Abstract
L-2-hydroxyglutaric aciduria (L2HGA) is a rare autosomal recessive neurometabolic disorder caused by the deficiency of L-2-hydroxyglutarate dehydrogenase (L2HGDH) enzyme. Dystonia, ataxia, pyramidal involvement and seizures are the common clinical manifestations. Coexisting behavioural problems and intellectual disability are also seen, however attention deficit hyperactivity disorder (ADHD) as the presenting clinical feature in L2HGA is rarely described. Here, we report a 5-year-old boy with behavioural problems and mild language delay. On clinical assessment, he fulfilled the diagnostic criteria for ADHD. His MR brain sequences showed classical finding of L2HGA—bilateral symmetrical T2-weighted hyperintensity involving subcortical white matter, basal ganglia and dentate nucleus. Urine analysis showed increased levels of 2-hydroxyglutaric acid and exome sequencing (targeted leukodystrophy panel) revealed homozygous likely pathogenic mutation in L2HGDH. He was started on high dose of riboflavin and levocarnitine and rehabilitative measures with which he had improvement in behavioural symptoms. This case illustrates the pivotol role of MR brain imaging in the diagnosis of inborn errors of metabolism.
Keywords: genetics, neurogenetics, neuroimaging, vitamins and supplements, Developmental paediatrics
Background
L-2-hydroxyglutaric aciduria (L2HGA) is a rare autosomal recessive disorder caused by the deficiency of the enzyme, L-2-hydroxyglutarate dehydrogenase (L2HGDH) and was first described by Duran et al in 1980.1 Since then, approximately only 110 cases of L2HGA were reported.2 MRI findings of the brain are very classical of this condition and metabolic parameters show increased levels of 2-hydroxyglutaric acid in urine, cerebrospinal fluid (CSF) and plasma.3 4 D-2-hydroxyglutaric aciduria (D2HGA), L2HGA and combined D-L2HGA can also show increased levels of 2-hydroxyglutaric acid in urine. Hence, diagnosis is confirmed with highly specific mass spectrometry analysis for detection of L-enantiomer or demonstration of homozygous or compound heterozygous mutations in the L2HGDH gene.3 5
The usual clinical presentation includes dystonia, ataxia, pyramidal involvement and seizures. They can also have concomitant behavioural problems and intellectual disability, however attention deficit hyperactivity disorder (ADHD) as an isolated clinical manifestation is rarely described.4 6–8 Evaluation for underlying cause of ADHD is usually pursued when additional neurological findings are elicited. Here, we describe a 5-year-old boy who presented to us with behavioural problems fulfilling the diagnostic criteria for combined type of ADHD and aetiological evaluation revealed L2HGA as the cause. Hence, metabolic and genetic evaluation of ADHD merits consideration depending on the clinical scenario. This child had a single episode of febrile seizures at 3 years of age which prompted us to investigate for underlying cause. Identifying this metabolic disorder is also important, as it is a treatable condition. He had good clinical improvement with high-dose riboflavin and levocarnitine at 1-year follow-up.
Case presentation
A 5-year-old boy presented to us with behavioural problems in the form of hyperactivity, anger outbursts, temper tantrums and inattention since 3 years of age. He was second of two siblings and was born of non-consanguineous parentage. He was delivered vaginally and had a perinatal history of umbilical cord prolapse, delayed cry and neonatal jaundice without any documented encephalopathy. His motor developmental milestones were normal. He was able to speak bisyllables at 1 year, had 50 word vocabulary at 3 years and joining of two words to form sentences by only 4 years of age suggestive of mild language delay. He had simple febrile seizures at 3 years age and since then he was on intermittent clobazam prophylaxis. Family history of febrile seizures for elder sister and intellectual disability for maternal aunt was present.
