Abstract
Objective:
Since hot flashes are a common symptom experienced by women with breast cancer, we sought to explore genetic predictors associated with response to acupuncture for the treatment of hot flashes.
Methods:
Using data from our completed randomized controlled trial (Clinicaltrials.gov identifier: NCT01005108) on hot flashes among breast cancer survivors who provided biomarker collection (N=108), we extracted and assayed DNA for single nucleotide polymorphisms (SNPs) in genes involved in neuro-transmission, thermo-regulation, and inflammation (ADORA1, COMT, TCL1A, and TRPV1). For our primary outcome we classified individuals with a 50% or more reduction in their hot flash composite score at the end of treatment as responders. We used Fisher’s exact test to identify individual and combined SNPs associated with treatment response.
Results:
Among women (N=57) who received acupuncture treatment (electro- or sham), we found that women who were carriers of at least one of these six genotypes (ADORA1 rs41264025-GA or rs16851029-GG or rs12744240-GT, COMT rs6269-GA, TCL1A rs2369049-GG, and TRPV1 rs8065080-TT) were more likely to respond to acupuncture for hot flashes than non-carriers (70.3% vs. 37.5%, p=0.035). These six genotypes were not associated with response in women (N=51) who received pharmacological hot flash treatment (gabapentin or placebo pill (37.5% vs. 37.5%, p=1.0).
Conclusions:
In this exploratory, proof of concept study, we identified six genotypes that may predict response to acupuncture for hot flashes in breast cancer survivors. If confirmed by future studies, these findings may inform the development of personalized acupuncture for managing hot flashes.
Keywords: breast neoplasm, hot flashes, genetics, acupuncture
Introduction
Hot flashes are one of the most common and distressing symptoms experienced by up to 73% of breast cancer survivors after cancer treatment.1–4 Hot flashes result when a thermo-regulatory problem occurs in the body, usually induced by a reduction in estrogen levels.5,6 Some cancer treatments, such as surgery, chemotherapy, and anti-estrogen therapies, disrupt estrogen synthesis and activity, which can result in severe hot flashes.1,7 Thus, having effective treatment options for hot flashes is important for improving the survivorship experience of breast cancer survivors.
Acupuncture, a non-pharmacological therapy, involves penetrating the skin with thin, solid, metallic needles that are manipulated by hand or electrical stimulation.8 It is used by cancer survivors at a higher rate than the general population9 and is considered safe with few side effects (e.g. needling pain, bruising).10 In a randomized controlled trial (RCT) with breast cancer survivors (N=190), Lesi et al. found that acupuncture along with self-care was better than self-care alone for hot flashes and quality of life.11 Findings from our completed RCT (N=120 breast cancer survivors) showed that acupuncture produced a reduction in hot flashes similar to gabapentin but with fewer side effects; in addition, the effects appeared to persist over time.12
Growing research has been dedicated to finding genetic biomarkers to improve cancer treatment-related symptoms.13 Given the potential of precision medicine for cancer treatment-related symptoms, the objective of this proof of concept study was to explore the association between selected candidate single nucleotide polymorphisms (SNPs) in genes involved in neuro-transmission [Catechol-O-methyltransferase (COMT)14,15 and Adenosine A1 Receptors (ADORA1)16–18] thermo-regulation [Transient Receptor Potential Cation Channel Subfamily V Member 1 (TRPV1)19,20], and inflammation [T-cell leukemia 1A (TCL1A)21,22] pathways and response to acupuncture for the treatment of hot flashes among breast cancer survivors. We hypothesized that these selected candidate SNPs would be associated with a positive response to acupuncture treatment for hot flashes.
