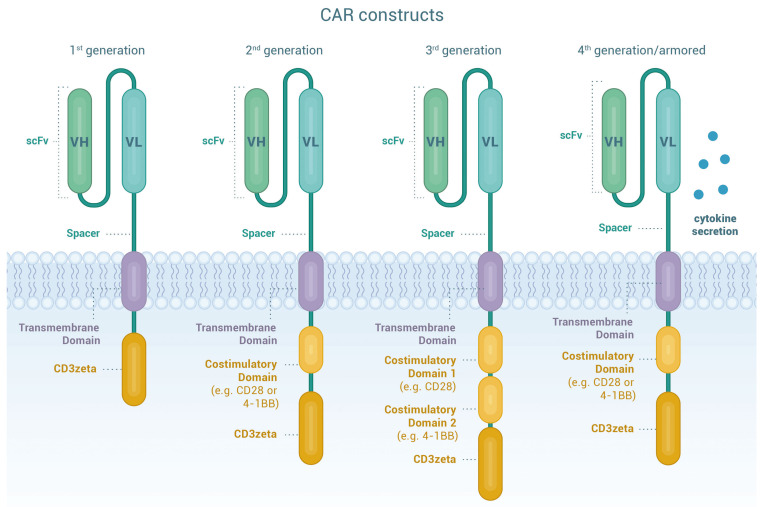
Figure 1.
Chimeric antigen receptor T cells. Chimeric antigen receptors (CAR) are designer proteins that redirect T cells towards a defined surface antigen on tumor cells. The CAR construct contains four essential components. The extracellular antigen recognition domain consists of a single chain variable fragment (scFv) commonly derived from the variable domains of the heavy and light chains (VH and VL) of monoclonal antibodies joined by a linker to provide flexibility and solubility and therefore improve antigen recognition and binding capacity. The hinge or spacer moiety based on Ig- (IgG1 or IgG4), CD8- or CD28-derived domains, provides flexibility, stability and the suitable length for optimal access to the target antigen. The transmembrane domain links the extracellular and intracellular domains of the CAR. It is based on CD3ζ, CD4, CD8α, CD28 or ICOS moieties, influences CAR stability and signaling and may also be involved in immune synapse arrangement. The last components of the CAR construct are the intracellular signaling domains. The activation domain is typically derived from the CD3ζ moiety of the T-cell receptor (first generation CAR), whereas co-stimulatory domains are derived from CD28, 4-1BB, OX40, CD27, or ICOS (second and third generation CAR). Co-stimulation results in intracellular signals that further optimize T-cell function, persistence and proliferation. Through additional genetic modifications, so called “armored” CAR T cells (CAR-T) (fourth generation CAR) secrete cytokines or express ligands to bolster CAR-T function or to overcome the immunosuppressive tumor microenvironment. Taken together, the molecular fine-tuning of pre-existing CAR components can greatly improve cellular migration, foster expansion and persistence of the CART and decrease toxicity.