Examination showed head circumference of 52 cms and weight of 18.8 kg which were normal for age. Power, tone and deep tendon reflexes were normal. No pyramidal, extrapyramidal or cerebellar findings were seen. He was subsequently evaluated by a multidisciplinary team comprising of speech language pathologist, clinical psychologist and occupational therapist. He satisfied the Diagnostic and Statistical Manual for Mental Disorders (DSM-5) criteria for combined type of ADHD. In INCLEN diagnostic tool for ADHD also, the presence of ADHD was confirmed. His social age was 3.2 years and social quotient was 61 suggestive of mild deficits in social adaptive skills by Vineland Social Maturity Scale. In receptive-expressive emergent language scale test (second edition), his receptive and expressive language ability was of 30–33 and 24–27 months, respectively. Behavioural issues reported were violent behaviour towards others, anger outbursts, temper tantrums, disobedience, adamancy, destructive and disruptive behaviour, inattention and hyperactivity.
Investigatons
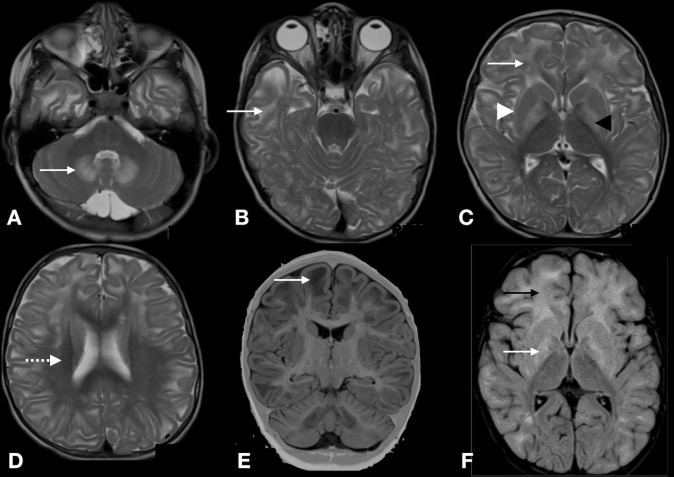
His MRI of the brain showed T2 weighted (T2W) hyperintensity of bilateral symmetrical white matter involving subcortical white matter with sparing of periventricular and deep white matter (centripetal pattern). Both caudate and putamen were hyperintense with rim being more hyperintense than the centre (caudate and putaminal rim sign). Globus pallidi was more hyperintense than striatum. Bilateral dentate nuclei were hyperintense without any cerebellar white matter involvement (figure 1). Fluid-attenuated inversion recovery (FLAIR) sequences showed hypointensity in the frontal and temporal subcortical white matter suggestive of rarefaction. In addition there was broadening of sulci.
Figure 1.
MRI of the brain. T2 weighted axial sequences show bilateral symmetric hyperintense signals involving dentate nuclei (A), subcortical U fibres of temporal lobe (B), frontal and occipital lobe (white arrow), putamen and caudate rim sign (white arrowhead) and more hyperintense globus pallidi (black arrowhead) (C), sparing of periventricular white matter (D), coronal T1 weighted sequence shows broadening of sulci and rarefaction (E) and fluid-attenuated inversion recovery axial sequence shows white matter hperintensity involving frontal white matter (black arrow) and basal ganglia (white arrow) (F).
Gas chromatography–mass spectrometry (GC–MS) analysis of urine showed elevated levels of 2-hydroxyglutaric acid (value 3991.9 mmol/mol of creatinine, analyte reference cut-off 433.03). Plasma amino acid and acylarnitine analysis showed normal levels of 2-hydroxyglutaric acid (value 9.4, normal range 4–35 µmol/mmol creatinine). His targeted leukodystrophy gene panel revealed homozygous likely pathogenic mutation in exon 3 of L2HGDH (c.293A>G) that resulted in the amino acid substitution of arginine for histidine at codon 98 (p.His98Arg). The in silico prediction tools to determine the pathogenicity of the variant was probably damaging by PolyPhen-2 and damaging by SIFT, LRT and MutationTaster2.