Methods
Study population
We used data from our completed RCT on hot flashes among breast cancer survivors (Clinicaltrials.gov identifier: NCT01005108).12 The full details of the completed RCT and the primary findings have been previously published.12 In brief, women with a history of stage I-III breast cancer, who reported at least two hot flashes per day for the previous seven days and had hot flashes for at least one month prior to enrollment, were recruited from the Abramson Cancer Center of the Hospital of the University of Pennsylvania (Philadelphia, PA). One hundred and twenty women were randomized to four arms (electro-acupuncture, sham acupuncture, gabapentin, and placebo pill). One hundred and eight women (90% of total) provided a peripheral blood sample, and of these, 84 (78%) women were White. Samples were banked at −80°C for genetic and biomarker analysis. For the exploratory analyses described in this paper, women were grouped into two arms: 1) acupuncture (electro- and sham) (N=57) and 2) pill (gabapentin and placebo) (N=51) due to the small sample size in each of the four arms (N range: 25 to 29). The Institutional Review Board of the University of Pennsylvania approved the study protocol.
Primary Outcome
The primary outcome was a 50% or more reduction in the weekly average hot flash composite score as measured by the Daily Hot Flash Diary. Each participant recorded the number and severity of daily hot flashes starting from Baseline until Week 12 and again for one week at Week 24. The composite score for each day was calculated by multiplying the number of mild, moderate, severe, or very severe hot flashes by 1, 2, 3, or 4, respectively, and adding the values.23 Based on previous research, we developed a dichotomous outcome that considered those individuals with a 50% or more reduction in their hot flash composite score at the end of treatment to be responders.24,25
SNP Genotyping and Selection
Based on existing literature of polymorphisms in genes that have been found to play a role in the mechanism of acupuncture, we selected 18 candidate SNPs in genes involved in neuro-transmission,14–18 thermo-regulation,19,20 and inflammation21,22 pathways. Participant DNA was extracted from buffy coat specimens using the Qiagen QiaAmp 96 DNA Blood Kit (Valencia, CA). SNPs were genotyped using the SNPlex or the OpenArray platform from Applied Biosystems (Foster City, CA). Given the small sample size in each arm, we selected SNPs that had at least a greater than 15% difference in treatment responders (the primary outcome) between the allele groups rather than relying on a p-value.
Statistical Analysis
Descriptive statistics were conducted to obtain the N (%) of participants having the specific SNP in each treatment group as well as the N (%) of participants who were classified as treatment responders. We used Fisher’s exact test to identify the individual and combined SNPs associated with response to treatment for hot flashes. Data analyses were conducted using STATA 15.0 for Windows (STATA Corporation, College Station, TX).
Results
Genotyping failure rates were <1.8%. All SNP distributions satisfied Hardy-Weinberg proportions and were consistent with reported reference SNP frequencies (data not shown). If the frequency for one of the genotypes was <5% of the population, we collapsed the SNPs genotypes into two categories. As shown in Table 1, six SNPs out of the initial 18 candidate SNPs had a difference of at least >15% in treatment responders between the allele groups: ADORA1 rs41264025-GA vs. GG; ADORA1 rs16851029-GG vs. TT/GT; ADORA1 rs12744240-GT vs. GG; COMT rs6269-GA vs. AA/GG; TCL1A rs2369049-GG vs. AA/GG; and TRPV1 rs8065080-TT vs. CT/CC.
