor tissues revealed CARD11 and TRAF3 mutations related to constitutive NF-kB activation, and the mutations were identified as possible resistance mechanisms to brentuximab vedotin.
Figure 2.
Mutational profiles of 14 patients in this trial. Samples for 14 patients, including four pairs of sequentially acquired samples, were subjected to targeted sequencing encompassing 120 lymphoma-associated genes. *Patient SNU22 was initially diagnosed as having extranodal NK/T-cell lymphoma according to the patient’s referral record from another hospital. CR: complete remission; PR: partial remission; SD: stable disease; PD: progressive disease; AITL: angioimmunoblastic T-cell lymphoma; PTCL: peripheral T-cell lymphoma; ENKTCL: extranodal NK/T-cell lymphoma, nasal type; EBV+ LPD: Epstein-Barr virus-positive Tcell lymphoproliferative disorders.
In conclusion, brentuximab vedotin alone demonstrated significant and durable clinical activity with a manageable toxicity profile in relapsed or refractory EBV-positive and CD30-positive non-Hodgkin lymphomas. However, the results of this study, including correlative outcomes, should be interpreted cautiously because of the small number of patients, the single-arm design as well as the heterogeneous group of diseases. Thus, brentuximab vedotin warrants further investigational study, either as monotherapy or in combination, on a larger scale in this patient population.
Supplementary Material
Acknowledgments
We thank the patients who participated in this trial and their families. We also thank Soyeon Kim, Ph.D., Sujung Huh, and Daye Paek for their contribution to the soluble CD30 experiments and Seonah Ha, Ph.D., who assisted in medical writing. Statistical analyses were supported by the Medical Research Collaborating Center (MRCC), Seoul National University, Seoul, Korea.
Funding Statement
Funding: this study was funded by Takeda Pharmaceuticals Co, Ltd. and supported by a grant from the Korean Health Technology R&D Project "Strategic Center of Cell and Bio Therapy for Heart, Diabetes & Cancer” through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (MHW) (grant number: HI-17C-2085).
References
- 1.Dupuis J, Emile JF, Mounier N, et al. Prognostic significance of Epstein-Barr virus in nodal peripheral T-cell lymphoma, unspecified: a Groupe d'Etude des Lymphomes de l'Adulte (GELA) study. Blood. 2006;108(13):4163-4169. [DOI] [PubMed] [Google Scholar]
- 2.Park S, Lee J, Ko YH, et al. The impact of Epstein-Barr virus status on clinical outcome in diffuse large B-cell lymphoma. Blood. 2007; 110(3):972-978. [DOI] [PubMed] [Google Scholar]
- 3.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586. [DOI] [PubMed] [Google Scholar]
- 4.Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29(18):2598-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30(18):2183-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012; 30(18): 2190-2196. [DOI] [PubMed] [Google Scholar]
- 7.Connors JM, Jurczak W, Straus DJ, et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin's lymphoma. N Engl J Med. 2018;378(4):331-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horwitz S, O'Connor OA, Pro B, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON- 2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobsen ED, Sharman JP, Oki Y, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015;125(9):1394-1402. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz SM, Advani RH, Bartlett NL, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014;123(20):3095-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duvic M, Tetzlaff MT, Gangar P, Clos AL, Sui D, Talpur R. Results of a phase II trial of brentuximab vedotin for CD30+ cutaneous T-cell lymphoma and lymphomatoid papulosis. J Clin Oncol. 2015; 33(32):3759-3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YH, Tavallaee M, Sundram U, et al. Phase II investigator-initiated study of brentuximab vedotin in mycosis fungoides and Sézary syndrome with variable CD30 expression level: a multi-institution collaborative project. J Clin Oncol. 2015;33(32):3750-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishikori M, Ohno H, Haga H, Uchiyama T. Stimulation of CD30 in anaplastic large cell lymphoma leads to production of nuclear factorkappaB p52, which is associated with hyperphosphorylated Bcl-3. Cancer Sci. 2005;96(8):487-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruss HJ, Boiani N, Williams DE, Armitage RJ, Smith CA, Goodwin RG. Pleiotropic effects of the CD30 ligand on CD30-expressing cells and lymphoma cell lines. Blood. 1994;83(8):2045-2056. [PubMed] [Google Scholar]
- 15.Wei W, Lin Y, Song Z, et al. A20 and RBX1 regulate brentuximab vedotin sensitivity in Hodgkin lymphoma models. Clin Cancer Res. 2020;26(15):4093-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.