Diagnosis
The diagnosis of L2HGA was considered as the first possibility based on the MRI as this condition has distinct imaging findings. Some of the closely mimicking radiological differentials which we considered were thiamine responsive basal ganglia disease (SLC19A3), Leigh syndrome, propionic acidemia, glutaric aciduria type 1 and Wilson disease.8 9 The diagnosis in this case was confirmed by urine GC–MS and genetic tests.
Treatment
He was started on riboflavin 60 mg/day (3 mg/kg dose), levocarnitine 1000 mg/day (50 mg/kg) and low-protein diet. Concomitantly, interventions for speech and language delay and behavioural problems were also started. Parents were educated to improve the desirable behaviours by positive reinforcement and remove the unfavourable consequences of bad behaviours. Strategies for reducing the impulsive behaviours and structured attention enhancing activities were given. Speech therapy for improving the prelinguistic skills and age appropriate literacy training was also provided.
Outcome and follow-up
He was evaluated at 6 months and at 1 year after starting the pharmacological therapy and multidisciplinary intervention. Parents reported a steady improvement in his behavioural symptoms during these visits. His temper tantrums, anger outbursts and destructive behaviours subsided. His attention span improved to 6 min from 2 min with significant reduction in impulsivity and hyperactivity. Head banging behaviour completely subsided and waiting behaviour also improved. He is now obeying instructions. His behavioural profile score from Vineland Adaptive Behaviour Scale-second edition also showed clinically significant improvement (figure 2). His language ability also became better with overall receptive and expressive language ability at ages 3–3 1/2 years. The current adaptive functional levels in various subdomains (communication, daily living skills, motor and socialisation) are given in table 1.
Figure 2.
The behavioural profile pre treatment and post treatment. Problem behaviours parts 1 and 2 of Vineland Adaptive Behaviour Scale-second edition were administered to the parents before and after 1 year treatment with riboflavin, levocarnitine and multidisciplinary therapy. The scores showed improvement in Maladaptive Behavioural Index and internalising and externalising behaviours.
Table 1.
Vineland Adaptive Behaviour Scale-second edition score summary on follow-up at 6.3 years of age
| Subdomains | Raw score | Age equivalent (years) | Adaptive level |
| Communication | |||
| Receptive | 25 | 2 | Moderately low |
| Expressive | 52 | 2.4 | Low |
| Written | 1 | 2.1 | Low |
| Daily living skills | |||
| Personal | 19 | 1.8 | Low |
| Domestic | 3 | 1.6 | Low |
| Community | 6 | 2.2 | Low |
| Socialisation | |||
| Interpersonal relationships | 26 | 1.3 | Low |
| Play and leisure time | 20 | 1.1 | Low |
| Coping skills | 11 | 2.2 | Low |
| Motor skills | |||
| Gross | 58 | 2.2 | Low |
| Fine | 27 | 2.4 | Low |
| Sum score | 231 | Low |
Discussion
In this case report, we discuss a rare aetiology for ADHD in a 5-year-old boy. The diagnosis of L2HGA was considered as the first possibility based on the MRI as this condition has distinct imaging findings.2 Some of the closely mimicking radiological differentials which we considered were thiamine responsive basal ganglia disease (SLC19A3), Leigh syndrome, propionic acidemia, Canavan disease, Kearns-Sayre syndrome, HMG-CoA lyase deficiency and Wilson disease.9 The diagnosis in this case was confirmed by urine GC–MS and genetic tests. Recognition of this rare neurometabolic entity is important since it is treatable with high-dose riboflavin.10 In this child, disease activity stabilised and his behavioural symptoms improved with riboflavin (3 mg/kg/day) and levocarnitine (50 mg/kg/day) along with multidisciplinary intervention on follow-up.