Table 1:
Association between individual single nucleotide polymorphisms and response to hot flash treatment
| Gene | Polymorphisma | Functional Consequence | Treatment Type: Acupuncture (N=57) | Treatment Type: Pill (N= 51) | ||||
|---|---|---|---|---|---|---|---|---|
| Participants N (%) | Responders N (%) | p-valueb | Participants N (%) | Responders N (%) | p-valueb | |||
| ADORA1c | rs41264025 | Exon 3′-UTRg variant | 0.14 | 0.28 | ||||
| GG | 52 (91) | 27 (56) | 42 (89) | 16 (40) | ||||
| GA | 5 (9) | 5 (100) | 5 (11) | 0 (0) | ||||
| rs16851029 | Exon 3′-UTRg variant | 1.00 | NA | |||||
| TT/GT | 55 (98) | 30 (59) | 48 (100) | 17 (37) | ||||
| GG | 1 (2) | 1 (100) | 0 (0) | 0 (0) | ||||
| rs12744240 | Exon 3”-UTRg variant | 0.13 | 0.71 | |||||
| GG | 48 (84) | 25 (56) | 40 (78) | 14 (36) | ||||
| GT | 9 (16) | 7 (88) | 11 (22) | 4 (44) | ||||
| COMTd | rs6269 | Intron variant | 0.17 | 0.15 | ||||
| AA/GG | 30 (53) | 15 (52) | 21 (42) | 10 (50) | ||||
| GA | 27 (47) | 17 (71) | 29 (58) | 8 (28) | ||||
| TCL1Ae | rs2369049 | Intron variant | 0.38 | 0.62 | ||||
| AA/AG | 51 (89) | 27 (57) | 43 (88) | 15 (36) | ||||
| GG | 6 (11) | 5 (83) | 6 (12) | 2 (50) | ||||
| TRPV1f | rs8065080 | Missense variant | 0.26 | 0.37 | ||||
| TT | 21 (38) | 14 (70) | 20 (42) | 9 (45) | ||||
| CT/CC | 35 (62) | 17 (53) | 28 (58) | 8 (31) | ||||
rs, reference sequence
Fisher’s Exact Test. Outcome is dichotomized hot flash composite score responder (>50% reduction in hot flashes) at end of treatment.
ADORA1, Adenosine A1 Receptors
COMT, Catechol-O-methyltransferase
TCL1A, T-cell leukemia 1A
TRPV1, Transient Receptor Potential Cation Channel Subfamily V Member 1
3’-UTR, three prime untranslated region
Since the proportion of women who were carriers of each SNP ranged from 1 to 52% (MAF ranges from 0.024 to 0.488), we classified women who were carriers of at least one of the six SNPs listed above as carriers of a potentially “responsive genotype”. Seventy percent of women in our population were carriers of this responsive genotype. Among women (N=57) who received acupuncture treatment, 70.2% were carriers, and among those who received pharmacological treatment (N=51), 84.3% were carriers.
Figure 1 illustrates the response to treatment (acupuncture or pill) by carrier status of the responsive genotype. Among women who received acupuncture treatment, we found that women who were carriers of at least one of these six SNPs (ADORA1 rs41264025-GA or rs16851029-GG or rs12744240-GT, COMT rs6269-GA, TCL1A rs2369049-GG, and TRPV1 rs8065080-TT) were more likely to respond to acupuncture for hot flashes than non-carriers (70.3% vs. 37.5%, p=0.035). To ensure these genotypes were not associated with response to any therapy or enrollment in a clinical trial, we repeated the analyses among women who received pharmacological hot flash treatment and did not find any evidence for predicting response (37.5% vs. 37.5%, p=1.0).
Figure 1.
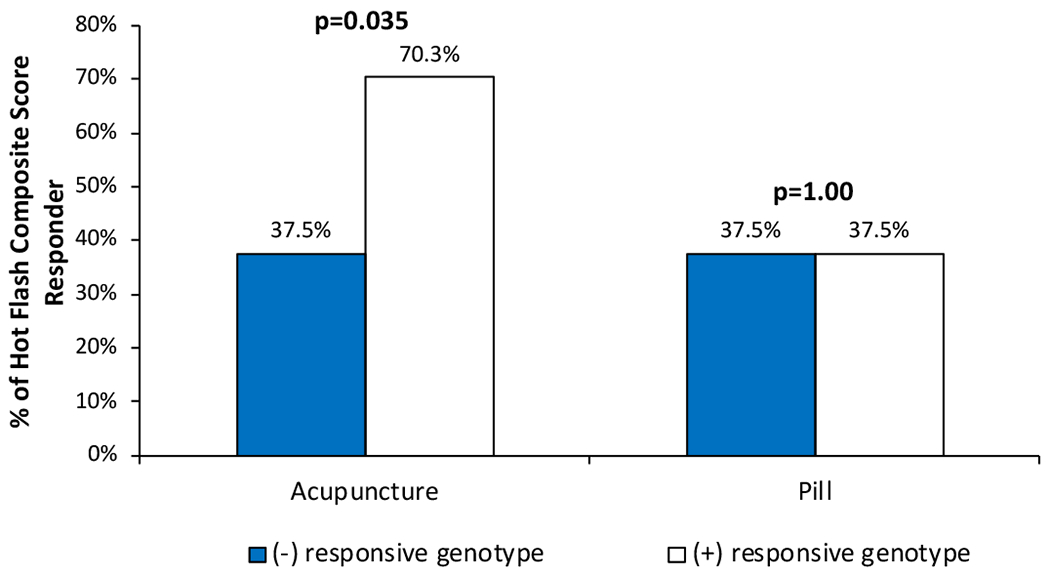
Response to hot flash treatment by presence or absence of the potentially “responsive genotype” (carrier of at least one of these six SNPs: ADORA1 rs41264025-GA or rs16851029-GG or rs12744240-GT, COMT rs6269-GA, TCL1A rs2369049-GG, and TRPV1 rs8065080-TT)
Discussion