First case of L2HGA was reported in 1980 by Duran et al, in a 5-year-old boy with developmental delay and growth insufficiency.1 It is an inherited metabolic disorder involving L-2-hydroxyglutaric acid pathway. The enzyme deficient is L2HGDH, a mitochondrial enzyme which acts on L-2 hydroxyglutarate for conversion to alpha-ketoglutarate (2-ketoglutarate).1 5 This enzyme is encoded in the L2HGDH gene located on chromosome 14 and consist of 10 exons. Flavin adenine dinucleotide (FAD) is the cofactor for this enzymatic reaction (figure 3). Homozygous/compound heterozygous mutation can result in the deficiency of enzyme L2HGDH and thereby hindering the metabolism of L-2-hydroxyglutaric acid. This will lead to accumulation of L-2-hydroxyglutaric acid in urine, plasma and CSF.10
Figure 3.
Metabolic pathway of L-2-hydroxyglutaric acid. FAD, flavin adenine dinucleotide; L2HGA, L-2-hydroxyglutaric acuduria; L-malate DH, L-malate dehydrogenase; NAD, nicotinamide adenine dinucleotide; TCA, tricarboxylic acid cycle.
Pathogenic mechanism of brain damage due to the abnormal elevation of L-2-hydroxyglutaric acid is poorly understood. However, impairment of energy metabolism due to elevated concentrations of lactate and Krebs cycle intermediates and inhibition of mitochondrial creatine kinase activity are proposed to explain the neurological manifestation in this condition. Impaired glutaminergic neurotransmission and oxidative stress are the other reasons hypothesised for the neurodegenerative pathology seen in L2HGA.10
L2HGA can be easily diagnosed based on the elevated levels of 2-hydroxyglutaric acid in urine.3 However, 2-hydroxyglutaric acid has L and D enantiomers and for distinguishing these two molecules, chiral differentiation with GC–MS or liquid chromatography–tandem mass spectrometry is required which is not widely available.10 The clinical presentation of D2HGA and L2HGA is different, hence when urine shows abnormally increased 2-hydroxyglutaric acid, the clinical picture should be taken into account while interpreting the urine GC–MS results. D2HGA manifests predominantly with epilepsy, hypotonia, psychomotor retardation, cortical blindness and cardiomyopathy. It is a severe form of encephalopathy and presents in neonatal period itself, whereas L2HGA mainly presents with developmental delay, ataxia, extrapyramidal involvement, spasticity, seizures and encephalopathy in early childhood. Neuropsychological manifestations includes intellectual disability, autism spectrum disorders, hyperactivity and other behavioural issues.1 3 10 Macrocephaly can be present in both types of hydroxyglutaric aciduria. Nocturnal myoclonus, nystagmus, task specific dystonia and optic atrophy are other manifestations of L2HGA.10
Intellectual disability and behavioural issues can variably accompany these patients, ADHD as a predominant clinical presentation without any accompanying neurological signs as seen in our case is rather rare. The only clue was the single episode of simple febrile seizures he had at 3 years of age. There are a very few reports of hyperactivity, autism spectrum disorder and depression associated with L2HGA that have been described, but a detailed description of the neurobehavioral aspects is unavailable.3 8 Kiykim et al studied 300 children with autism, and 9 were found to have inborn errors of metabolism, of whom 2 had L2HGA.11Here, through this case report we have highlighted the developmental, communication and behavioural problems comprehensively using standardised assessment tools.
In L2HGA, the MRI features are classic with bilateral symmetrical white matter hyperintensity mainly involving subcortical white matter, with sparing of periventricular white matter in T2W sequences. Basal ganglia and dentate nuclei are also T2 hyperintense. The outer rim of the putamen and caudate is more hyperintense and is referred to as putaminal and caudate rim sign.3 9 FLAIR sequences show hypointense subcortical white matter suggestive of rarefaction and high water content. The centrally located deep white matter, internal capsule, corpus callosum and periventricular white matter are spared, hence the pattern of leukoencephalopathy is termed centripetal distribution.3 The brainstem and cerebellar white matter are also characteristically uninvolved. Additionally, magnetic resonance spectroscopy in some studies showed small lipid-lactate peak with mild reduction of N-acetylaspartate.4 The presence of atypical regression, microcephaly, macrocephaly, seizures, abnormal neurologic examination, or to exclude intracranial manifestations associated with genetic syndromes are the indications for MRI in the evaluation of ASD and ADHD. MRI can point towards the underlying etiology especially inherited metabolic disorders in these conditions.11 In this case, MRI was suggestive of L2HGA as his neuroimaging findings were similar to other cases reported in the literature.3 12 Hence, MRI play a key role in the diagnosis of L2HGA and other neurometabolic disorders.
The treatment tried for L2HGA includes high-dose riboflavin 100–200 mg/day or flavin adenine dinucleotide (FAD) and levocarnitine 50–100 mg/kg/day. Protein restricted diet is also helpful. The treatment response mainly depends on the residual enzyme activity and riboflavin acts by enhancing the action of FAD.2 12 13 In this patient, we had started high-dose riboflavin (60 mg/day) and levocarnitine (1000 mg/day). Coenzyme Q10 (400 mg/day) even though was tried showed no proven benefit. On follow-up after 6 months and 1 year, while on medications, parents reported improvement in behavioural symptoms which was also reflected in the behavioural assessment. The improvement noticed could also have been contributed by the multidisciplinary interventions given by psychologist and speech and language pathologists.
An important aspect which should be considered during long term is the risk for central nervous system (CNS) tumours in these patients.14 Astrocytoma is the predominant CNS tumour reported in them between the ages of 9 and 12 years, and high-grade glioma is more common in children above 12 years.15 Hence, follow-up neuroimaging is important. The other complications includes dystonia, ataxia, developmental delay, intellectual disability, epilepsy and paresis of limbs.7 But our patient had only ADHD as manifestation without other complications.
Conclusion
ADHD as a unique presentation of L2HGA is very rare. This case highlights the importance of aetiological evaluation of children who manifests with behavioural problems as the predominant clinical presentation. MRI findings are highly specific and can facilitate an early diagnosis of this rare treatable entity.
Psychologist perspectives.
Speech and language therapist perspectives
Speech and language pathologists have a crucial role in assisting in the diagnosis of ADHD and assessment of language skills, listening, speaking, reading and writing in these children. Parents brought their 5-year-old son with a medical diagnosis of L2HGA. He had combined type of ADHD and language delay, an unusual presentation of this rare metabolic disorder. On assessment, child was able to comprehend and express all the basic concepts and use two-word utterances for communication. He had a receptive language age of 30–33 months and expressive language age of 24–27 months. The recommendations given were to develop prelinguistic skills and regular speech and language interventions at home and with therapist. On review after 1 year, there was significant improvement in the child’s receptive and expressive language at ages 3–31/2 years. His behaviour also improved and was co-operative for various interventions. Parents were advised to continue speech and language therapy and also to initiate age-appropriate literacy training.
Learning points.
L-2-hydroxyglutaric aciduria (L2HGA) is a rare autosomal recessive neurometabolic disorder manifesting in early childhood as spasticity, dystonia, developmental delay and seizures.
Attention deficit hyperactivity disorder as a presentation of L2HGA is rarely described; however, comorbid language and behavioural disorders are reported.
MRI features are very characteristic of this condition and diagnosis is established by urine gas chromatography–mass spectrometry or by genetic tests.
Although not curable, the recognition of this entity is important as treatment with riboflavin and levocarnitine has shown some beneficial effects.
Footnotes
Contributors: JG: study design, acquisition of data and drafting of manuscript. PS: acquisition of data and drafting the manuscript. KVS: critical revision and supervision of manuscript. SS: study concept, design, drafting of manuscript and supervision and critical revision of manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Parents/guardians consent obtained.