Considering the prevalence, significance, and impact of hot flashes among breast cancer survivors,1–4 treatment options that apply a precision medicine framework to optimize hot flash management are needed. In this exploratory, proof of concept study, we identified six SNPs (ADORA1 rs41264025-GA or rs16851029-GG or rs12744240-GT, COMT rs6269-GA, TCL1A rs2369049-GG, and TRPV rs8065080-TT) present in 70% of our population that may predict response to acupuncture for hot flashes in breast cancer survivors.
While we were not able to identify literature on genetic predictors associated with acupuncture response to hot flashes, previous research has demonstrated that polymorphisms in genes involved in neuro-transmission,14–18 thermo-regulation,19,20 and inflammation21,22 have been found to play a role in the mechanism of acupuncture. In particular, COMT is involved in neuro-transmission by regulating dopamine catabolism and playing a key role in prefrontal cortex processes associated with the placebo effect such as reward, pain, memory, and learning.14,15 We recently found that a polymorphism in COMT was associated with response to acupuncture for the management of pain symptoms in women with breast cancer.26
Additionally, ADORA1 is a neuromodulator with inhibitory function, such as anti-nociceptive properties.18 From previous animal model research, acupuncture has been shown to activate ADORA1 pathways through increased adenosine concentrations at acupoints, which may mediate the local anti-nociceptive effects and improve neuropathic pain.16,17 Further, the effect of acupuncture has been found to be influenced by the activation of mast cells in acupoints via TRPV1 and ADORA1 pathways.27–29 Additionally, TRPV1 plays a role in thermo-regulation; mice exposed to a TRPV1 agonist exhibit vasomotor symptoms-like responses, such as a drop in core body temperature and cold-seeking behavior.20 These findings support that the underlying biological mechanisms associated with acupuncture’s effect though ADORA1 and TRPV1 pathways may explain the response to acupuncture for hot flashes.
Further, TCL1A signals influence proinflammatory cytokines and chemokines through its interactions with Akt kinase and its involvement with cell proliferation, stabilizing mitochondrial membrane potential, and promoting cell survival.22,30 Previous studies have identified four SNPs in high linkage disequilibrium and close to TCL1A, including rs2369049, that are associated with estradiol-induced TCL1A expression, musculoskeletal adverse events in women treated with AIs, and IL-17 production.21,22,31 Further, Bao et al. found that acupuncture appeared to reduce peripheral circulating IL-17 in breast cancer survivors.32 Our findings, in line with these previous studies, suggest biological plausibility that SNP rs2369049 may be involved in the mechanism of acupuncture response via inflammatory pathways.
Given the multiple comparison and exploratory nature of these post-hoc analyses, our findings are primarily useful for hypothesis generation and may be at risk for false positives. However, we did not see any association between the potentially responsive genotype and response to treatment by those in the pill group suggesting that the responsive genotype may be unique to the acupuncture process. Due to the small sample size, we had to combine the electro-acupuncture and sham acupuncture into one group. As acupuncture is a complex intervention involving both the process of delivery and needling specificity, future research may help uncover a genetic signature that predicts response to different types of acupuncture.