References
- 1.Duran M, Kamerling JP, Bakker HD, et al. L-2-Hydroxyglutaric aciduria: an inborn error of metabolism? J Inherit Metab Dis 1980;3:109–12. 10.1007/BF02312543 [DOI] [PubMed] [Google Scholar]
- 2.Seijo-Martínez M, Navarro C, Castro del Río M, et al. L-2-Hydroxyglutaric aciduria: clinical, neuroimaging, and neuropathological findings. Arch Neurol 2005;62:666–70. 10.1001/archneur.62.4.666 [DOI] [PubMed] [Google Scholar]
- 3.Steenweg ME, Jakobs C, Errami A, et al. An overview of L-2-hydroxyglutarate dehydrogenase gene (L2HGDH) variants: a genotype-phenotype study. Hum Mutat 2010;31:380–90. 10.1002/humu.21197 [DOI] [PubMed] [Google Scholar]
- 4.Muthusamy K, Sudhakar SV, Christudass CS, et al. Clinicoradiological spectrum of L-2-Hydroxy glutaric aciduria: typical and atypical findings in an Indian cohort. J Clin Imaging Sci 2019;9:3. 10.25259/JCIS-9-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Schaftingen E, Rzem R, Veiga-da-Cunha M. L: -2-Hydroxyglutaric aciduria, a disorder of metabolite repair. J Inherit Metab Dis 2009;32:135–42. 10.1007/s10545-008-1042-3 [DOI] [PubMed] [Google Scholar]
- 6.Barth PG, Hoffmann GF, Jaeken J, et al. L-2-Hydroxyglutaric acidemia: a novel inherited neurometabolic disease. Ann Neurol 1992;32:66–71. 10.1002/ana.410320111 [DOI] [PubMed] [Google Scholar]
- 7.Zübarioğlu T, Yalçınkaya C, Oruç Çiğdem, et al. Evaluation of clinical, neuroradiologic, and genotypic features of patients with L-2-hydroxyglutaric aciduria. Turk Pediatri Ars 2020;55:290–8. 10.14744/TurkPediatriArs.2019.06926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topçu M, Aydin OF, Yalçinkaya C, et al. L-2-Hydroxyglutaric aciduria: a report of 29 patients. Turk J Pediatr 2005;47:1–7. [PubMed] [Google Scholar]
- 9.Mohammad SS, Angiti RR, Biggin A, et al. Magnetic resonance imaging pattern recognition in childhood bilateral basal ganglia disorders. Brain Commun 2020;2:fcaa178. 10.1093/braincomms/fcaa178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kranendijk M, Struys EA, Salomons GS, et al. Progress in understanding 2-hydroxyglutaric acidurias. J Inherit Metab Dis 2012;35:571–87. 10.1007/s10545-012-9462-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiykim E, Zeybek CA, Zubarioglu T, et al. Inherited metabolic disorders in Turkish patients with autism spectrum disorders. Autism Res 2016;9:217–23. 10.1002/aur.1507 [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz K. Riboflavin treatment in a case with L-2-hydroxyglutaric aciduria. Eur J Paediatr Neurol 2009;13:57–60. 10.1016/j.ejpn.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 13.Samuraki M, Komai K, Hasegawa Y, et al. A successfully treated adult patient with L-2-hydroxyglutaric aciduria. Neurology 2008;70:1051–2. 10.1212/01.wnl.0000287141.90944.95 [DOI] [PubMed] [Google Scholar]
- 14.Aghili M, Zahedi F, Rafiee E. Hydroxyglutaric aciduria and malignant brain tumor: a case report and literature review. J Neurooncol 2009;91:233–6. 10.1007/s11060-008-9706-2 [DOI] [PubMed] [Google Scholar]
- 15.Patay Z, Mills JC, Löbel U, et al. Cerebral neoplasms in L-2 hydroxyglutaric aciduria: 3 new cases and meta-analysis of literature data. AJNR Am J Neuroradiol 2012;33:940–3. 10.3174/ajnr.A2869 [DOI] [PMC free article] [PubMed] [Google Scholar]