Conclusions
Despite this study’s limitations, our findings in this exploratory, proof of concept study suggest that six genotypes related to neuro-transmission, thermo-regulation, and inflammation pathways may predict response to acupuncture for the treatment of hot flashes. Future validation of these findings in an independent study with an adequate sample size is warranted and has the potential to personalize the integration of acupuncture based on host genetics to optimize hot flash management.
Acknowledgements:
We would like to thank all the breast cancer survivors, clinicians, and research staff who contributed to this project.
Sources of funding:
Research related to the development of this paper was supported in part by the National Cancer Institute grants to Dr. Jun J. Mao (K23 AT004112), the University of Pennsylvania Abramson Cancer Center (P30-CA016520) and the Memorial Sloan Kettering Cancer Center (P30-CA008748) as well as the Translational and Integrative Medicine Research Fund at Memorial Sloan Kettering Cancer Center. The funding sources were not involved in the study design; collection, analysis and interpretation of data; writing of the report; or decision to submit the article for publication.
Footnotes
Presentation: Accepted for Best of SIO oral presentation at the Society for Integrative Oncology 2018 15th International Conference; Scottsdale, AZ; October 29, 2018.
Financial disclosures/conflicts of interest: None reported.
References
- 1.Carpenter JS, Johnson D, Wagner L, Andrykowski M. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncol Nurs Forum. 2002;29(3):E16–25. [DOI] [PubMed] [Google Scholar]
- 2.Marino JL, Saunders CM, Emery LI, Green H, Doherty DA, Hickey M. Nature and severity of menopausal symptoms and their impact on quality of life and sexual function in cancer survivors compared with women without a cancer history. Menopause. 2014;21(3):267–274. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter JS, Andrykowski MA, Cordova M, et al. Hot flashes in postmenopausal women treated for breast carcinoma: Prevalence, severity, correlates, management, and relation to quality of life. Cancer. 1998;82(9):1682–1691. [PubMed] [Google Scholar]
- 4.Chandwani KD, Heckler CE, Mohile SG, et al. Hot flashes severity, complementary and alternative medicine use, and self-rated health in women with breast cancer. Explore. 2014;10(4):241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stearns V, Ullmer L, Lopez JF, Smith Y, Isaacs C, Hayes D. Hot flushes. Lancet. 2002;360(9348):1851–1861. [DOI] [PubMed] [Google Scholar]
- 6.Freedman RR. Physiology of hot flashes. Am J Hum Biol. 2001;13(4):453–464. [DOI] [PubMed] [Google Scholar]
- 7.Bernhard J, Luo W, Ribi K, et al. Patient-reported outcomes with adjuvant exemestane versus tamoxifen in premenopausal women with early breast cancer undergoing ovarian suppression (TEXT and SOFT): A combined analysis of two phase 3 randomised trials. Lancet Oncol. 2015;16(7):848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao JJ, Kapur R. Acupuncture in primary care. Prim Care. 2010;37(1):105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao JJ, Palmer CS, Healy KE, Desai K, Amsterdam J. Complementary and alternative medicine use among cancer survivors: A population-based study. J Cancer Surviv. 2011;5(1):8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White A A cumulative review of the range and incidence of significant adverse events associated with acupuncture. Acupunct Med. 2004;22(3):122–133. [DOI] [PubMed] [Google Scholar]
- 11.Lesi G, Razzini G, Musti MA, et al. Acupuncture as an integrative approach for the treatment of hot flashes in women with breast cancer: A prospective multicenter randomized controlled trial (AcCliMaT). J Clin Oncol. 2016;34(15):1795–1802. [DOI] [PubMed] [Google Scholar]
- 12.Mao JJ, Bowman MA, Xie SX, Bruner D, DeMichele A, Farrar JT. Electroacupuncture versus gabapentin for hot flashes among breast cancer survivors: A randomized placebo-controlled trial. J Clin Oncol. 2015;33(31):3615–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCall MK, Stanfill AG, Skrovanek E, Pforr JR, Wesmiller SW, Conley YP. Symptom science: Omics supports common biological underpinnings across symptoms. Biol Res Nurs. 2018;20(2):183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butts SF, Freeman EW, Sammel MD, Queen K, Lin H, Rebbeck TR. Joint effects of smoking and gene variants involved in sex steroid metabolism on hot flashes in late reproductive-age women. J Clin Endocrinol Metab. 2012;97(6):E1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young EE, Kelly DL, Shim I, Baumbauer KM, Starkweather A, Lyon DE. Variations in COMT and NTRK2 influence symptom burden in women undergoing breast cancer treatment. Biol Res Nurs. 2017;19(3):318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman N, Chen M, Fujita T, et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosc. 2010;13(7):883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren W, Tu W, Jiang S, Cheng R, Du Y. Electroacupuncture improves neuropathic pain: Adenosine, adenosine 5′-triphosphate disodium and their receptors perhaps change simultaneously. Neural Regen Res. 2012;7(33):2618–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawynok J Adenosine receptor targets for pain. Neurosc. 2016;338:1–18. [DOI] [PubMed] [Google Scholar]
- 19.Alawi K, Keeble J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol Ther. 2010;125(2):181–195. [DOI] [PubMed] [Google Scholar]
- 20.Krull AA, Larsen SA, Clifton DK, Neal-Perry G, Steiner RA. A comprehensive method to quantify adaptations by male and female mice with hot flashes induced by the neurokinin B receptor agonist senktide. Endocrinol. 2017;158(10):3259–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingle JN, Schaid DJ, Goss PE, et al. Genome-wide associations and functional genomic studies of musculoskeletal adverse events in women receiving aromatase inhibitors. J Clin Oncol. 2010;28(31):4674–4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu M, Wang L, Bongartz T, et al. Aromatase inhibitors, estrogens and musculoskeletal pain: Estrogen-dependent T-cell leukemia 1A (TCL1A) gene-mediated regulation of cytokine expression. Breast Cancer Res. 2012;14(2):R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001; 19(23):4280–4290. [DOI] [PubMed] [Google Scholar]
- 24.Freeman EW, Ensrud KE, Larson JC, et al. Placebo improvement in pharmacologic treatment of menopausal hot flashes: time course, duration, and predictors. Psychosom Med. 2015;77(2):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: A randomized controlled trial. JAMA. 2003;289(21):2827–2834. [DOI] [PubMed] [Google Scholar]
- 26.Genovese TJ, Mao JJ. Genetic Predictors of Response to Acupuncture for Aromatase Inhibitor-Associated Arthralgia Among Breast Cancer Survivors. Pain Med. 2019;20(1):191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao HY, Hsieh CL, Huang CP, Lin YW. Electroacupuncture attenuates CFA-induced inflammatory pain by suppressing Nav1.8 through S100B, TRPV1, opioid, and adenosine pathways in mice. Sci Rep. 2017;7:42531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang M, Wang X, Xing B, et al. Critical roles of TRPV2 channels, histamine H1 and adenosine A1 receptors in the initiation of acupoint signals for acupuncture analgesia. Sci Rep. 2018;8(1):6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen HC, Chen MY, Hsieh CL, Wu SY, Hsu HC, Lin YW. TRPV1 is a responding channel for acupuncture manipulation in mice peripheral and central nerve system. Cell Physiol Biochem. 2018;49(5):1813–1824. [DOI] [PubMed] [Google Scholar]
- 30.Laine J, Kunstle G, Obata T, Sha M, Noguchi M. The protooncogene TCL1 is an Akt kinase coactivator. Mol Cell. 2000;6(2):395–407. [DOI] [PubMed] [Google Scholar]
- 31.Ho MF, Bongartz T, Liu M, et al. Estrogen, SNP-dependent chemokine expression and selective estrogen receptor modulator regulation. Mol Endocrinol. 2016;30(3):382–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bao T, Cai L, Giles JT, et al. A dual-center randomized controlled double blind trial assessing the effect of acupuncture in reducing musculoskeletal symptoms in breast cancer patients taking aromatase inhibitors. Breast Cancer Res Treat. 2013;138(1):167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]


